Submitted:
18 August 2023
Posted:
18 August 2023
You are already at the latest version
Abstract
Keywords:
Introduction
- (1)
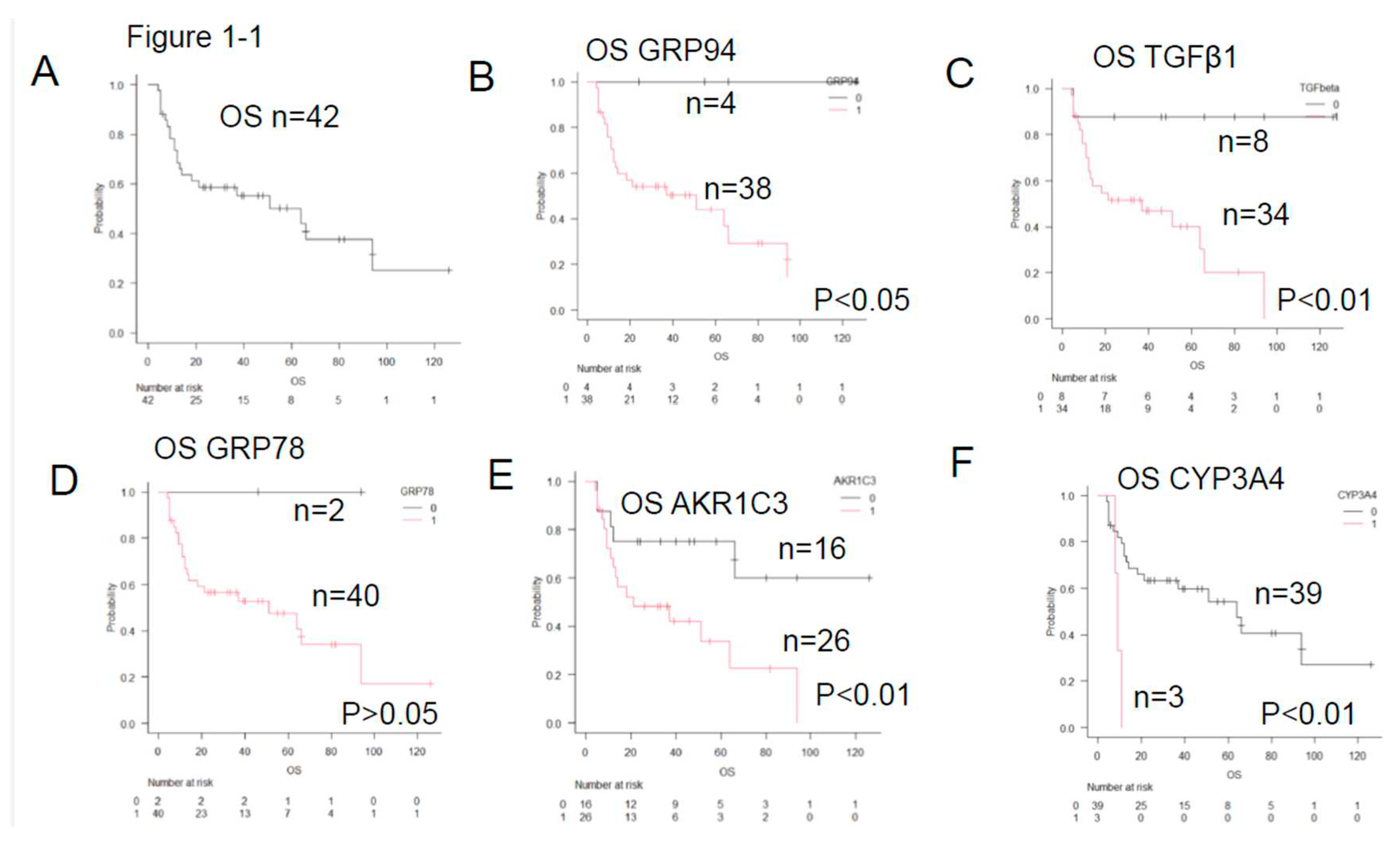
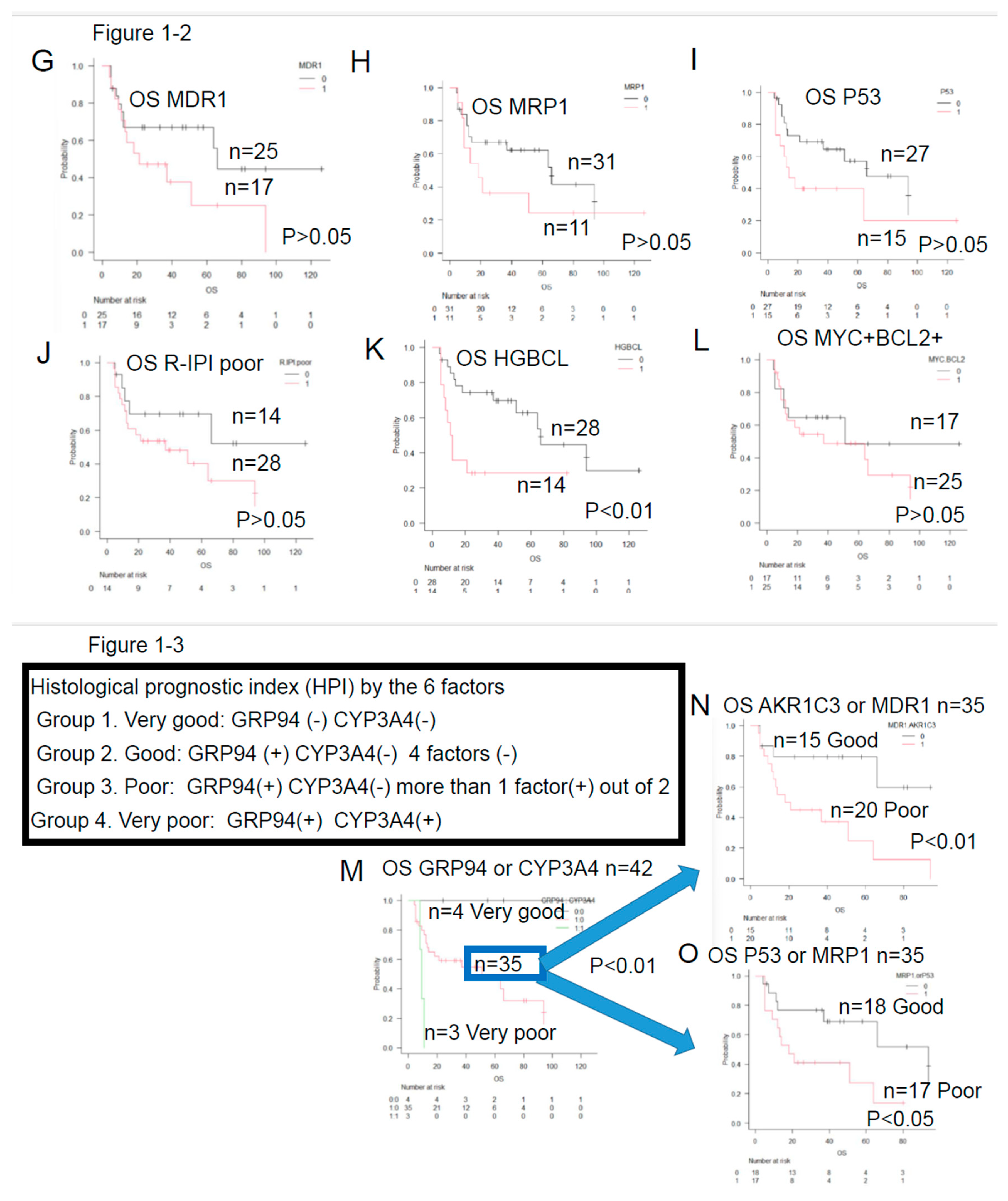
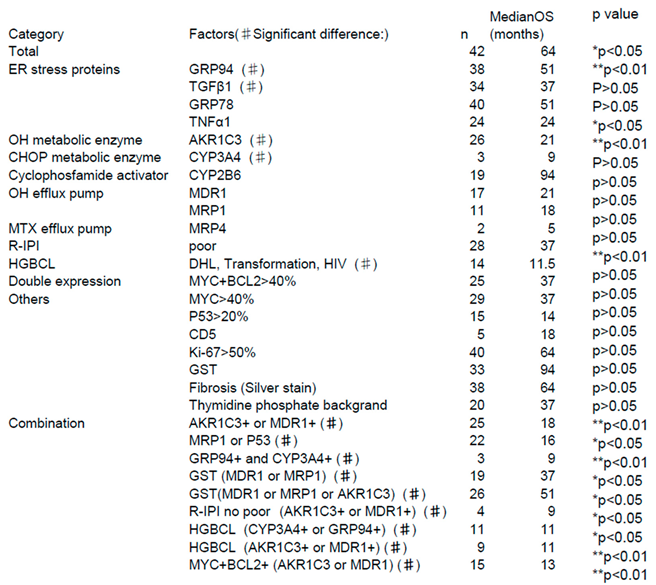
- ER stress proteins: glucose-regulated protein 94 (GRP94)4)-6), GRP787)8), transforming growth factor β1 (TGFβ1)9)10), and tumor necrosis factor α1 (TNFα1)11). They act to overcome various stressful conditions in the tumor microenvironment, including hypoxia, hypoglycemia, dysregulation of homeostasis, altered cellular metabolism.
- (2)
- AS enzymes involved in anticancer drug metabolism, the following three enzymes were selected: Aldo-keto reductase family 1 member C3 (AKR1C3)12)-16), CYP3A417)18), and CYP2B619). AKR1C3 lowers the activities of hydroxyl doxorubicin (H) and oncovin (O) (HO of CHOP)14). ,The risk for disease progression and death increases in patients with LBCL carrying AKR1C312). CYP3A4 inactivates many anticancer drugs including CHOP. Therefore, intratumoral drugs, such as PTCL, may be further inactivated. Expression of CYP3A4 as a predictor of response to chemotherapy in peripheral T-cell lymphomas17). As a result, the efficacy of these drugs may be lowered, which leads to the development of drug resistance17)18). CYP62B6 activates cyclophosphamide19).
- (3)
- Anticancer drug efflux pumps : multidrug resistance protein 1 (MDR1)20)-22), multidrug resistance-associated protein 1 (MRP1)23)24), and MRP425). MDR1 and MRP1 found on cell membranes are hydroxyl doxorubicin(H) and oncovin (O) (HO of CHOP) efflux pumps. Overexpression of MDR1 and MRP1 leads to the development of drug resistance in tumors20). LBCL patients without MDR1 have a good prognosis21).
- (4)
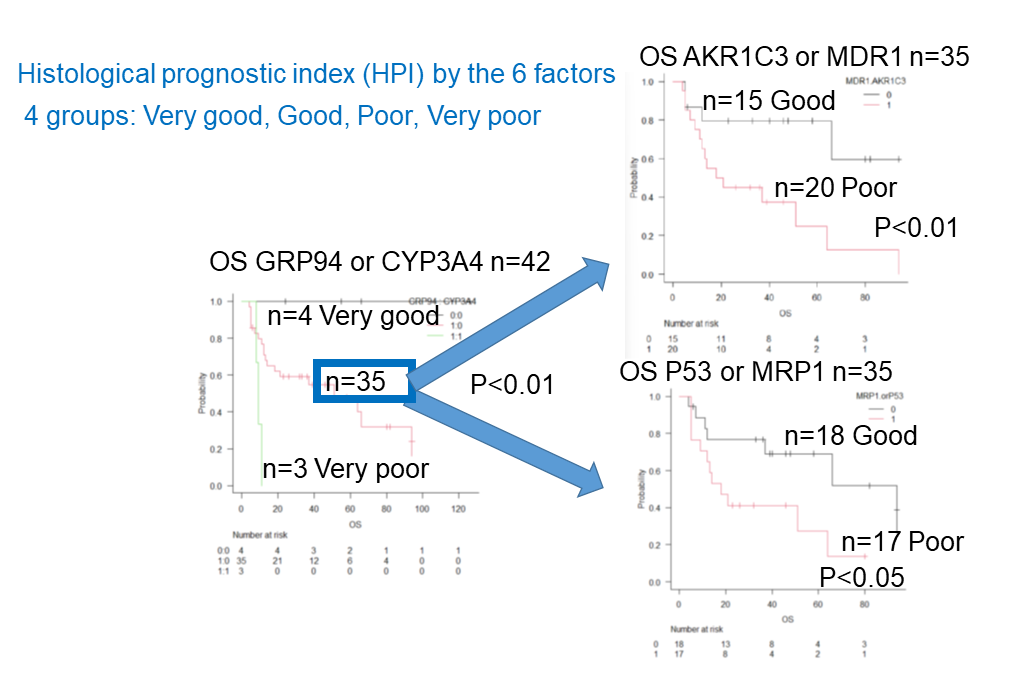
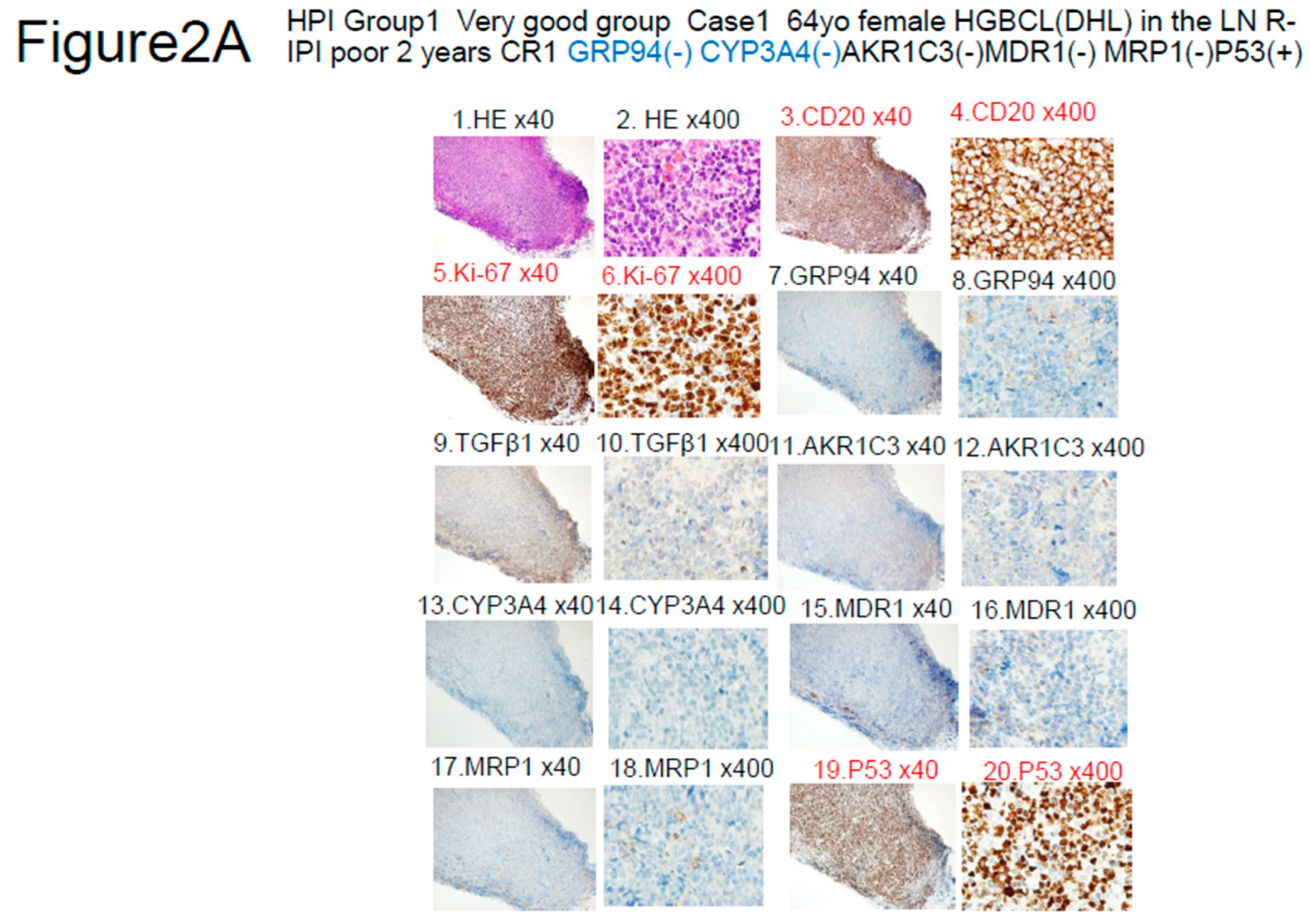
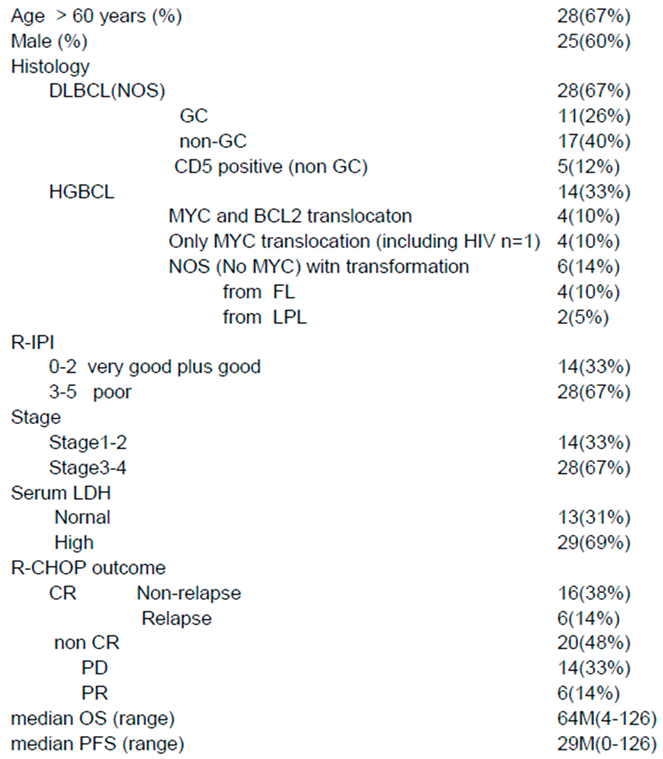
- Other items include the revised International prognostic Index (R-IPI)-poor and high-grade B-cell lymphoma (HGBCL) , such as double-hit lymphoma (DHL),MYC translocation LBCL, follicular lymphoma transformation, lymphoplasmacytic lymphoma transformation, and HIV-related Burkitt lymphoma.. In addition, double expression (MYC and BCL2), p5326), Ki-6726), CD5, glutathione-S-transferase (GST)27), presence/absence of fibrosis, and thymidine phosphate28) were also investigated.
Material and methods
Patients and Sample Collection
Immuno-histochemical staining
Statistical Analysis
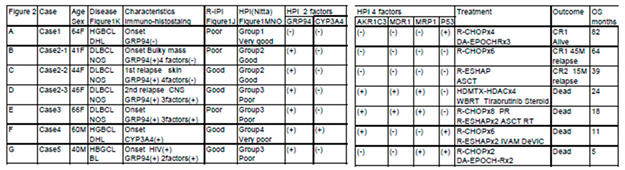
Results
Odds ratio by Logistic regression
Discussion
Patents
Author Contributions
Acknowledgments
Conflicts of Interest
Ethics statement
Patient and Public Involvement
References
- Bertrand Coiffier, Cle´mentine Sarkozy. Diffuse large B-cell lymphoma: R-CHOP failure—what to do? Hematology Am Soc Hematol Educ Program. 2016, Dec 2 (1):366-378. [CrossRef]
- R. Schmitz, G.W. Wright, D.W. Huang, C.A. et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N Engl J Med. 2018, April 12;378(15): 1396-1407.
- Bjoern Chapuy, Jaegil Kim, Atanas Kamburov et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med. 2018, May 24(5): 679–690. [CrossRef]
- Jerina Boelens, Jean, Philippe Jais, Barbara Vanhoecke et al. ER stress in diffuse large B cell lymphoma: GRP94 is a possible biomarker in germinal center versus activated B-cell type. Leukemia Research 2013,37:3-8. [CrossRef]
- JiWoong Kim, Yea Bin Cho, Sukmook Lee. Cell Surface GRP94 as a Novel Emerging Therapeutic Target for Monoclonal Antibody Cancer Therapy. Cells, 2021 Mar17, 10(3), 670. https://doi.org/10.3390/cells10030670. [CrossRef]
- Xiaofeng Duan, Stephen Iwanowycz, Soo Ngoi, Megan Hill, Qiang Zhao, Bei Liu. Molecular Chaperone GRP94/GP96 in Cancers. Oncogenesis and Therapeutic Target. Front. Oncol., 09 2021, 09 April. Volume 11, Article 629846.. https://doi.org/10.3389/fonc.2021.62984. [CrossRef]
- Ana Mozos , Gaël Roué, Armando López-Guillermo et sl. The expression of the endoplasmic reticulum stress sensor BiP/GRP78 predicts response to chemotherapy and determines the efficacy of proteasome inhibitors in diffuse large b-cell Lymphoma. Am J Pathol, 2011. 179(5): 2601–2610, https://doi.org/10.1016/j.ajpath.2011.07.031. Epub 2011 Sep 9. [CrossRef]
- 8. Hua-chuan Zheng, Hiroyuki Takahashi, Xiao-han Li et al. . Overexpression of GRP78 and GRP94 are markers for aggressive behavior and poor prognosis in gastric carcinomas. Human Pathology 2008,39,1042–1049. [CrossRef]
- Ashraf M. El-Hefnia, Nashwa M. et al. Prognostic Utility of Transforming Growth Factor Beta-1 in Diffuse Large Cell Non-Hodgkin Lymphoma. J Hematol. 4(1):131-136. 2015. [CrossRef]
- Sulsal Haque and John C. Morris. Transforming growth factor-b: A therapeutic target for cancer. HUMAN VACCINES & IMMUNOTHERAPEUTICS, VOL. 13, NO. 8, 1741–1750, 2017. [CrossRef]
- Shoko Nakayama, Taiji Yokote, Motomu Tsuji, et al. TNF-a Receptor 1 Expression Predicts Poor Prognosis of Diffuse Large B-cell Lymphoma, Not Otherwise Specified. Am J Surg Pathol, 2014, Aug;38(8):1138-46. [CrossRef]
- Heather L, Gustafson. Song Yao et al.. Genetic polymorphisms in oxidative stress related genes are associated with outcomes following treatment for aggressive B cell non-Hodgkin lymphoma. Am J Hematol.2014 89(6): 639–645. [CrossRef]
- Yang Liu, Siyu He, Ying Chen et al. Overview of AKR1C3: Inhibitor Achievements and Disease Insights. J. Med. Chem., 2020. 63, 11305−11329. [CrossRef]
- Trevor M. Penning, Sravan Jonnalagadda, Paul C. Trippier, Tea Lanišnik Rižner... Aldo-Keto Reductases and Cancer Drug Resistance. Pharmacol Rev 2021 Jul y 73(3)1150–1171. [CrossRef]
- Wei Xiong, Jing Zhao, Hongliang Yu. Elevated Expression of AKR1C3 Increases Resistance of Cancer Cells to Ionizing Radiation via Modulation of Oxidative Stress. November 24, 2014. https://doi.org/10.1371/journal.pone.0111911. [CrossRef]
- Donya Moradi Manesh, Jad El-Hoss, Kathryn Evans, Jennifer Richmond et al. AKR1C3 is a biomarker of sensitivity to PR-104 in preclinical models of T-cell acute lymphoblastic leukemia. Blood.;126(10):1193-1202, 2015. [CrossRef]
- Cristina Rodríguez-Antona, Susanna Leskelä, Magdalena Zajac et al. Expression of CYP3A4 as a predictor of response to chemotherapy in peripheral T-cell lymphomas. Blood. 2007,110:3345-3351. [CrossRef]
- 18. Laura Molenaar-Kuijsten, Dorieke E M Van Balen , Jos H Beijnen, Neeltje Steeghs, Alwin D R Huitema. A Review of CYP3A Drug-Drug Interaction Studies: Practical Guidelines for Patients Using Targeted Oral Anticancer Drugs. Front Pharmacol . 2021 Aug 30;12:670862. https://doi.org/10.3389/fphar.2021.670862. [CrossRef]
- Ibrahim El-Serafi, Parvaneh Afsharian, Ali Moshfegh, Moustapha Hassan, Ylva Terelius. Cytochrome P450 Oxidoreductase Influences CYP2B6 Activity in Cyclophosphamide Bioactivation. PLOS ONE, November 6, 2015. https://doi.org/10.1371/journal.pone.0141979. [CrossRef]
- Robert W. Robey, Kristen M. Pluchino, Matthew D. Hall et al. Revisiting the role of ABC transporters in multidrug- resistant cancer. Nature Reviews Cancer 2018, 18: 452–464. [CrossRef]
- Charalambos Andreadis, Phyllis A. Gimotty, Peter Wahl et al. Members of the glutathione and ABC-transporter families are associated with clinical outcome in patients with diffuse large B-cell lymphoma. Blood. 2007,109:3409-341. [CrossRef]
- Ilaria Genovesea, Andrea Ilarib, Yehuda G. Assarafaf, Francesco Fazi, Gianni Colotti. Not only P-glycoprotein: Amplification of the ABCB1-containing chromosome region 7q21 confers multidrug resistance upon cancer cells by coordinated overexpression of an assortment of resistance-related proteins. Drug Resistance Updates Volume 32, May 2017, Pages 23-46. [CrossRef]
- Éva Bakos, László Homolya. Portrait of multifaceted transporter, the multidrug resistance-associated protein 1 (MRP1/ABCC1). Eur J Physiol 2017, 453:621–641. https://doi.org/10.1007/s00424-006-0160-8, 2007. [CrossRef]
- Jamie F. Lu, Deep Pokharel, Mary Bebawy. MRP1 and its role in anticancer drug resistance. Drug Metabolism Reviews, 2015, 47:4, 406-419. [CrossRef]
- Tony Huynh, Murray D. Norris, Michelle Haber, Michelle J. Henderson. ABCC4/MRP4: a MYCN regulated transporter and potential therapeutic target in neuroblastoma. Front Oncol. 2012, Dec 19;2:178. https://doi.org/10.3389/fonc. 2012.00178. [CrossRef]
- Tarek N El-Bolkainy, Mohamed N El-Bolkainy, Hussein M Khaled et al. Evaluation of MIB-1 and p53 Overexpression as Risk Factors in Large Cell Non-Hodgkin Lymphoma in Adults. Journal of the Egyptian Nat. Cancer Inst., Vol. 19, No. 4, 2007, December: 231-238.
- Danyelle M Townsend, Kenneth D Tew. The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene 2003, 22, 7369–7375. [CrossRef]
- Xingcao Nie, Peter M Clifford, Rekha Bhat, Rebecca Heintzelman, Mini Abraham, J Steve Hou. Thymidine phosphorylase expression in B-cell lymphomas and its significance: a new prognostic marker? Anal Quant Cytopathol Histpathol. 2013 Dec;35(6):301-5. 2013.
- Y Kanda TECHNICAL REPORT Investigation of the freely available easy-to-use software‘EZR’ for medical statistics. Bone Marrow Transplantation 48, 452–458. 2013. [CrossRef]
- Nobuya Hiraoka, Jiro Kikuchi, Takahiro Yamauchi et al.. Purine Analog-Like Properties of Bendamustine Underlie. Rapid Activation of DNA Damage Response and Synergistic Effects with Pyrimidine Analogues in Lymphoid Malignancies. PLoS One.; 2014, 9(3): e90675. [CrossRef]
- Sungpil Cho, Meiling Lu, Xiaolong He et al. .Notch1 regulates the expression of the multidrug resistance gene ABCC1/MRP1 in cultured cancer cells. PNAS vol. 108 no. 51:20778–20783, 2011. [CrossRef]
- Hisham Qosa,, David S. Miller, Piera Pasinelli, Davide Trotti. Regulation of ABC Efflux Transporters at Blood-Brain Barrier in Health and Neurological Disorders. Brain Res. 2015, Dec. 2, 1628(Pt B): 298–316. https://doi.org/10.1016/j.brainres.2015.07.005. [CrossRef]
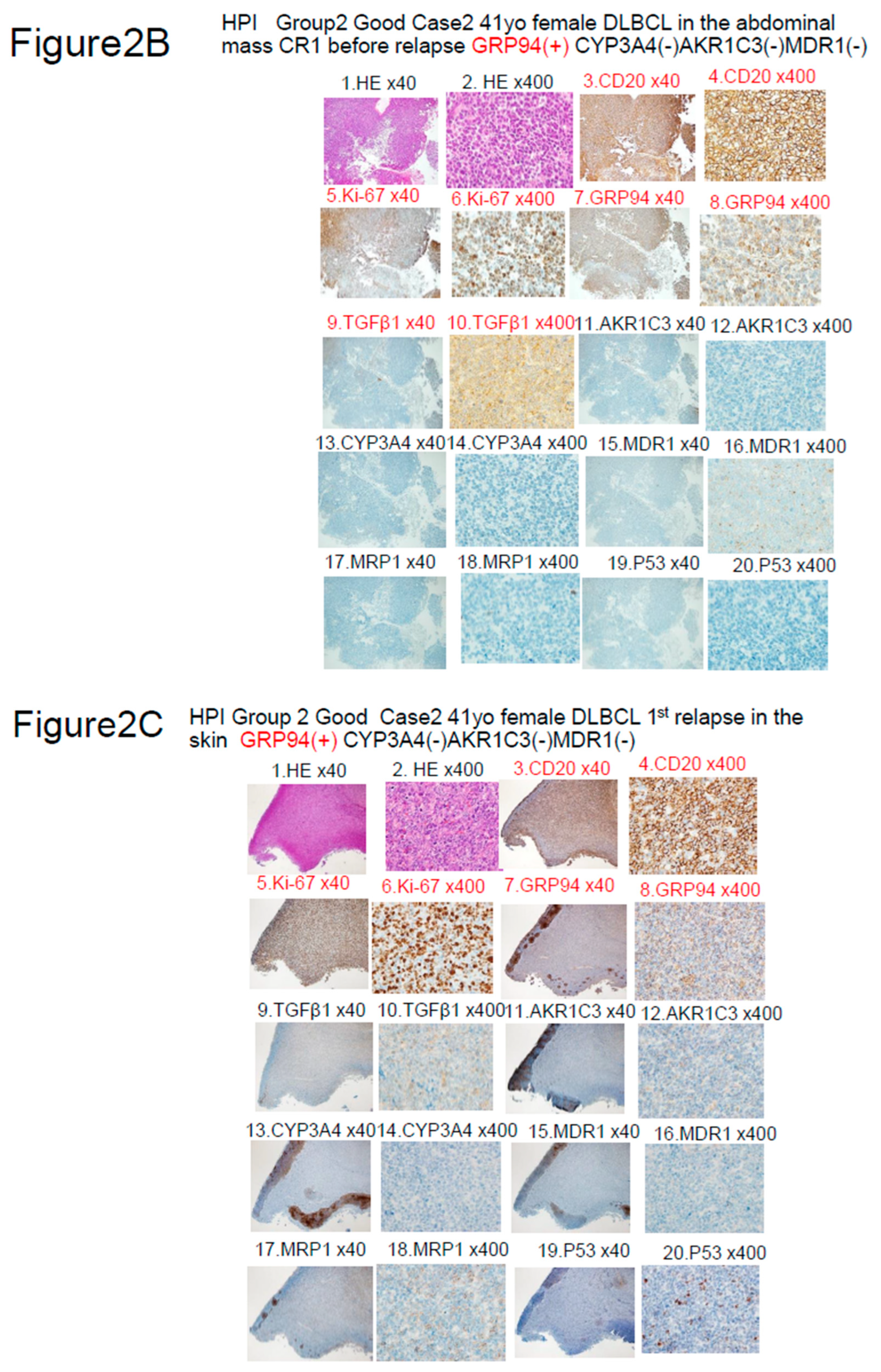
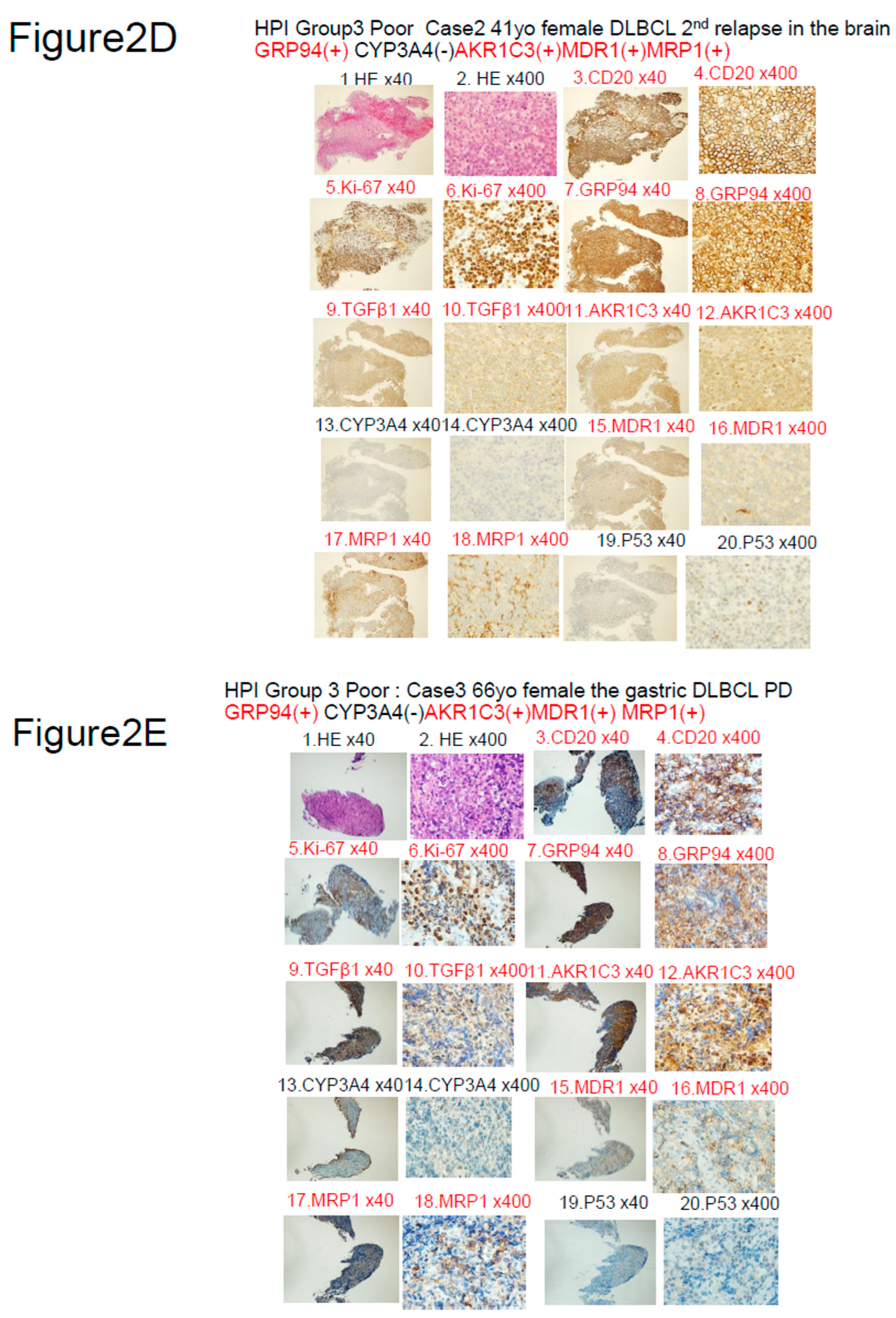
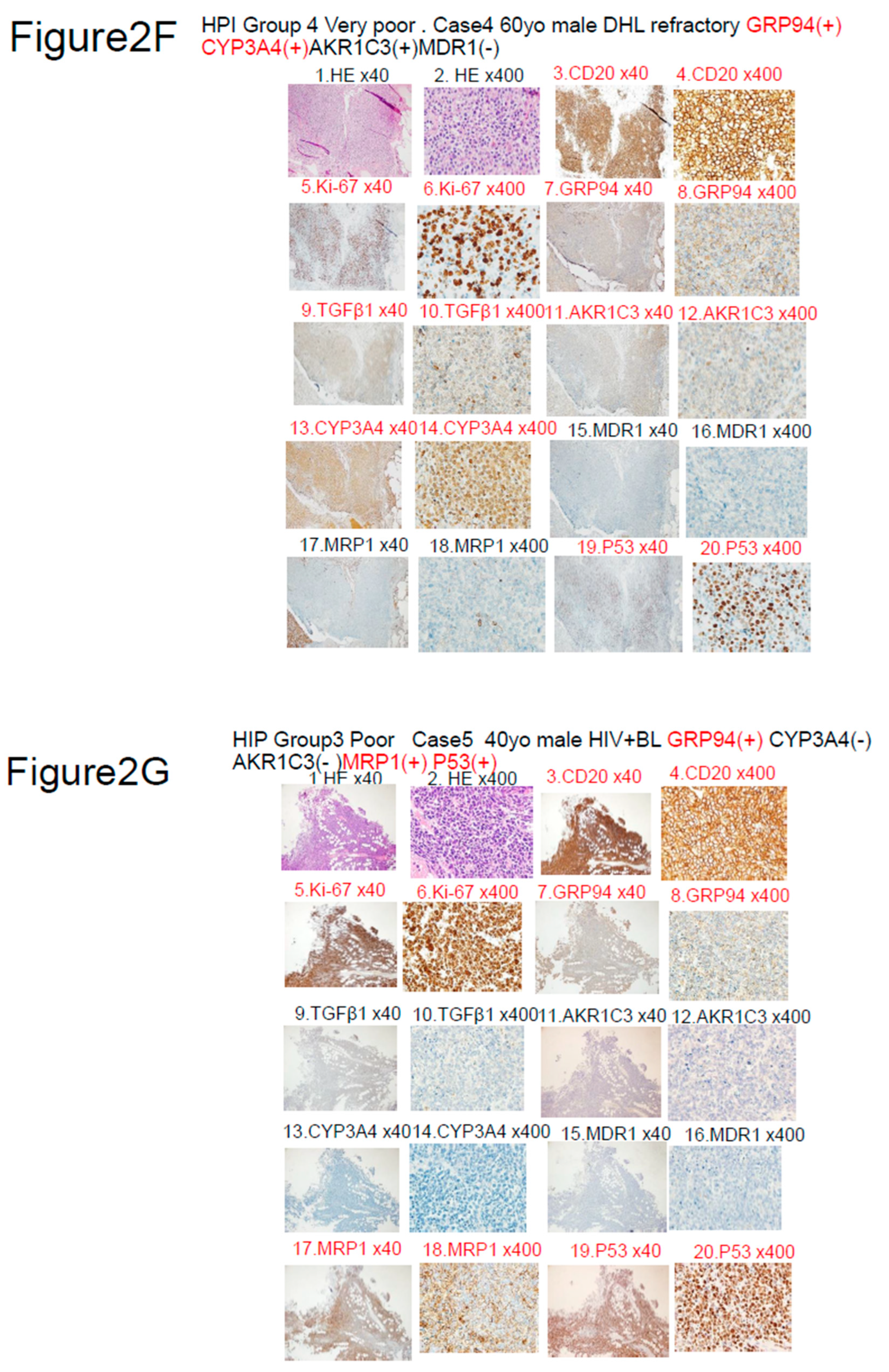
 |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





