Submitted:
18 August 2023
Posted:
22 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell culture
2.3. Western blot analysis
2.4. Real-time PCR analysis
2.5. Measurement of PGE2 release
2.6. Measurement of intracellular ROS
2.7. Transient transfection with siRNAs
2.8. Cell viability
2.9. Statistical analysis of data
3. Results
3.1. SiNPs induce COX-2 expression and PGE2 production in HPAEpiCs
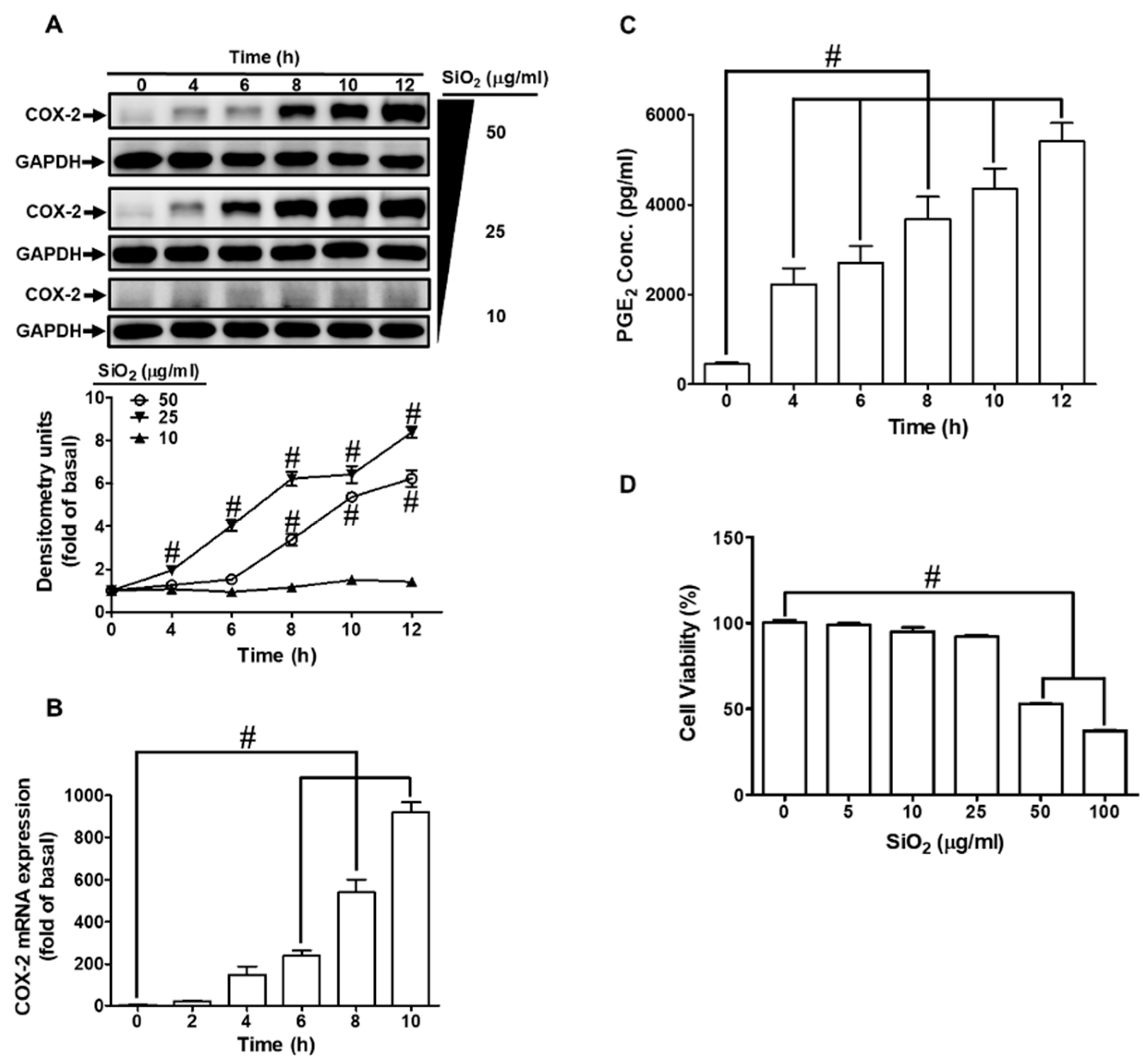
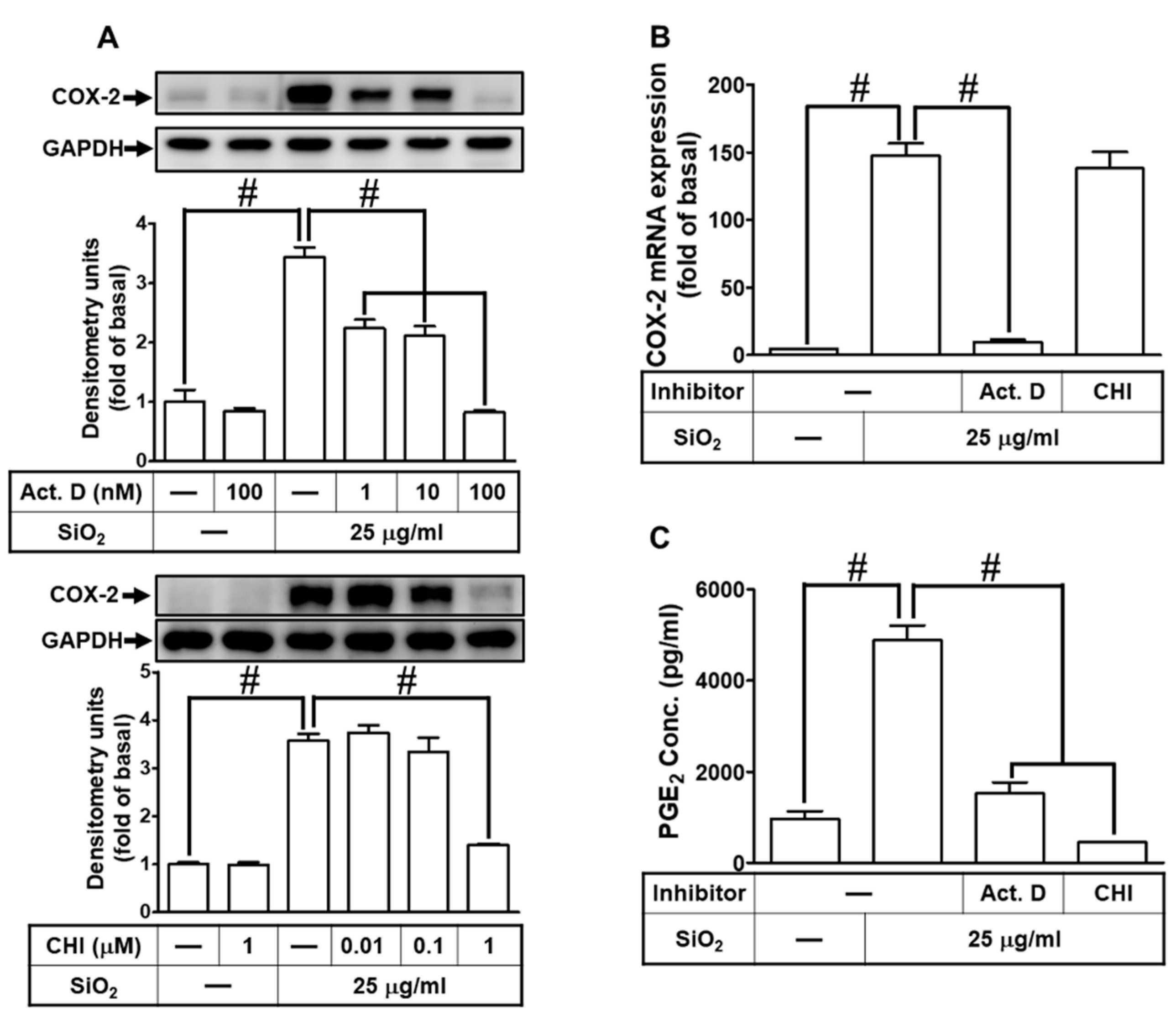
3.2. SiNPs induce COX-2 expression mediated through transcription and translation in HPAEpiCs
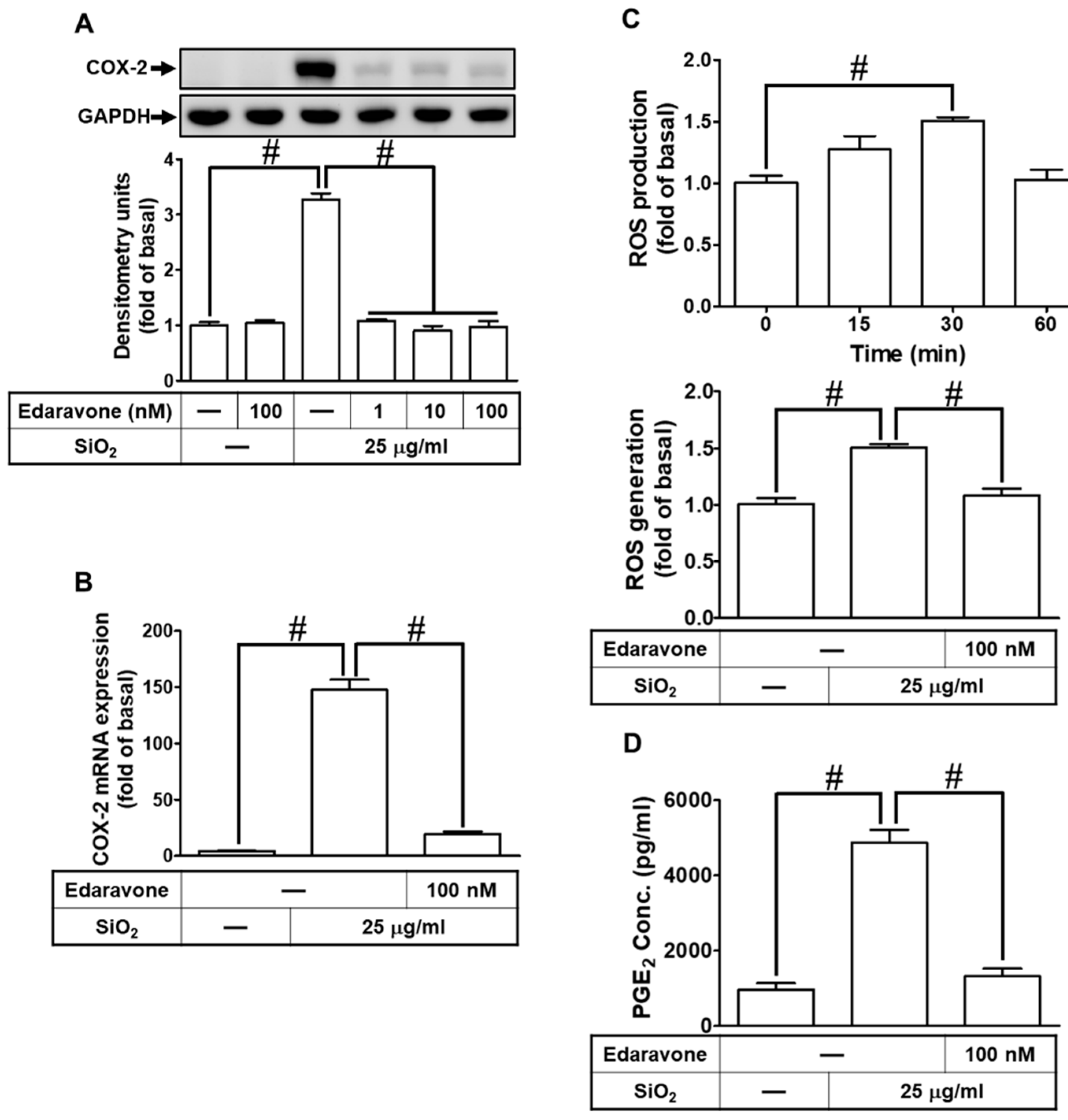
3.3. ROS generation participates in SiNPs-induced COX-2 expression and PGE2 synthesis in HPAEpiCs
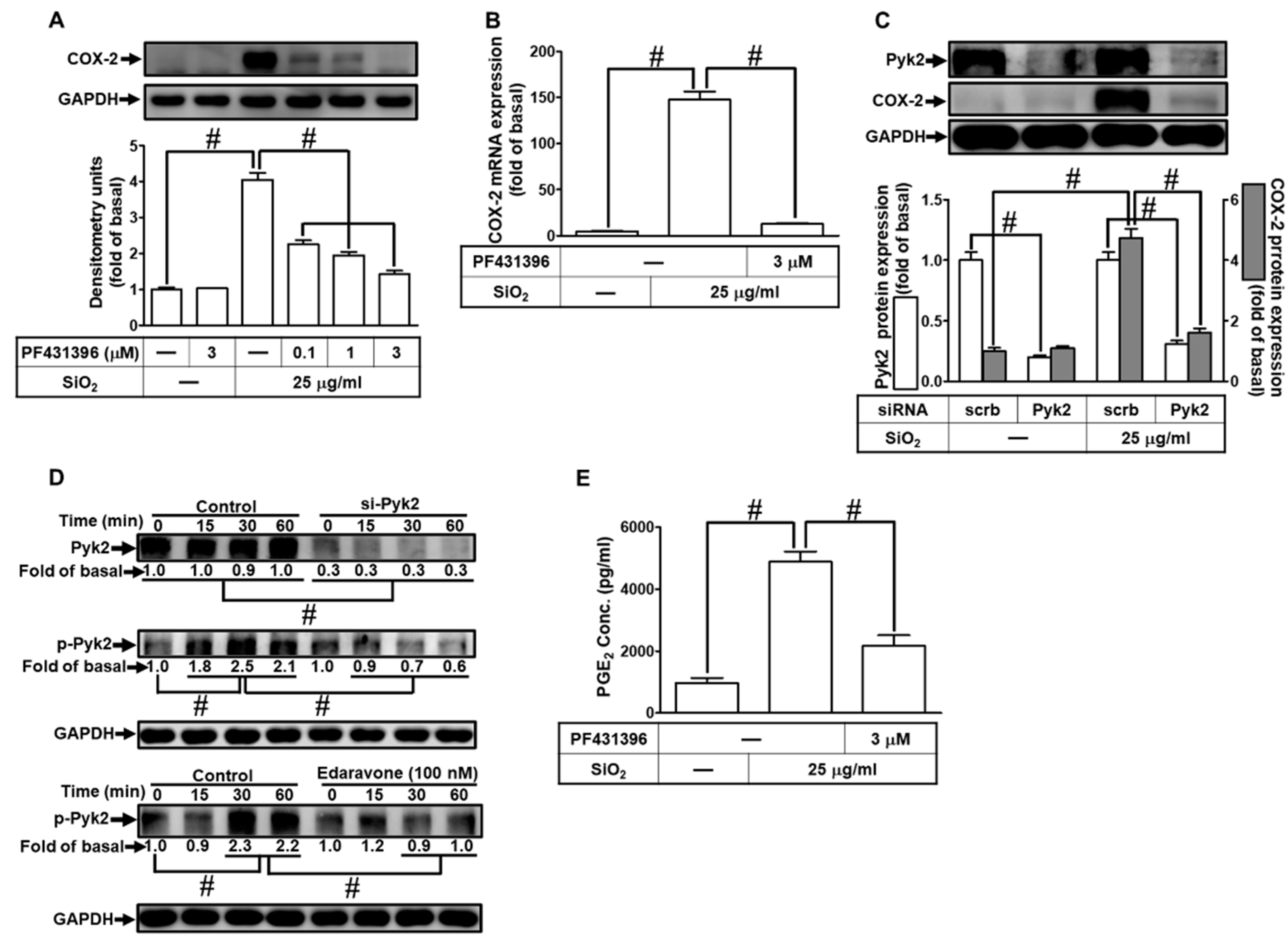
3.4. SiNPs induce COX-2 expression and PGE2 synthesis through the activation of Pyk2 in HPAEpiCs
3.5. EGFR is necessary for SiNPs-induced COX-2 expression and PGE2 synthesis
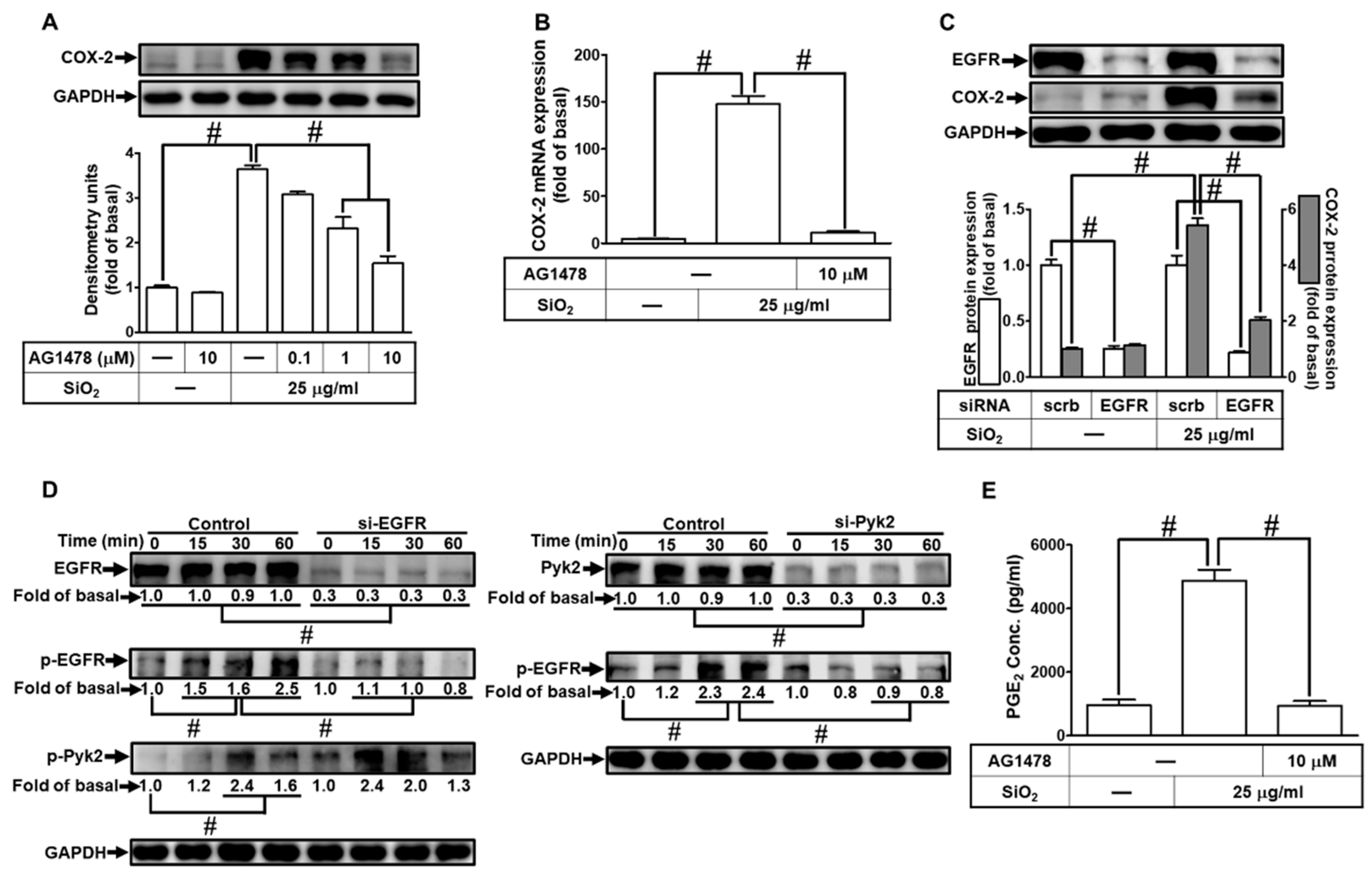
3.6. PI3K/Akt pathway participates in SiNPs-induced COX-2 expression and PGE2 synthesis in HPAEpiCs
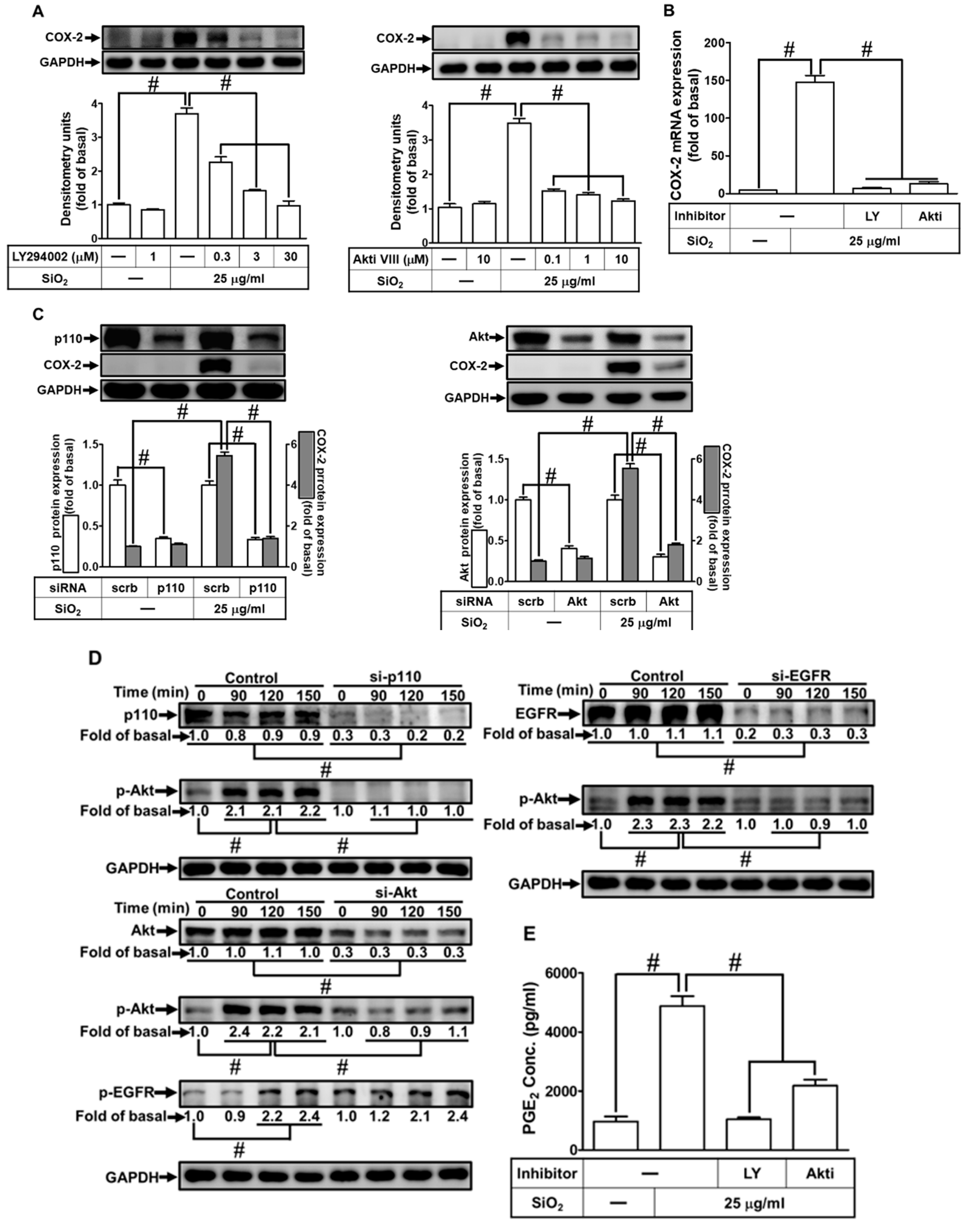
3.7. SiNPs induce COX-2 expression through p38 MAPK activation
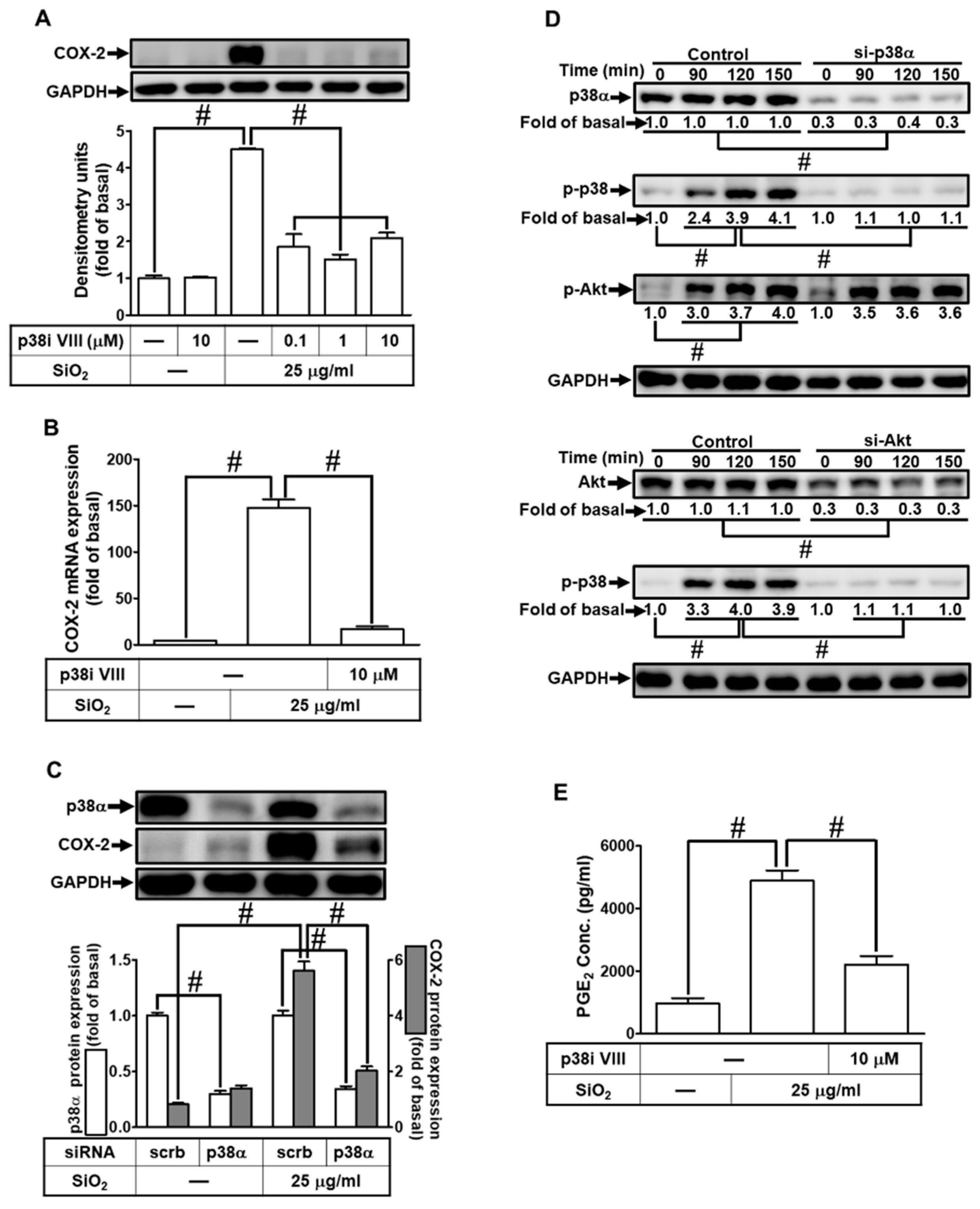
3.8. SiNPs induce COX-2 expression and PGE2 secretion via JNK1/2
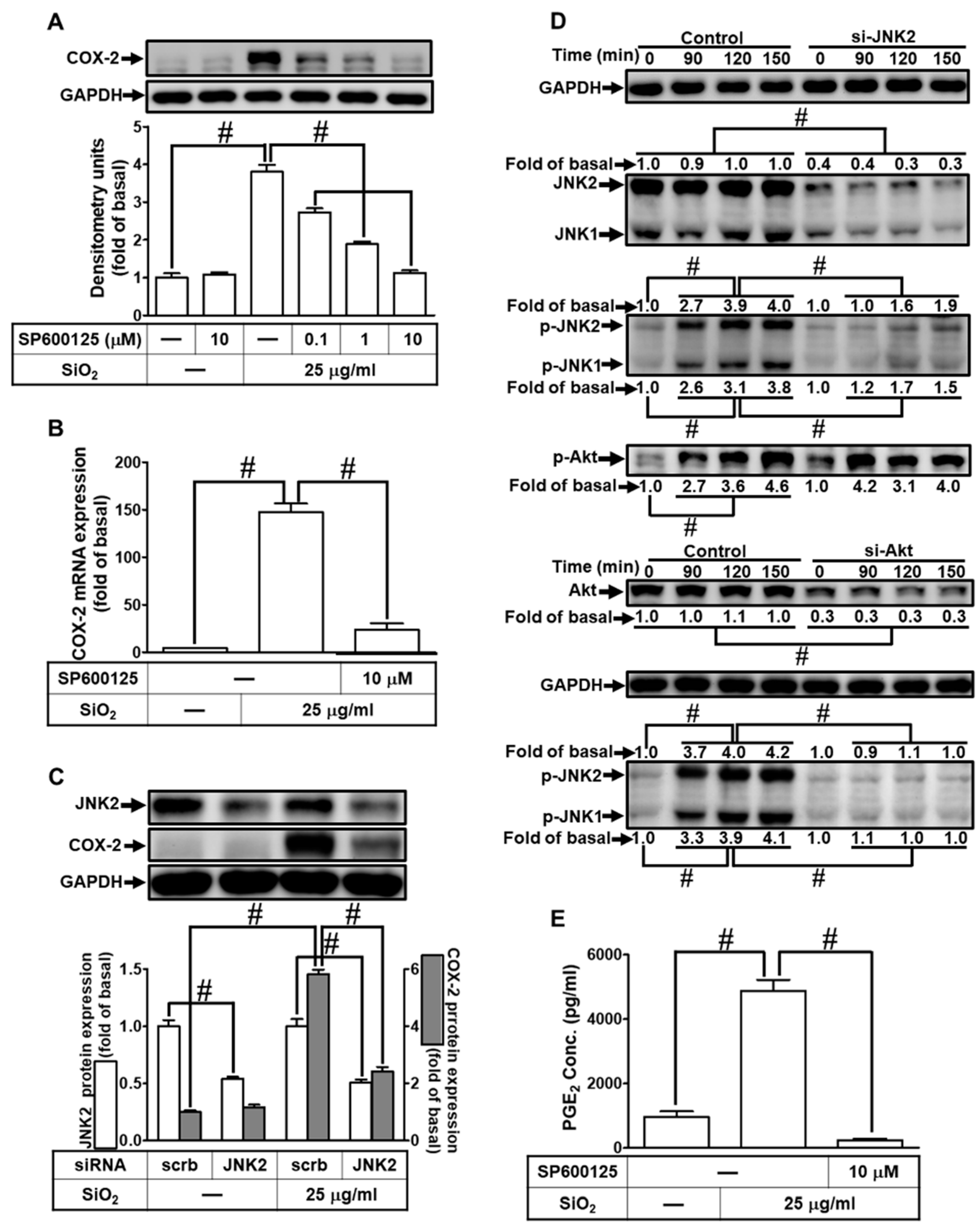
3.9. SiNPs induce COX-2 expression via FoxO1.
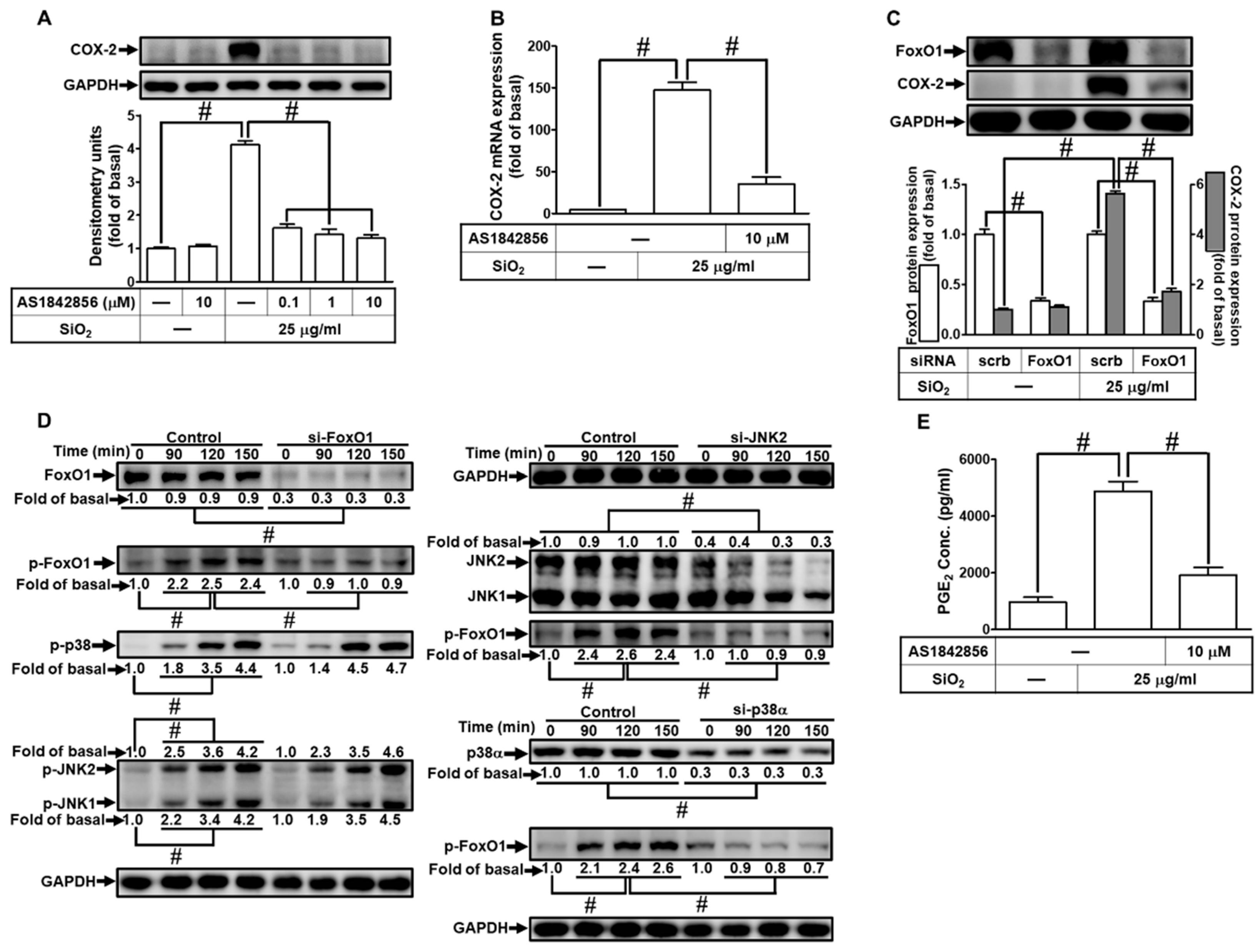
3.10. SiNPs induce COX-2 expression via AP-1 in HPAEpiCs
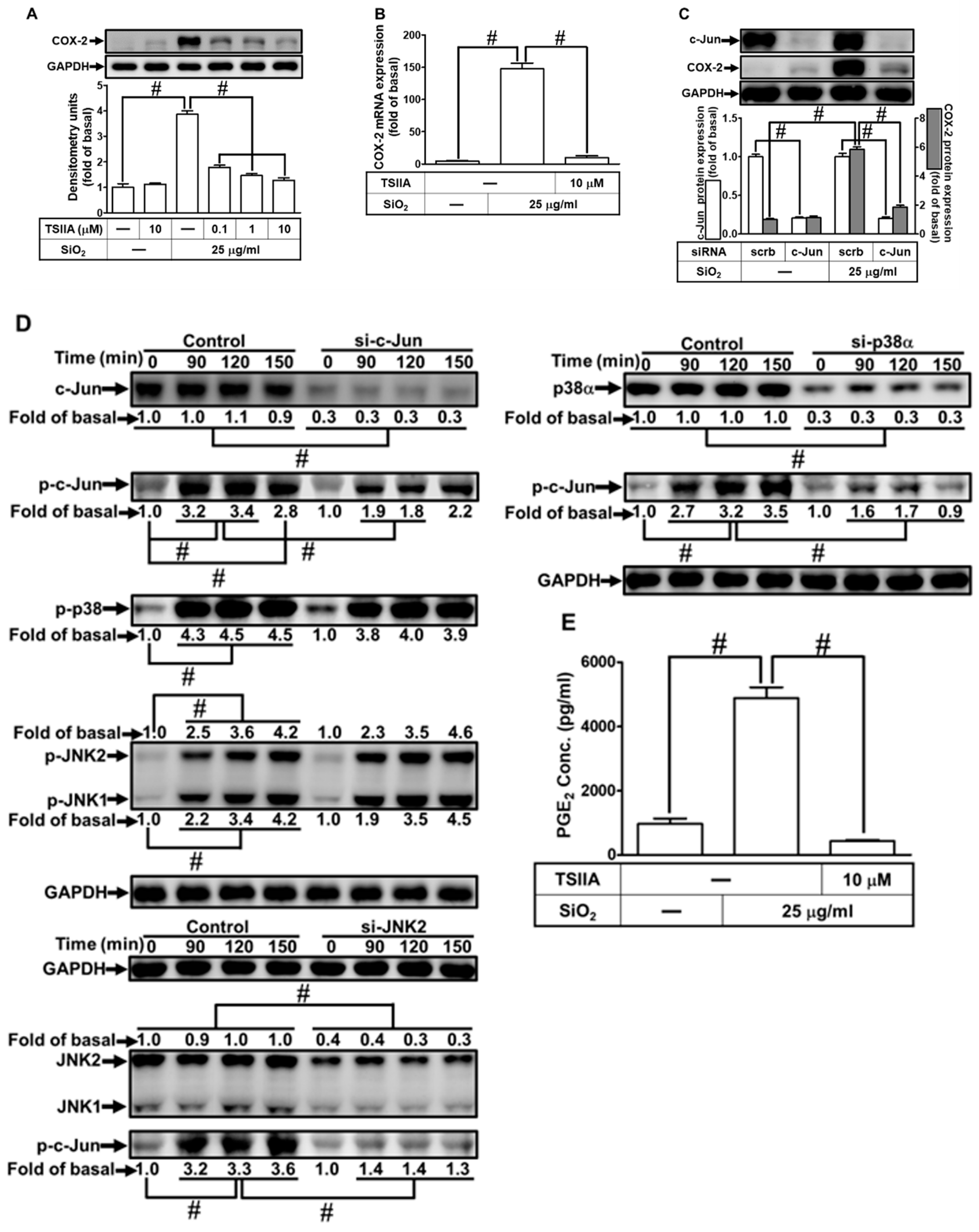
4. Discussion
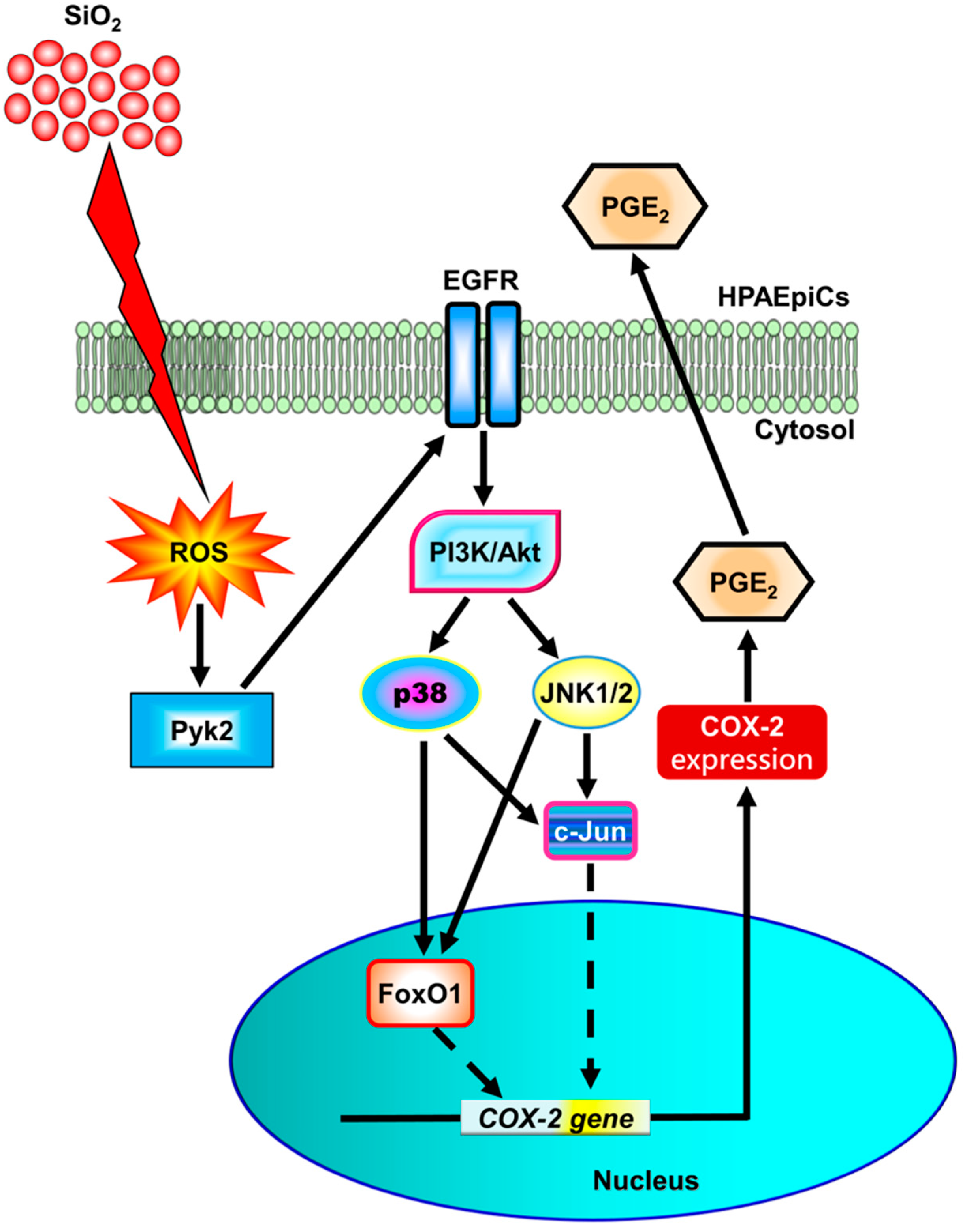
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oberdörster, G. Safety assessment for nanotechnology and nanomedicine: concepts of nanotoxicology. J. Intern. Med. 2010, 267, 89–105. [Google Scholar] [CrossRef]
- Salata, O.V. Applications of nanoparticles in biology and medicine. J. Nanobiotechnology 2004, 2, 3–3. [Google Scholar] [CrossRef]
- Croissant, J.G.; Butler, K.S.; Zink, J.I.; Brinker, C.J. Synthetic amorphous silica nanoparticles: toxicity, biomedical and environmental implications. Nat. Rev. Mater. 2020, 5, 886–909. [Google Scholar] [CrossRef]
- Ge, C.; Peters, S.; Olsson, A.; Portengen, L.; Schüz, J.; Almansa, J.; Behrens, T.; Pesch, B.; Kendzia, B.; Ahrens, W. , et al. Respirable crystalline silica exposure, smoking, and lung cancer subtype risks. A pooled analysis of case-control studies. Am. J. Respir. Crit. Care Med. 2020, 202, 412–421. [Google Scholar] [CrossRef]
- Inoue, M.; Sakamoto, K.; Suzuki, A.; Nakai, S.; Ando, A.; Shiraki, Y.; Nakahara, Y.; Omura, M.; Enomoto, A.; Nakase, I. Size and surface modification of silica nanoparticles affect the severity of lung toxicity by modulating endosomal ROS generation in macrophages. Part. Fibre. Toxicol. 2021, 18, 21–21. [Google Scholar] [CrossRef]
- Lee, K.; Lee, J.; Kwak, M.; Cho, Y.-L.; Hwang, B.; Cho, M.J.; Lee, N.G.; Park, J.; Lee, S.-H.; Park, J.-G. Two distinct cellular pathways leading to endothelial cell cytotoxicity by silica nanoparticle size. J. Nanobiotechnology 2019, 17, 24. [Google Scholar] [CrossRef]
- Panas, A.; Comouth, A.; Saathoff, H.; Leisner, T.; Al-Rawi, M.; Simon, M.; Seemann, G.; Dössel, O.; Mülhopt, S.; Paur, H.-R. Silica nanoparticles are less toxic to human lung cells when deposited at the air-liquid interface compared to conventional submerged exposure. Beilstein. J. Nanotechnol. 2014, 5, 1590–1602. [Google Scholar] [CrossRef]
- Dubois, R.N.; Abramson, S.B.; Crofford, L.; Gupta, R.A.; Simon, L.S.; Van De Putte, L.B.; Lipsky, P.E. Cyclooxygenase in biology and disease. FASEB J. 1998, 12, 1063–1073. [Google Scholar] [CrossRef]
- Crofford, L.J. COX-1 and COX-2 tissue expression: implications and predictions. J. Rheumatol. Suppl. 1997, 49, 15–19. [Google Scholar]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Park, G.Y.; Christman, J.W. Involvement of cyclooxygenase-2 and prostaglandins in the molecular pathogenesis of inflammatory lung diseases. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 290, L797–L805. [Google Scholar] [CrossRef] [PubMed]
- Taha, R.; Olivenstein, R.; Utsumi, T.; Ernst, P.; Barnes, P.J.; Rodger, I.W.; Giaid, A. Prostaglandin H synthase 2 expression in airway cells from patients with asthma and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2000, 161, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Roca-Ferrer, J.; Pujols, L.; Agusti, C.; Xaubet, A.; Mullol, J.; Gimferrer, J.M.; Picado, C. Cyclooxigenase-2 levels are increased in the lung tissue and bronchial tumors of patients with chronic obstructive pulmonary disease. Arch. Bronconeumol. 2011, 47, 584–589. [Google Scholar] [CrossRef]
- Lin, C.-C.; Lee, I.T.; Yang, Y.-L.; Lee, C.-W.; Kou, Y.R.; Yang, C.-M. Induction of COX-2/PGE2/IL-6 is crucial for cigarette smoke extract-induced airway inflammation: Role of TLR4-dependent NADPH oxidase activation. Free Radic. Biol. Med. 2010, 48, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Hsiao, L.D.; Shih, Y.F.; Hsu, C.K.; Hu, C.Y.; Yang, C.M. Thrombin Induces COX-2 and PGE2 Expression via PAR1/PKCα/MAPK-Dependent NF-κB Activation in Human Tracheal Smooth Muscle Cells. Mediators Inflamm. 2022, 2022, 4600029. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Xia, Y.; Niu, P.; Jiang, L.; Duan, J.; Yu, Y.; Zhou, X.; Li, Y.; Sun, Z. Silica nanoparticles induce oxidative stress, inflammation, and endothelial dysfunction in vitro via activation of the MAPK/Nrf2 pathway and nuclear factor-κB signaling. Int. J. Nanomedicine 2015, 10, 1463–1477. [Google Scholar] [CrossRef]
- Guo, C.; Yang, M.; Jing, L.; Wang, J.; Yu, Y.; Li, Y.; Duan, J.; Zhou, X.; Li, Y.; Sun, Z. Amorphous silica nanoparticles trigger vascular endothelial cell injury through apoptosis and autophagy via reactive oxygen species-mediated MAPK/Bcl-2 and PI3K/Akt/mTOR signaling. Int. J. Nanomedicine 2016, 11, 5257–5276. [Google Scholar] [CrossRef]
- Eom, H.-J.; Choi, J. Oxidative stress of silica nanoparticles in human bronchial epithelial cell, Beas-2B. Toxicol. In Vitro 2009, 23, 1326–1332. [Google Scholar] [CrossRef]
- Gong, C.; Tao, G.; Yang, L.; Liu, J.; He, H.; Zhuang, Z. The role of reactive oxygen species in silicon dioxide nanoparticle-induced cytotoxicity and DNA damage in HaCaT cells. Mol. Biol. Rep. 2012, 39, 4915–4925. [Google Scholar] [CrossRef]
- Park, E.-J.; Park, K. Oxidative stress and pro-inflammatory responses induced by silica nanoparticles in vivo and in vitro. Toxicol. Lett. 2009, 184, 18–25. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-mediated cellular signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965–4350965. [Google Scholar] [CrossRef] [PubMed]
- El-Hashim, A.Z.; Khajah, M.A.; Renno, W.M.; Babyson, R.S.; Uddin, M.; Benter, I.F.; Ezeamuzie, C.; Akhtar, S. Src-dependent EGFR transactivation regulates lung inflammation via downstream signaling involving ERK1/2, PI3Kδ/Akt and NF-κB induction in a murine asthma model. Sci. Rep. 2017, 7, 9919. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.; Kim, D.H.; Chae, I.G.; Lee, J.K.; Lee, S.; Jeong, C.H.; Chun, K.S. Silicon dioxide nanoparticles induce COX-2 expression through activation of STAT3 signaling pathway in HaCaT cells. Toxicol. In Vitro 2018, 52, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Cheng, J.-C.; Chang, H.-M.; Leung, P.C.K. COX2 and PGE2 mediate EGF-induced E-cadherin-independent human ovarian cancer cell invasion. Endocr. Relat. Cancer 2014, 21, 533–543. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev : MMBR 2011, 75, 50–83. [Google Scholar] [CrossRef]
- Yang, C.C.; Hsiao, L.D.; Su, M.H.; Yang, C.M. Sphingosine 1-phosphate induces cyclooxygenase-2/prostaglandin E2 expression via PKCα-dependent mitogen-activated protein kinases and NF-κB cascade in human cardiac fibroblasts. Front. Pharmacol. 2020, 11, 569802. [Google Scholar] [CrossRef]
- Yang, C.M.; Yang, C.C.; Hsiao, L.D.; Yu, C.Y.; Tseng, H.C.; Hsu, C.K.; Situmorang, J.H. Upregulation of COX-2 and PGE2 induced by TNF-α mediated through TNFR1/MitoROS/PKCα/P38 MAPK, JNK1/2/FoxO1 cascade in human cardiac fibroblasts. J. Inflamm. Res. 2021, 14, 2807–2824. [Google Scholar] [CrossRef]
- Kang, Y.-J.; Wingerd, B.A.; Arakawa, T.; Smith, W.L. Cyclooxygenase-2 gene transcription in a macrophage model of inflammation. J. Immunol. 2006, 177, 8111–8122. [Google Scholar] [CrossRef]
- Yang, C.C.; Hsiao, L.D.; Wang, C.Y.; Lin, W.N.; Shih, Y.F.; Chen, Y.W.; Cho, R.L.; Tseng, H.C.; Yang, C.M. HO-1 upregulation by kaempferol via ROS-dependent Nrf2-ARE cascade attenuates lipopolysaccharide-mediated intercellular cell adhesion molecule-1 expression in human pulmonary alveolar epithelial cells. Antioxidants (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Hsu, S.-Y.; Morris, R.; Cheng, F. Signaling pathways regulated by silica nanoparticles. Molecules 2021, 26, 1398. [Google Scholar] [CrossRef]
- Fubini, B.; Hubbard, A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radic. Biol. Med. 2003, 34, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Learoyd, J.; Meliton, A.Y.; Leff, A.R.; Zhu, X. Inhibition of Pyk2 blocks lung inflammation and injury in a mouse model of acute lung injury. Respir. Res. 2012, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Bierman, A.; Yerrapureddy, A.; Reddy, N.M.; Hassoun, P.M.; Reddy, S.P. Epidermal growth factor receptor (EGFR) regulates mechanical ventilation-induced lung injury in mice. Transl. Res. 2008, 152, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Vallath, S.; Hynds, R.E.; Succony, L.; Janes, S.M.; Giangreco, A. Targeting EGFR signalling in chronic lung disease: therapeutic challenges and opportunities. Eur. Respir. J. 2014, 44, 513. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, H.; Su, F.; Li, J.; Ma, X.; Gong, P. Relationship between epidermal growth factor receptor (EGFR) mutation and serum cyclooxygenase-2 Level, and the synergistic effect of celecoxib and gefitinib on EGFR expression in non-small cell lung cancer cells. Int. J. Clin. Exp. Pathol. 2015, 8, 9010–9020. [Google Scholar]
- Kang, Y.-J.; Mbonye, U.R.; DeLong, C.J.; Wada, M.; Smith, W.L. Regulation of intracellular cyclooxygenase levels by gene transcription and protein degradation. Prog. Lipid Res. 2007, 46, 108–125. [Google Scholar] [CrossRef]
- Ye, N.; Ding, Y.; Wild, C.; Shen, Q.; Zhou, J. Small molecule inhibitors targeting activator protein 1 (AP-1). J. Med. Chem. 2014, 57, 6930–6948. [Google Scholar] [CrossRef]
- Sato, T.; Shimosato, T.; Klinman, D.M. Silicosis and lung cancer: current perspectives. Lung Cancer (Auckl). 2018, 9, 91–101. [Google Scholar] [CrossRef]
- Liao, C.-M.; Wu, B.-C.; Cheng, Y.-H.; You, S.-H.; Lin, Y.-J.; Hsieh, N.-H. Ceramics manufacturing contributes to ambient silica air pollution and burden of lung disease. Environ. Sci. Pollut. Res. Int. 2015, 22, 15067–15079. [Google Scholar] [CrossRef]
- Paiva, C.N.; Bozza, M.T. Are reactive oxygen species always detrimental to pathogens? Antioxid. Redox. Signal. 2014, 20, 1000–1037. [Google Scholar] [CrossRef]
- Joshi, G.N.; Goetjen, A.M.; Knecht, D.A. Silica particles cause NADPH oxidase-independent ROS generation and transient phagolysosomal leakage. Mol. Biol. Cell 2015, 26, 3150–3164. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kundu, J.; Chae, I.G.; Lee, J.K.; Heo, J.S.; Chun, K.S. Titanium dioxide nanoparticles induce COX-2 expression through ROS generation in human periodontal ligament cells. J. Toxicol. Sci. 2019, 44, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Di Cioccio, V.; Strippoli, R.; Bizzarri, C.; Troiani, G.; Cervellera, M.N.; Gloaguen, I.; Colagrande, A.; Cattozzo, E.M.; Pagliei, S.; Santoni, A. , et al. Key role of proline-rich tyrosine kinase 2 in interleukin-8 (CXCL8/IL-8)-mediated human neutrophil chemotaxis. Immunology 2004, 111, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Wang, W.; Luo, W.; Zhang, W.; Cheng, Y.; Huang, J.; Wang, J.; Dai, X.; Fang, S.; Chao, J. CircHECTD1 mediates pulmonary fibroblast activation via HECTD1. Ther. Adv. Chronic Dis. 2019, 10, 2040622319891558. [Google Scholar] [CrossRef] [PubMed]
- Jeon, D.; Kim, H.; Nam, K.; Oh, S.; Son, S.H.; Shin, I. Cytotoxic effect of nano-SiO2 in human breast cancer cells via modulation of EGFR signaling cascades. Anticancer Res. 2017, 37, 6189–6197. [Google Scholar]
- Wang, Z. Transactivation of epidermal growth factor receptor by G protein-coupled receptors: recent progress, challenges and future research. Int. J. Mol. Sci. 2016, 17, 95. [Google Scholar] [CrossRef]
- Cattaneo, F.; Guerra, G.; Parisi, M.; De Marinis, M.; Tafuri, D.; Cinelli, M.; Ammendola, R. Cell-surface receptors transactivation mediated by g protein-coupled receptors. Int. J. Mol. Sci. 2014, 15, 19700–19728. [Google Scholar] [CrossRef]
- Frank, G.D.; Eguchi, S. Activation of tyrosine kinases by reactive oxygen species in vascular smooth muscle cells: significance and involvement of EGF receptor transactivation by angiotensin II. Antioxid. Redox. Signal. 2003, 5, 771–780. [Google Scholar] [CrossRef]
- Stark, A.-K.; Sriskantharajah, S.; Hessel, E.M.; Okkenhaug, K. PI3K inhibitors in inflammation, autoimmunity and cancer. Curr. Opin. Pharmacol. 2015, 23, 82–91. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Panas, A.; Comouth, A.; Saathoff, H.; Leisner, T.; Al-Rawi, M.; Simon, M.; Seemann, G.; Dössel, O.; Mülhopt, S.; Paur, H.-R. Silica nanoparticles are less toxic to human lung cells when deposited at the air–liquid interface compared to conventional submerged exposure. Beilstein J. Nanotechnol. 2014, 5, 1590–1602. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, Y.; Graves, D.T. FOXO transcription factors: their clinical significance and regulation. Biomed. Res. Int. 2014, 2014, 925350–925350. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




