1. Introduction
Steam-gas treatment with corrosion inhibitors is widely used for the preservation of industrial products during long-term storage or transportation in conditions of high humidity and aggressive atmosphere [
1,
2,
3,
4,
5,
6]. The vast majority of existing volatile corrosion inhibitors are adsorption-type inhibitors (VCIA) based on aliphatic carboxylic acids and amines. As a rule, these are monofunctional compounds having in their composition a carboxylic R =CO(OH) or an amine R-NH2 group of atoms through which a chemical bond with a metal is carried out. Such compounds form monolayer coatings that prevent the adsorption of aggressive substances on the surface of metals, but they do not have high barrier and mechanical properties to protect against corrosion in conditions of long-term storage, high temperature and humidity. A distinctive feature of VCI is the selective chemisorption of molecules on various metals. Amine–based inhibitors are effective for metals having free d-orbitals (Fe, Cu, Zn) capable of forming donor-acceptor bonds [
7,
8,
9] with undivided pairs of nitrogen electrons. Compounds containing a carboxyl group of atoms form strong chemical bonds with hydroxylated surfaces of light metals (Al, Mg) as a result of the condensation reaction of acid protons and hydroxyl groups on the metal surface [
10,
11]. Volatile polymer-type corrosion inhibitors (VСIP) based on organosilanes can form a polymer coating on the surface of metals [
12,
13] and mineral substrates with the formation of strong chemical bonds. Most VСIA compounds have low partial pressures at a deposition temperature of 10
-7-10
-5 at., which significantly complicates the process of metal inhibition. VCIP partial pressures can vary in any range below atmospheric due to the use of azeotropic mixtures. This makes it possible to obtain polymer layers with a thickness of hundreds of nanometers on the surface of metals at relatively low temperatures. Currently, there are a large number of organosilanes suitable for vapor-gas deposition on metals. The main condition is the presence of -Si(OR)
3 groups of atoms in organic compounds. This greatly facilitates the preparation of inhibitory compositions for obtaining polymer coatings with desired properties. Our studies [
14] have shown that the polymerization rate of silanols increases significantly in the presence of multifunctional aliphatic, carboxylic and phosphonic acids. The most suitable for these purposes is ethylene glycol, which has two groups in its composition –OH, capable of participating in polycondensation reactions with VS molecules. In this connection, vinyltrimethoxysilane (VS) and ethylene glycol (EG) were chosen as the main component of polymer mixtures.The technology of deposition of VS from the gas phase can be used for deposition of powder corrosion inhibitors on metals having extremely low vapor pressures at operating temperatures of 100-150°C. The possibility of precipitation of HEDP and 1.2.3 benzotriazole (BTA) on steel from organic solutions together with VS has been investigated. It was found that solutions of HEDP in ethanol and BTA in isopropanol evaporate azeotropically during boiling in a wide range of concentrations. The role of organosilanes is to cross-link inhibitor molecules with VS molecules during their joint deposition on the surface of metals. The subject of the present research is the study of the mechanism of vapor-gas deposition of VS and powder inhibitors on the iron surface using XPS and optical spectroscopy methods. Based on the obtained research results, to develop optimal technologies for the deposition of VS with polymerization promoters and powder coatings with high barrier properties.
2. Experimental
2.1. Materials and solutions
The following materials were used for steam - gas deposition
- -
Vinyltrimethoxysilane (VS), (WitcoCo); CH2 = CH −Si (OCH 3)3
- -
Ethylene Glycol (EG), ( Chem.avangard); HO-CH2-CH2-OH
- -
1-Hydroxy Ethylidene-1,1-Diphosphonic Acid (HEDP), (Chem.Rus) CH3C(OH)(H2PO3)2
- -
1.2.3 Benzotriazole (BTA),(Chem.Russia); C6H5N3
- -
Butanol (Bt), (Chem.Russia); C4H9OH
- -
Toluene (Th), (Chem.Russia); C₆H₅CH₃
- -
Isopropanol (IPA),C3H8OH ( Chem.avangard)
2.2. Surface treatment of samples before research
In this work, samples of carbon steel of the St.3 were used. During X-ray photoelectron spectroscopy, the samples had the shape of rectangular steel plates measuring 10x10 mm, which were pre-cleaned with Smirdex micron great 1000 sandpaper, degreased with acetone and processed in ethanol. After deposition of the coatings, the samples were processed in an ultrasonic bath with distilled water to remove the layers physically adsorbed from the vapor phase.
2.3. Deposition of polymer coatings from the vapor - gas phase
Vapor-gas deposition was carried out in a sealed chamber with a volume of 3 liters, equipped with a thermometer and a disk heater for evaporation of working solutions from measuring cylinders
3. Experimental technique
3.1. Optical research
Optical studies were carried out using an AvaSpec-256 spectrophotometer. The range of wavelengths studied was 200-1160 nm with an optical resolution of 0.5-6.4 nm. The source of radiation was a UV lamp with excitation wavelength of 400.2 nm and radiation intensity of 743 mW/cm2. Absorption spectra of the components of the mixtures were obtained in optical glass cuvettes (Chem.Rus). Fluorescence of polymer coatings was studied on a spectrometer in Scope mode on mirror-polished samples of 3x5 cm in size. Chemically, the composition and thickness of the coating were evaluated by calibration absorption spectra and XPS analysis methods.
3.2. X-ray photoelectron spectroscopy study
XPS measurements were performed using OMICRON ESCA+ spectrometer (Omicron NanoTechnology, Taunusstein, Germany) with the Al-anode (the radiation energy 1486.6 eV and power 252 W). The pass energy of the analyzer was set at 20 eV, and in some cases at 10 eV to increase the resolution. To take into account the charge of the samples, the position of the XPS peaks was standardized by the C1s peak of the hydrocarbon impurities from the atmosphere, which binding energy Eb was taken equal to 285.0 eV. The base pressure in the analyzer chamber was kept no higher than 8x10
-10 mbar. The spectra were deconvoluted into components after subtraction of the background determined by the Shirley method [
16]. The element ratios were calculated using integral intensities under the peaks, taking into account the photoionization cross sections of the corresponding electron shells [
17].Using the integrated intensity of the peaks and the MultiQuant program [
18]. The thicknesses of the layers formed on the surface were calculated with allowance for the mean free path of the electrons, according to the Cumpson and Seah equation [
19].
4. Experimental
4.1. Optical research
The chemical activity of VS is determined by the presence of three methoxy groups on the atom of silicon atoms, which are easily hydrolyzed to form RSi(OH)
3 silanols. As a result of the polycondesation reaction, a chemisorption monolayer with a strong chemical bond Me-O-Si-R(OH) is formed on the hydroxylated surface of metals. Subsequent layers form three–dimensional cyclic siloxane structures [–O–SiR
2–O-]n, which are the basis of the polymer coating. Thus, for the polymerization of organosilanes from the vapor-gas phase on metal surfaces, the hydrolysis and polycondensation reactions must proceed sequentially. In this mechanism of polymerization, it is necessary to have two evaporators for water and VS molecules in the deposition chamber (DC). Since the boiling points of water and VS differ by an order of magnitude, it is necessary to use azeotropic .mixtures to equalize their partial pressures in the chamber. According to [
15], such a mixture can be a 40-45% solution of VS in butane (Bt). Optical spectroscopy was used to determine the concentration of butanol in water.
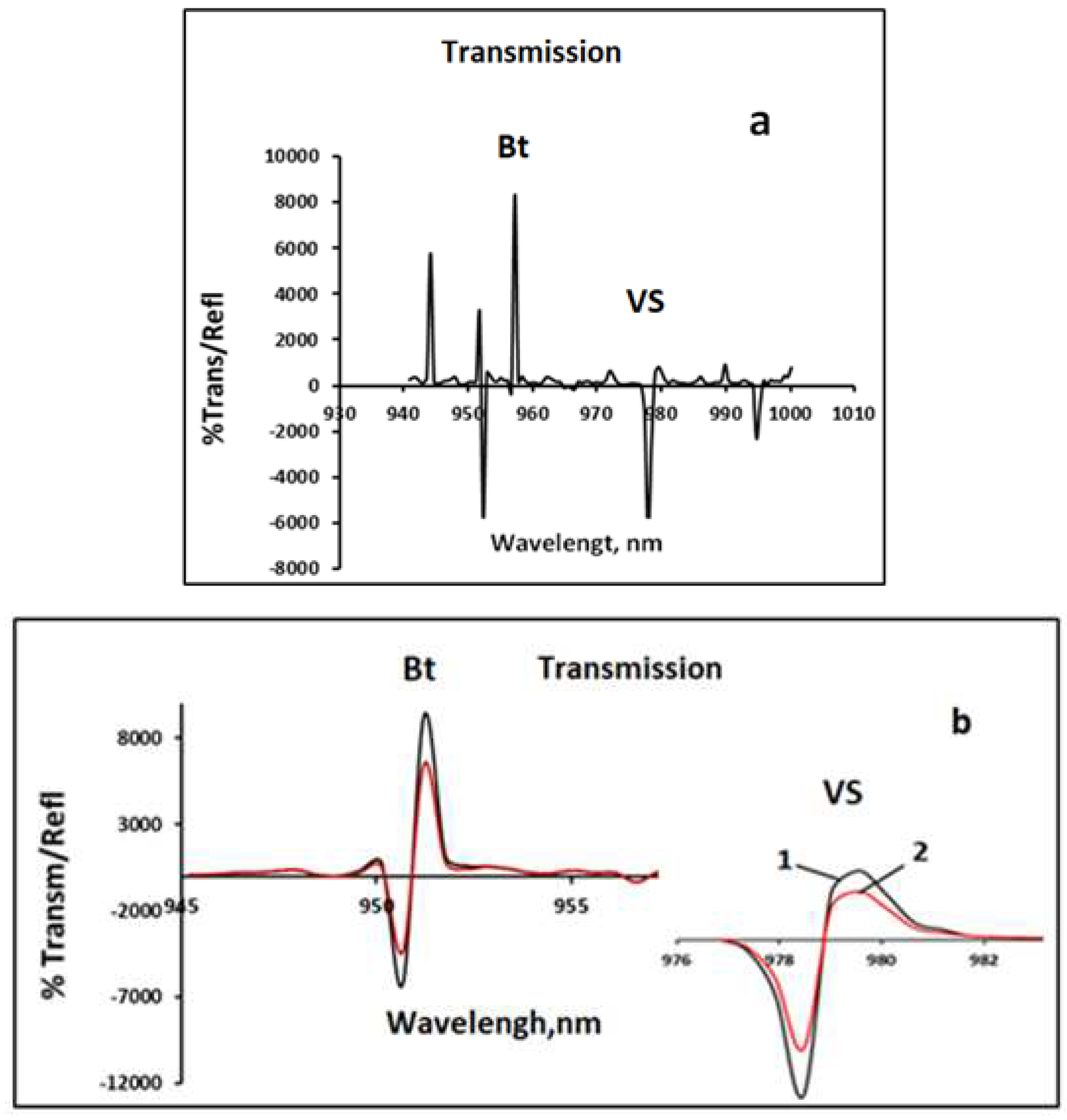
Figure 1 shows calibration absorption spectra of a 40% solution of VS in butanol (a) and after holding the solution in the evaporator for 30 min at T =95°C (b).
As a result of evaporation of the solution, the concentration of the initial components changes.
Table 1 shows the data the mass loss of the substance after holding the solution in the evaporator for 60 minutes at E = 95°C.
From the data given in the table, it can be seen that the evaporation of the components from the solution occurs in almost an equivalent amount, that is, its composition is close to asiotropic.
When siloxane coatings are deposited on metals from aqueous solutions, various additives in the form of corrosion inhibitors and promoters accelerating the polymerization of organosilanes are used to improve their protective properties. Only inhibitors containing at least two hydroxyl groups of atoms capable of participating in polycondensation reactions are suitable for the formation of a siloxane coating from aqueous solutions. Monofunctional inhibitors are suppressors of chains of polymer structures and interfere with the polymerization of trialkosilanes. For iron, (HEDP), ethylene glycol (EG), 1.2.3 benzotriazole (BTA) can be effective promoters and corrosion inhibitors. The most suitable promoter for VS vapor deposition may be ethylene glycol, since it is a liquid of simple chemical structure and is widely available. However, its high boiling point makes it difficult to use in combination with aqueous compositions. According to [
15], ethylene glycol with toluene forms an azeotropic mixture with a boiling point of 110.8. In this connection there is a possibility of equalization of partial pressures of vapors of water, VS and ethylene glycol in the deposition chamber (DC) during vapor-gas deposition of siloxane coatings. Optical spectroscopy was used to determine the concentration of the components of the working mixtures.
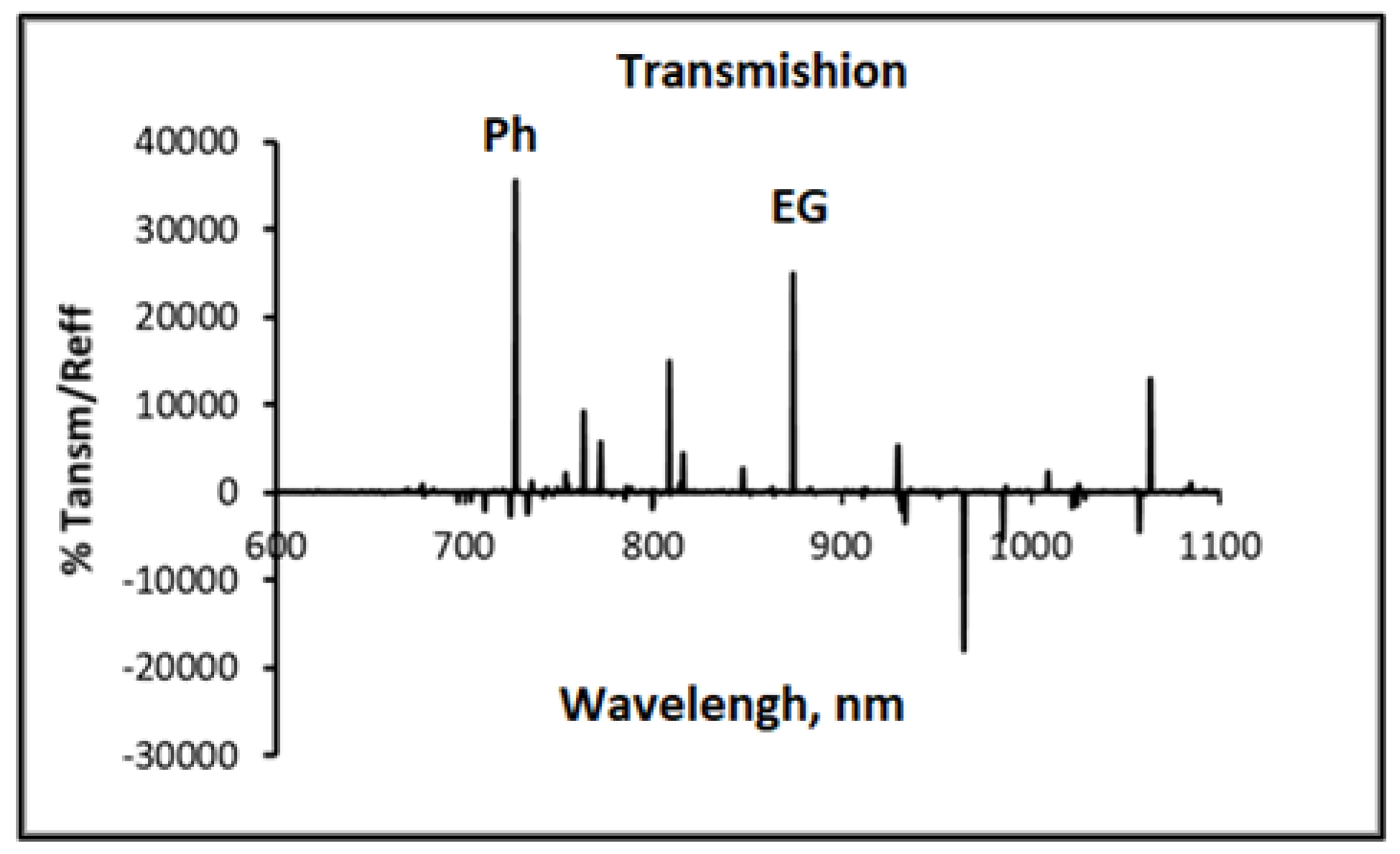
Figure 2 shows the calibration curves that were used for calculations.
Table 2 shows the data on the loss of EG and phenol in evaporators during combined-cycle deposition of VS at T = 120° C and 60 minutes of deposition.
Due to the fact that the temperature of coating deposition becomes higher, ethylene glycol in the amount of 70% vol. is added to the evaporator with water. According to [
22] at these temperatures, the vapor phase of this mixture contains 90-95% water.
Table 3 shows the composition of working solutions in evaporators and partial pressures of VS and EG in the deposition chamber at T=120°C and 60 minutes of deposition time.
Partial pressures P in the deposition chamber were calculated using the Mendeleev-Clapeyron formula.
where µ is the molar mass of the substance, g ; m is the loss of the substance in the evaporator, g; n is the molar fraction of the substance; V is the volume of the chamber, l.
Saturated vapor pressure of ethylene glycol and VS at T=100°s C according to[
22] is 10
-3-.10
-4 atm, which is about three orders of magnitude less than the pressure in the deposition chamber during deposition of polymer coatings. It is clear that without the use of azeotropic solutions it is impossible to obtain polymer coatings on metals with the help of vapor-gas deposition of organosilanes.
4.2. XPS research
4.2.1. Studies of vapor-gas deposition of VS on iron in a two-component mixture of VS+H2O
Vapor-gas deposition was carried out at atmospheric pressure for 60 minutes at T = 95°C. The vapor - gas mixture was obtained from two evaporators containing water and a 40% mixture of VS in butanol. The chemical composition of the surface of the samples was studied after coating, ultrasonic cleaning in distilled water and air drying.
Table 4 shows the chemical composition of the siloxane coating obtained from the vapor-gas phase in VS+ H
2O.
Significant amounts of silicon and carbon atoms belonging to the deposited organosilane films are detected on the X-ray spectra of the samples. In addition, a small amount of iron hydroxides is present in the composition of the coating.
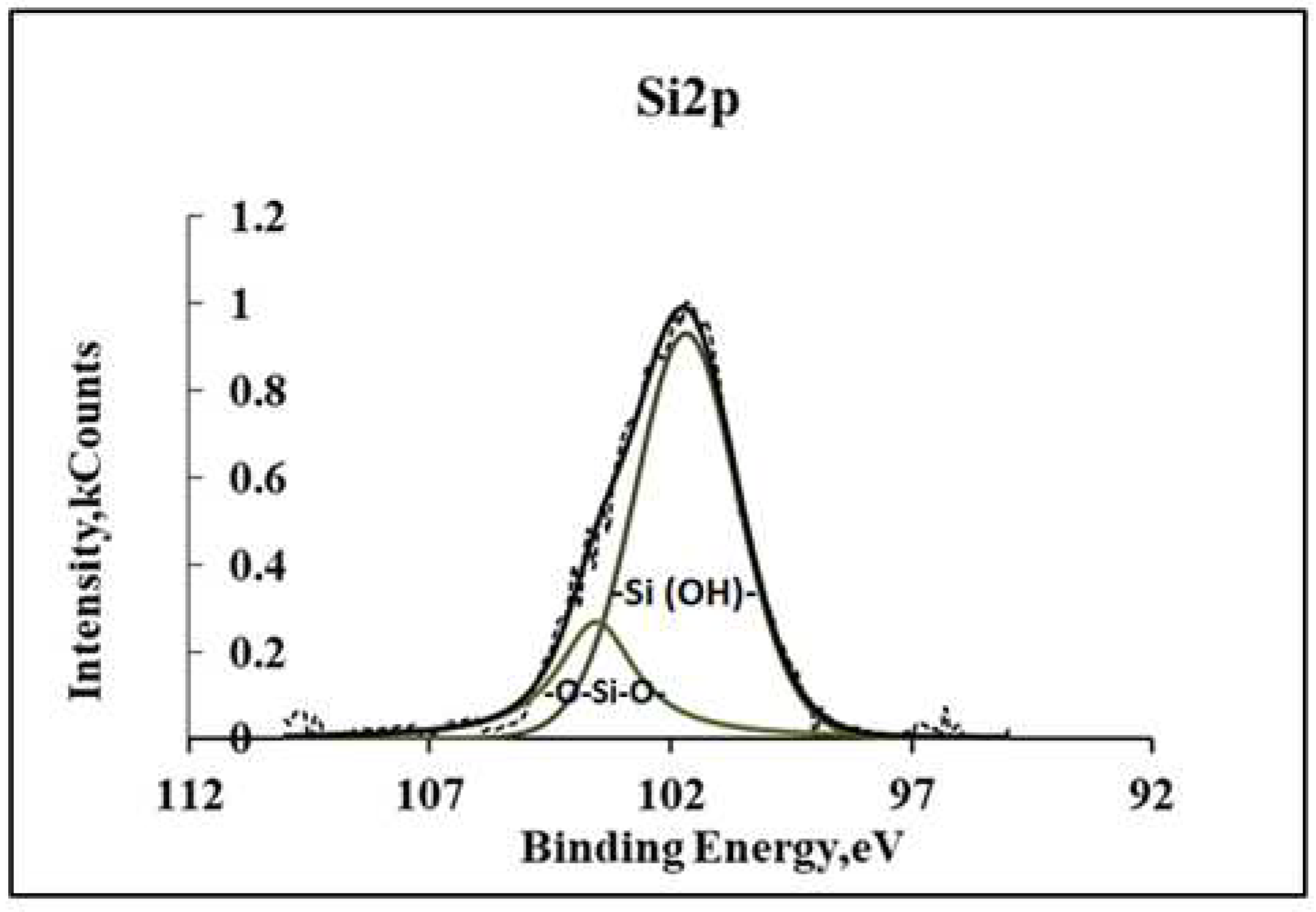
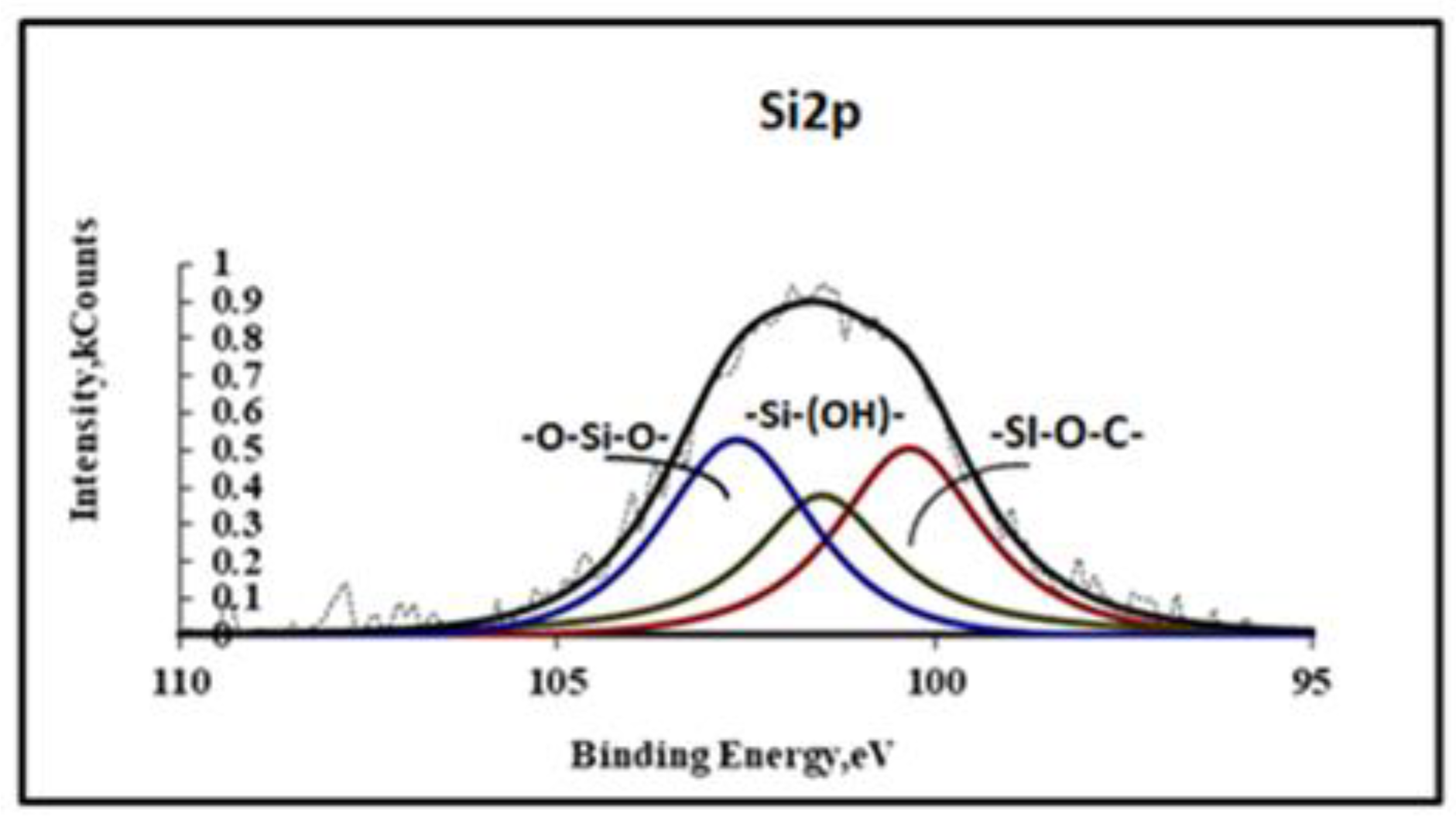
Figure 3 shows the XPS spectra of Si2p, on which two peaks can be distinguished, belonging to the silanol and siloxane groups of polymer coating.
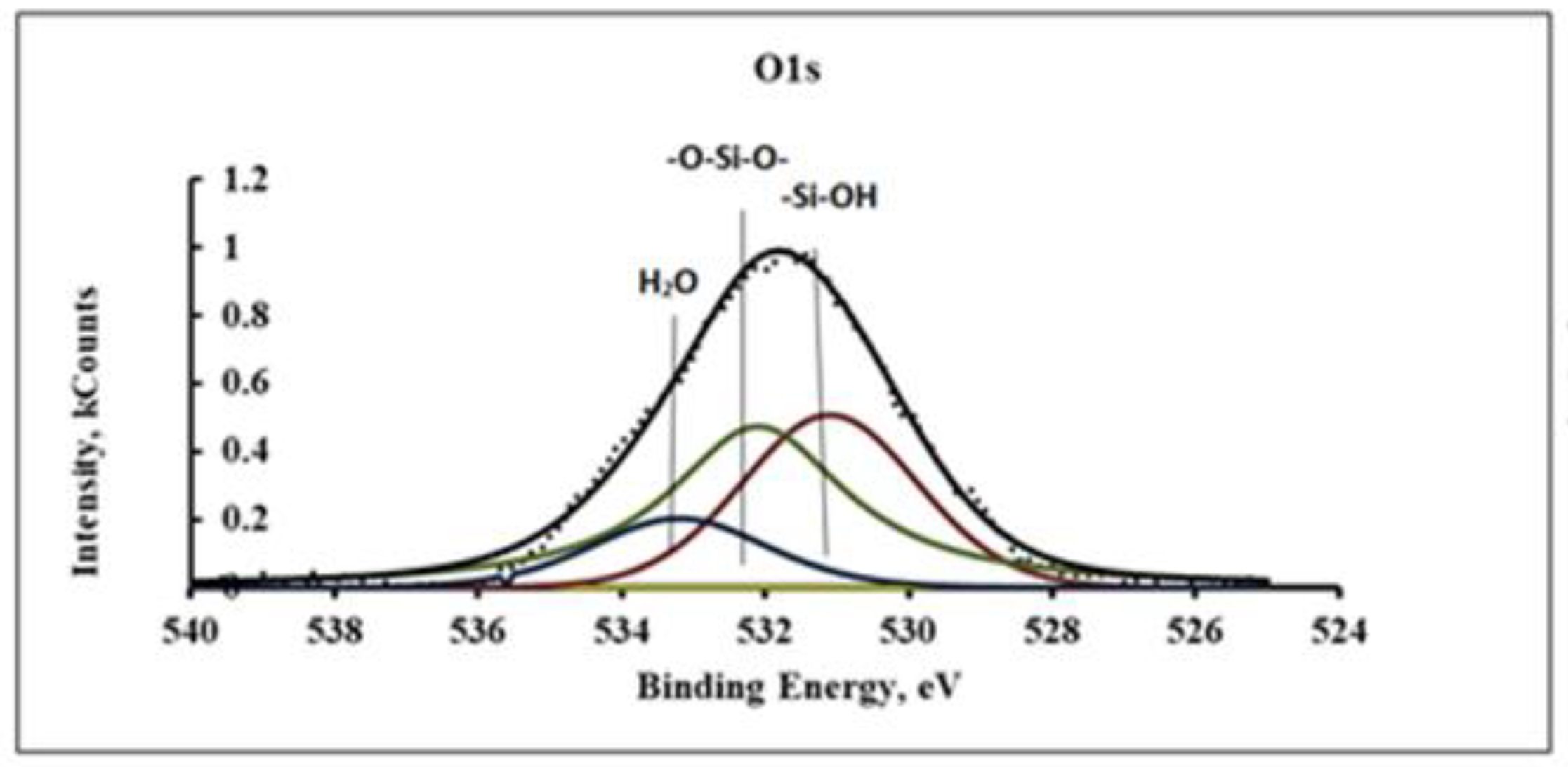
From these spectra it can be seen that a significant number of silanol groups did not participate in the polymerization of the coating. On the O1s spectra (
Figure 4) three peaks can be distinguished related to silanol in VS, siloxane structures and water molecules, respectively.
It can be seen from the spectra shown in
Figure 2 and
Figure 3 that the composition of the siloxane coating includes significant amounts of silanols and water. After annealing in the furnace at T = 150°C for 60 minutes, the final polymerization of the coating occurs with the formation of cyclic siloxane structures, the spectra of which are shown in
Figure 5.
Two symmetrical peaks of silicon and oxygen belonging to siloxane coating structures are visible on this spectrum.
4.2.2. Studies of vapor-gas deposition of VS on iron in a three-component mixture of VS + EG + H2O
Vapor-gas deposition was carried out from three evaporators at T = 120°C for 60 minutes. The composition of the mixture in the evaporators is shown in
Table 3.
Figure 6 shows the XPS spectra of Si2p siloxane coating.
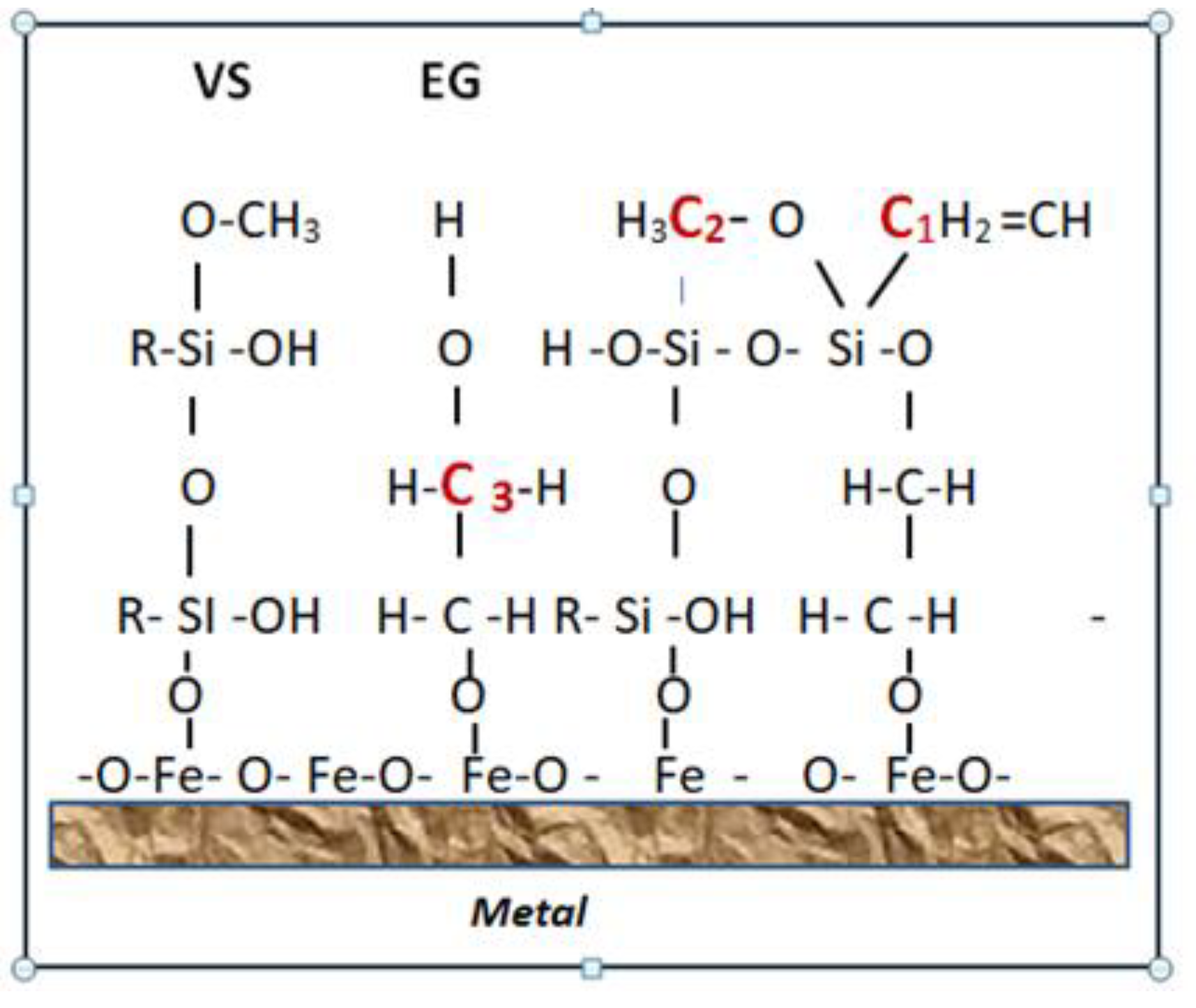
In these spectra , in addition to silanol and siloxane structures , there is a peak with an energy of 101.6 eV belonging to silicon atoms chemically bound to ethylene glycol molecules . Ethylene glycol having two functional groups of atoms at the end of the molecule forms bridging bonds between the molecules, contributing to the polymerization of the silaxane coating.
When comparing the peaks on the spectrum, it can be seen that the number of silicon atoms associated with siloxane structures and ethylene glycol is approximately the same. This indicates the high chemical activity of ethylene glycol during polymerization of siloxane coating. The number of free silanol groups in the coating is significantly less than in the absence of ethylene glycol, This is also facilitated by higher vapor-gas deposition temperatures.
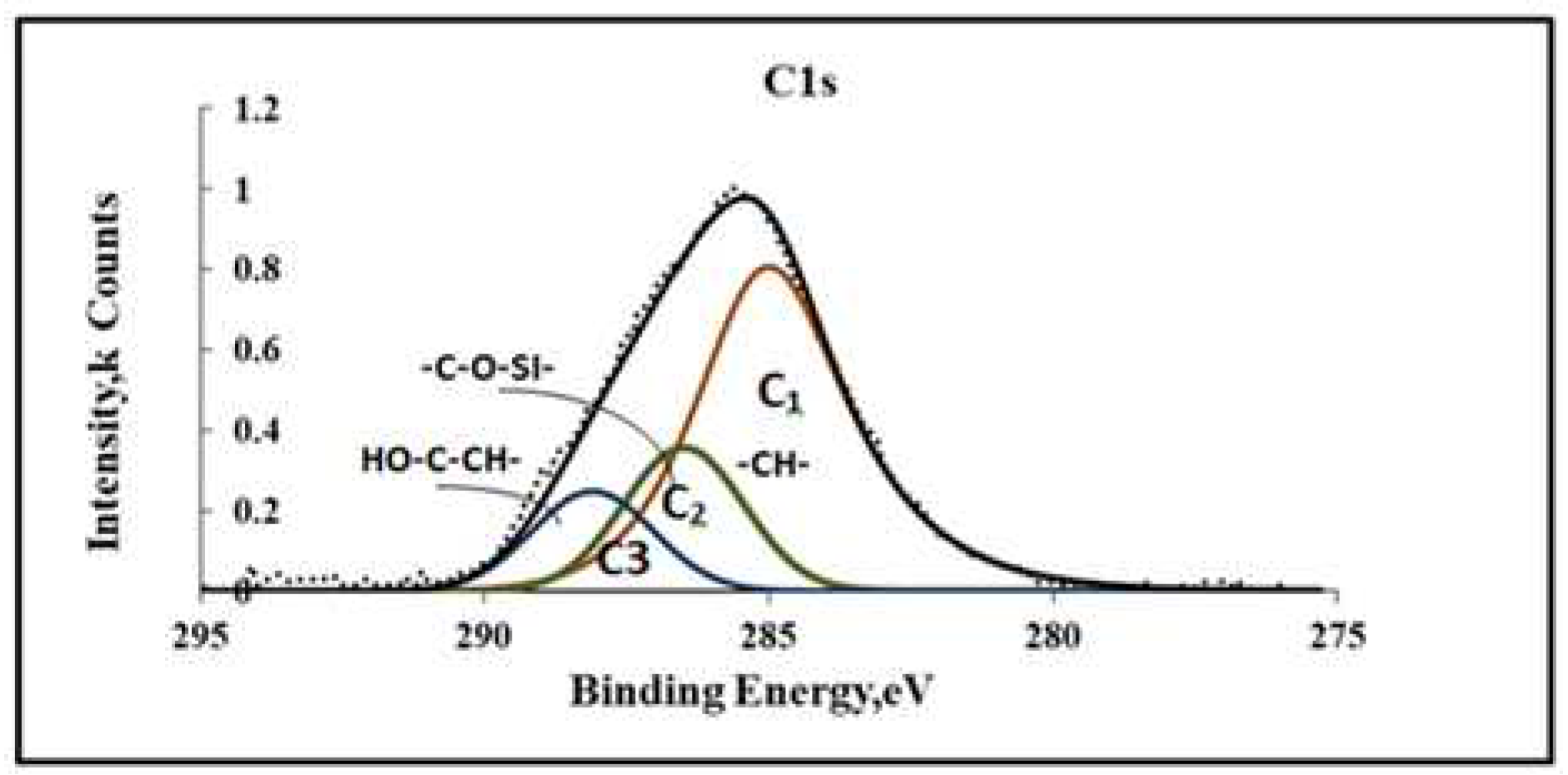
On the C1S spectra (
Figure 7) ethylene glycol molecules can include a peak with an energy of 288.2 eV belonging to carbon in the C–OH groups. In the structural diagram (
Figure 8), it is designated as a C3 atom.
The presence of methoxy groups in VS (C2) and ethoxy groups EC (C3) in the coating indicates incomplete hydrolysis and condensation of VS+EG molecules during precipitation.
During high-temperature annealing, further polymerization of the silaxane coating occurs as a result of condensation of the remaining hydroxy structures.
4.2.3. Investigation of vapor-gas deposition of VS with powder inhibitors
Cyclic azoles and phosphonic acids are widely used as volatile corrosion inhibitors of metals. The authors [
12,
23] conducted studies of vapor-gas deposition of phosphonic acids on low-carbon steel and magnesium alloys. In [
24,
25,
26,
27,
28,
29], the vapor deposition of BTA on copper, aluminum and magnesium alloys. In all cases, the thickness of the coatings was several monolayers. Obviously, the barrier properties of such coatings are not great. The ohmic resistance of charge transfer through such films is usually 1-10 kOhm [
20,
30], which is several orders of magnitude less than that of siloxal coatings[
12,
14].In this regard, it is of interest to co-precipitate these inhibitors with VS to form a composite coating with increased barrier properties. Powder inhibitors BTA and HEDP have low vapor pressures of ~10
-7-10
-8 at, so it is necessary to convert them into such a state that will reduce the evaporation temperature. Such a possibility can be realized if evaporation is carried out from aqueous or organic solutions. The vapor-gas deposition of BTA and HEDP on iron from solutions of these inhibitors in isopraponol (IPA) was investigated. The deposition was carried out from two sources, the composition of working mixtures in which is given in
Table 5.
Preliminary studies have shown that no precipitate is formed during the evaporation of BTA and HEDP solutions, i.e. the inhibitors are completely evaporated during vapor deposition. XPS studies showed that in the absence of VS during vapor deposition of BTA and HEDP, thin loose layers weakly bound to the surface of iron are formed on the iron surface. In the presence of VS, siloxane coatings containing significant amounts of HEDP and BTA are formed. The chemical composition of these coatings is given in
Table 6.
A significant number of inhibitors in the coating is due to the fact that they are polymerization promoters and are actively embedded in the siloxane lattice as shown in the structural diagram of
Figure 9.
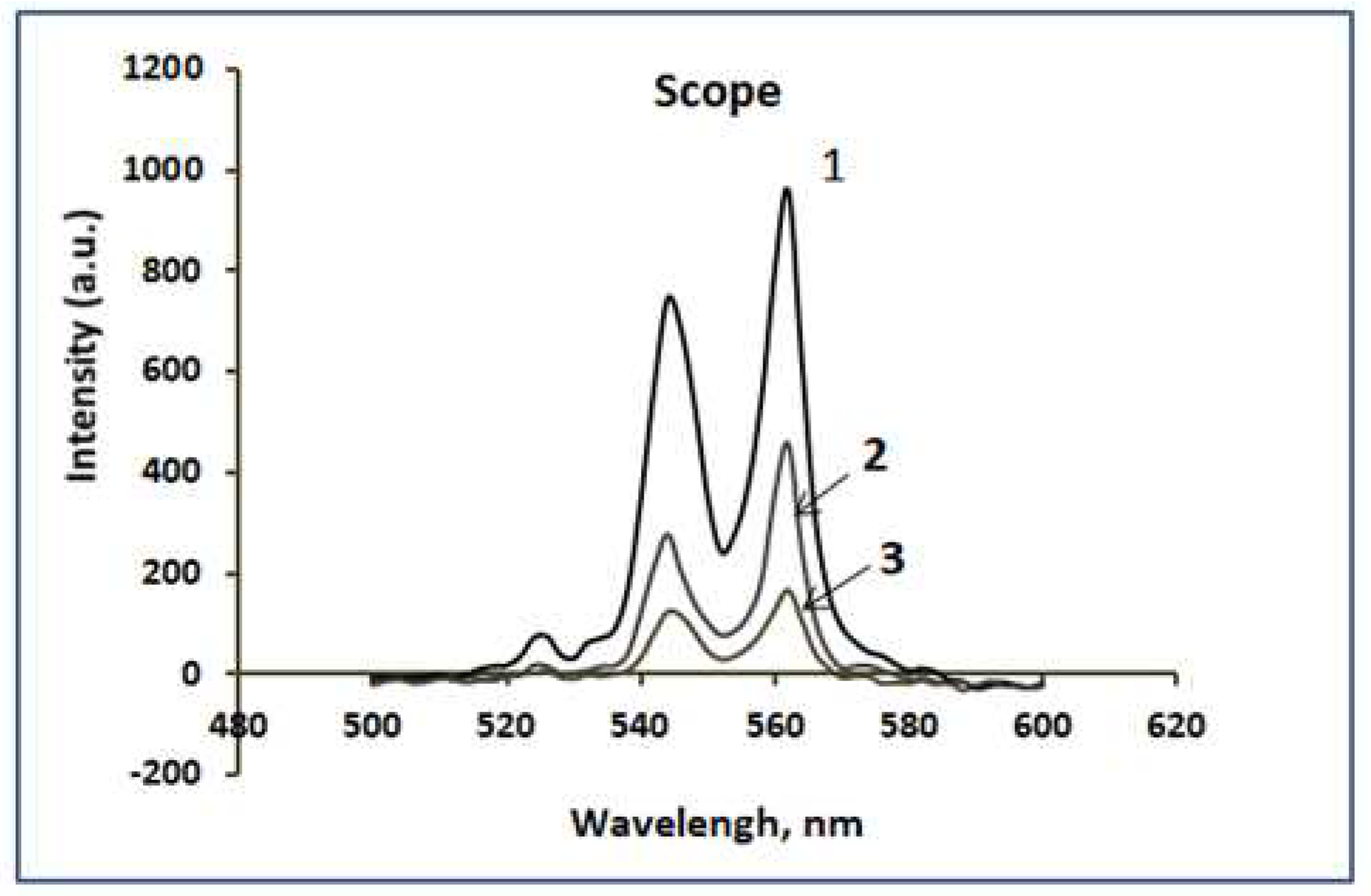
Using optical spectroscopy, the thicknesses of siloxane coatings obtained by vapor-gas deposition of VS with and without polymerization promoters were determined. Intense peaks associated with the fluorescence of siloxane coatings are detected on the spectra of samples obtained in the Scope mode . The intensity of these peaks directly depends on the thickness of the coatings.
Figure 10 shows the fluorescence spectra of siloxane coatings on iron obtained by vapor-gas deposition of VS with additives of polymerization promoters EG, HEDP and BTA.
The thickness of the VS coatings with additives EG, HEDP and BTA was 450, 210 and 120 nm, respectively. Without additives, the coating thickness was 90-100 nm. From the above results, it can be seen that the most effective promoters of VS polymerization are EG and HEDP, which have 2 and 4 chemically active functional groups of atoms, respectively. BTA has little effect on the deposition rate of siloxane coatings, but it is an effective corrosion inhibitor, especially for copper.
5. Conclusions
As a result of the conducted research, the compositions of azeotropic mixtures were developed to equalize the partial pressures of the components of the vapor phase during the deposition of VS on iron. A method for determining the partial pressures of the vapor phase using optical spectroscopy of components of working mixtures has been developed . The main factors influencing the kinetics of steam-gas deposition of VS on iron were determined using XPS and optical spectroscopy. It has been found that the addition of ethylene glycol and phosphonic acid HEDP to the vapor phase of VS significantly accelerates the polymerization of organosilanes on the surface of metals. With the help of structural models, an explanation of the interaction of VS with polymerization promoters EG is given. A new method of vapor-gas deposition of non-volatile powder inhibitors on metals is proposed. Studies of steam-gas deposition of the most well-known powder corrosion inhibitors BTA and HEDP on iron have been carried out. It has been established that BTA and HEDP dissolved in organic solvents are transferred to the metal surface during evaporation in the form of chemisorbed and Vandervaals layers with a thickness of several nanometers. To obtain dense structured films of BTA an HEDP on the surface of metals, a technique for crosslinking Vandervaals layers with siloxanes during their joint deposition with VS has been developed.
References
- H. Sugimura, A. Hozumi, T. Kameyama, O. Takai. Organosilane self-assembled monolayers formed at the vapour/solid interface // Surface and Interface Analysis; 2002; V. 34; (1); pp. 550–554. https://doi.org/10.1002/sia.1358. [CrossRef]
- M.A Petrunin, L.B Maksaeva, T.A Yurasova, E.V Terekhova, V.A Kotenev, E.N Kablov, A.Yu Tsivadze, The directional formation and protective effect of selfassembling vinyl siloxane nanolayers on copper surface, J. Prot. Met. Phys. Chem. Surf. 48 (2012) 656–664. https://doi.org/10.1134/S2070205112060081. [CrossRef]
- Goncharova, A.Yu. Luchkin, I.A. Archipushkin, N.N. Andreev,* Yu.I. Kuznetsov, Vapor-phase protection of steel by inhibitors based on salts of higher carboxylic acids ,Int. J. Corros. Scale Inhib., 2019, 8, no. 3, 586–599 586. https://doi.org/10.1179/026708404225016481. [CrossRef]
- A.Yu. Luchkin, O.A. Goncharova, N.N. Andreev and Yu.I. Kuznetsov, Steel protection by treatment by vapors of octadecylamine, benzotriazole and their mixture at elevated temperature, Korroz.: Mater., Zashch. (Corrosion: Materials, Protection), 2017, no. 12, 20–27 (in Russian).
- D. Zhang, Z. An, Q. Pan, L. Gao and G. Zhou, Volatile corrosion inhibitor film formation on carbon steel surface and its inhibition effect on the atmospheric corrosion of carbon steel, Appl. Surf. Sci., 2006, 253, no. 3, 1343–1348. [CrossRef]
- V. Saini and H. Kumar, “Study of amine as vapour phase corrosion inhibitors for mild steel under different aggressive atmospheric conditions at high temperature”, Int. J. Eng. Inn. Tech., 2014, 3, 248–256.
- Yu. Kuznetsov, O. Goncharova, A. Luchkin, S. Vesely and N. Andreev, Vapor-phase protection of metals from atmospheric corrosion by low-volatile organic inhibitors,Eurocorr, 2018, Paper 121167.
- A.I. Altsybeeva, V.V. Burlov, N.S. Fedorova, T.M. Kuzinova and G.F. Palatik,“Volatile inhibitors of atmospheric corrosion of ferrous and nonferrous metals. I. Physical and chemical aspects of selection of starting reagents and synthetic routes”,Int. J. Corros. Scale Inhib., 2012, 1, no. 1, 51–64. https://doi.org/10.17675/2305-6894-2012-1-1-051-064. [CrossRef]
- Bastidas, D. M., Cano, E., and Mora, E. M. (2005a). Volatile corrosion inhibitors: an overvew. Anticorrosion. Mater Methods 52, 71–77. https://doi.org/10.1108/00035590510584771. [CrossRef]
- R. Hu, S. Zhang, J. Bu, C. Lin, G. Song, Recent progress in corrosion protection of magnesium alloys by organic coatings, Prog. Org. Coat. 73 (2012) 129–141. [CrossRef]
- N.P. Andreeva1, Yu.I. Kuznetsov1 and Kh.S. Shikhaliev2 The use of ellipsometry for studying the adsorption of organic corrosion inhibitors from aqueous solutions on metals. Review. Part 2. Adsorption of salts of organic acids and azoles.
- Yu.B. Makarychev*, A.Yu. Luchkin, O.Yu. Grafov., N.N. Andreev, Vapor-phase deposition of polymer siloxane coatings on the surface of copper and low-carbon steel, International Journal of Corrosion and Scale Inhibition, V.11, № 3, с. 980-1000 DOI.
- Makarychev Yu B., Vapor-Gas Deposition of Polymer Coatings on Metals and Mineral Carriers.Journal of Biomedical Research and Environmental Sciences, MDPI (Basel, Switzerland, 2022, тoм 15, № 19, с. 6625. [CrossRef]
- Natalia Gladkikh⁎, Yuriy Makarychev, Marina Maleeva, Maxim Petrunin, Ludmila Maksaeva,Alevtina Rybkina, Andrei Marshakov, Yurii Kuznetsov, Synthesis of thin organic layers containing silane coupling agents and azole on the surface of mild steel. Synergism of inhibitors for corrosion protection of underground pipelines, Progress in Organic Coatings, Volume 132, July 2019, Pages 481-489. [CrossRef]
- Azeotropic mixtures / Ageev E. P. // Big Russian Encyclopedia [Electronic resource]. —2016.
- D.A. Shirley, High-resolution X-ray photoemission spectrum of the valence bands of gold, Phys. Rev. B 5 (1972) 4709–4713. [CrossRef]
- M. Mohai, XPS MultiQuant: multimodel XPS quantification software, Surf. Interface Anal. 36 (2004) 828–832. [CrossRef]
- R.A. Waldo, An Iteration Procedure to Calculate Film Compositions and Thicknesses in Electron-Probe Microanalysis, San Francisco Press, San-Francisco, 1988.
- Mohai M., Bertoti I. Calculation of overlayer thickness on curved surfaces based on XPS intensities. // Surf. Interface Anal., 2004, V. 36, № 8, P. 805-808. [CrossRef]
- H. Amar a, A. Tounsi b,*, A. Makayssi b. Derja a J. Benzakour a, A. Outzourhit , Corrosion inhibition of Armco iron by 2-mercaptobenzimidazole in sodium chloride 3% media, Corrosion Science 49 (2007) 2936–2945. [CrossRef]
- N. Poongothai, P. Rajendran, M. Natesan and N. Palaniswamy, “Wood bark oils as vapour phase corrosion inhibitors for metals in NaCl and SO2 environments”, Indian J. Chem. Technol., 2005, 12, 641–647.
- Lyubitov Yu. N. Saturated steam // Physical Encyclopedia : Т. 3, p.848.
- Ishizaki, T.; Okido, M.; Masuda, Y.; Saito, N.; Sakamoto, M. Corrosion Resistant Performances of Alkanoic and Phosphonic Acids Derived Self-Assembled Monolayers on Magnesium Alloy AZ31 by Vapor-Phase Method. Langmuir 2011, 27, 6009–6017.[PubMed] . [CrossRef]
- Goncharova, O.A.; Andreev, N.N.; Luchkin, A.Y.; Kuznetsov, Y.I.; Andreeva, N.P.; Vesely, S.S., Protection of copper by treatment with hot vapors of octadecylamine, 1,2,3-benzotriazole, and their mixtures. Mater. Corros. 2018, 70, 161–168. [CrossRef]
- Zhang, H.-L.; Zhang, D.-Q.; Gao, L.-X.; Liu, Y.-Y.; Yan, H.-B.; Wei, S.-L.; Ma, T.-F. Vapor phase assembly of benzotriazole and octadecylamine complex films on aluminum alloy surface. J. Coatings Technol. Res. 2020, 18, 435–446. [CrossRef]
- Goncharova, O.A.; Luchkin, A.Y.; Kuznetsov, Y.I.; Andreev, N.N.; Andreeva, N.P.; Vesely, S.S. Octadecylamine, 1,2,3-benzotriazole and a mixture thereof as chamber inhibitors of steel corrosion. Int. J. Corros. Scale Inhib. 2018, 2, 203–212. [CrossRef]
- Luchkin, A.; Goncharova, O.; Arkhipushkin, I.; Andreev, N.; Kuznetsov, Y. The effect of oxide and adsorption layers formed in 5-Chlorobenzotriazole vapors on the corrosion resistance of copper. J. Taiwan Inst. Chem. Eng. 2020, 117, 231–241. [CrossRef]
- Luchkin, A.Y.; Goncharova, O.A.; Andreeva, N.P.; Kasatkin, V.E.; Vesely, S.S.; Kuznetsov, Y.I.; Andreev, N.N. Mutual Effects of Components of Protective Films Applied on Steel in Octadecylamine and 1,2,3-Benzotriazole Vapors. Materials 2021, 14, 7181. [CrossRef]
- Kazanskii, L.P.; Selyaninov, I.A. XPS of 1,2,3-benzotriazole nanolayers formed on iron surface. Prot. Met. Phys. Chem. Surfaces, 2010, 46, 797–804. [CrossRef]
- Goncharova O.A., Luchkin 30.A.Y., Senchikhin I.N., Makarychev Y.B., Luchkina V.A., Dement’eva O.V., Vesely S.S., Andreev N.N,. Structuring of Surface Films Formed on Magnesium in Hot Chlorobenzotriazole Vapors, Materials, (Basel, Switzerland), тoм 15, № 19, с. 6625. [CrossRef]
Figure 1.
Calibration spectra of 40% VS solution in butanol (a);on spectra( b) 1-initial solution,2-after 60 minutes of exposure at T= 95° C.
Figure 1.
Calibration spectra of 40% VS solution in butanol (a);on spectra( b) 1-initial solution,2-after 60 minutes of exposure at T= 95° C.
Figure 2.
Calibration absorption spectra of a solution containing: 60%Ph+40%EG.
Figure 2.
Calibration absorption spectra of a solution containing: 60%Ph+40%EG.
Figure 3.
XPS spectra of Si2p siloxane coating deposited from a vapor-gas mixture of VS+H2O at T=95°C.
Figure 3.
XPS spectra of Si2p siloxane coating deposited from a vapor-gas mixture of VS+H2O at T=95°C.
Figure 4.
XPS spectra of O1s siloxane coating deposited from a vapor-gas mixture of VS+H2O at T=95°C.
Figure 4.
XPS spectra of O1s siloxane coating deposited from a vapor-gas mixture of VS+H2O at T=95°C.
Figure 5.
XPS spectra of Si2p (a) and O1s (b) siloxane coating after annealing in the furnace at T=150°C for 60 minutes.
Figure 5.
XPS spectra of Si2p (a) and O1s (b) siloxane coating after annealing in the furnace at T=150°C for 60 minutes.
Figure 6.
XPS spectra of O1s siloxane coating deposited from a vapor-gas mixture in VS+ E G+H2O at T=120°C.
Figure 6.
XPS spectra of O1s siloxane coating deposited from a vapor-gas mixture in VS+ E G+H2O at T=120°C.
Figure 7.
XPS spectra of C1S siloxane coating deposited from a vapor-phase mixture of VS+ EG+H2O at T=120°C.
Figure 7.
XPS spectra of C1S siloxane coating deposited from a vapor-phase mixture of VS+ EG+H2O at T=120°C.
Figure 8.
Structural diagram of a siloxane coating on iron obtained in a vapor-gas mixture of VS + EG.
Figure 8.
Structural diagram of a siloxane coating on iron obtained in a vapor-gas mixture of VS + EG.
Figure 9.
Structural model of siloxane coating with HEDP.
Figure 9.
Structural model of siloxane coating with HEDP.
Figure 10.
Fluorescence spectra of siloxane coatings on iron obtained by vapor-gas deposition of VS with additives of polymerization promoters: 1-EG, 2-HEDP, 3- BTA.
Figure 10.
Fluorescence spectra of siloxane coatings on iron obtained by vapor-gas deposition of VS with additives of polymerization promoters: 1-EG, 2-HEDP, 3- BTA.
Table 1.
Loss of mass of the substance after holding the solution in the evaporator.
Table 1.
Loss of mass of the substance after holding the solution in the evaporator.
| Chemical compound |
Wavelength, nm |
Intensity of the initial spectrum,
% Transm/Refl |
Spectrum Intensity
after evaporation,
% Transm/Refl |
Mass loss of substance, % vol |
| VS 40 % |
978.5 |
8260 |
6240 |
33.2 |
| Bt 60% |
956.4 |
6940 |
4260 |
39.6 |
Table 2.
Loss of mass of the substance after holding the solution in the evaporator.
Table 2.
Loss of mass of the substance after holding the solution in the evaporator.
| Chemical compound |
Wavelength, nm |
Intensity of the initial spectrum
% Transm/Refl |
Spectrum Intensity
after evaporation
% Transm/Refl |
Mass loss of substance,
% vol |
| EG ( 40 %) |
882.3 |
20560 |
15740 |
22.5 |
| Ph ( 60%) |
726.4 |
34520 |
18630 |
45.1 |
Table 3.
Compositions of working solutions in evaporators and partial pressures of VS and EG in the deposition chamber at T = 120°C.
Table 3.
Compositions of working solutions in evaporators and partial pressures of VS and EG in the deposition chamber at T = 120°C.
| Evaporators |
Boiling point, °C |
Loss of components
of the mixture m, g |
Partial pressures P, at
|
| 1.VS(60% )+ Bt |
108.5 |
2.52 |
0.26 |
| 2.EG(40%)+ Ph |
110.8 |
1.62 |
0.16 |
| 3.H2O(30 %)+ EG |
112.3 |
3 5 |
0.63 |
Table 4.
Chemical composition of siloxane coating obtained from the vapor-gas phase in VS+ H2O.
Table 4.
Chemical composition of siloxane coating obtained from the vapor-gas phase in VS+ H2O.
| Composition of mixtures |
Si, % ат |
C , % at |
Fe, % at |
O, % at |
| VS+H2O |
11.4 |
52.1 |
3.1 |
33.4 |
Table 5.
The composition of the working mixtures in the evaporators.
Table 5.
The composition of the working mixtures in the evaporators.
| Inhibitor |
Evaporator 1 |
Evaporator 2 |
Inhibitor concentration in IPA, M/l |
Temperature
in evaporators, °C |
| HEDP |
VS(60%)+Bt |
HEDP+IPA |
0,1 |
95 |
| BTA |
VS(60%)+Bt |
BTA+IPA |
0,1 |
95 |
Table 6.
Chemical composition of siloxane coatings deposited from VS + HEDP and VS+BTA vapors.
Table 6.
Chemical composition of siloxane coatings deposited from VS + HEDP and VS+BTA vapors.
| Coating composition |
Si,% at |
P,%at |
C,%at |
N,%at |
O,%at |
| VS +HEDP |
9.2 |
6.1 |
43,6 |
- |
42.1 |
| VS+BTA |
10.4 |
- |
44.7 |
5.5 |
39.4 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).