Submitted:
16 August 2023
Posted:
18 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Computational Details and Methodology
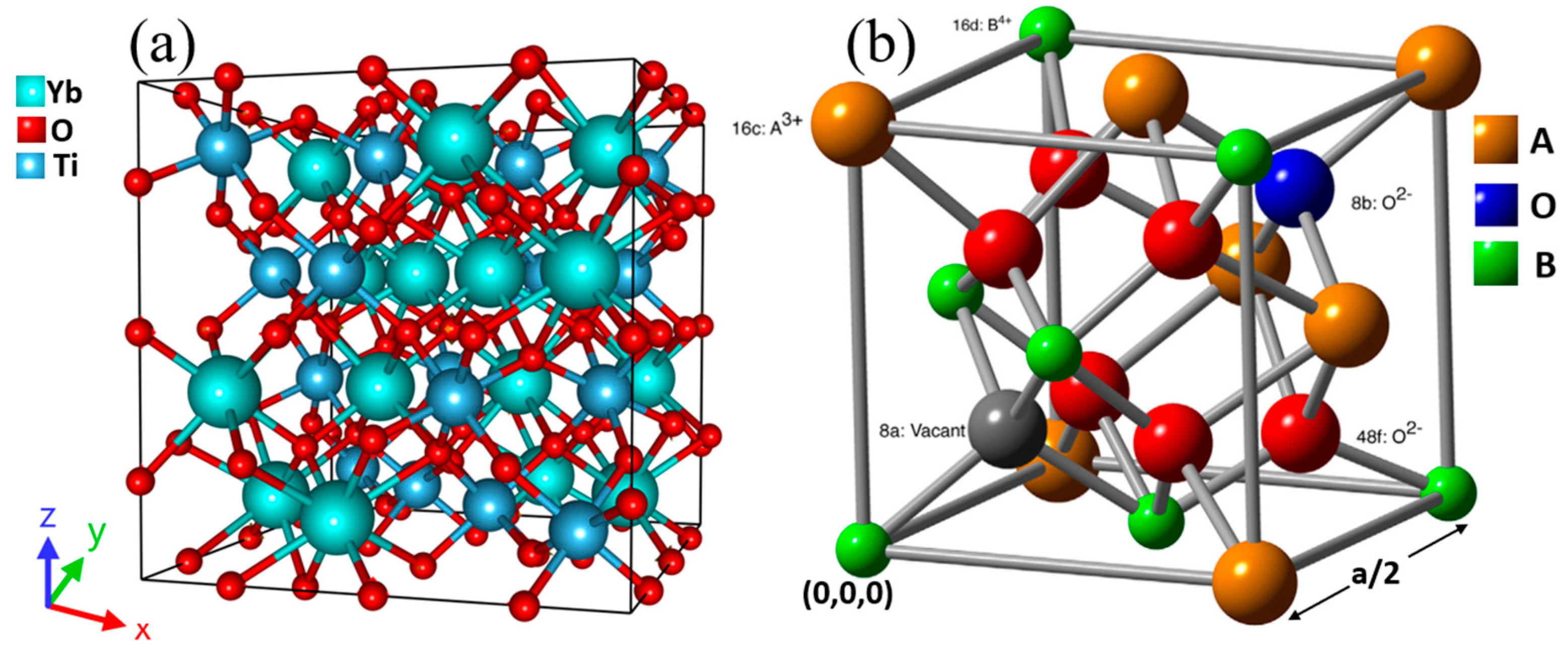
2.1. Crystal Structure
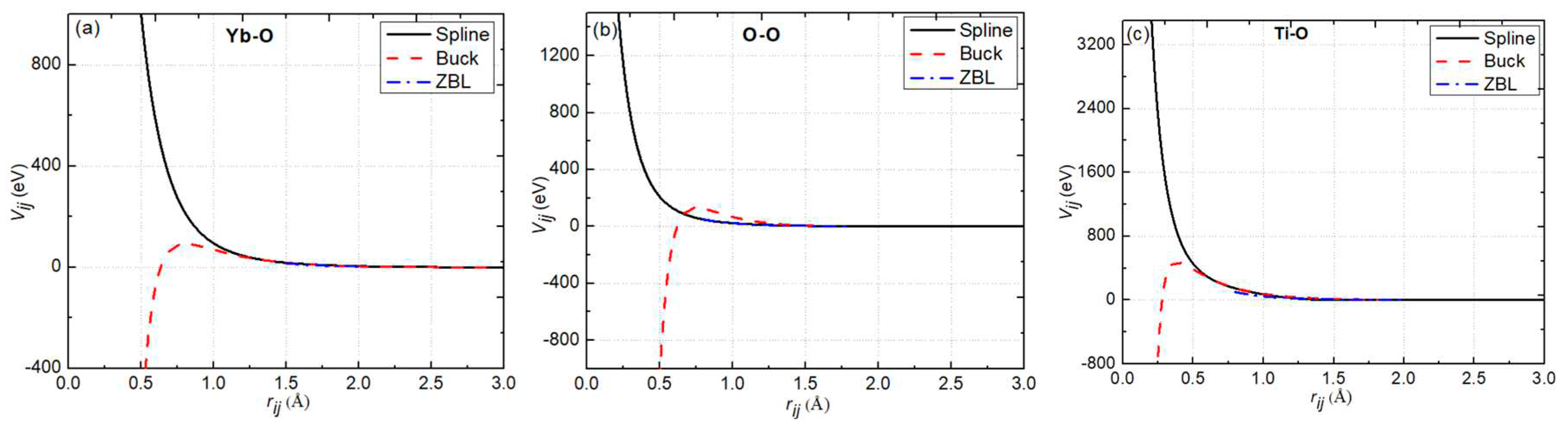
2.2. Interatomic Potential
2.3. Displacement Cascades Simulations
3. Results and Discussion
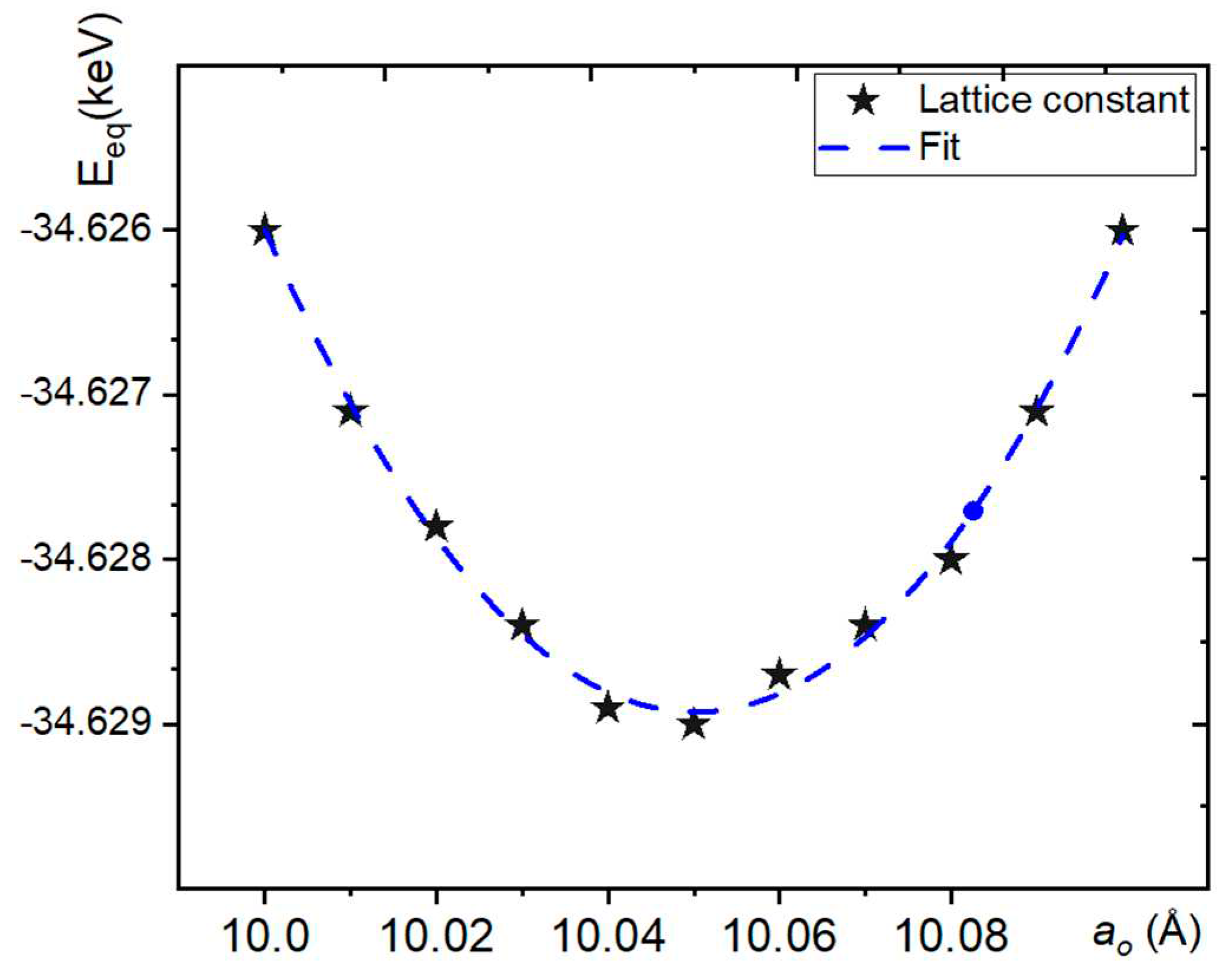
3.1. Calculation for Equilibrium LATTIce Constant
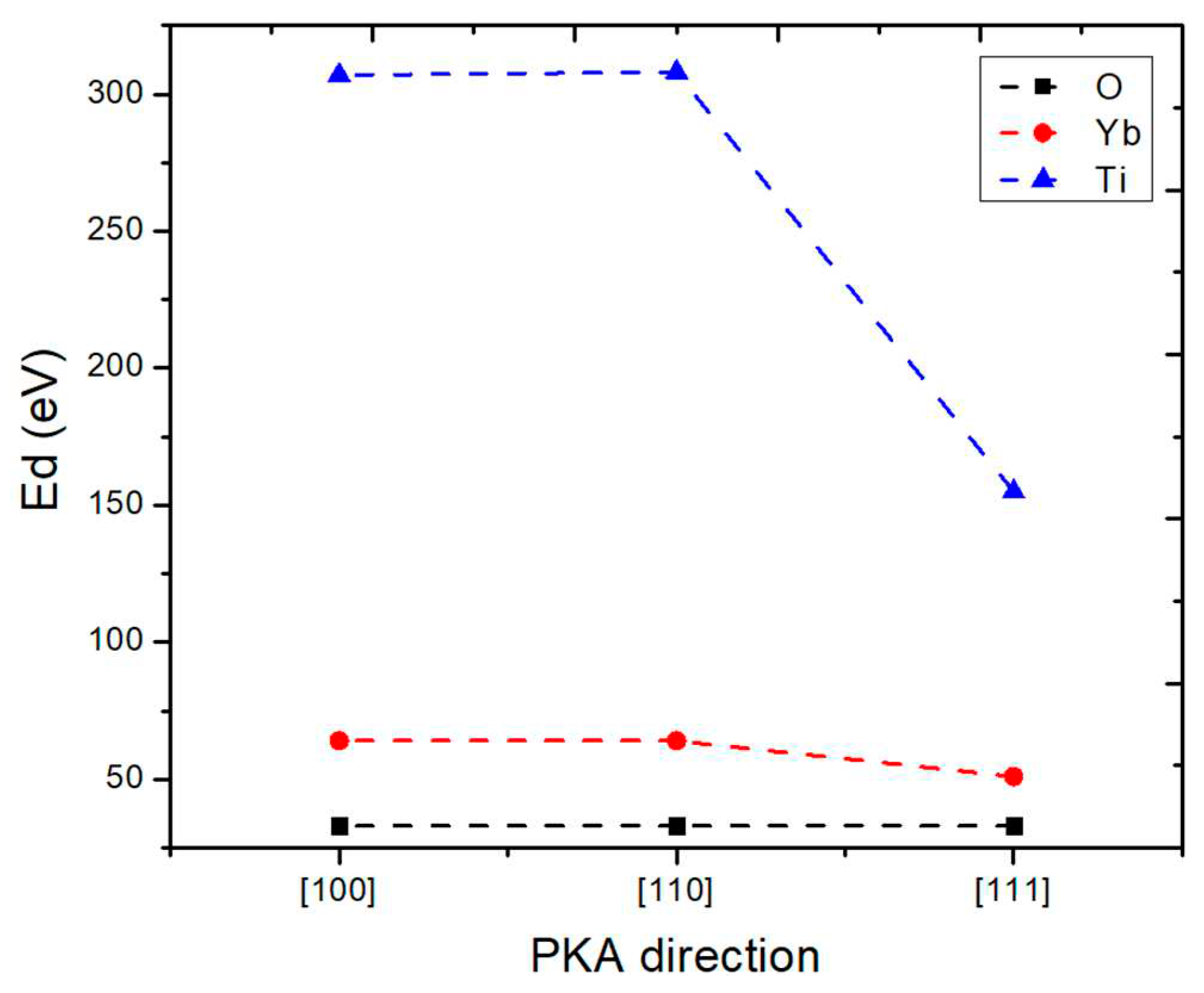
3.2. Threshold Displacement Energy (Ed)
3.3. Effect of Displacement Cascades on Energy and Direction of PKA
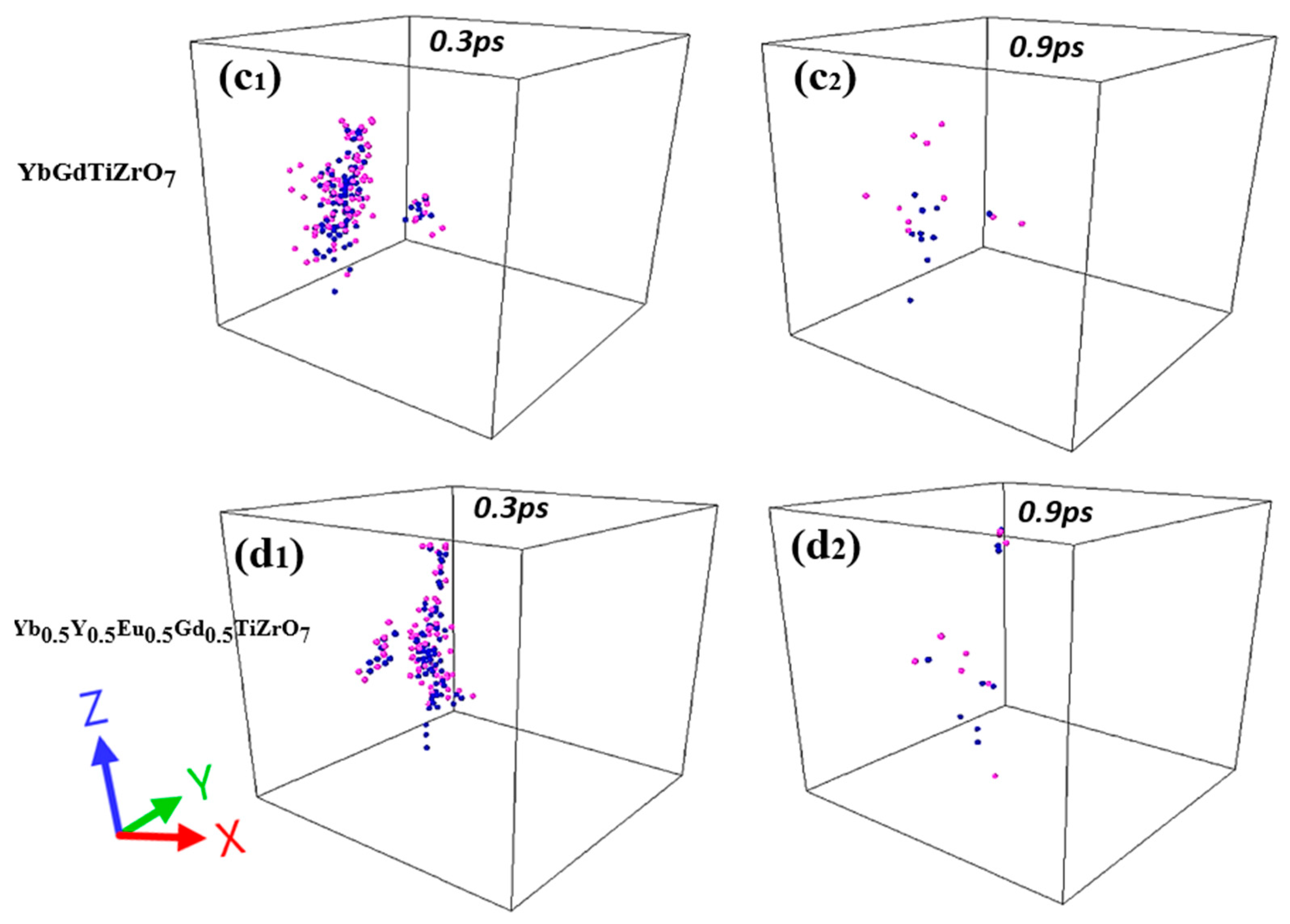
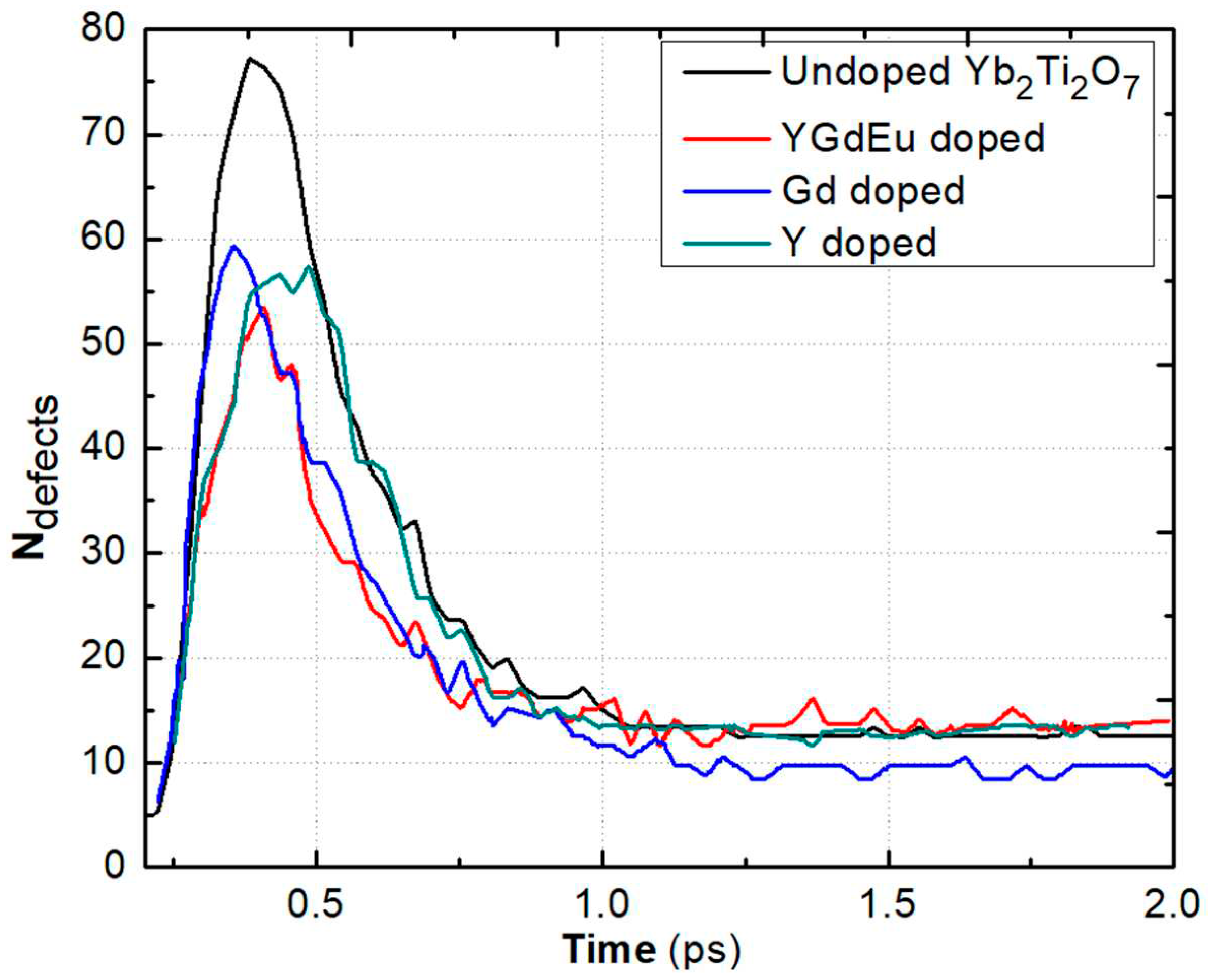
3.4. Displacement Cascades in HEPy
4. Conclusion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- B. Raj, M. Vijayalakshmi, P.R. Vasudeva Rao, K.B.S. Rao, Challenges in materials research for sustainable nuclear energy, MRS Bull. 33 (2008) 327–337.
- P.W. Beck, NUCLEAR ENERGY IN THE TWENTY CENTURY: Examination of a Contentious Subject, Annu. Rev. Energy Environ. 24 (1999) 113–37.
- N. Mahmood, Danish, Z. Wang, B. Zhang, The role of nuclear energy in the correction of environmental pollution: Evidence from Pakistan, Nucl. Eng. Technol. 52 (2020) 1327–1333. [CrossRef]
- T. Abram, S. Ion, Generation-IV nuclear power: A review of the state of the science, Energy Policy. 36 (2008) 4323–4330. 4323–4330. [CrossRef]
- K. Dungan, R.W.H. Gregg, K. Morris, F.R. Livens, G. Butler, Assessment of the disposability of radioactive waste inventories for a range of nuclear fuel cycles: Inventory and evolution over time, Energy. 221 (2021) 119826. [CrossRef]
- J. Amiard, Nuclear Waste Disposal Methods, Manag. Radioact. Waste. (2021) 23–66. [CrossRef]
- L. Kong, I. Karatchevtseva, Y. Zhang, T. Wei, The incorporation of Nd or Ce in CaZrTi2O7 zirconolite: Ceramic versus glass-ceramic, J. Nucl. Mater. 543 (2021) 152583. [CrossRef]
- Z. Liu, K. Liu, X. Zheng, Y. Wang, X. Sun, P. Xue, C. Li, M. Du, Formulation of Poly(ionic liquids)@COF Nanotrap for Efficient Perrhenate Sequestration from Alkaline Nuclear Waste, Chem. Mater. 34 (2022) 5452–5456. [CrossRef]
- X. Guo, S. Gin, G.S. Frankel, Review of corrosion interactions between different materials relevant to disposal of high-level nuclear waste, Npj Mater. Degrad. 4 (2020). [CrossRef]
- G.S. Frankel, J.D. Vienna, J. Lian, X. Guo, S. Gin, S.H. Kim, J. Du, J. V Ryan, J. Wang, W. Windl, C.D. Taylor, J.R. Scully, Recent Advances in Corrosion Science Applicable To Disposal of High-Level Nuclear Waste, Chem. Rev. 121 (2021) 12327–12383. [CrossRef]
- and, H.F.S. Xu Huifang, Yifeng Wang, Pihong Zhao, William L. Bourcier, Richard Van Konynenburg, Investigation of Pyrochlore-Based U-Bearing Ceramic Nuclear Waste : Uranium Leaching Test and TEM Observation, Environ. Sci. Technol. 38 (2004) 1480–1486.
- M. Dholakia, S. Chandra, S.M. Jaya, Comparative study of radiation damage in Gd2Zr2O7 and Y2Ti2O7 crystal: A molecular dynamics investigation, AIP Conf. Proc. 2265 (2020) 30373. [CrossRef]
- Y. Zhang, Ceramic Materials for Nuclear Energy Applications, JOM. 71 (2019) 4806–4807. [CrossRef]
- J. Marra, Advanced ceramic materials for next-generation nuclear applications, IOP Conf. Ser. Mater. Sci. Eng. 18 (2011) 16001. [CrossRef]
- W.H. Lee, J.H. Cheong, Potential radiological hazard and options to cope with consequences from recycling of activated metal waste disposed of at a near-surface disposal facility, Ann. Nucl. Energy. 152 (2021) 107993. [CrossRef]
- M.L. Hand, M.C. Stennett, N.C. Hyatt, Rapid low temperature synthesis of a titanate pyrochlore by molten salt mediated reaction, J. Eur. Ceram. Soc. 32 (2012) 3211–3219. [CrossRef]
- M. Dholakia, S. Chandra, Structural stability of titanate pyrochlores undergoing radiation damage, Comput. Mater. Sci. 201 (2022) 110889. [CrossRef]
- L. Minervini, R.W. Grimes, K.E. Sickafus, Disorder in pyrochlore oxides, J. Am. Ceram. Soc. 83 (2000) 1873–1878. [CrossRef]
- L. Minervini, R.W. Grimes, K.E. Sickafus, Disorder in pyrochlore oxides, J. Am. Ceram. Soc. 83 (2000) 1873–1878. [CrossRef]
- Y. Zhang, Machine learning the lattice constant of cubic pyrochlore compounds, Int. J. Appl. Ceram. Technol. 18 (2021) 661–676. [CrossRef]
- B.J. Kennedy, B.A. Hunter, C.J. Howard, Structural and Bonding Trends in Tin Pyrochlore Oxides, J. Solid State Chem. 130 (1997) 58–65. [CrossRef]
- H. Sakai, K. Yoshimura, H. Ohno, H. Kato, S. Kambe, R.E. Walstedt, T.D. Matsuda, Y. Haga, Y. Onuki, Superconductivity in a pyrochlore oxide, Cd2Re2O7, J. Phys. Condens. Matter. 13 (2001). [CrossRef]
- L. Dong, Y. Li, R. Devanathan, W. Setyawan, F. Gao, Low energy ion-solid interactions and chemistry effects in a series of pyrochlores, J. Am. Ceram. Soc. 100 (2017) 3132–3144. [CrossRef]
- A. Chartier, C. Meis, J.P. Crocombette, L.R. Corrales, W.J. Weber, Atomistic modeling of displacement cascades in (formula presented) pyrochlore, Phys. Rev. B - Condens. Matter Mater. Phys. 67 (2003) 1–13. [CrossRef]
- K.R. Whittle, M.G. Blackford, R.D. Aughterson, G.R. Lumpkin, N.J. Zaluzec, Ion irradiation of novel yttrium / ytterbium-based pyrochlores : The effect of disorder, Acta Mater. 59 (2011) 7530–7537. [CrossRef]
- A. Archer, H.R. Foxhall, N.L. Allan, J.R.W. Shearer, D.S.D. Gunn, J.H. Harding, I.T. Todorov, K.P. Travis, J.A. Purton, Multiple cascade radiation damage simulations of pyrochlore, Mol. Simul. 47 (2021) 273–283. [CrossRef]
- T. Subramani, A. Voskanyan, K. Jayanthi, M. Abramchuk, A. Navrotsky, A Comparison of Order-Disorder in Several Families of Cubic Oxides, Front. Chem. 9 (2021) 1–21. [CrossRef]
- A. Annamareddy, J. Eapen, J. Eapen, Decoding ionic conductivity and reordering in cation-disordered pyrochlores Subject Areas : Author for correspondence :, Philos. Trans. A. 379 (2021).
- Z. Teng, Y. Tan, H. Zhang, High-entropy pyrochlore a2b2o7 with both heavy and light rare-earth elements at the a site, Materials (Basel). 15 (2022) 1–8. [CrossRef]
- H. Xiang, Y. Xing, F. zhi Dai, H. Wang, L. Su, L. Miao, G. Zhang, Y. Wang, X. Qi, L. Yao, H. Wang, B. Zhao, J. Li, Y. Zhou, High-entropy ceramics: Present status, challenges, and a look forward, 2021. [CrossRef]
- M. Widom, Modeling the structure and thermodynamics of high-entropy alloys, J. Mater. Res. 33 (2018).
- Q. Zhi, X. Tan, Z. Liu, Y. Liu, Q. Zhang, Y. Chen, M. Li, Effect of Zr content on microstructure and mechanical properties of lightweight Al2NbTi3V2Zrx high entropy alloy, Micron. 144 (2021) 103031. [CrossRef]
- G. Ji, Z. Zhou, F. Meng, X. Yang, R. Sheng, J. Qiao, P.K. Liaw, M. Li, L. Jiang, S. Chen, Y. Tong, Effect of Zr addition on the local structure and mechanical properties of Ti–Ta–Nb–Zr refractory high-entropy alloys, J. Mater. Res. Technol. 19 (2022) 4428–4438. [CrossRef]
- L.. Brixner, Preparation and Properties of the Ln2Ti20 -Type Rare Earth Titanates, Inorg. Chem. 3 (1964) 1065–1067. [CrossRef]
- Z. Wang, G. Zhou, D. Jiang, S. Wang, Recent development of A2B2O7 system transparent ceramics, J. Adv. Ceram. 7 (2018) 289–306. [CrossRef]
- M.M. Azeem, Q. Wang, Y. Zhang, S. Liu, M. Zubair, Effect of Grain Boundary on Diffusion of P in Alpha-Fe: A Molecular Dynamics Study, Front. Phys. 7 (2019) 1–7. [CrossRef]
- M.M. Azeem, Z. Li, Q. Wang, A. Hussian, Molecular Dynamics Simulation Study on the Possible Factors Affecting Stability of ODS Steel, in: IOP Conf. Ser. Mater. Sci. Eng., 2018: p. 012003. [CrossRef]
- M. Mustafa Azeem, K. Abd El Gawad, M. Zubair, S.A. Ibrahim, M. Ado, G. Mehdi, Radiation damage effects in oxide dispersion strengthened steel alloys, in: Int. Conf. Nucl. Eng. Proceedings, ICONE, 2019. [CrossRef]
- M. Mustafa Azeem, Z. Li, Q. Wang, M. Zubair, Molecular dynamics studies and irradiation effects in ODSS alloys, Int. J. Nucl. Energy Sci. Technol. 12 (2018) 381–399. [CrossRef]
- M.M. Azeem, Q. Wang, Z. Li, Y. Zhang, Dislocation-oxide interaction in Y2O3 embedded Fe: A molecular dynamics simulation study, Nucl. Eng. Technol. 52 (2020) 337–343. [CrossRef]
- M.M. Azeem, Z. Li, Q. Wang, Q.M.N. Amjad, M. Zubair, O.M.H. Ahmed, Classical molecular dynamics study for defect sink behavior in oxide dispersed strengthened alloys, in: Proc. 2018 15th Int. Bhurban Conf. Appl. Sci. Technol. IBCAST 2018, 2018: pp. 12–15. [CrossRef]
- A.M. Mustafa, Z. Li, L. Shao, Molecular Dynamics Simulations of Damage Cascades Creation in Oxide-Particle-Embedded Fe., in: 25th Int. Conf. Nucl. Eng., 2017: pp. 1–5. [CrossRef]
- M.M. Azeem, Q. Wang, M. Zubair, Atomistic simulations of nanoindentation response of irradiation defects in iron, Sains Malaysiana. 48 (2019) 2029–2039. [CrossRef]
- M.M. Azeem, D. Yun, M. Zubair, Atomic insights on interaction mechanism of dislocation with void/impurity/ precipitates in bcc iron, Int. Conf. Nucl. Eng. Proceedings, ICONE. 2 (2021) 1–7. [CrossRef]
- G. Vinothkumar, S. Rengaraj, P. Arunkumar, S.W. Cha, K.S. Babu, and La 3 + ) - Doped Cerium Oxide Nanoparticles for Enhanced Multienzyme-Mimetic and Hydroxyl Radical Scavenging Activity, (2019). [CrossRef]
- A. Garbout, S. Bouattour, M. Ellouze, A.W. Kolsi, Synthesis, FT-IR and X-ray diffraction investigations of gadolinium-substituted pyrochlore oxide Gd1.82Cs0.18Ti2O6.82 via a sol-gel process, J. Alloys Compd. 425 (2006) 88–95. [CrossRef]
- D.S.D. Gunn, N.L. Allan, H. Foxhall, J.H. Harding, J.A. Purton, W. Smith, M.J. Stein, I.T. Todorov, K.P. Travis, Novel potentials for modelling defect formation and oxygen vacancy migration in Gd 2Ti 2O 7 and Gd 2Zr 2O 7 pyrochlores, J. Mater. Chem. 22 (2012) 4675–4680. [CrossRef]
- R. Devanathan, W.J. Weber, Insights into the radiation response of pyrochlores from calculations of threshold displacement events, J. Appl. Phys. 98 (2005). [CrossRef]
- X.J. Wang, H.Y. Xiao, X.T. Zu, Y. Zhang, W.J. Weber, Ab initio molecular dynamics simulations of ion–solid interactions in Gd 2 Zr 2 O 7 and Gd 2 Ti 2 O 7, J. Mater. Chem. C. 1 (2013) 1665–1673.
- E.R. Aluri, A.P. Grosvenor, An X-ray absorption spectroscopic study of the effect of bond covalency on the electronic structure of Gd2Ti2-xSnxO 7, Phys. Chem. Chem. Phys. 15 (2013) 10477–10486. [CrossRef]
- S. Zheng, S. Wang, First-principles design of refractory high entropy alloy VMoNbTaW, Entropy. 20 (2018). [CrossRef]
- K. Lipkina, A. Savchenko, M. Skupov, A. Glushenkov, A. Vatulin, O. Uferov, Y. Ivanov, G. Kulakov, S. Ershov, S. Maranchak, A. Kozlov, E. Maynikov, K. Konova, Metallic inert matrix fuel concept for minor actinides incineration to achieve ultra-high burn-up, J. Nucl. Mater. 452 (2014) 378–381. [CrossRef]
- M.K. Anupama, B. Rudraswamy, Effect of Gd3+- Cr3+ ion substitution on the structural, electrical and magnetic properties of Ni - Zn ferrite nanoparticles, in: IOP Conf. Ser. Mater. Sci. Eng., 2016. [CrossRef]
- L. Chen, X. Su, Y. Li, First-Principles Study on Cation-Antisite Defects of Stannate and Titanate Pyrochlores, OALib. 01 (2014) 1–8. [CrossRef]
- Y. Yang, W. Wang, G.-. Y. Gan, X.-. F. Shi, B.-. Y. Tang, Structural, mechanical and electronic properties of (TaNbHfTiZr)C high entropy carbide under pressure: ab initio investigation, Phys. B. 550 (2018).
- W. Akhtar, M.M. Azeem, M.B. Khan, M. Science, Atomistic Insights into the Irradiation Effects in Molybdenum, Int. J. Eng. Work. 9 (2022) 187–192.
- A. Ullah, Q. Wang, Y. Song, M.M. Azeem, M. Ado, M. Sohail, Continuous Stiffness Measurements and Nanoindentation Studies of bcc Mo: An Atomistic Approach, Trans. Indian Inst. Met. 75 (2022) 1555–1561. [CrossRef]
- S.A. Ibrahim, Q. Wang, Y. Zhang, M. Ado, G.D. Chung, M.M. Azeem, Molecular dynamics simulation of strengthening dependence on precipitate Cr composition in Fe-15at.%Cr alloy, Micron. 131 (2020) 102823. [CrossRef]
- A. Ullah, W. Qingyu, M. Ado, M.M. Azeem, A. Shah, Effect of Concentration of Mo on the Mechanical behavior of γ UMo: an Atomistic Study, Pet. Chem. Ind. Int. 4 (2021) 67–71. [CrossRef]
- M.M. Azeem, D. Yun, M. Zubair, Atomic Insights on Interaction Mechanism of Dislocation With Void/Impurity/Precipitates in BCC Iron, in: Proc. 2021 28th Int. Conf. Nucl. Eng., 2021. [CrossRef]
- A.K.D. Willie, H. Zhao, M. Mustafa Azeem, T. Svetlana, Analysis of Burnup effects and Its Integrity Assessment in the Interim of Irradiation with Molecular Dynamics, MRS Adv. 5 (2020) 1799–1810. [CrossRef]
- S.A. Ibrahim, Q. Wang, M. Ado, M. Mustafa Azeem, Z. Abbati, Irradiation effects of Fe-Cr alloys, in: Int. Conf. Nucl. Eng. Proceedings, ICONE, 2019. [CrossRef]
- G. Mehdi, N. Ali, S. Hussain, A.A. Zaidi, A. Hussain Shah, M.M. Azeem, Design and fabrication of automatic single axis solar tracker for solar panel, in: 2019 2nd Int. Conf. Comput. Math. Eng. Technol. ICoMET 2019, 2019. [CrossRef]
- A.M.M. Eltayeb Yousif, Zhang Zhijian, Tian Zhao-fe, A Simulation of Small Break Loss of Coolant Accident (SB-LOCA) in AP1000 Nuclear Power Plant Using RELAP5-MV, 25th Int. Conf. Nucl. Eng. 5 (2017) 1–5. [CrossRef]
- Ahmed, F.I. Habbani, A.M. Mustafa, E.M.A. Mohamed, A.M. Salih, F. Seedig, Quality Assessment Statistic Evaluation of X-Ray Fluorescence via NIST and IAEA Standard Reference Materials, World J. Nucl. Sci. Technol. 07 (2017) 121–128. [CrossRef]
- M.M. Azeem, I. Jamil, M. Khan, Multiscale Modeling of Radiation Damage in Oxide Dispersed Strengthened Steel Alloys : A Perspective, Int. J. Eng. Work. 9 (2022) 193–200.
- M.M. Azeem, Z. Li, Q. Wang, A. Hussian, Molecular Dynamics Simulation Study on the Possible Factors Affecting Stability of ODS Steel, IOP Conf. Ser. Mater. Sci. Eng. 389 (2018) 12003. [CrossRef]
- M. Zubair, A. Wakeel, M.M. Azeem, A. Tabbassum, Computational analysis of high carbon steel for optimal design, Proc. 18th Int. Bhurban Conf. Appl. Sci. Technol. IBCAST 2021. (2021) 10–14. [CrossRef]
- M. V. Talanov, V.M. Talanov, Structural Diversity of Ordered Pyrochlores, Chem. Mater. 33 (2021) 2706–2725. [CrossRef]
- T. Connor, O. Cheong, T. Bornhake, A.C. Shad, R. Tesch, M. Sun, Z. He, A. Bukayemsky, V.L. Vinograd, Pyrochlore Compounds From Atomistic Simulations, Front. Chem. 9 (2021) 1–14. [CrossRef]
- R.C. Ewing, W.J. Weber, J. Lian, Nuclear waste disposal-pyrochlore (A 2B 2O 7): Nuclear waste form for the immobilization of plutonium and “minor” actinides, J. Appl. Phys. 95 (2004) 5949–5971. [CrossRef]
- K. Momma, F. Izumi, VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data, J. Appl. Crystallogr. 44 (2011) 1272–1276. [CrossRef]
- K.B. Wiberg, A Scheme for Strain Energy Minimization. Application to the Cycloalkanes, J. Am. Chem. Soc. 87 (1965) 1070–1078. [CrossRef]
- V. Kocevski, G. Pilania, B.P. Uberuaga, Modeling Disorder in Pyrochlores and Other Anion-Deficient Fluorite Structural Derivative Oxides, Front. Chem. 9 (2021) 1–10. [CrossRef]
- B.C. Chakoumakos, Systematics of the pyrochlore structure type, ideal A2B2X6Y, J. Solid State Chem. 53 (1984) 120–129. [CrossRef]
- K.E. Sickafus, L. Minervini, R.W. Grimes, J.A. Valdez, M. Ishimaru, F. Li, K.J. McClellan, T. Hartmann, Radiation tolerance of complex oxides, Science (80-. ). 289 (2000) 748–751. [CrossRef]
- S. Plimpton, B. Hendrickson, A New Parallel Method for Molecular Dynamics Simulation of Macromolecular Systems, J. Comput. Chem. 17 (1996) 326–337. [CrossRef]
- A. Stukowski, Visualization and analysis of atomistic simulation data with OVITO the Open Visualization Tool, Model. Simul. Mater. Sci. Eng. 18 (2010) 015012. [CrossRef]
- M. Dholakia, S. Chandra, S.M. Jaya, A comparative study of topology and local disorder in Y2O3, Y2TiO5, and Y2Ti2O7 crystals Manan, J. Alloys Compd. 739 (2018) 1037–1047. [CrossRef]
- L. Minervini, R.W. Grimes, Y. Tabira, R.L. Withers, K.E. Sickafus, The oxygen positional parameter in pyrochlores and its dependence on disorder, Philos. Mag. A Phys. Condens. Matter, Struct. Defects Mech. Prop. 82 (2002) 123–135. [CrossRef]
- G. Balducci, J. Kašpar, P. Fornasiero, M. Graziani, M.S. Islam, Surface and reduction energetics of the CeO2-ZrO2 catalysts, J. Phys. Chem. B. 102 (1998) 557–561. [CrossRef]
- B.A. Luty, M.E. Davis, I.G. Tironi, W.F. Van Gunsteren, A comparison of particle-particle, particle-mesh and ewald methods for calculating electrostatic interactions in periodic molecular systems, Mol. Simul. 14 (1994) 11–20. [CrossRef]
- J.F. Ziegler, J.P. Biersack, The Stopping and Range of Ions in Matter BT - Treatise on Heavy-Ion Science: Volume 6: Astrophysics, Chemistry, and Condensed Matter, in: D.A. Bromley (Ed.), Springer US, Boston, MA, 1985: pp. 93–129. [CrossRef]
- M.W.D. Cooper, M.J.D. Rushton, R.W. Grimes, A many-body potential approach to modelling the thermomechanical properties of actinide oxides, J. Phys. Condens. Matter. 26 (2014). [CrossRef]
- M. Ado, Q. Wang, S.A. Ibrahim, S.O. Adede, Effect of radiation and substitution of Ce4+ at Zr site in Y4Zr3O12 using collision cascades: a molecular dynamics simulation study, J. Nucl. Sci. Technol. 00 (2022) 1–10. [CrossRef]
- S. Plimpton, Fast parallel algorithms for short-range molecular dynamics, J. Comput. Phys. 117 (1995) 1–19. [CrossRef]
- M. Duvail, P. Vitorge, R. Spezia, Building a polarizable pair interaction potential for lanthanoids ( III ) in liquid water : A molecular dynamics study of structure and dynamics of the whole series Building a polarizable pair interaction potential for lanthanoids „ III … in liquid water, (2009). [CrossRef]
- R. Nakamoto, B. Xu, C. Xu, H. Xu, L. Bellaiche, Properties of rare-earth iron garnets from first principles, Phys. Rev. B. 95 (2017) 024434. [CrossRef]
- W. Lee, S.Y. Chen, E. Tseng, A. Gloter, C.L. Chen, Study of defect structure in ferromagnetic nanocrystalline CeO2: Effect of ionic radius, J. Phys. Chem. C. 120 (2016) 14874–14882. [CrossRef]
- H. Yamamura, H. Nishino, K. Kakinuma, K.N. Þ, Crystal Phase and Electrical Conductivity in the Pyrochlore Type, J. Ceram. Sociery Japan. 11 (2003) 902–906.
- M.A. Subramanian, G. Aravamudan, G. V. Subba Rao, Oxide pyrochlores - A review, Prog. Solid State Chem. 15 (1983) 55–143. [CrossRef]
- L. Dong, Y. Li, R. Devanathan, F. Gao, Molecular dynamics simulation of the structural, elastic, and thermal properties of pyrochlores, RSC Adv. 6 (2016) 41410–41419. [CrossRef]
- L. Dong, W. Setyawan, Y. Li, R. Devanathan, F. Gao, Molecular dynamics simulation of low-energy recoil events in titanate pyrochlores, RSC Adv. 7 (2017) 35403–35410. [CrossRef]
- A.E. Sand, K. Nordlund, S.L. Dudarev, Radiation damage production in massive cascades initiated by fusion neutrons in tungsten, J. Nucl. Mater. 455 (2014) 207–211. [CrossRef]
- N. Chen, D. Huang, E.R. Heller, D.A. Cardimona, F. Gao, Atomistic simulation of displacement damage and effective nonionizing energy loss in InAs, Phys. Rev. Mater. 5 (2021). [CrossRef]
- R. Devanathan, W.J. Weber, F. Gao, Atomic scale simulation of defect production in irradiated 3C-SiC, J. Appl. Phys. 90 (2001) 2303–2309. [CrossRef]
- B.P. Mandal, K. Bhattacharyya, J.H. Zain, V. Sudarsan, S. Nigam, C. Nayak, A.K. Tyagi, Variation of structural disorder in Zr substituted Y2Sn2O7: Its impact on photocatalysis, J. Solid State Chem. 303 (2021) 122472. [CrossRef]
- L. Cai, A.L. Arias, J.C. Nino, The tolerance factors of the pyrochlore crystal structure, J. Mater. Chem. 21 (2011) 3611–3618. [CrossRef]
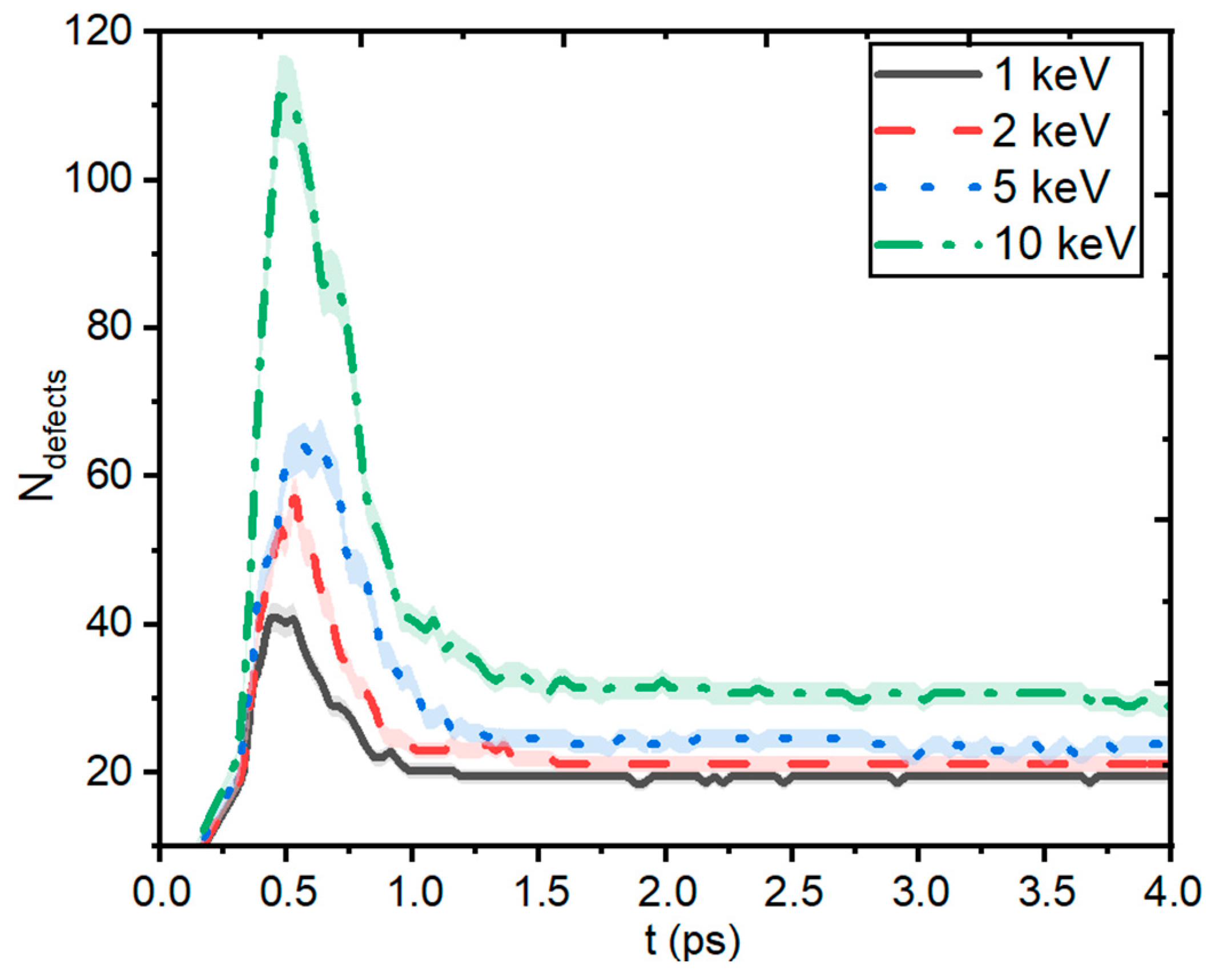
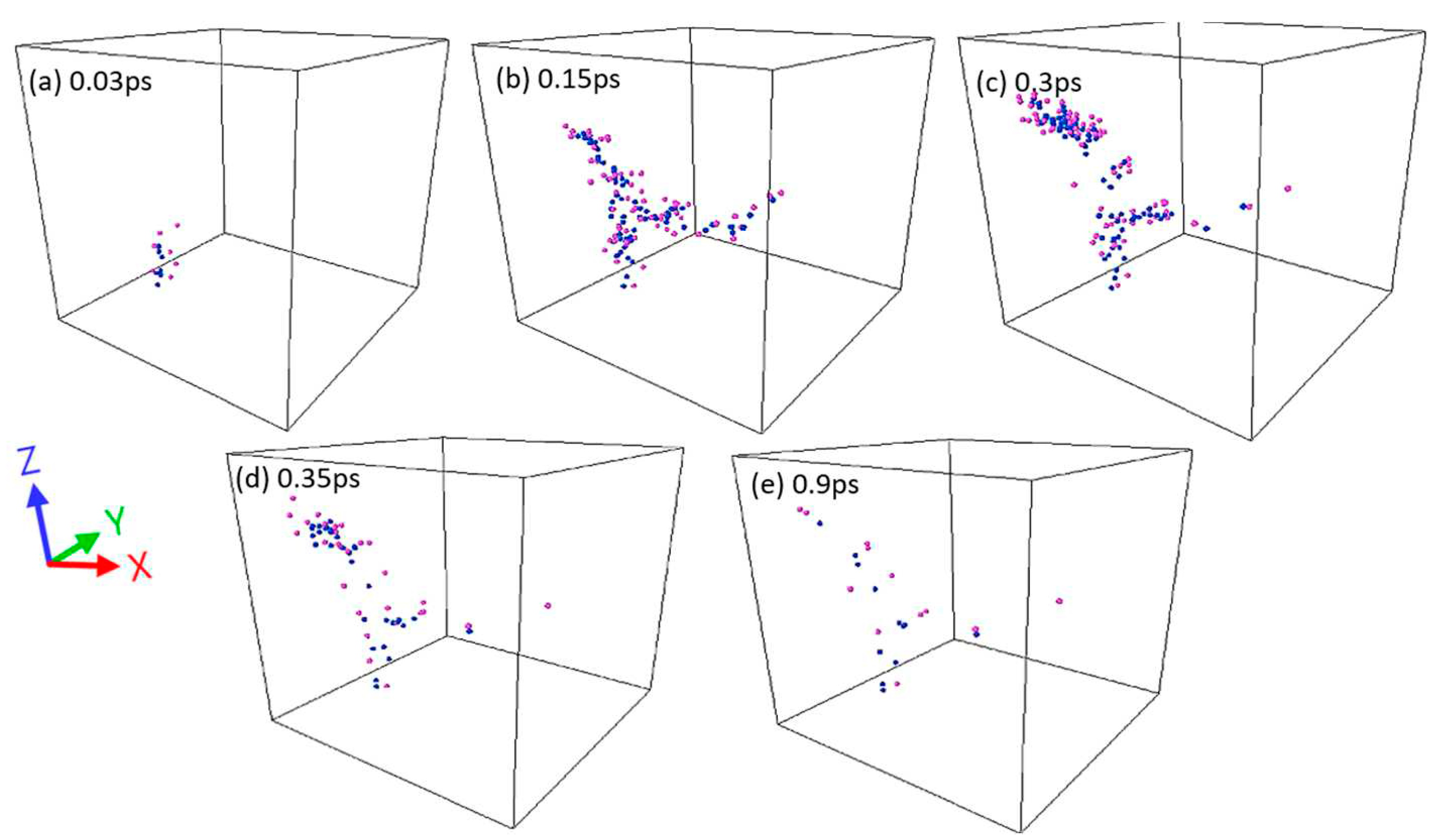
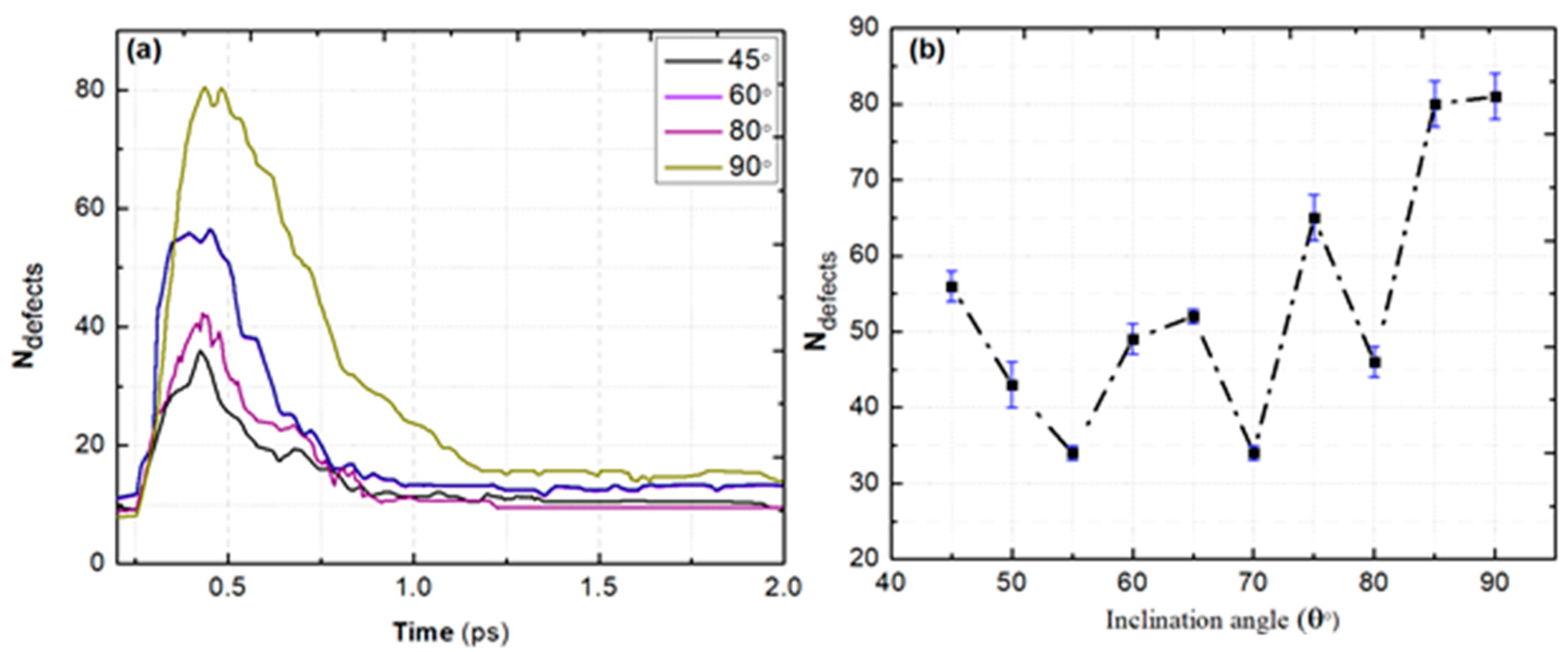
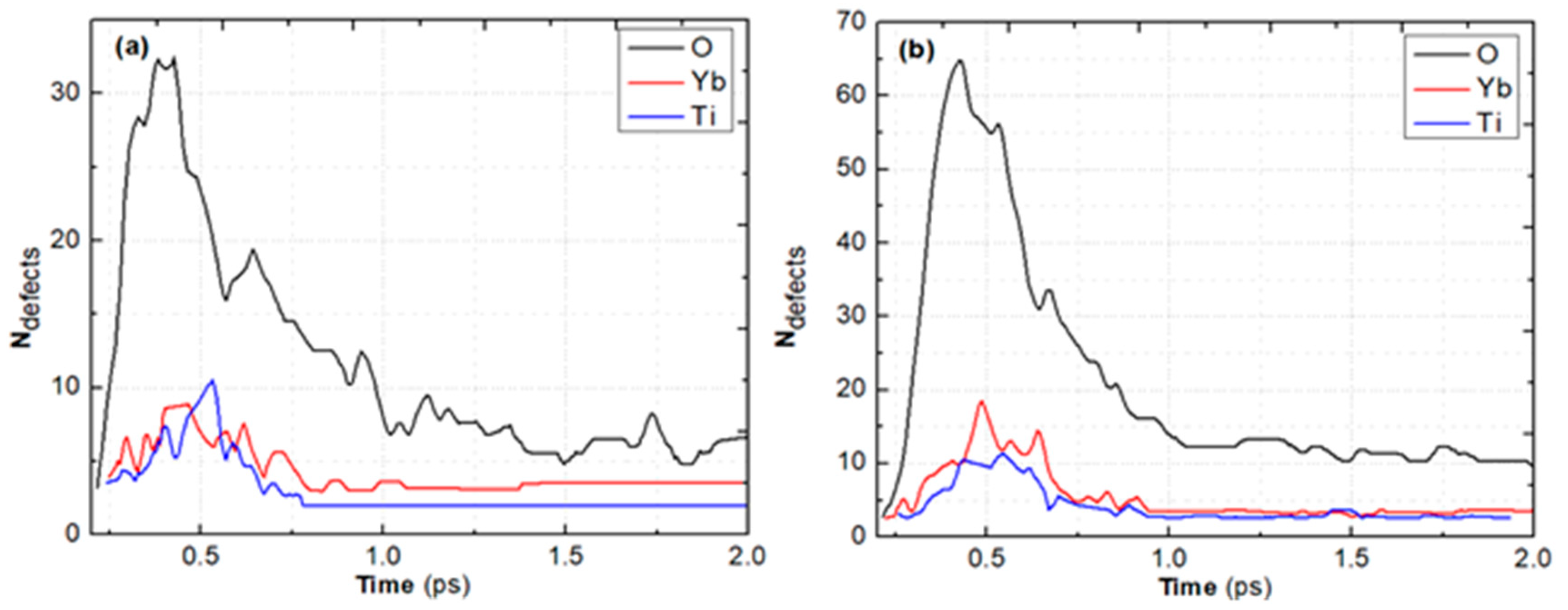

| Pair (s) | eV) | Å) | ) | Å) | Å) | ||||
| Yb-O | 1649.80 | 0.3386 | 16.57 | 10.40 | -8.38 | 3.08 | -0.55 | 0.6 | 1.3 |
| Ti-O | 2131.40 | 0.3038 | 0.400 | 10.62 | -14.80 | -14.74 | -6.28 | 0.3 | 2.0 |
| O-O | 9547.96 | 0.2192 | 32.00 | 9.355 | -10.71 | 6.23 | -1.68 | 0.8 | 2.1 |
| Types of PKA | PKA direction | Ed (eV) | Types of PKA | PKA direction | Ed (eV) | |
|---|---|---|---|---|---|---|
| Yb | [100] | 60 | Ti | [100] | 1182 | |
| [110] | 60 | [110] | 1177 | |||
| [111] | 66 | [111] | 625 | |||
| Y | [100] | 146 | Zr | [100] | 430 | |
| [110] | 146 | [110] | 431 | |||
| [111] | 174 | [111] | 426 | |||
| Gd | [100] | 93 | O | [100] | 1769 | |
| [110] | 93 | [110] | 1521 | |||
| [111] | 84 | [111] | 343 | |||
| Eu | [100] | 85 | ||||
| [110] | 85 | |||||
| [111] | 128 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




