1. Introduction
Bone cement is a type of injectable orthopedic biomaterial with self-curing properties [
1,
2,
3]. During surgery, bone cement is used in a flowable and injectable state, and it polymerizes and solidifies within the body to provide sufficient support to the implant site [
4,
5,
6]. Clinically used or research-oriented bone cements can be broadly classified into two categories based on the material types: 1. Organic material bone cement, represented by polymethyl methacrylate cement (PMMA), 2. inorganic material bone cement, represented by calcium phosphate cement (CPC) and calcium sulfate cement (CSC) [
7]. PMMA bone cement is a biologically inert material that is unlikely to cause adverse reactions in the human body. It can be injected and rapidly shaped during surgery, and after solidification, it provides strong biomechanical strength, offering sufficient stability to the bone defect site [
8]. On the other hand, inorganic material bone cements like CPC and CSC have longer curing times, poorer injectability, and lower mechanical strength compared to PMMA bone cement [
9,
10,
11]. There have been reports of CSC causing recurrent fractures in the original fractured vertebrae when applied to unstable bone fractures. Inorganic material bone cements are primarily used for bone filling and repair in non-load-bearing areas, such as oral and maxillofacial surgery and neurosurgery [
12]. As a result, PMMA bone cement has gradually become the “gold standard” [
13].
To prevent and treat bacterial infections in orthopedic implant surgeries, researchers first proposed the concept of antibiotic loaded bone cement (ALBC) [
14,
15,
16]. This involves adding a certain amount of antibiotics to PMMA bone cement to prevent and treat bacterial infections. In 1977, Klemmet et al. used ALBC which containing gentamicin to treat osteomyelitis [
17]. Since the concept of ALBC was first introduced in the 1970s, research in this field has continuously progressed and achieved remarkable results [
18,
19,
20]. Currently, there are several commercially available ALBC loaded with gentamicin, and surgeons often add antibiotics such as gentamicin and vancomycin to the bone cement during surgery as needed [
21]. However, the use of ALBC has also presented some issues. One of the main concerns is the brust release of antibiotics, where the concentration of antibiotics in the local environment falls below the minimum inhibitory concentration after just one week of release [
22,
23]. This rapid decrease in antibiotic concentration can potentially lead to the development of bacterial resistance, making the treatment less effective over time [
24].
In addition to physically mixing antibiotics into bone cement, antibacterial motifs can also be covalently bonded or physical adsorption to the bone cement matrix and permanently modify the surface properties of the cement, which is known as non-leaching antibacterial bone cement (NLBC). Unlike ALBC, fixed antibacterial motifs are usually connected to the polymer skeleton, which exhibit strong chemical stability and can produce long-term antibacterial activities. Non-leaching antibacterial, also known as contact-kill, involves the use of active substances on the surface of materials to disrupt bacterial cell membranes, thereby inhibiting or killing adhered bacteria [
25,
26,
27]. The antimicrobial motifs used in this context range from natural biological molecules such as antimicrobial peptides (AMPs) to synthetic chemical substances such as quaternary ammonium compounds (QACs) and heterocyclic compounds [
28,
29].
The aim of this review is to provide a comprehensive overview of the different approaches adopted to impart antibacterial motif covalently bonded or physical adsorption to PMMA-based acrylic bone cement, starting from innovative non-leaching antibacterial cement formulations to different antibacterial activity detection methods, highlighting the ongoing issues in this area.
2. Bone Cement with Quaternary Ammonium Compounds (QACs) as Co-Monomers
QACs are commonly used antimicrobial motifts [
30]. Recently, there have been significant advances in research on acrylic bone cement containing QACs.
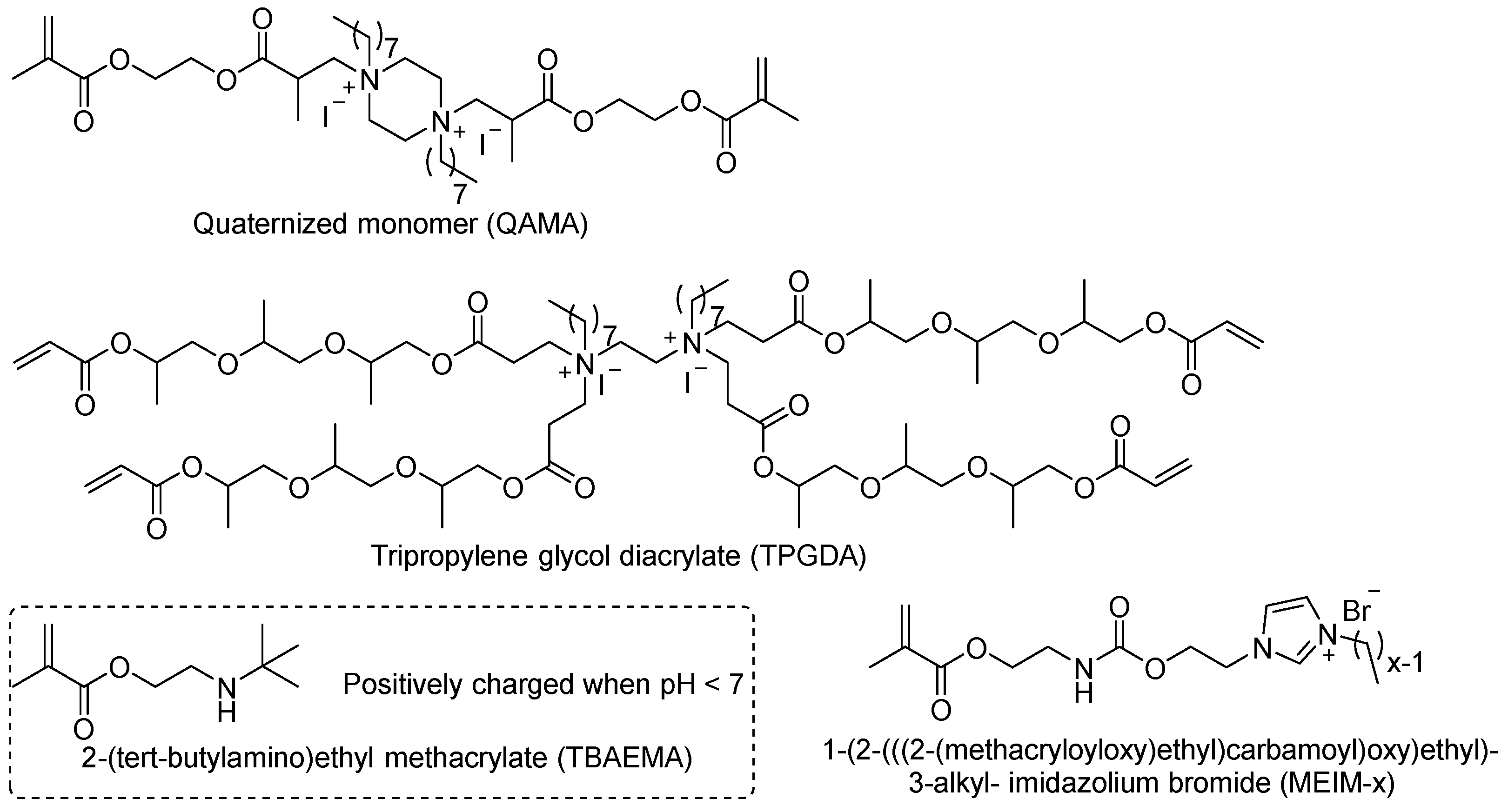
Deb et al. developed a novel acrylic monomer called quaternized EGDMA-piperazine octyl ammonium iodide (QAMA) that contains two acrylic structures for polymerization and two quaternary ammonium salt motifs for antimicrobial activity [
31]. QAMA monomer was added as a liquid component to form the cement. The cement containing 15% QAMA did not exhibit a zone of inhibition, but bacterial growth was inhibited on the cement surface, indicating non-leaching antimicrobial activity. The mechanism of action of this antimicrobial monomer involves contact killing of bacteria without releasing bioactive agents. Singh et al. synthesized a dendrimer quaternary ammonium acrylate comonomer with four arms, tri-propylene glycol diacrylate (TPGDA), which provides four acrylate polymerizable motifs [
32]. They ground TPGDA-containing bone cement into powder and added it to bacterial culture medium, resulting in bacterial killing. Although this grinding pretreatment significantly increased the surface area of the antimicrobial surface, which may differ from the cement’s morphology during use, it demonstrated the potential efficacy of dendrimer QACs as antimicrobial agents in bone cement.
Miyazaki et al. developed 2-(tert-butylamino)ethyl methacrylate (TBAEMA), which containing an amino group in the side chain [
33]. In their study, the authors considered TBAEMA as a quaternary ammonium compound, but we believe TBAEMA is a secondary amine antimicrobial compound (as shown in the
Figure 1). The results showed that antimicrobial activity increased with the higher content of TBAEMA. The authors claimed that the hydrolyzed amino motifs could produce antimicrobial activity. These compounds at low doses may indeed exhibit antimicrobial activity, but the material’s surface antimicrobial activity contributes more significantly to bacterial reduction. This amino motif of TBAEMA is positively charged in the acidic microenvironment of bacterial infection, and it can function similarly to quaternary ammonium salts. Additionally, since the author claims that TBAEMA is a quaternary ammonium salt monomer, we classify this compound as belonging to the quaternary ammonium salt category.
He et al. synthesized a series of acrylate co-monomers containing imidazolium quaternary ammonium salts, 1-(2-(((2-(methacryloyloxy)ethyl)carbamoyl)oxy)ethyl)-3-alkyl-imidazolium bromide (MEIM-x), with varying alkyl chain lengths [
34]. The research found that these monomers exhibited antibacterial activity against
Staphylococcus aureus and
Escherichia coli, and the addition of 5% MEIM-x monomer to bone cement showed good antimicrobial activity. Compared to several previously reported quaternary ammonium co-monomers, this bone cement demonstrated better mechanical properties.
It is important to note that the antimicrobial activity assessment methods in these studies differ, making it challenging to compare the antimicrobial properties of the newly discovered compounds. It is necessary to establish a universal antibacterial test standard. Due to the promising antimicrobial characteristics of these quaternary ammonium monomers, further comprehensive comparisons in subsequent research are necessary.
3. Bone Cement with Side-Chain Cyclic Organic Molecules as Co-Monomers
Wang synthesized a series of copolymers, p(MMA-co-BA), through free radical polymerization with varying ratios of methyl methacrylate (MMA) and borneol acrylate (BA) monomers (
Figure 2) [
35]. The research indicated that p(MMA-co-10% BA) exhibited good antimicrobial activity, while p(MMA-co-25% BA) with 25% BA showed 99.7% antimicrobial activity against
Escherichia coli. This result demonstrated that utilizing polymer surface stereochemistry is an advanced strategy for antimicrobial adhesion.
Methacrylate derived from benzothiazole (BTTMA) has also been shown to possess potential antimicrobial activity [
36]. He et al. incorporated BTTMA as a co-monomer to prepare benzothiazole covalently cross-linked bone cement, and it was proven that this composite cement exhibited antimicrobial activity [
37]. More recently, Fu and Chu developed a novel acrylate monomer containing a heterocyclic compound, nitrofurfuryl methacrylate (NFMA), and prepared copolymer-modified bone cement, p(NFMA-co-MMA) [
38]. This p(NFMA-co-MMA) cement demonstrated not only antimicrobial activity but also good biocompatibility and mechanical properties.
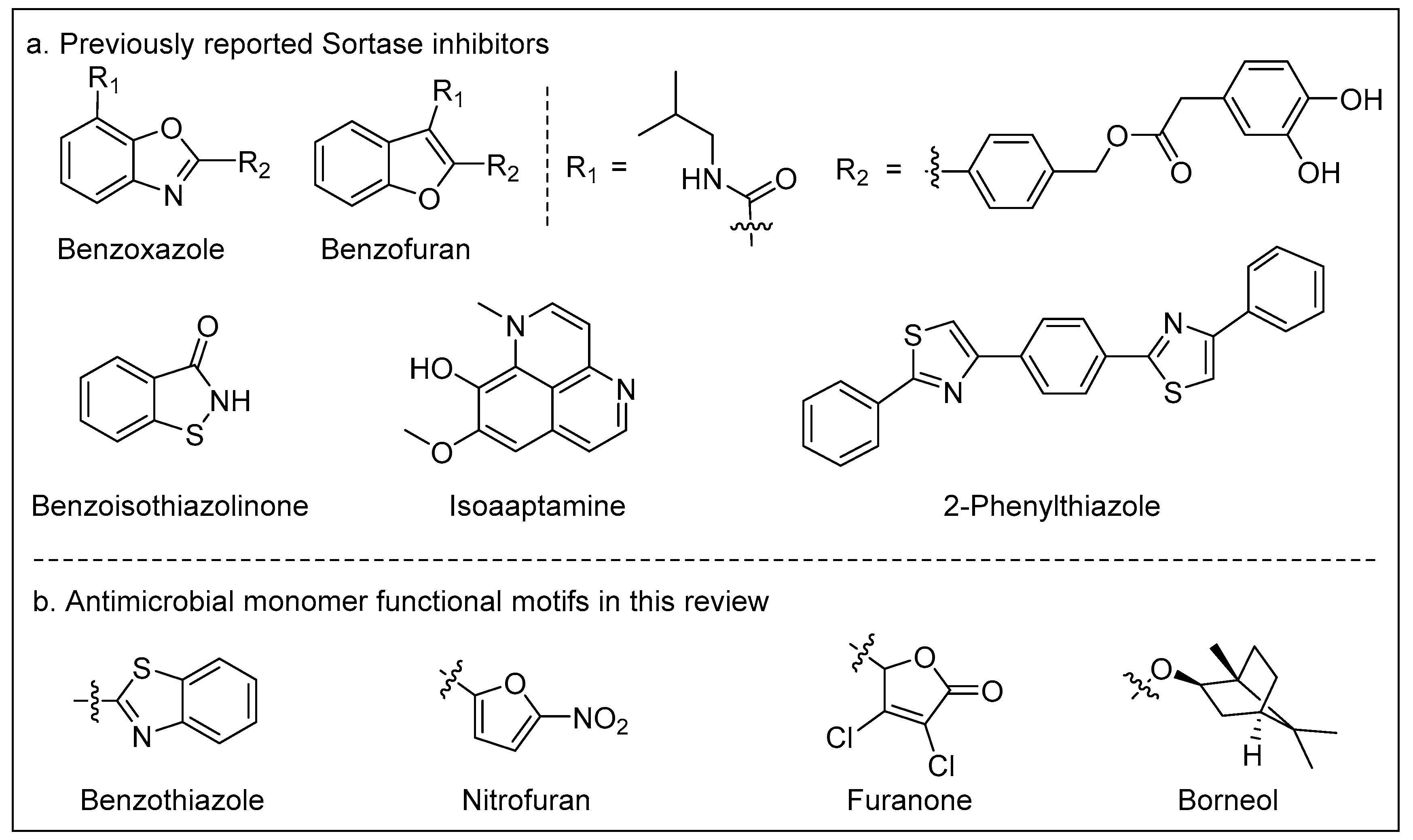
The enzymes on the surface of bacteria are related to bacterial growth, such as Sortase A on the surface of Gram-positive bacteria [
39]. Recently, inhibitors of these enzymes have become prominent molecules in the research of novel antibacterial drugs [
40,
41]. As shown in the Fig 3a, there are some reported Sortase inhibitors that contain cyclic molecular structures, such as benzofuran and benzothiazole. Interestingly, some of the monomers with surface antibacterial activity that have been reported also contain cyclic molecular structures. We speculate that these cyclic motifs in monomers might interact with enzymes on the bacterial surface (
Figure 3b). However, this cannot explain why they exhibit antibacterial activity against Gram-negative bacteria.
Subsequent research may be needed to investigate enzyme activity to further understand whether these cyclic compounds have enzyme inhibition. By evaluating the enzyme inhibitory properties, researchers can gain a better understanding of how these compounds interfere with bacterial growth and infection. Overall, exploring the enzyme inhibitory properties of these cyclic organic compounds will be a valuable avenue for further research to better harness their antimicrobial potential and develop innovative strategies for combating bacterial infections.
4. Other Promising Copolymer Monomers
In addition to the aforementioned antimicrobial copolymer-based bone cements, several novel antimicrobial copolymer monomers have been developed for potential application in bone cement. Xie et al. have made continuous and outstanding efforts in this area, developing a series of acrylate monomers containing furanone for dental resin materials (
Figure 4) [
42,
43]. In their research, the copolymerization of monomers with one or two arms not only imparted the resin with antimicrobial activity but also improved its compressive strength. These unique properties are crucial for bone cement as it is often implanted in load-bearing regions such as the spine.
Sydlik et al. also conducted research on covalently cross-linked drug-loaded bone cement [
44]. They developed three acrylate monomers containing drug molecules (
Figure 4). Although antimicrobial properties were not studied, the addition of these novel monomers also enhanced the mechanical properties of the bone cement. They found that drug monomers cross-linked by amide bonds permanently cured within the bone cement. These findings serve as inspiration for the development of new antimicrobial monomers with excellent mechanical properties and stability.
5. NLBC Based on Physical Adsorption
The antibacterial NLBC based on physical adsorption is mainly to load some antibacterial motifs on the carrier in advance, such as nanoparticles, polymers, etc., and then add them to the PMMA solid phase agent as an additive, so the antibacterial groups in this NLBC are compatible with bone cement. There is no covalent connection, which belongs to physical doping.
Tang et al. investigated the antibacterial properties of quaternized chitosan (hydroxypropyltrimethyl ammonium chloride chitosan, HACC)-doped PMMA bone cement [
45]. They found that PMMA loaded with 26% HACC could prevent staphylococci (including antibiotic-resistant strains) from forming biofilms on the surface of bone cement and down-regulate the expression of virulence-related genes of antibiotic-resistant staphylococci, suggesting that HACC doped PMMA is an effective potential antibacterial materials. In further research, Tang found that this new type of bone cement, especially the cement containing 20% HACC, has good osteogenic activity and mechanical properties [
46]. Although the incorporation of HACC will slightly reduce the compressive strength, it is still suitable for spongy bone.
Xie et al. synthesized structures containing antibacterial furanone derivatives, and coated Al
2O
3 particles through covalent linkage, and prepared antibacterial alumina particles with different loadings of antibacterial furanone derivatives [
47]. They mixed antibacterial Al
2O
3 particles into traditional PMMA bone cement for research. Experiments showed that the flexural strength of the new bone cement was 10% higher than that of PMMA cement, and the flexural modulus was increased by 18%. Antibacterial activity against
Staphylococcus aureus increased by 67%, and against
Escherichia coli by 84%. The leaching experiment of antibacterial furanone derivatives showed that there was no leaching antibacterial component in this modified bone cement, indicating that the antibacterial property depended on the furanone derivatives in its own structure.
6. Discusses the Testing Methods for Surface Antimicrobial Activity
Currently, there is a lack of standardized testing methods for surface antimicrobial activity, leading to difficulties in comparing the results from different studies. Therefore, it is important to have a discussion on this topic and provide guidance for researchers working on non-leaching antimicrobial materials. Additionally, it is suggested that the potential antimicrobial cements discussed in this article should be compared using the same testing method.
In Deb’s study, they utilized scanning electron microscopy (SEM) to directly observe bacteria on the material surface. Specifically, samples (1 cm × 1 cm) were immersed in a specific concentration of bacterial solution and incubated [
31]. The samples were then washed with PBS to remove non-adherent bacteria, fixed with glutaraldehyde on the material surface, immersed in distilled water, silver-sputtered after freeze-drying, and finally observed using SEM. This method provides direct evidence of the material’s ability to inhibit bacteria by directly observing the material surface. It is considered a powerful testing method for material research under suitable conditions. However, the drawback is that the sample processing is complex, the testing period is long, and it may not be suitable for screening new antimicrobial materials. Additionally, this method requires the use of SEM, which has a higher level of technical expertise. In Xie’s study, they conducted antimicrobial studies on disc-shaped specimens of antimicrobial bone cement by co-culturing them with bacteria. The change in bacterial count in the bacterial solution was observed under a microscope to compare their antimicrobial properties.
Figure 5.
Deb et al. utilized SEM to observe bacteria on the surface of bone cement (
a) Bacterial growth on PMMA cement. (
b) No bacterial growth on 15% QAMA containing cement [
32].
Figure 5.
Deb et al. utilized SEM to observe bacteria on the surface of bone cement (
a) Bacterial growth on PMMA cement. (
b) No bacterial growth on 15% QAMA containing cement [
32].
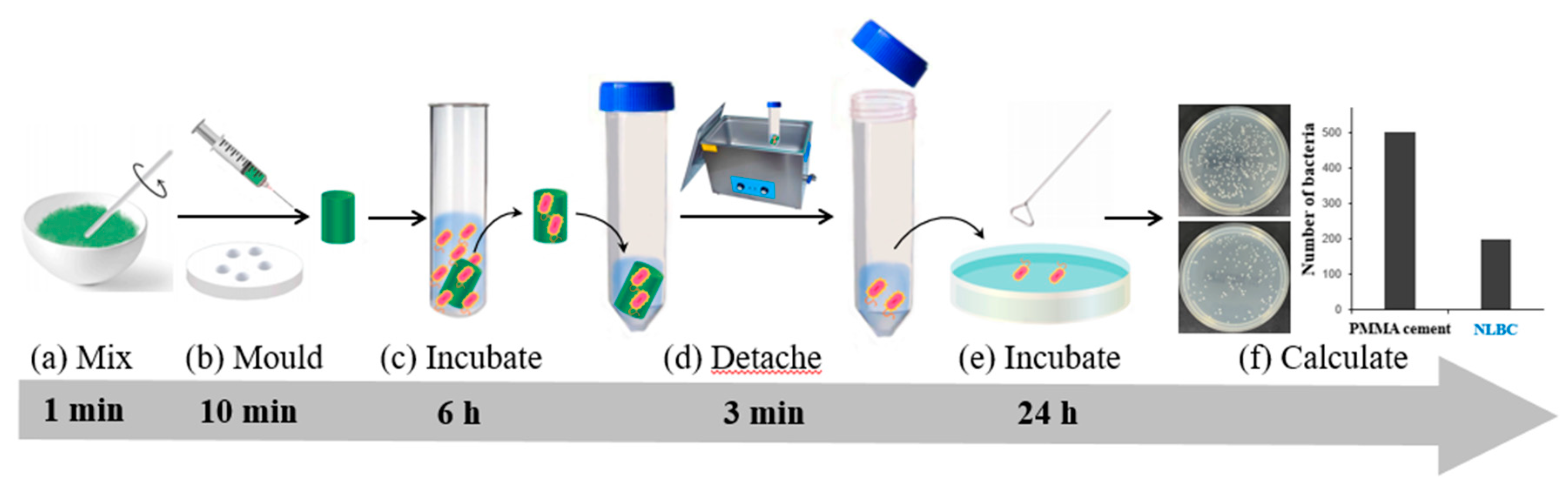
The following methods described also involve indirect approaches to studying the antimicrobial properties of materials and share some similarities. In Singh’s study of antimicrobial bone cement containing a novel co-monomer TPGDA, the cement was ground into powder and added to a bacterial solution for incubation. A certain amount of the bacterial solution was then spread on agar plates for cultivation. The bacterial colonies on the plates were counted to compare the antimicrobial activity of bone cement with different TPGDA contents. This method provides antimicrobial results that reflect the material’s intrinsic activity and hold reference value. However, it also has limitations; for instance, bone cement acts as a complete piece within the body, not as ground powder. Therefore, determining antimicrobial activity using powdered bone cement may not accurately represent its real behavior in the body. He et al. used the Direct Contact Test (DCT) to assess the surface antimicrobial properties of bone cement. Specifically, bone cement was prepared as thin sheet-like specimens, and bacterial solution was dropped onto the specimens for incubation. After rinsing with water to remove floating bacteria, adhered bacteria were brushed off the surface and spread onto agar plates. Colony counting was performed after cultivation to calculate and compare the material’s surface antimicrobial activity. Chu’s group also employed DCT for bone cement antimicrobial studies. Different from the thin sheet-like specimens, they used cylindrical specimens similar to those used for mechanical testing in ISO 5833. After culturing the specimens with bacterial solution, removing floating bacteria with water, and detaching surface adhered bacteria using ultra sonication, agar plates were used for colony counting.
Figure 6 illustrates a typical DCT method for testing the antibacterial activities of bone cement surfaces.
After comprehensive analysis of the methods mentioned above, researchers have adopted various approaches to test the antimicrobial activities of NLBC. These include directly observing changes in bacterial quantity and colony counting using agar plates. These methods may yield different results under different conditions, hence caution is necessary when comparing the antimicrobial properties of different materials. Among these methods, the approach of directly observing bacterial quantity changes, as demonstrated in studies by Deb and Xie, can directly illustrate the material’s ability to inhibit bacteria. However, it involves intricate procedures and demands a high level of technical proficiency. Conversely, the method of colony counting using agar plates, as employed in research by Singh, He, Chu, and others, is relatively straightforward and adaptable. Nonetheless, due to the utilization of powdered forms of the materials for testing, it may not accurately mirror the materials’ actual antimicrobial performance in practical usage settings. We assert that the DCT method represents a user-friendly and widely applicable technique for assessing material surface antimicrobial activity. Whether using He’s thin sheet specimens or Chu’s cylindrical specimens, both methodologies generate rapid antimicrobial activity data with high reproducibility. The chief advantage lies in the uncomplicated design of the experimental apparatus, making it suitable for most laboratory settings. Hence, when choosing testing methodologies, it is imperative to consider research objectives and practical constraints. Comprehensive comparisons between diverse methods are essential to obtain more precise assessments of antimicrobial activities.
7. Summary and Perspectives
The NLBC formed by incorporating covalently or non-covalently attached antimicrobial motifs in bone cement, without the addition of antibiotics, represents a non-traditional and innovative approach to antibacterial bone cement. It belongs to a new class of surface contact-killing antibacterial bone cement, showing significant potential for various applications. Currently, the research on antimicrobial motifs mainly focuses on quaternary ammonium salts and cyclic organic molecules. However, we believe that there should be broader investigations into new types of antimicrobial motifs. For instance, Aparicio et al. recently developed a novel method for immobilizing antimicrobial peptides on titanium surfaces, which can be used to prevent bacterial infections in dentistry and orthopedics [
47]. The immobilization of antimicrobial peptides on coating surfaces can provide inspiration for the study of NLBC containing antimicrobial peptides, focusing on the methods of peptide incorporation and stability, which are crucial aspects of the research. Additionally, there has been significant research on antimicrobial halamine compounds, which exhibit broad-spectrum antimicrobial properties and biocompatibility. However, the mechanical properties, antimicrobial activity, and particularly the biocompatibility towards osteoblasts of halamine-containing bone cement have not been adequately evaluated.
On the other hand, although NLBC has long-term antibacterial activity, it might not effectively address acute infections that have already occurred. Therefore, a comprehensive evaluation of the performance of NLBC doped with antibiotics is essential. Recently, some studies have demonstrated the ability of NLBC to release antibiotics effectively [
48]. Nevertheless, further research is needed, including the investigation of different types of NLBC doped with various antibiotics in terms of release kinetics, antimicrobial activity, and duration of effectiveness. Furthermore, we believe that research on the mechanism of surface sterilization should be prioritized. The antibacterial mechanism of quaternary ammonium salts involves disrupting cell membranes through positive charges, while the antimicrobial mechanism of cyclic organic molecules remains unclear. Although we speculate that it may be related to the inhibition of enzymatic activity on the cell surface, direct evidence is lacking. Studies in this area will help us understand the mechanisms of surface sterilization and promote the development of new types of NLBC. Thus, the development and application of NLBC present an innovative solution to antibacterial bone cement, offering promising avenues for diverse applications and holding the potential to play a significant role in clinical treatment.
Author Contributions
Methodology, J.-J.C., Y.X., Z.G.; validation, Y.X., Y.-H.X. and G.Z.; formal analysis, Z.G., Y.-C.K. and Y.X.; investigation, Z.G., Y.-H.X., Y.-C.K.; resources, Z.G. and Y.-C.K.; writing-original draft preparation, Y.X., Z.G.; writing-review and editing, Y.X., Z.G.; visualization, Y.X., Z.G.; supervision, J.-J.C.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Hefei Key Common Technology Research and Major Scientific Achievements Project (2021YL004), University Natural Science Project of Anhui Province (KJ2021A0348), Fund of Anhui Medical University (2022xkj108), Health research Project of Anhui Province (AHWJ2022b091).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available upon request. The data presented in this study
are available on request from the corresponding author.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hosseini, S. A Review of Bone Cements as Injectable Materials for Treatment of Bone-Related Diseases: Current Status and Future Developments. J Res in Orthop Sci 2022, 9, 1–14. [Google Scholar] [CrossRef]
- Liu, W.; Huan, Z.; Wu, C.; Zhou, Z.; Chang, J. High-strength calcium silicate-incorporated magnesium phosphate bone cement with osteogenic potential for orthopedic application. Compos B Eng 2022, 247, 110324. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, Y.; Liu, J.; Han, J.; Cui, Z.; Wu, S.; Liang, Y.; Zhu, S.; Ge, X.; Li, Z. Gelatin/gentamicin sulfate-modified PMMA bone cement with proper mechanical properties and high antibacterial ability. Mater Res Express 2022, 9, 035405. [Google Scholar] [CrossRef]
- Magnan, B.; Bondi, M.; Maluta, T.; Samaila, E.; Schirru, L.; Dall’Oca, C. Acrylic bone cement: current concept review. Musculoskelet Surg 2013, 97, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Cohn, D.; Sloutski, A.; Elyashiv, A.; Varma, V.B.; Ramanujan, R. In Situ Generated Medical Devices. Adv Healthc Mater 2019, 8, e1801066. [Google Scholar] [CrossRef]
- Soleymani Eil Bakhtiari, S.; Bakhsheshi-Rad, H.R.; Karbasi, S.; Tavakoli, M.; Hassanzadeh Tabrizi, S.A.; Ismail, A.F.; Seifalian, A.; RamaKrishna, S.; Berto, F. Poly (Methyl Methacrylate) Bone Cement, Its Rise. Growth, Downfall and Future. Polym Int 2021, 70, 1182–1201. [Google Scholar] [CrossRef]
- Gong, Y.; Zhang, B.; Yan, L. A preliminary review of modified polymethyl methacrylate and calcium-based bone cement for improving properties in osteoporotic vertebral compression fractures. Front Mater 2022, 9, 912713. [Google Scholar] [CrossRef]
- Sa, Y.; Yang, F.; Wang, Y.; Wolke, J.G.C.; Jansen, J.A. Modifications of Poly(Methyl Methacrylate) Cement for Application in Orthopedic Surgery. Adv Exp Med Biol 2018, 1078, 119–134. [Google Scholar] [PubMed]
- Gong, T.; Wang, Z.; Zhang, Y.; Sun, C.; Yang, Q.; Troczynski, T.; Häfeli, U.O. Preparation, characterization, release kinetics, and in vitro cytotoxicity of calcium silicate cement as a risedronate delivery system. J Biomed Mater Res A 2014, 102, 2295–2304. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.J.; Wu, H.Y. Study of C3S/Nano-HAp/collagen composite bone cement. Curr Nanosci 2014, 10, 212–216. [Google Scholar] [CrossRef]
- Hablee, S.; N Razali, N.; SF Alqap, A.; Sopyan, I. Recent developments on injectable calcium phosphate bone cement. Recent Pat Mater Sci 2016, 9, 72–94. [Google Scholar] [CrossRef]
- Weiss, D.D.; Sachs, M.A.; Woodard, C.R. Calcium phosphate bone cements: a comprehensive review. J Long Term Eff Med Implants 2003, 13, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Rohmiller, M.T.; Schwalm, D.; Glattes, R.C.; Elalayli, T.G.; Spengler, D.M. Evaluation of calcium sulfate paste for augmentation of lumbar pedicle screw pullout strength. Spine J 2002, 2, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, H.W.; Engelbrecht, H. Uber die Depotwirkung einiger Antibiotica bei Vermischung mit dem Kunstharz Palacos [Depot effects of various antibiotics mixed with Palacos resins]. Chirurg 1970, 41, 511–515. [Google Scholar] [PubMed]
- Wahlig, H.; Dingeldein, E. Antibiotics and bone cements. Experimental and clinical long-term observations. Acta Orthop Scand 1980, 51, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Duncan, CP.; Masri, B. Antibiotic depots. J Bone Joint Surg Br 1993, 75, 349–350. [Google Scholar] [CrossRef]
- Klemm, K.; Dingeldein, E.; Wahlig, H. Gentamycin-PMMA-Kugeln bei chronischen Knocheninfektionen. Langenbecks Arch Surg 1977, 345, 631–631. [Google Scholar] [CrossRef]
- Wahlig, H.; Dingeldein, E.; Buchholz, H.W.; Buchholz, M.; Bachmann, F. Pharmacokinetic study of gentamicin-loaded cement in total hip replacements. Comparative effects of varying dosage. J Bone Joint Surg Br 1984, 66, 175–179. [Google Scholar] [CrossRef]
- Tootsi, K.; Heesen, V.; Lohrengel, M.; Enz, A.E.; Illiger, S.; Mittelmeier, W.; Lohmann, C.H. The use of antibiotic-loaded bone cement does not increase antibiotic resistance after primary total joint arthroplasty. Knee Surg Sports Traumatol Arthrosc 2022, 30, 3208–3214. [Google Scholar] [CrossRef] [PubMed]
- Sabater-Martos, M.; Verdejo, M.A.; Morata, L.; Muñoz-Mahamud, E.; Guerra-Farfan, E.; Martinez-Pastor, J.C.; Soriano, A. Antimicrobials in polymethylmethacrylate: from prevention to prosthetic joint infection treatment: basic principles and risk of resistance. Arthroplasty 2023, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Berberich, C.E.; Josse, J.; Laurent, F.; Ferry, T. Dual antibiotic loaded bone cement in patients at high infection risks in arthroplasty: Rationale of use for prophylaxis and scientific evidence. World J Orthop 2021, 12, 119–128. [Google Scholar] [CrossRef]
- Moojen, D.J.; Hentenaar, B.; Vogely, H.C.; Verbout, A.J.; Castelein, R.M.; Dhert, W.J. In vitro release of antibiotics from commercial PMMA beads and articulating hip spacers. J Arthroplasty 2008, 23, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Al Thaher, Y.; Perni, S.; Prokopovich, P. Nano-carrier based drug delivery systems for sustained antimicrobial agent release from orthopaedic cementous material. Adv Colloid Interface Sci 2017, 249, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Berberich, C.; Sanz-Ruiz, P. Risk assessment of antibiotic resistance development by antibiotic-loaded bone cements: is it a clinical concern? EFORT Open Rev 2019, 4, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S. Antibacterial properties of resin composites and dentin bonding systems. Dent Mater 2003, 19, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Almaroof, A.; Niazi, S.A.; Rojo, L.; Mannocci, F.; Deb, S. Influence of a polymerizable eugenol derivative on the antibacterial activity and wettability of a resin composite for intracanal post cementation and core build-up restoration. Dent Mater 2016, 32, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.R.; Fonseca, A.C.; Mendonça, P.V.; Branco, R.; Serra, A.C.; Morais, P.V.; Coelho, J.F. Recent Developments in Antimicrobial Polymers: A Review. Materials (Basel) 2016, 9, 599. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Liu, F.; He, J. Effect of polymerizable quaternary ammonium monomer MEIM-x’s alkyl chain length and content on bone cement’s antibacterial activity and physicochemical properties. J Mech Behav Biomed Mater 2018, 87, 279–287. [Google Scholar] [CrossRef]
- Lewis, G. Antibiotic-free antimicrobial poly (methyl methacrylate) bone cements: A state-of-the-art review. World J Orthop 2022, 13, 339–353. [Google Scholar] [CrossRef]
- Jiao, Y.; Niu, L.N.; Ma, S.; Li, J.; Tay, F.R.; Chen, J.H. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog Polym Sci 2017, 71, 53–90. [Google Scholar] [CrossRef]
- Deb, S.; Doiron, R.; Disilvio, L.; Punyani, S.; Singh, H. PMMA bone cement containing a quaternary amine comonomer with potential antibacterial properties. J Biomed Mater Res B Appl Biomater 2008, 85, 130–139. [Google Scholar] [CrossRef]
- Abid, C.K.; Jain, S.; Jackeray, R.; Chattopadhyay, S.; Singh, H. Formulation and characterization of antimicrobial quaternary ammonium dendrimer in poly(methyl methcarylate) bone cement. J Biomed Mater Res B Appl Biomater 2017, 105, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Maeda, T.; Miyazaki, T. Preparation of bioactive and antibacterial PMMA-based bone cement by modification with quaternary ammonium and alkoxysilane. J Biomater Appl 2021, 36, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Liu, F.; He, J. Synthesis of imidazolium-containing mono-methacrylates as polymerizable antibacterial agents for acrylic bone cements. J Mech Behav Biomed Mater 2017, 74, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Qian, Z.; Luo, L.; Yuan, Q.; Guo, X.; Tao, L.; Wei, Y.; Wang, X. Antibacterial Adhesion of Poly(methyl methacrylate) Modified by Borneol Acrylate. ACS Appl Mater Interfaces 2016, 8, 28522–28528. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Lao, C.; Luo, S.; Liu, F.; Huang, Q.; He, J.; Lin, Z. Mechanical and antibacterial properties of benzothiazole-based dental resin materials. J Biomater Sci Polym Ed 2018, 29, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Liu, F.; Yu, B.; He, J. Preparation of antibacterial acrylic bone cement with methacrylate derived from benzothiazole. J Mech Behav Biomed Mater 2021, 117, 104403. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Li, C.; Guo, J.; Xu, Y.; Fu, Y. Preparation of new bio-based antibacterial acrylic bone cement via modification with a biofunctional monomer of nitrofurfuryl methacrylate. POLYM CHEM-UK 2022, 13, 4675–4683. [Google Scholar] [CrossRef]
- Cascioferro, S.; Raffa, D.; Maggio, B.; Raimondi, M.V.; Schillaci, D.; Daidone, G. Sortase A Inhibitors: Recent Advances and Future Perspectives. J Med Chem 2015, 58, 9108–9123. [Google Scholar] [CrossRef]
- Alharthi, S.; Alavi, S.E.; Moyle, P.M.; Ziora, Z.M. Sortase A (SrtA) inhibitors as an alternative treatment for superbug infections. Drug Discov Today 2021, 26, 2164–2172. [Google Scholar] [CrossRef]
- Jaudzems, K.; Kurbatska, V.; Jekabsons, A.; Bobrovs, R.; Rudevica, Z.; Leonchiks, A. Targeting Bacterial Sortase A with Covalent Inhibitors: 27 New Starting Points for Structure-Based Hit-to-Lead Optimization. ACS Infect Dis 2020, 6, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Howard, L.; Guo, X.; Chong, V.J.; Gregory, R.L.; Xie, D. A novel antibacterial resin composite for improved dental restoratives. J Mater Sci Mater Med 2012, 23, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Almousa, R.; Anderson, G.G. Developing a novel antibacterial dental resin composite with improved properties. J Compos Mater 2019, 53, 002199831983913. [Google Scholar] [CrossRef]
- Wright, Z.M.; Pandit, A.M.; Holt, B.D.; Sydlik, S.A. Therapeutic Methacrylic Comonomers for Covalently Controlled Release from Mechanically Robust Bone Cement: Kinetics and Structure–Function Relationships. Macromolecules 2019, 52, 3775–3786. [Google Scholar] [CrossRef]
- Tan, H.; Peng, Z.; Li, Q.; Xu, X.; Guo, S.; Tang, T. The use of quaternised chitosan-loaded PMMA to inhibit biofilm formation and downregulate the virucence-associated gene expression of antibiotic-resistant staphylococcus. Biomaterials 2012, 33, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Ao, H.; Ma, R.; Tang, T. Quaternised chitosan-loaded polymethylmethacrylate bone cement: biomechanical and histological evaluations. J Orthop Translat 2013, 1, 57–66. [Google Scholar] [CrossRef]
- Chen, Y.; Caneli, G.; Xie, D. A PMMA bone cement with improved antibacterial function and flexural strength. J Biomater Sci Polym Ed 2022, 33, 1398–1414. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Xu, Y.; Kan, Y.; Li, H.; Guo, R.; Han, L.; Bu, W.; Chu, J. Comparison of antibacterial activity and biocompatibility of non-leaching nitrofuran bone cement loaded with vancomycin, gentamicin, and tigecycline. J Orthop Surg Res 2023, 18, 569. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).