Submitted:
17 August 2023
Posted:
18 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Synthesis of Naturally Occurring Metabolites
3. Synthesis of Isotope-labelled Metabolites
4. Synthesis of Pharmaceutical Drug Metabolites
5. Synthesis of Metabolite-like Compounds
6. Discussion
8. Future Directions
Funding
Acknowledgments
Conflicts of Interest
References
- Schreiber, S.L. Small molecules: the missing link in the central dogma. Nat. Chem. Biol. 2005, 1(2), 64–66. [Google Scholar] [CrossRef] [PubMed]
- McKnight, S.L. Back to the future: molecular biology meets metabolism. In: Cold Spring Harbor Symposia on Quantitative Biology 2011, 76, 403-411. Cold Spring Harbor Laboratory Press. [CrossRef]
- Kell, D.B.; Oliver, S. The metabolome 18 years on: a concept comes of age. Metabolomics 2016, 12, 148. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics by Gas Chromatography-Mass Spectrometry: the combination of targeted and untargeted profiling. Curr. Protoc. Mol. Biol. 2017, 114, 30.4.1–30.4.32. [Google Scholar] [CrossRef] [PubMed]
- Plumb, R.S.; Gethings, L.A.; Rainville, P.D.; Isaac, G.; Trengove, R.; King, A.M.; Wilson, I.D. Advances in high throughput LC/MS based metabolomics: A review. Trends Anal. Chem. 2023, 116954. [Google Scholar] [CrossRef]
- Emwas, A.H.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Gowda, G.N.; Raftery, D.; Alahmari, F.; Jaremko, L.; Jaremko, M.; Wishart, D.S. NMR spectroscopy for metabolomics research. Metabolites 2019, 9(7), 123. [Google Scholar] [CrossRef]
- Guijas, C; Montenegro-Burke, J.R.; Domingo-Almenara, X.; Palermo, A.; Warth, B.; Hermann, G.; Koellensperger, G.; Huan, T.; Uritboonthai, W.; Aisporna, A.E.; Wolan, D.W.; Spilker, M.E.; Benton, H.P.; Siuzdak, G. METLIN: A Technology Platform for Identifying Knowns and Unknowns. Anal. Chem. 2018, 90, 3156–3164. [Google Scholar] [CrossRef]
- Wishart, D.S.; Cheng, L.L.; Copié, V.; Edison, A.S.; Eghbalnia, H.R.; Hoch, J.C.; Gouveia, G.J.; Pathmasiri, W.; Powers, R.; Schock, T.B.; Sumner, L.W. NMR and metabolomics—A roadmap for the future. Metabolites 2022, 12(8), 678. [Google Scholar] [CrossRef]
- Hoch, J.C.; Baskaran, K.; Burr, H.; Chin, J.; Eghbalnia, H.R.; Fujiwara, T.; Gryk, M.R.; Iwata, T.; Kojima, C.; Kurisu, G.; Maziuk, D.; Miyanoiri, Y.; Wedell, J.R.; Wilburn, C.; Yao, H.; Yokochi, M. Nucleic Acids Res. 2023, 51(D1), D368–D376. [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic Acids Res. 2014, 42(D1), D199–D205. [Google Scholar] [CrossRef]
- Hastings, J.; Owen, G.; Dekker, A.; Ennis, M.; Kale, N.; Muthukrishnan, V.; Turner, S.; Swainston, N.; Mendes, P.; Steinbeck, C. ChEBI in 2016: Improved services and an expanding collection of metabolites. Nucleic Acids Res. 2016, 44(D1), D1214–D1219. [Google Scholar] [CrossRef]
- van Santen, J.A.; Jacob, G.; Singh, A.L.; Aniebok, V.; Balunas, M.J.; Bunsko, D.; Neto, F.C.; Castaño-Espriu, L.; Chang, C.; Clark, T.N.; Little, J.L.C.; Delgadillo, D.A.; Dorrestein, P.C.; Duncan, K.R.; Egan, J.M.; Galey, M.M.; Haeckl, F.P.J.; Hua, A.; Hughes, A.H.; Iskakova, D.; Khadilkar, A.; Lee, J.H.; Lee, S.; LeGrow, N.; Dennis, Y. Liu, D.Y.; Macho, J.M.; McCaughey, C.S.; Medema, M.H.; Neupane, R.P.; O’Donnell, T.J.; Paula, J.S.; Sanchez, L.M.; Shaikh, A.F.; Soldatou, S.; Terlouw, B.R.; Tran, T.A.; Valentine, M.; van der Hooft, J.J.J.; Vo, D.A.; Wang, M.; Wilson, D.; Zink, K.E.; Linington, R.G. The Natural Products Atlas: An Open Access Knowledge Base for Microbial Natural Products Discovery. ACS Cent. Sci. 2019, 5, 1824–1833. [Google Scholar] [PubMed]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L; Berjanskii, M. HMDB 5.0: the human metabolome database for 2022. Nucleic Acids Res. 2022, 50(D1), D622–D631. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Oler, E.; Peters, H.; Guo, A.; Girod, S.; Han, S.; Saha, S.; Lui, V.W.; LeVatte, M.; Gautam, V.; Kaddurah-Daouk, R.; Karu, N. MiMeDB: the Human Microbial Metabolome Database. Nucleic Acids Res. 2023, 51(D1), D611–D620. [Google Scholar] [CrossRef]
- Sajed, T.; Marcu, A.; Ramirez, M.; Pon, A.; Guo, A.; Knox, C.; Wilson, M.; Grant, J.; Djoumbou, Y.; Wishart, D. ECMDB 2.0: A richer resource for understanding the biochemistry of E. coli. Nucleic Acids Res. 2016, 44(D1), D495–D501. [Google Scholar] [CrossRef]
- Huang, W.; Luke, K. Brewer, L.K.; Jace W. Jones, J.W.; Angela T. Nguyen, A.T.; Ana Marcu, A.; David S. Wishart, D.S.; Amanda G. Oglesby-Sherrouse, A.G.; Maureen A. Kane, M.A.; Angela Wilks, A. PAMDB: a comprehensive Pseudomonas aeruginosa metabolome database. Nucleic Acids Res. 2018, 46(D1), D575–D580. [Google Scholar] [CrossRef] [PubMed]
- Moumbock, A.F.A.; Gao, M.; Qaseem, A.; Li, J.; Kirchner, P.A.; Ndingkokhar, B.; Bekono, B.D.; Simoben, C.V.; Babiaka, S.M.; Malange, Y.I.; Sauter, F.; Zierep, P.; Ntie-Kang, F.; Günther, S. StreptomeDB 3.0: an updated compendium of streptomycetes natural products. Nucleic Acids Res. 2021, 49(D1), D600–D604. [Google Scholar] [CrossRef]
- Jones, M.R.; Pinto, E.; Torres, M.A.; Dörr, F.; Mazur-Marzec, H.; Szubert, K.; Tartaglione, L.; Dell’Aversano, C.; Miles, C.O.; Beach, D.G.; McCarron, P.; Sivonen, K.; Fewer, D.P.; Jokela, J.; Janssen, E.M.L. CyanoMetDB, a comprehensive public database of secondary metabolites from cyanobacteria. Water Res. 2021, 196, 117017. [Google Scholar] [CrossRef]
- Wang, D.G.; Wang, C.Y.; Hu, J.Q.; Wang, J.J.; Liu, W.C.; Zhang, W.J.; Du, X.R.; Wang, H.; Zhu, L.L.; Sui, H.Y.; Li, Y.Z.; Wu, C. Constructing a Myxobacterial Natural Product Database to Facilitate NMR-Based Metabolomics Bioprospecting of Myxobacteria. Anal. Chem. 2023, 95, 12, 5256–5266. [Google Scholar] [CrossRef]
- Ramirez-Gaona, M.; Marcu, A.; Pon, A.; Guo, A.C.; Sajed, T.; Wishart, N.A.; Karu, N.; Djoumbou Feunang, Y.; Arndt, D.; Wishart, D.S. YMDB 2.0: a significantly expanded version of the yeast metabolome database. Nucleic Acids Res. 2017, 45, D440–D445. [Google Scholar] [CrossRef]
- Foroutan, A.; Fitzsimmons, C.; Mandal, R.; Piri-Moghadam, H.; Zheng, J.; Guo, A.; Li, C.; Guan, L.L.; Wishart, D.S. The Bovine Metabolome. Metabolites 2020, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Takeshi Ara, T.; Sakurai, N.; Takahashi, S.; Waki, N.; Suganuma, H.; Aizawa, K.; Matsumura, Y.; Kawada, T.; Shibata, D. TOMATOMET: A metabolome database consists of 7118 accurate mass values detected in mature fruits of 25 tomato cultivars. Plant Direct. 2021, 5, e00318. [Google Scholar] [CrossRef]
- Link, H.; Kochanowski, K; Sauer, U. Systematic identification of allosteric protein-metabolite interactions that control enzyme activity in vivo. Nat. Biotechnol. 2013, 31, 357–361. [Google Scholar] [CrossRef]
- Piazza, I.; Kochanowski, K.; Cappelletti, V.; Fuhrer, T.; Noor, E.; Sauer, U.; Picotti, P. A map of protein-metabolite interactions reveals principles of chemical communication. Cell 2018, 172(1), 358–372. [Google Scholar] [CrossRef] [PubMed]
- Rinschen, M.R.; Ivanisevic, J.; Giera, M.; Siuzdak, G. Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 2019, 20(6), 353–367. [Google Scholar] [CrossRef]
- Buescher, J.M.; Antoniewicz, M.R.; Boros, L.G.; Burgess, S.C.; Brunengraber, H.; Clish, C.B.; DeBerardinis, R.J.; Feron, O.; Frezza, C., Ghesquiere, B.; Gottlieb, E.; Hiller, K., Jones, R.G.; Kamphorst, J.J.; Kibbey, R.G.; Kimmelman, A.C.; Locasale, J.W.; Lunt, S.Y.; Maddocks, O.D.K.; Malloy, C.; Metallo, C.M., Meuillet, E.J.; Munger, J.; Nöh, K.; Rabinowitz, J.D.; Ralser, M.; Sauer, U.; Stephanopoulos, G.; St-Pierre, J.; Tennant, D.A.; Wittmann, C.; Vander Heiden, M.G.; Vazquez, A.; Vousden, K.; Young, J.D. Zamboni, N.; Fendt, S.M. A roadmap for interpreting 13C metabolite labeling patterns from cells. Curr. Opin. Biotechnol. 2015, 34, 189–201. [CrossRef] [PubMed]
- Di Yu, D.; Zhou, L.; Liu, X.; Xu, G. Stable isotope-resolved metabolomics based on mass spectrometry: Methods and their applications. Trends Anal. Chem. 2023, 160, 116985. [Google Scholar] [CrossRef]
- Han, S.; Van Treuren, W.; Fischer, C.R.; Merrill, B.D.; DeFelice, B.C.; Sanchez, J.M.; Higginbottom, S.K.; Guthrie, L.; Fall, L.A.; Dodd, D.; Fischbach, M.A.; Sonnenburg, J.I. A metabolomics pipeline for the mechanistic interrogation of the gut microbiome. Nature 2021, 595, 415–420. [Google Scholar] [CrossRef]
- Schultheisz, H.L.; Szymczyna, B.R.; Scott, L.G.; Williamson, J.R. Pathway Engineered Enzymatic de novo Purine Nucleotide Synthesis. ACS Chem Biol. 2008, 3(8), 499–511. [Google Scholar] [CrossRef]
- Rowbotham, J.S.; Ramirez, M.A.; Lenz, O.; Reeve, H.A.; Vincent, K.A. Bringing biocatalytic deuteration into the toolbox of asymmetric isotopic labelling techniques. Nat. Commun. 2020, 11(1), 1454. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Snyder, S.A. Chasing molecules that were never there: misassigned natural products and the role of chemical synthesis in modern structure elucidation. Angew. Chem. Int. Ed. 2005, 44(7), 1012–1044. [Google Scholar] [CrossRef] [PubMed]
- Sunazuka, T.; Hirose, T.; Omura, S. Efficient Total Synthesis of Novel Bioactive Microbial Metabolites. Acc. Chem. Res. 2008, 41(2), 302–314. [Google Scholar] [CrossRef]
- Nicolaou, K.C. Organic synthesis: the art and science of replicating the molecules of living nature and creating others like them in the laboratory. Proc. R. Soc. A 2014, 470, 20130690. [Google Scholar] [CrossRef]
- Wohlgemuth, R. Route Selection and Reaction Engineering for Sustainable Metabolite Synthesis. React. Chem. Eng. 2023, Advance Article. [Google Scholar] [CrossRef]
- Demain, A.L. From natural products discovery to commercialization: a success story. J. Ind. Microbiol. Biotechnol. 2006, 33, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Hoff, B.; Plassmeier, J.; Blankschien, M.; Letzel, A.C.; Kourtz, L.; Schröder, H.; Koch, W.; Zelder, O. Unlocking Nature's Biosynthetic Power—Metabolic Engineering for the Fermentative Production of Chemicals. Angew. Chem. Int. Ed. 2021, 60(5), 2258–2278. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Eun, H.; Prabowo, C.P.S.; Cho, S.; Lee, S.Y. Metabolic and cellular engineering for the production of natural products. Curr. Opin. Biotechnol. 2022, 77, 102760. [Google Scholar] [CrossRef]
- Wohlgemuth, R. 2022. Selective biocatalytic defunctionalization of raw materials. ChemSusChem 2022, 15(9), e202200402. [Google Scholar] [CrossRef]
- Kaiser, R. Scent of the Vanishing Flora. ISBN 13: 978-3-906390-64-2, Wiley-VHCA AG, Zürich, Switzerland, 2010.
- Walsh, C.T.; Tang, Y. Natural product biosynthesis – Chemical logic and enzymatic machinery. Royal Society of Chemistry, London, UK, 2017.
- Alcántara, A.R.; Dominguez de Maria, P.; Littlechild, J.A.; Schürmann, M.; Sheldon, R.A.; Wohlgemuth, R. Biocatalysis as key to sustainable industrial chemistry. ChemSusChem 2022, 15(9), e202102709. [Google Scholar] [CrossRef]
- Wohlgemuth, R. Tools and ingredients for the biocatalytic synthesis of metabolites. Biotechnol. J. 2009, 4(9), 1253–1265. [Google Scholar] [CrossRef]
- Wohlgemuth, R. Horizons of systems biocatalysis and renaissance of metabolite synthesis. Biotechnol. J. 2018, 13(6), 1700620. [Google Scholar] [CrossRef] [PubMed]
- Oberg, N.; Zallot, R.; Gerlt, J.A. EFI-EST, EFI-GNT, and EFI-CGFP: Enzyme Function Initiative (EFI) Web Resource for Genomic Enzymology Tools. J. Mol. Biol. 2023, 435, 168018. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, C.; Liao, J.C. (Eds.) Industrial Biotechnology: Microorganism. First Edition, Wiley-VCH, Weinheim, Germany, 2017. ISBN: 978-3-527-34179-5.
- Wittmann, C.; Liao, J.C. (Eds.) Industrial Biotechnology: Products and Processes. First Edition, Wiley-VCH, Weinheim, Germany, 2017. ISBN: 978-3-527-34181-8.
- Lee, S.Y.; Nielsen, J.; Stephanopoulos, G. (Eds.) Metabolic Engineering - Concepts and Applications. First Edition, Wiley-VCH, Weinheim, Germany, 2021. ISBN: 978-3-527-34662-2.
- Flickinger, M.C. (Ed.) Encyclopedia of Industrial Biotechnology: Bioprocess, Bioseparation, and Cell Technology. Vol. 1-7, John Wliey & Sons, Hoboken, New Jersey, USA, 2010. ISBN: 978-0-471-79930-6.
- Wohlgemuth, R.; Littlechild, J. Complexity reduction and opportunities in the design, integration and intensification of biocata-lytic processes for metabolite synthesis. Front. Bioeng. Biotechnol. 2022, 10, 958606. [Google Scholar] [CrossRef]
- Wohlgemuth, R. Biocatalysis–Key enabling tools from biocatalytic one-step and multi-step reactions to biocatalytic total synthesis. New Biotechnol. 2021, 60, 113–123. [Google Scholar] [CrossRef]
- Wentrup, C. Origins of Organic Chemistry and Organic Synthesis. Eur. J. Org. Chem. 2022, e202101492. [Google Scholar] [CrossRef]
- Wöhler, F. Ueber künstliche Bildung des Harnstoffs. Ann. Phys. 1828, 88(2), 253–256. [Google Scholar] [CrossRef]
- Kolbe, H. Beiträge zur Kenntnis der gepaarten Verbindungen. Ann. Chem. Pharm. 1845, 54(2), 145–188. [Google Scholar] [CrossRef]
- Sheehan, J.C.; Henery-Logan, K.R. The total synthesis of penicillin V. J. Am. Chem. Soc. 1957, 79, 5, 1262–1263. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Vourloumis, D.; Winssinger, N.; Baran, P.S. The art and science of total synthesis at the dawn of the twenty-first century. Angew. Chem. Int. Ed. 2000, 39(1), 44–122. [Google Scholar] [CrossRef]
- Veitch, G.E.; Boyer, A.; Ley, S.V. The Azadirachtin Story. Angew. Chem. Int. Ed. 2008, 47, 9402–9429. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Rigol, S. Perspectives from nearly five decades of total synthesis of natural products and their analogues for biology and medicine. Nat. Prod. Rep. 2020, 37(11), 1404–1435. [Google Scholar] [CrossRef] [PubMed]
- Min, L.; Han, J.C.; Zhang, W.; Gu, C.C.; Zou, Y.P.; Li, C.C. Strategies and Lessons Learned from Total Synthesis of Taxol. Chem. Rev. 2023, 123, 8, 4934–4971. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Snyder, S.A. Chasing molecules that were never there: misassigned natural products and the role of chemical synthesis in modern structure elucidation. Angew. Chem. Int. Ed. 2005, 44, 1012–1044. [Google Scholar] [CrossRef] [PubMed]
- Peters, D.S.; Pitts, C.R.; McClymont, K.S.; Stratton, T.P.; Bi, C.; Baran, P.S. Ideality in Context: Motivations for Total Synthesis. Acc. Chem. Res. 2021, 54, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Cardwell, H.M.E.; Cornforth, J.W.; Duff, S.R.; Holtermann, H.; Robinson, R. Total synthesis of androgenic hormones. Chem. Ind. 1951, 20, 389–90. [Google Scholar]
- Woodward, R.B.; Sondheimer, F.; Taub, D. The total synthesis of cholesterol. J. Am. Chem. Soc. 1951, 73(7), 3548–3548. [Google Scholar] [CrossRef]
- Eschenmoser, A.; Wintner, C.E. Natural Product Synthesis and Vitamin B12: Total synthesis of vitamin B12 provided a framework for exploration in several areas of organic chemistry. Science 1977, 196(4297), 1410–1420. [Google Scholar] [CrossRef]
- Woodward, R.B. The total synthesis of vitamin B12. Pure Appl. Chem. 1973, 33(1), 145–178. [Google Scholar] [CrossRef] [PubMed]
- Kishi, Y. Palytoxin: an inexhaustible source of inspiration - personal perspective. Tetrahedron 2002, 58, 6239–6258. [Google Scholar] [CrossRef]
- Sears, J.E.; Boger, D.L. Total Synthesis of Vinblastine, Related Natural Products, and Key Analogues and Development of Inspired Methodology Suitable for the Systematic Study of Their Structure−Function Properties. Acc. Chem. Res. 2015, 48, 653–662. [Google Scholar] [CrossRef]
- Baran, P.S.; Maimone, T.J.; Richter, J.M. Total synthesis of marine natural products without using protecting groups. Nature 2007, 446, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Pasteur, L. Mémoire sur la fermentation appelée lactique. Comptes Rendus Chimie 1857, 45, 913–916. [Google Scholar]
- Buchner, E. Alkoholische Gährung ohne Hefezellen. Berichte der Deutschen Chemischen Gesellschaft 1897, 30, 117–124. [Google Scholar] [CrossRef]
- Krebs, H.A.; Henseleit, K. Untersuchungen über die Harnstoffbildung im Tierkörper. Klin. Wochenschr. 1932, 11, 757–759. [Google Scholar] [CrossRef]
- Krebs, H.A., Henseleit, K. Untersuchungen über die Harnstoffbildung im Tierkörper. II. Klin. Wochenschr. 1932, 11, 1137–1139. [CrossRef]
- Amato, A.; Becci, A.; Beolchini, F. Citric acid bioproduction: the technological innovation change. Crit. Rev. Biotechnol. 2020, 40(2), 199–212. [Google Scholar] [CrossRef] [PubMed]
- Elander, R.P. Industrial production of β-lactam antibiotics. Appl. Microbiol. Biotechnol. 2003, 61, 385–392. [Google Scholar] [CrossRef]
- Sanchez, S.; Rodríguez-Sanoja, R.; Ramos, A.; Demain. A.L. Our microbes not only produce antibiotics, they also overproduce amino acids. J. Antibiotics 2018, 71, 26–36. [Google Scholar] [CrossRef]
- Vandamme, E.J.; Revuelta, J.L. Industrial Biotechnology of Vitamins, Biopigments, and Antioxidants. Wiley-VCH, Weinheim, Germany, 2016. [CrossRef]
- Meyer, H.P.; Robins, K.T. Large scale bioprocess for the production of optically pure L-carnitine. Monatsh. Chemie 2005, 136, 1269–1277. [Google Scholar] [CrossRef]
- Ramírez-Rendon, D.; Passari, A.K.; Ruiz-Villafán, B.; Rodríguez-Sanoja, R.; Sánchez, S.; Demain, A.L. Impact of novel microbial secondary metabolites on the pharma industry. Appl. Microbiol. Biotechnol. 2022, 106, 1855–1878. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83(3), 770–803. [Google Scholar] [CrossRef] [PubMed]
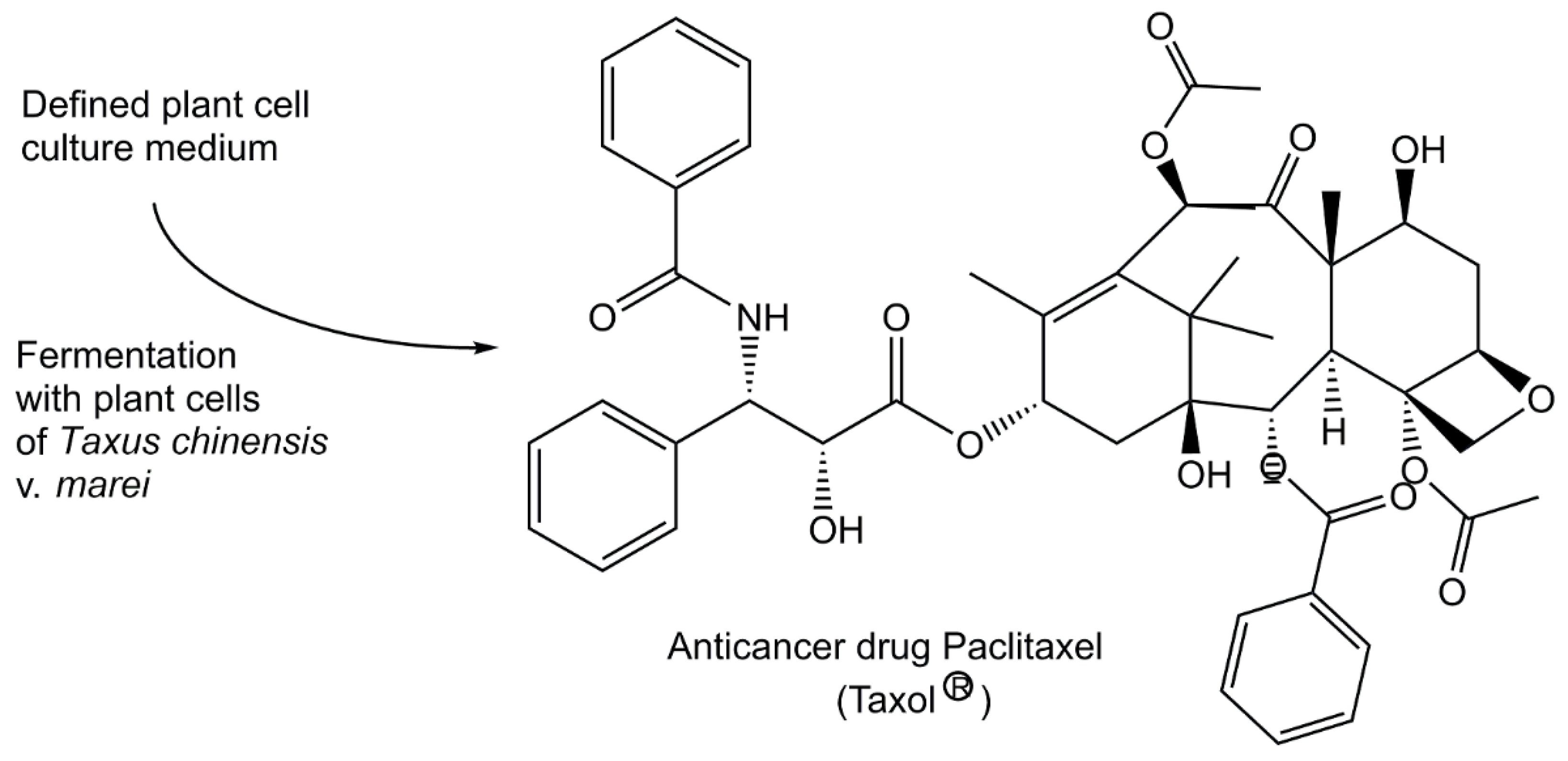
- Bringi, V.; Kadkade, P.G.; Prince, C.L.; Roach, B.L. Enhanced production of taxol and taxanes by cell cultures of Taxus species. US 2013/0017582 A1, 2013.
- Sharma, A.; Bhatia, S.K.; Banyal, A.; Chanana, I.; Kumar, A.; Chand, D.; Kulshrestha, S.; Kumar, P. An Overview on Taxol Production Technology and Its Applications as Anticancer Agent. Biotechnol. Bioprocess Engin. 2022, 27, 706–728. [Google Scholar] [CrossRef]
- Omura, S. A Splendid Gift from the Earth: The Origins and Impact of the Avermectins (Nobel Lecture). Angew. Chem. Int. Ed. 2016, 55, 10190–10209. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y. Artemisinin—A Gift from Traditional Chinese Medicine to the World (Nobel Lecture). Angew. Chem. Int. Ed. 2016, 55, 10210–10226. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, M.A.; Walsh, C.T. Antibiotics for Emerging Pathogens. Science 2009, 325(5944), 1089–1093. [Google Scholar] [CrossRef]
- Lewis, K. The Science of Antibiotic Discovery. Cell 2020, 181, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B., Moreira, R.; Li, Y.; Luzhetskyy, A.; Medema, M.H.; Pernodet, J.L.; Stadler, M.; Tormo, J.R.; Genilloud, O.; Truman, A.W.; Weissman, K.J.; Takano, E.; Sabatini, S.; Stegmann, E.; Brötz-Oesterhelt, H.; Wohlleben, W.; Seemann, M.; Empting, M.; Hirsch, A.K.H.; Loretz, B.; Lehr, C.M.; Titz, A.; Herrmann, J.; Jaeger, T.; Alt, S.; Hesterkamp, T.; Winterhalter, M.; Schiefer, A.; Pfarr, K.; Hoerauf, A.; Graz, H.; Graz, M.; Lindvall, M.; Ramurthy, S.; Karlén, A.; van Dongen, M.; Petkovic, H.; Keller, A.; Peyrane, F.; Stefano Donadio, S.; Fraisse, L.; Piddock, L.J.V.; Gilbert, I.H.; Moser, H.E.; Müller, R.. Towards the sustainable discovery and development of new antibiotics. Nat Rev Chem 2021, 5, 726–749. [CrossRef]
- Meyer, H.P.; Eichhorn, E.; Hanlon, S.; Lütz, S.; Schürmann, M.; Wohlgemuth, R.; Coppolecchia, R. The use of enzymes in organic synthesis and the life sciences: perspectives from the Swiss Industrial Biocatalysis Consortium (SIBC). Cat. Sci. Technol. 2013, 3(1), 29–40. [Google Scholar] [CrossRef]
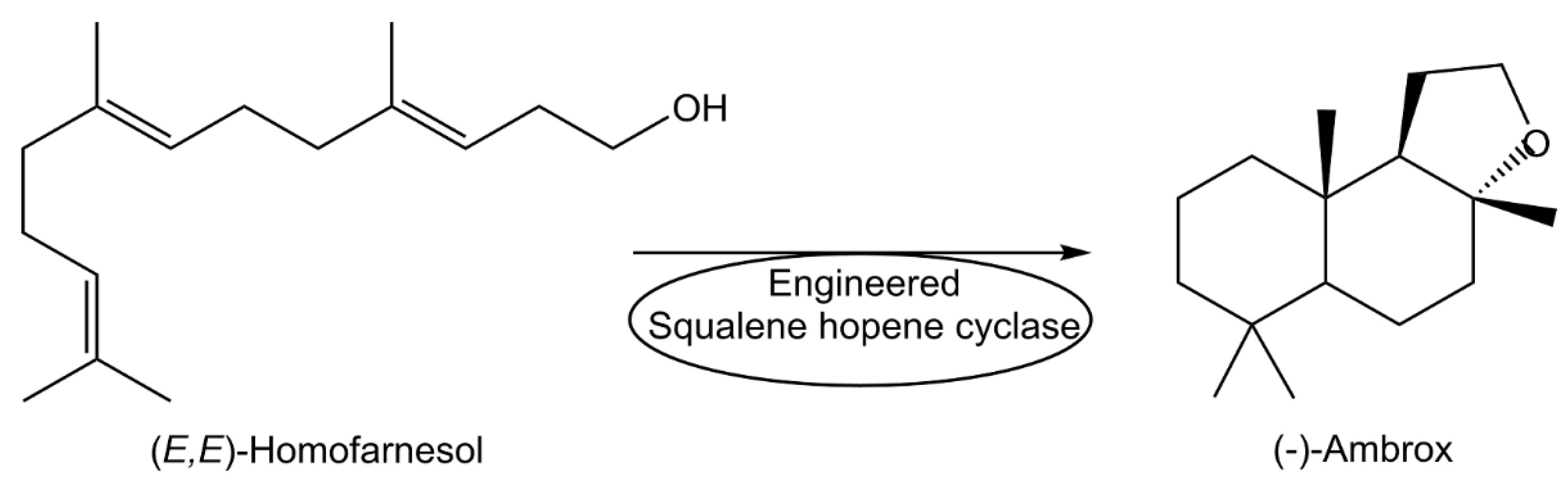
- Eichhorn, E.; Locher, E.; Guillemer, S.; Wahler, D.; Fourage, L.; Schilling, B. 2018. Biocatalytic process for (−)-Ambrox production using squalene hopene cyclase. Adv. Synth. Cat. 2018, 360(12), 2339–2351. [Google Scholar] [CrossRef]
- Calvillo, A.; Pellicer, T.; Carnicer, M.; Planas, A. Bioprocess Strategies for Vitamin B12 Production by Microbial Fermentation and Its Market Applications. Bioengineering 2022, 9, 365. [Google Scholar] [CrossRef]
- Bühlmann, P.; Pretsch, E.; Bakker, E. Carrier-Based Ion-Selective Electrodes and Bulk Optodes. 2. Ionophores for Potentiometric and Optical Sensors. Chem. Rev. 1998, 98, 1593−1687. [Google Scholar] [CrossRef]
- Jani, P.; Emmert, J.; Wohlgemuth, R. Process analysis of macrotetrolide biosynthesis during fermentation by means of direct infusion LC-MS. Biotechnol. J. 2008, 3(2), 202–208. [Google Scholar] [CrossRef] [PubMed]
- Cuartero, M.; Colozza, N.; Fernández-Pérez, B.M.; Crespo, G.A. Why ammonium detection is particularly challenging but insightful with ionophore-based potentiometric sensors – an overview of the progress in the last 20 years. Analyst 2020, 145, 3188–3210. [Google Scholar] [CrossRef] [PubMed]
- Gauss, D.; Schoenenberger, B.; Wohlgemuth, R. Chemical and enzymatic methodologies for the synthesis of enantiomerically pure glyceraldehyde 3-phosphates. Carbohydrate Res. 2014, 389, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Molla, G.S.; Kinfu, B.M.; Chow, J.; Streit, W.; Wohlgemuth, R.; Liese, A. Bioreaction engineering leading to efficient synthesis of L-glyceraldehyd-3-phosphate. Biotechnol. J. 2017, 12(3), 1600625. [Google Scholar] [CrossRef]
- Richter, N., Neumann, M., Liese, A., Wohlgemuth, R., Eggert, T. and Hummel, W., 2009. Characterisation of a Recombinant NADP-Dependent Glycerol Dehydrogenase from Gluconobacter oxydans and its Application in the Production of L-Glyceraldehyde. ChemBioChem 2009, 10(11), 1888–1896. [CrossRef]
- Richter, N.; Neumann, M.; Liese, A.; Wohlgemuth, R.; Weckbecker, A.; Eggert, T.; Hummel, W. Characterization of a whole-cell catalyst co-expressing glycerol dehydrogenase and glucose dehydrogenase and its application in the synthesis of L-glyceraldehyde. Biotechnol. Bioeng. 2010, 106(4), 541–552. [Google Scholar] [CrossRef] [PubMed]
- Gauss, D.; Sánchez-Moreno, I.; Oroz-Guinea, I.; García-Junceda, E.; Wohlgemuth, R. Phosphorylation catalyzed by dihydroxyacetone kinase. Eur. J. Org. Chem. 2018, 23, 2892–2895. [Google Scholar] [CrossRef]
- Hardt, N.; Kinfu, B.M.; Chow, J.; Schoenenberger, B.; Streit, W.R.; Obkircher, M.; Wohlgemuth, R. Biocatalytic Asymmetric Phosphorylation Catalyzed by Recombinant Glycerate-2-Kinase. ChemBioChem 2017, 18(15), 1518–1522. [Google Scholar] [CrossRef] [PubMed]
- Shaeri, J.; Wohlgemuth, R.; Woodley, J.M. Semiquantitative process screening for the biocatalytic synthesis of D-xylulose-5-phosphate. Org. Proc. Res. Dev. 2006, 10(3), 605–610. [Google Scholar] [CrossRef]
- Shaeri, J.; Wright, I.; Rathbone, E.B.; Wohlgemuth, R.; Woodley, J.M. Characterization of enzymatic D-xylulose 5-phosphate synthesis. Biotechnol. Bioeng. 2008, 101(4), 761–767. [Google Scholar] [CrossRef] [PubMed]
- Hardt, N.; Kind, S.; Schoenenberger, B.; Eggert, T.; Obkircher, M.; Wohlgemuth, R. Facile synthesis of D-xylulose-5-phosphate and L-xylulose-5-phosphate by xylulokinase-catalyzed phosphorylation. Biocat. Biotrans. 2020, 38(1), 35–45. [Google Scholar] [CrossRef]
- Hardt, N.; Kind, S.; Schoenenberger, B.; Eggert, T.; Obkircher, M.; Wohlgemuth, R. Kinase-Catalysed Phosphorylations of Xylulose Substrates and Synthesis of Xylulose-5-Phosphate Enantiomers. In: Applied Biocatalysis, Whittall, J.; Sutton, P.W. (Eds.) John Wiley & Sons, Hoboken, USA, 2021, 397-401.
- Schoenenberger, B.; Kind, S.; Meier, R.; Eggert, T.; Obkircher, M.; Wohlgemuth, R. Efficient biocatalytic synthesis of D-tagatose 1, 6-diphosphate by LacC-catalysed phosphorylation of D-tagatose 6-phosphate. Biocat. Biotrans. 2020, 38(1), 53–63. [Google Scholar] [CrossRef]
- Schoenenberger, B.; Kind, S.; Meier, R.; Eggert, T.; Obkircher, M.; Wohlgemuth, R. Kinase-Catalysed Phosphorylations of Ketohexose Phosphates and LacC-Catalysed Synthesis of D-Tagatose-1, 6-Diphosphate Lithium Salt. In: Applied Biocatalysis, Whittall, J.; Sutton, P.W. (Eds.) John Wiley & Sons, Hoboken, USA, 2021, 393-397.
- Krevet, S.; Shen, L.; Bohnen, T.; Schoenenberger, B.; Meier, R.; Obkircher, M.; Bangert, K.; Koehling, R.; Allenspach, E.; Wohlgemuth, R.; Siebers, B. Enzymatic synthesis of 2-keto-3-deoxy-6-phosphogluconate by the 6-phosphogluconate-dehydratase from Caulobacter crescentus. Front. Bioeng. Biotechnol. 2020, 8, 185. [Google Scholar] [CrossRef]
- Shen, L.; Kohlhaas, M.; Enoki, J.; Meier, R.; Schönenberger, B.; Wohlgemuth, R.; Kourist, R.; Niemeyer, F.; van Niekerk, D.; Bräsen, C.; Niemeyer, J. Snoep, J.; Siebers, B. A combined experimental and modelling approach for the Weimberg pathway optimisation. Nat. Commun. 2020, 11(1), 1098. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; Köhling, R.; Schönenberger, B.; Kouril, T.; Esser, D.; Bräsen, C.; Siebers, B.; Wohlgemuth, R. One-step synthesis of 2-keto-3-deoxy-D-gluconate by biocatalytic dehydration of D-gluconate. J. Biotechnol. 2014, 191, 69–77. [Google Scholar] [CrossRef]
- Schoenenberger, B.; Wszolek, A.; Milesi, T.; Brundiek, H.; Obkircher, M.; Wohlgemuth, R. Synthesis of Nω-Phospho-L-arginine by Biocatalytic Phosphorylation of L-Arginine. ChemCatChem 2017, 9(1), 121–126. [Google Scholar] [CrossRef]
- Schoenenberger, B.; Wszolek, A.; Milesi, T.; Brundiek, H.; Obkircher, M.; Wohlgemuth, R. Phosphoramidates by Kinase-Catalysed Phosphorylation and Arginine Kinase-Catalysed Synthesis of N𝛚-Phospho-L-Arginine. In: Applied Biocatalysis, Whittall, J.; Sutton, P.W. (Eds.) John Wiley & Sons, Hoboken, USA, 2021, 401-407.
- Schoenenberger, B.; Wszolek, A.; Meier, R.; Brundiek, H.; Obkircher, M.; Wohlgemuth, R. Recombinant AroL-Catalyzed Phosphorylation for the Efficient Synthesis of Shikimic Acid 3-Phosphate. Biotechnol. J. 2018, 13(8), 1700529. [Google Scholar] [CrossRef]
- Schoenenberger, B.; Wszolek, A.; Meier, R.; Brundiek, H.; Obkircher, M.; Wohlgemuth, R. Shikimate Kinase-Catalysed Phosphorylations and Synthesis of Shikimic Acid 3-Phosphate by AroL-Catalysed Phosphorylation of Shikimic Acid. Applied Biocatalysis, Whittall, J.; Sutton, P.W. (Eds.) John Wiley & Sons, Hoboken, USA, 2021, 386-393.
- Schell, U.; Wohlgemuth, R.; Ward, J.M. Synthesis of pyridoxamine 5′-phosphate using an MBA: pyruvate transaminase as biocatalyst. J. Mol. Catal. B: Enzymatic 2009, 59(4), 279–285. [Google Scholar] [CrossRef]
- Schoenenberger, B.; Wszolek, A.; Meier, R.; Brundiek, H.; Obkircher, M.; Wohlgemuth, R. Biocatalytic asymmetric Michael addition reaction of l-arginine to fumarate for the green synthesis of N-(([(4S)-4-amino-4-carboxy-butyl] amino) iminomethyl)-L-aspartic acid lithium salt (L-argininosuccinic acid lithium salt). RSC Adv. 2017, 7(77), 48952–48957. [Google Scholar] [CrossRef]
- Schoenenberger, B.; Wszolek, A.; Meier, R.; Brundiek, H.; Obkircher, M.; Wohlgemuth, R. Biocatalytic Asymmetric Aza-Michael Addition Reactions and Synthesis of L-Argininosuccinate by Argininosuccinate Lyase ARG4-Catalysed Aza-Michael Addition of L-Arginine to Fumarate. In: Applied Biocatalysis, Whittall, J.; Sutton, P.W. (Eds.) John Wiley & Sons, Hoboken, USA, 2021, 204-210.
- Calvin, M. The Path of Carbon in Photosynthesis: The carbon cycle is a tool for exploring chemical biodynamics and the mechanism of quantum conversion. Science 1962, 135(3507), 879–889. [Google Scholar] [CrossRef]
- Rising, K.A.; Schramm, V.L. Enzymatic Synthesis of NAD+ with the Specific Incorporation of Atomic Labels. J. Am. Chem. Soc. 1994, 116, 6531–6536. [Google Scholar] [CrossRef]
- Tran, A.; Yokose, R.; Cen, Y. Chemo-enzymatic synthesis of isotopically labelled nicotinamide riboside. Org. Biomol. Chem. 2018, 16, 3662–3671. [Google Scholar] [CrossRef]
- Khoroshilov, A.V. Production of stable isotopes of light elements: past, present and future. J. Phys.: Conf. Ser. 2018, 1099, 012002. [Google Scholar] [CrossRef]
- Fan, T.W.M.; Lane, A.N. Applications of NMR spectroscopy to systems biochemistry. Progr. NMR Spectroscopy 2016, 92–93, 18–53. [Google Scholar] [CrossRef] [PubMed]
- Yu, D., Zhou, L., Liu, X. and Xu, G. Stable isotope-resolved metabolomics based on mass spectrometry: Methods and their applications. Trends Anal. Chem. 2023, 160, 116985. [CrossRef]
- Giraudeau, P. Quantitative NMR spectroscopy of complex mixtures. Chem. Commun., 2023, 59, 6627–6642. [Google Scholar] [CrossRef]
- Wohlgemuth, R.; Waespe-Sarcevic, N.; Seelig, J. Bilayers of Phosphatidylglycerol. A Deuterium and Phosphorus NuclearMagnetic Resonance Study of the Head-Group Region. Biochemistry 1980, 19, 3315–3321. [Google Scholar] [CrossRef]
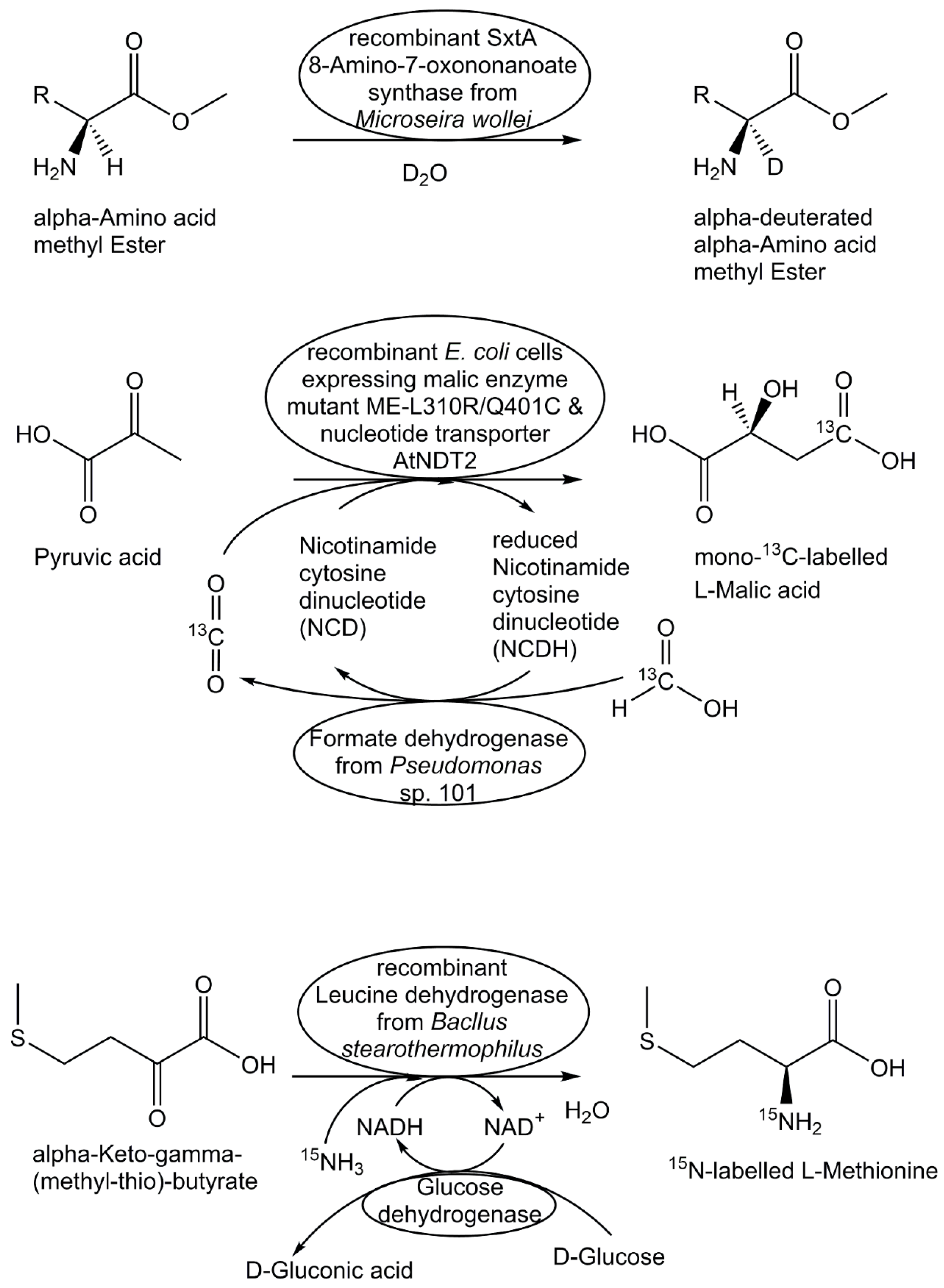
- Chun, S.W.; Narayan, A.R.H. Biocatalytic, Stereoselective Deuteration of α-Amino Acids and Methyl Esters. ACS Catal. 2020, 10(13), 7413–7418. [Google Scholar] [CrossRef]
- Doyon, T.J.; Buller, A.R. Site-Selective Deuteration of Amino Acids through Dual-Protein Catalysis. J. Am. Chem. Soc. 2022, 144, 16, 7327–7336. [Google Scholar] [CrossRef]
- Rowbotham, J.S.; Ramirez, M.A.; Lenz, O.; Reeve, H.A.; Vincent, K.A. Bringing biocatalytic deuteration into the toolbox of asymmetric isotopic labelling techniques. Nat. Commun. 2020, 11, 1454. [Google Scholar] [CrossRef]
- Rowbotham, J.S.; Hardy, A.P.; Reeve, H.A.; Vincent, K.A. Synthesis of [4S-2H] NADH, [4R-2H] NADH, [4-2H2] NADH and [4-2H] NAD+ cofactors through heterogeneous biocatalysis in heavy water J. Label. Compd. Radiopharm. 2021, 64, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lou, Y.; Wang, L.; Wang, Z.; Xu, W.; Ma, W.; Chen, Z.; Chen, X.; Wu, Q. Rational Design of Biocatalytic Deuteration Platform of Aldehydes. ACS Catal. 2021, 11, 21, 13348–13354. [Google Scholar] [CrossRef]
- Tolbert, T.J.; Williamson, J.R. Preparation of Specifically Deuterated RNA for NMR Studies Using a Combination of Chemical and Enzymatic Synthesis. J. Am. Chem. Soc. 1996, 118, 7929–7940. [Google Scholar] [CrossRef]
- Bennett, B.D.; Yuan, J.; Kimball, E.H.; Rabinowitz, J.D. Absolute quantitation of intracellular metabolite concentrations by an isotope ratio-based approach. Nat. Protoc. 2008, 3(8), 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Sauer, U. Metabolic networks in motion: 13C-based flux analysis. Molecular Systems Biol. 2006, 2, 62. [Google Scholar] [CrossRef] [PubMed]
- Arrivault, S.; Guenther, M.; Fry, S.C.; Fuenfgeld, M.F.F.F.; Veyel, D.; Mettler-Altmann, T.; Stitt, M.; Lunn, J.E. Synthesis and Use of Stable-Isotope-Labelled Internal Standards for Quantification of Phosphorylated Metabolites by LC–MS/MS. Anal. Chem. 2015, 87, 13, 6896–6904. [Google Scholar] [CrossRef]
- Eisenreich, W.; Schwarz, M.; Cartayrade, A.; Arigoni, D.; Zenk, M.H.; Bacher, A. The deoxyxylulose phosphate pathway of terpenoid biosynthesis in plants and microorganisms. Chem. Biol. 1998, 5(9), R221–R233. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, S.; Serianni, A.S. Labeling monosaccharides with stable isotopes. Methods Enzymol. 2015, 565, 423–458. [Google Scholar] [CrossRef] [PubMed]
- Goux, W.J.; Rench, L.; Weber, D.S. Stereoselective synthesis of stable isotope labeled L-α-amino acids: The enzymatic preparation of 13C-labeled L-glutamic acids. J. Label. Compd. Radiopharm. 1993, 33(3), 181–193. [Google Scholar] [CrossRef]
- Maeda, H.; Takata, K.; Toyoda, A.; Niitsu, T.; Iwakura, M.; Shibata, K. Production of L-[3-13C] serine from [13C] formaldehyde and glycine using an enzyme system combined with tetrahydrofolate regeneration. J. Ferment. Bioeng. 1997, 83(1), 113–115. [Google Scholar] [CrossRef]
- Jemielity, J.; Kańska, M.; Kański, R. Enzymatic Synthesis of [1-13C]-and [1-14C]-L-Phenyl-Alanine. Isot. Environ. Health Stud. 1998, 34(4), 335–339. [Google Scholar] [CrossRef]
- Akita, H.; Suzuki, H.; Doi, K.; Ohshima, T. Efficient synthesis of D-branched-chain amino acids and their labeled compounds with stable isotopes using D-amino acid dehydrogenase. Appl. Microbiol. Biotechnol. 2014, 98, 1135–1143. [Google Scholar] [CrossRef]
- Van Raad, D.; Huber, T.; Otting, G. Improved spectral resolution of [13C,1H]-HSQC spectra of aromatic amino acid residues in proteins produced by cell-free synthesis from inexpensive 13C-labelled precursors. J. Biomol. NMR 2023. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liu, Y.; Wang, Q.; Wang, X.; Li, Q.; Liu, W.; Zhao, Z.K. Non-natural Cofactor and Formate-Driven Reductive Carboxylation of Pyruvate. Angew. Chem. Int. Ed. 2020, 59(8), 3143–3146. [Google Scholar] [CrossRef]
- Morgan, K.D. The use of nitrogen-15 in microbial natural product discovery and biosynthetic characterization. Front. Microbiol. 2023, 14, 1174591. [Google Scholar] [CrossRef]
- Chiriaca, M.; Lupan, I.; Popa, F.; Palibroda, N.; Popescu, O. Enzymatic synthesis of some 15N-labelled L-amino acids. Isot. Environ. Health Stud. 2010, 46(2), 249–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Asam, S.; Chen, J.; Ehrmann, M.; Rychlik, M. Production of Four 15N-Labelled Cobalamins via Biosynthesis Using Propionibacterium freudenreichii. Front. Microbiol. 2021, 12, 713321. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; Assempour, N.; Iynkkaran, I.; Liu, Y.; Maciejewski, A.; Gale, N.; Wilson, A.; Chin, L.; Cummings, R.; Le, D.; Pon, A.; Knox, C.; Wilson, M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46(D1), D1074–D1082. [Google Scholar] [CrossRef]
- Clayton, T.A.; Baker, D.; Lindon, J.C.; Everett, J.R.; Nicholson, J.K. Pharmacometabonomic identification of a significant host-microbiome metabolic interaction affecting human drug metabolism. Proc. Natl. Acad. Sci. 2009, 106(34), 14728–14733. [Google Scholar] [CrossRef]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Wegmann, R.; Goodman, A. L. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature 2019, 570, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Javdan, B.; Lopez, J.G.; Chankhamjon, P.; Lee, Y.C.J.; Hull, R.; Wu, Q.; Wang, X.; Chatterjee, S.; Donia, M.S. Personalized mapping of drug metabolism by the human gut microbiome. Cell 2020, 181, 1661–1679.e22. [Google Scholar] [CrossRef] [PubMed]
- Heinken, A.; Hertel, J.; Acharya, G.; Ravcheev, D.A.; Nyga, M.; Okpala, O.E.; Hogan, M.; Magnúsdóttir, S.; Martinelli, F.; Nap, B.; Preciat, G.; Edirisinghe, J.N.; Henry, C.S.; Fleming, R.M.T.; Thiele, I. Genome-scale metabolic reconstruction of 7,302 human microorganisms for personalized medicine. Nat. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Fura, A.; Shu, Y.Z.; Zhu, M.; Hanson, R.L.; Roongta, V.; Humphreys, W.G. Discovering Drugs through Biological Transformation: Role of Pharmacologically Active Metabolites in Drug Discovery. J. Med. Chem. 2004, 47(18), 4339–4351. [Google Scholar] [CrossRef]
- Rautio, J.; Meanwell, N.A.; Di, L.; Hageman, M.J. The expanding role of prodrugs in contemporary drug design and development. Nat. Rev. Drug Discov. 2018, 17, 559–587. [Google Scholar] [CrossRef]
- Schadt, S.; Bister, B.; Chowdhury, S.K.; Funk, C.; Hop, C.E.C.A.; Humphreys, W.G.; Igarashi, F.; James, A.D.; Kagan, M.; Khojasteh, S.C.; Nedderman, A.N.R.; Prakash, C.; Runge, F.; Scheible, H.; Spracklin, D.K.; Swart, P.; Tse, S.; Yuan, J.; Obach, R.S. A Decade in the MIST: Learnings from Investigations of Drug Metabolites in Drug Development under the “Metabolites in Safety Testing” Regulatory Guidance. Drug Metab. Dispos. 2018, 46(6), 865–878. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration (FDA), Safety Testing of Drug Metabolites, 2020. https://www.fda.gov/media/72279/download.
- Luffer-Atlas, D.; Obach, R.S.; Smith, D.A. A MIST conception: what has been learned from twenty years of human metabolite safety assessment? Med. Chem. Res. 2023. [Google Scholar] [CrossRef]
- Chhatrapati Bisen, A.; Nashik Sanap, S.; Agrawal, S.; Biswas, A.; Sankar Bhatta, R. Chemical metabolite synthesis and profiling: Mimicking in vivo biotransformation reactions. Bioorganic Chemistry 2023, 139, 106722. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.; Geier, M.; Hanlon, S.P.; Nidetzky, B.; Glieder, A. Human enzymes for organic synthesis. Angew. Chem. Int. Ed. 2018, 57(41), 13406–13423. [Google Scholar] [CrossRef] [PubMed]
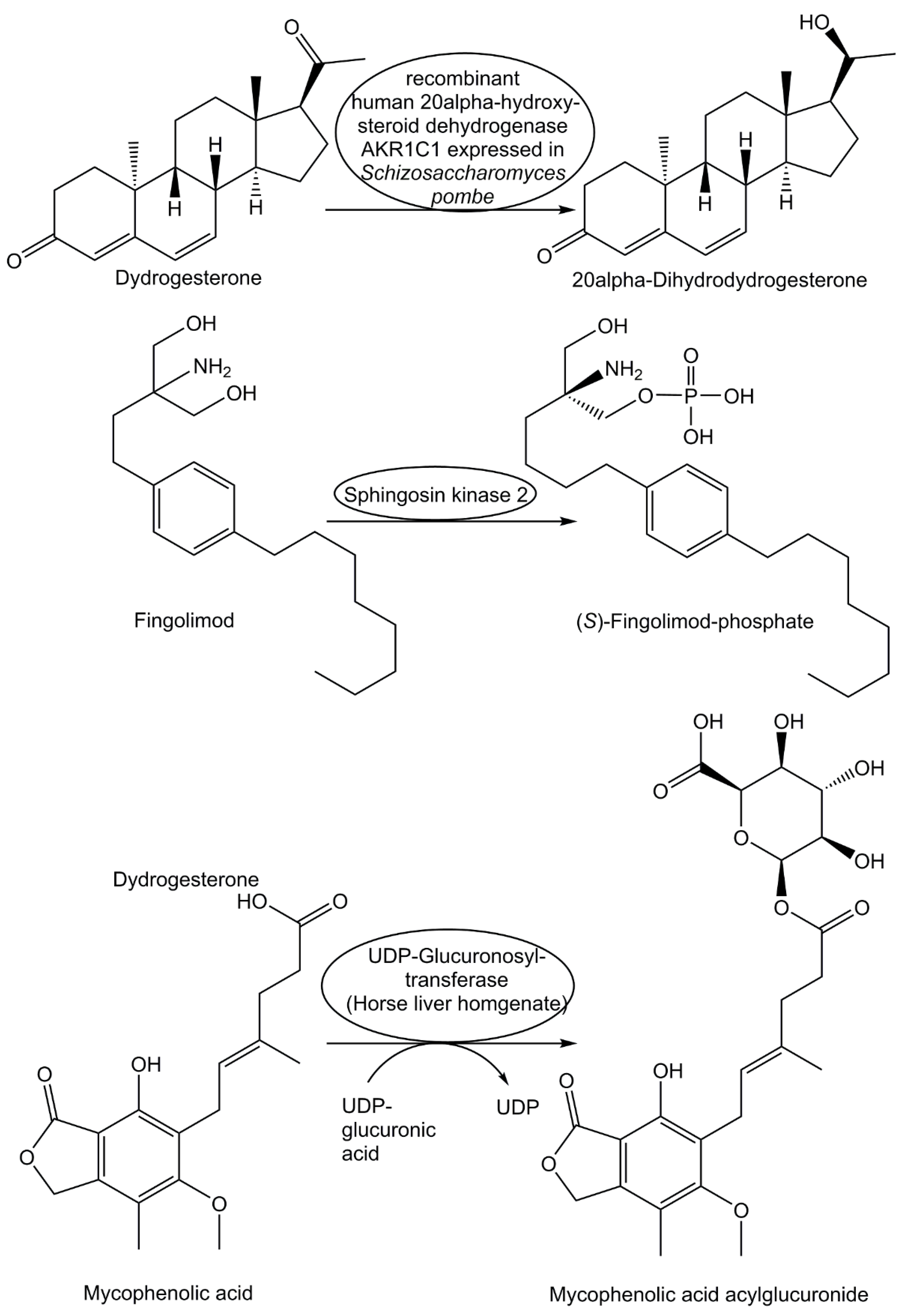
- Naumann, J.M.; Zöllner, A.; Drăgan, C.A.; Messinger, J.; Adam, J.; Bureik, M. Biotechnological Production of 20-alpha-Dihydrodydrogesterone at Pilot Scale. Appl. Biochem. Biotechnol. 2011, 165, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Billich, A.; Baumruker, T.; Heining, P.; Schmouder, R.; Francis, G.; Aradhye, S.; Burtin, P. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug Discov. 2010, 9(11), 883–897. [Google Scholar] [CrossRef] [PubMed]
- Kittelmann, M.; Rheinegger, U.; Espigat, A.; Oberer, L.; Aichholz, R.; Francotte, E.; Ghisalba, O. Preparative Enzymatic Synthesis of the Acylglucuronide of Mycophenolic Acid. Adv. Synth. Catal. 2003, 345, 825–829. [Google Scholar] [CrossRef]
- Rautio, J.; Kumpulainen, H.; Heimbach, T.; Oliyai, R.; Oh, D.; Järvinen, T.; Savolainen, J. Prodrugs: design and clinical applications. Nat. Rev. Drug Discov. 2008, 7, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Wolff, N.A.; Burckhardt, B.C.; Burckhardt, G.; Oellerich, M.; Armstrong, V.W. Mycophenolic acid (MPA) and its glucuronide metabolites interact with transport systems responsible for excretion of organic anions in the basolateral membrane of the human kidney. Nephrol. Dial. Transplant. 2007, 22, 2497–2503. [Google Scholar] [CrossRef]
- Park, B.K.; Boobis, A.; Clarke, S.; Goldring, C.E.P.; Jones, D.; Kenna, J.G.; Lambert, C.; Laverty, H.G.; Naisbitt, D.J.; Nelson, S.; Nicoll-Griffith, D.A.; Obach, R.S.; Routledge, P.; Smith, D.A.; Tweedie, D.J.; Vermeulen, N.; Williams, D.P.; Wilson, I.D.; Baillie, T.A. Managing the challenge of chemically reactive metabolites in drug development. Nat. Rev. Drug Discov. 2011, 10, 292–306. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, Y.; Ohe, T.; Ogawa, M.; Takahashi, K.; Nakamura, S.; Mashino, T. Development of Novel Diclofenac Analogs Designed to Avoid Metabolic Activation and Hepatocyte Toxicity. ACS Omega 2020, 5, 32608–32616. [Google Scholar] [CrossRef]
- Guengerich, F.P. A history of the roles of cytochrome P450 enzymes in the toxicity of drugs. Toxicol. Res. 2021, 37, 1–23. [Google Scholar] [CrossRef]
- Dahlin, D.C.; Miwa, G.T.; Lu, A.Y.; Nelson, S.D. N-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation pro-duct of acetaminophen. Proc. Natl. Acad. Sci. 1984, 81(5), 1327–1331. [Google Scholar] [CrossRef]
- Bender, R.P.; Lindsey, Jr., R.H.; Burden, D.A.; Osheroff, N. N-Acetyl-p-benzoquinone Imine, the Toxic Metabolite of Acetaminophen, Is a Topoisomerase II Poison. Biochemistry 2004, 43, 3731–3739. [CrossRef]
- Ertl, P.; Roggo, S.; Schuffenhauer, A. Natural Product-likeness Score and Its Application for Prioritization of Compound Libraries. J. Chem. Inf. Model, 2008; 48 (1), 168–174. [Google Scholar] [CrossRef]
- Tay, D.W.P.; Yeo, N.Z.X.; Adaikkappan, K.; Lim, Y.H.; Ang, S.J. 67 million natural product-like compound database generated via molecular language processing. Sci Data 2023, 10(1), 296. [Google Scholar] [CrossRef]
- Dobson, P.D.; Patel, Y.; Kell, D.B. Metabolite-likeness’ as a criterion in the design and selection of pharmaceutical drug libraries. Drug Discovery Today 2009, 14(1–2), 31–40. [Google Scholar] [CrossRef] [PubMed]
- O'Hagan, S.; Kell, D.B. Understanding the foundations of the structural similarities between marketed drugs and endogenous human metabolites. Front. Pharmacol. 2015, 6, 105. [Google Scholar] [CrossRef] [PubMed]
- O′Hagan, S.; Swainston, N.; Handl, J.; Kell, D.B. A ‘rule of 0.5’ for the metabolite-likeness of approved pharmaceutical drugs. Metabolomics 2015, 11, 323–339. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83(3), 770–803. [Google Scholar] [CrossRef]
- Ertl, P. Substituents of life: The most common substituent patterns present in natural products. Bioorg. Med. Chem. 54 (2022) 116562. [CrossRef] [PubMed]
- Shultz, M.D. Two Decades under the Influence of the Rule of Five and the Changing Properties of Approved Oral Drugs. J. Med. Chem. 2019, 62, 1701–1714. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.G.; Wobst, J.J. A Decade of FDA-Approved Drugs (2010−2019): Trends and Future Directions. J. Med. Chem. 2021, 64, 2312–2338. [Google Scholar] [CrossRef]
- Young, R.J.; Flitsch, S.L.; Grigalunas, M.; Leeson, P.D.; Quinn, R.J.; Turner, N.J.; Waldmann, H. The Time and Place for Nature in Drug Discovery. JACS Au 2022, 2, 2400–2416. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Rajpal, D.K.; Brown, J.R. Human microbial metabolites as a source of new drugs. Drug Discov. Today 2016, 21(4), 692–698. [Google Scholar] [CrossRef]
- Li, H.; Ranhotra, H.S.; Mani, S.; Dvořák, Z.; Sokol, H.; Müller, R. Human microbial metabolite mimicry as a strategy to expand the chemical space of potential drugs. Drug Discov. Today 2020, 25(9), 1575–1579. [Google Scholar] [CrossRef]
- Dvorák, Z.; Kopp, F.; Costello, C.M.; Kemp, J.S.; Li, H.; Vrzalová, A.; Stepánková, M.; Bartonková, I.; Jiskrová, E.; Poulíková, K.; Vyhlídalová, B.; Nordstroem, L.U.; Karunaratne, C.V.; Ranhotra, H.S.; Mun, K.S.; Naren, A.P.; Murray, I.A.; Perdew, G.H.; Brtko, J.; Toporova, L.; Schön, A.; Wallace, B.D.; Walton, W.G.; Redinbo, M.R.; Sun, K.; Beck, A.; Kortagere, S.; Neary, M.C.; Chandran, A.; Vishveshwara, S.; Cavalluzzi, M.M.; Lentini, G.; Cui, J.Y.; Gu, H.; March, J.C.; Chatterjee, S.; Matson, A.; Wright, D.; Flannigan, K.L.; Hirota, S.A.; Sartor, R.B.; Mani, S. Targeting the pregnane X receptor using microbial metabolite mimicry. EMBO Mol. Med. 2020, 12, e11621. [Google Scholar] [CrossRef]
- Xue, Y.P.; Cao, C.H.; Zheng, Y.G. Enzymatic asymmetric synthesis of chiral amino acids. Chem. Soc. Rev. 2018, 47, 1516–1561. [Google Scholar] [CrossRef] [PubMed]
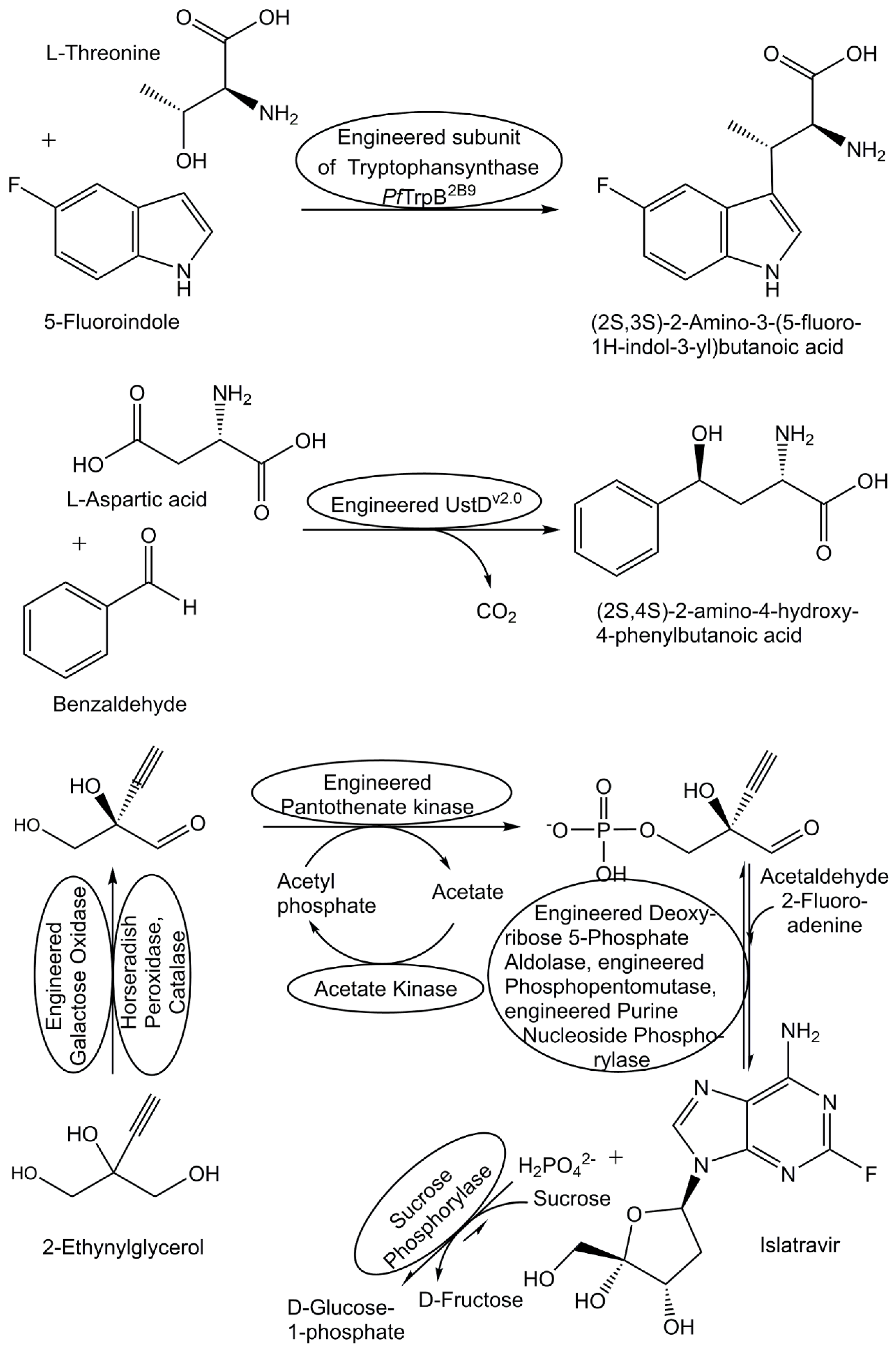
- Herger, M., van Roye, P., Romney, D.K., Brinkmann-Chen, S., Buller, A.R. and Arnold, F.H. Synthesis of β-branched tryptophan analogues using an engineered subunit of tryptophan synthase. J. Am. Chem. Soc. 2016, 138(27), 8388–8391. [CrossRef]
- Ellis, J.M.; Campbell, M.E.; Kumar, P.; Geunes, E.P.; Bingman, C.A.; Buller, A.R. Biocatalytic synthesis of non-standard amino acids by a decarboxylative aldol reaction. Nat. Catal. 2022, 5, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Alfonzo, E.; Das, A.; Arnold, F.H. New Additions to the Arsenal of Biocatalysts for Noncanonical Amino Acid Synthesis. Curr. Opin. Green Sustain. Chem. 2022, 38, 100701. [Google Scholar] [CrossRef] [PubMed]
- Löwe, J.; Dietz, K.J.; Gröger, H. From a Biosynthetic Pathway toward a Biocatalytic Process and Chemocatalytic Modifications: Three-Step Enzymatic Cascade to the Plant Metabolite cis-(+)-12-OPDA and Metathesis-Derived Products. Adv. Sci. 2020, 7(13), 1902973. [Google Scholar] [CrossRef]
- Westarp, S.; Kaspar, F.; Neubauer, P.; Kurreck, A. Industrial potential of the enzymatic synthesis of nucleoside analogs: existing challenges and perspectives. Curr. Opin. Biotechnol. 2022, 78, 102829. [Google Scholar] [CrossRef]
- Cosgrove, S.C.; Miller, G.J. Advances in biocatalytic and chemoenzymatic synthesis of nucleoside analogues, Expert Opin. Drug Discov. 2022, 17(4), 355–364. [Google Scholar] [CrossRef]
- Trung, M.N.; Kieninger, S.; Fandi, Z.; Qiu, D.; Liu, G.; Mehendale, N.K.; Saiardi, A.; Jessen, H.; Keller, B.; Fiedler, D. Stable Isotopomers of myo-Inositol Uncover a Complex MINPP1-Dependent Inositol Phosphate Network. ACS Cent. Sci. 2022, 8(12), 1683–1694. [Google Scholar] [CrossRef]
- Shen, B. A New Golden Age of Natural Products Drug Discovery. Cell 2015, 163, 1297–1300. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G. Atanasov, A.G., Zotchev, S.B., Dirsch, V.M. and Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20(3), 200–216. [Google Scholar] [CrossRef]
- Reetz, M.T.; Sun, Z.; Qu, G. Enzyme Engineering: Selective Catalysts for Applications in Biotechnology, Organic Chemistry, and Life Science. Wile-VCH, Weinhem, Germany, 2023. ISBN 978-3-527-35033-9.
- Chen, K.; Arnold, F.H. Engineering new catalytic activities in enzymes. Nat. Catal. 2020, 3(3), 203–213. [Google Scholar] [CrossRef]
- Zetzsche, L.E.; Chakrabarty, S.; Narayan, A.R. The transformative power of biocatalysis in convergent synthesis. J. Am. Chem. Soc. 2022, 144(12), 5214–5225. [Google Scholar] [CrossRef]
- Stout, C.N.; Wasfy, N.M.; Chen, F.; Renata, H. Charting the Evolution of Chemoenzymatic Strategies in the Syntheses of Complex Natural Products. J. Am. Chem. Soc. 2023. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Deng, H.; Renata, H. Chemoenzymatic approaches for exploring structure–activity relationship studies of bioactive natural products. Nat. Synth. 2023, 2, 708–718. [Google Scholar] [CrossRef]
- Hermann, J.C., Marti-Arbona, R., Fedorov, A.A., Fedorov, E., Almo, S.C., Shoichet, B.K. and Raushel, F.M. Structure-based activity prediction for an enzyme of unknown function. Nature 2007, 448(7155), 775–779.
- Zallot, R.; Oberg, N.; Gerlt, J.A. The EFI web resource for genomic enzymology tools: leveraging protein, genome, and metagenome databases to discover novel enzymes and metabolic pathways. Biochemistry 2019, 58(41), 4169–4182. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Wetmore, K.M.; Waters, R.J.; Callaghan, M.; Ray, J.; Liu, H.; Kuehl, J.V.; Melnyk, R.A.; Lamson, J.S.; Suh, Y.; Carlson, H.K.; Esquivel, Z.; Sadeeshkumar, H.; Chakraborty, R.; Zane, G.M.; Rubin, B.E.; Wall, J.D.; Visel, A.; Bristow, J.; Blow, M.J.; Arkin, A.P.; Deutschbauer, A.M. Mutant phenotypes for thousands of bacterial genes of unknown function. Nature 2018, 557, 503–509. [Google Scholar] [CrossRef]
- Robinson, S.L.; Piel, J.; Sunagawa, S. A roadmap for metagenomic enzyme discovery. Nat. Prod. Rep., 2021, 38, 1994–2023. [Google Scholar] [CrossRef]
- Caputi, L.; Franke, J.; Farrow, S.C.; Chung, K.; Payne, R.M.; Nguyen, T.D.; Dang, T.T.T.; Soares Teto Carqueijeiro, I.; Koudounas, K.; Dugé de Bernonville, T.; Ameyaw, B.; Jones, D.M.; Vieira, I.J.C.; Courdavault, V.; O’Connor, S.E. Missing enzymes in the biosynthesis of the anticancer drug vinblastine in Madagascar periwinkle. Science 2018, 360(6394), 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Shaw, S.; Augustijn, H.E.; Reitz, Z.L.; Biermann, F.; Alanjary, M.; Fetter, A.; Terlouw, B.R.; Metcalf, W.W.; Helfrich, E.J.N.; van Wezel, G.P.; Medema, M.H.; Weber, T. antiSMASH 7.0: new and improved predictions for detection, regulation, chemical structures and visualisation. Nucleic Acids Res. 2023, 51, W46–W50. [Google Scholar] [CrossRef] [PubMed]
- Caesar, L.K.; Montaser, R.; Keller, N.P.; Kelleher, N.L. Metabolomics and genomics in natural products research: complementary tools for targeting new chemical entities. Nat. Prod. Rep. 2021, 38(11), 2041–2065. [Google Scholar] [CrossRef]
- Scherlach, K.; Hertweck, C. Mining and unearthing hidden biosynthetic potential. Nat. Commun. 2021, 12(1), 3864. [Google Scholar] [CrossRef] [PubMed]
- Klapper, M.; Hübner, A.; Ibrahim, A.; Wasmuth, I.; Borry, M.; Haensch, V.G.; Zhang, S.; Al-Jammal, W.K.; Suma, H.; Fellows Yates, J.A.; Frangenberg, J.; Velsko, I.M.; Chowdhury, S.; Herbst, R.; Bratovanov, E.V.; Dahse, H.M.; Horch, T.; Hertweck, C.; González Morales, M.R.; Straus, L.G.; Vilotijevic, I.; Warinner, C.; Stallforth, P. Natural products from reconstructed bacterial genomes of the Middle and Upper Paleolithic. Science 2023, 380(6645), 619–624. [Google Scholar] [CrossRef]
- Caesar, L.K.; Butun, F.A.; Robey, M.T.; Ayon, N.J.; Gupta, R.; Dainko, D.; Bok, J.W.; Nickles, G.; Stankey, R.J.; Johnson, D.; Mead, D.; Cank, K.B.; Earp, C.E.; Raja, H.A.; Oberlies, N.H.; Keller, N.P.; Kelleher, N.L. Correlative metabologenomics of 110 fungi reveals metabolite–gene cluster pairs. Nat. Chem. Biol. 2023, 19, 846–854. [Google Scholar] [CrossRef]
- Smanski, M.J.; Zhou, H.; Claesen, J.; Shen, B.; Fischbach, M.; Voigt, C.A. Synthetic biology to access and expand nature’s chemical diversity. Nat. Rev. Microbiol. 2016, 14(3), 135–149. [Google Scholar] [CrossRef]
- Erb, T.J.; Patrik R Jones, P.R.; Bar-Even, A. Synthetic metabolism: metabolic engineering meets enzyme design. Curr. Opin. Chem. Biol. 2017, 37, 56–62. [Google Scholar] [CrossRef]
- Yi, J.; Li, Z. Artificial multi-enzyme cascades for natural product synthesis. Curr. Opin. Biotechnol. 2022, 78, 102831. [Google Scholar] [CrossRef]
- Huffman, M.A.; Fryszkowska, A.; Alvizo, O.; Borra-Garske, M.; Campos, K.R.; Canada, K.A.; Devine, P.N.; Duan, D.; Forstater, J.H.; Grosser, S.T.; Halsey, H.M.; Hughes, G.J.; Jo, J.; Joyce, L.A.; Kolev, J.N.; Liang, J.; Maloney, K.M.; Mann, B.F.; Marshall, N.M.; McLaughlin, M.; Moore, J.C.; Murphy, G.S.; Nawrat, C.C.; Nazor, J.; Novick, S.; Patel, N.R.; Rodriguez-Granillo, A.; Robaire, S.A.; Sherer, E.C.; Truppo, M.D.; Whittaker, A.M.; Verma, D.; Xiao, L.; Xu, Y.; Yang, H. Design of an in vitro biocatalytic cascade for the manufacture of islatravir. Science 2019, 366(6470), 1255–1259. [Google Scholar] [CrossRef]
- McIntosh, J.A.; Benkovics, T.; Silverman, S.M.; Huffman, M.A.; Kong, J.; Maligres, P.E.; Itoh, T.; Yang, H.; Verma, D.; Pan, W.; Ho, H.I.; Vroom, J.; Knight, A.M.; Hurtak, J.A.; Klapars, A.; Fryszkowska, A.; Morris, W.J.; Strotman, N.A.; Murphy, G.S.; Maloney, K.M.; Fier, P.S. Engineered ribosyl-1-kinase enables concise synthesis of molnupiravir, an antiviral for COVID-19. ACS Cent. Sci. 2021, 7(12), 1980–1985. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, J.A.; Liu, Z.; Andresen, B.M.; Marzijarani, N.S.; Moore, J.C.; Marshall, N.M.; Borra-Garske, M.; Obligacion, J.V.; Fier, P.S.; Peng, F.; Forstater, J.H.; Winston, M.S.; An, C.; Chang, W.; Lim, J.; Huffman, M.A.; Miller, S.P.; Tsay, F.R.; Altman, M.D.; Lesburg, C.A.; Steinhuebel, D.; Trotter, B.W.; Cumming, J.N.; Northrup, A.; Bu, X.; Mann, B.F.; Biba, M.; Hiraga, K.; Murphy, G.S.; Kolev, J.N.; Makarewicz, A.; Pan, W.; Farasat, I.; Bade, R.S.; Stone, K.; Duan, D.; Alvizo, O.; Adpressa, D.; Guetschow, E.; Hoyt, E.; Regalado, E.L.; Castro, S.; Rivera, N.; Smith, J.P.; Wang, F.; Crespo, A.; Verma, D.; Axnanda, S.; Dance, Z.E.X.; Devine, P.N.; Tschaen, D.; Canada, K.A.; Bulger, P.G.; Sherry, B.D.; Truppo, M.D.; Ruck, R.T.; Campeau, L.C.; Bennett, D.J.; Humphrey, G.R.; Campos, K.R.; Maddess, M.L. A kinase-cGAS cascade to synthesize a therapeutic STING activator. Nature 2022, 603(7901), 439–444. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




