Introduction
Neonates and infants hospitalized in the neonatal intensive care nursery (NICU) frequently require blood product transfusions, but clinical practices vary widely. Many very low birth weight (VLBW) infants receive packed red blood cell (RBC) or platelet transfusions during their initial NICU stay, with incidence inversely proportional to gestational age at birth 1,2. Many critically ill infants are also transfused with plasma or cryoprecipitate to promote coagulation 3,4. These blood product transfusions are most often prophylactic, with clinical decisions made in response to numerical blood count values, as opposed to therapeutic transfusions in the context of active bleeding.
Emerging evidence has shown that some transfusion practices are harmful for certain NICU patients, such as platelet transfusions in preterm infants 5,6. More broadly, transfusion reactions can occur with virtually all blood products 7 and these reactions are likely under-diagnosed, under-estimated, and under-reported in pediatric patients 3. Our intention was to establish optimal transfusion guidelines for our division and neonatal network, including 19 hospitals, based on a review of currently available literature.
Platelet Transfusions
Platelets facilitate hemostasis and have important roles in inflammation, immunity, and vascular biology 8. Thrombocytopenia, defined as a platelet count less than 150,000 platelets/µL blood, most often occurs secondary to infection or other systemic pathology in NICU patients 9. Platelet transfusions are given most frequently to prevent major bleeding in thrombocytopenic patients, rather than in response to active bleeding 10. VLBW neonates are at increased risk for thrombocytopenia and are frequently transfused with platelets in the first 7 days of life 11.
Platelet transfusion practices for neonates varies widely. Historically, clinical decision-making has been driven by concerns of increased risk of intraventricular hemorrhage (IVH) or other forms of major bleeding in thrombocytopenic infants. Platelet transfusion thresholds vary by country and institution, but have often been ~50,000/µL or higher for patients with critical illness 11. However, studies have refuted any correlation between thrombocytopenia severity and risk of IVH 11, and most neonates with severe thrombocytopenia (platelet count < 50,000 platelets/µL blood) do not have any episodes of major hemorrhage (e.g., Grade 3 or 4 IVH, pulmonary hemorrhage, or abdominal hemorrhage) 10. Most neonates with significant IVH had bleeding prior to developing severe thrombocytopenia.
Several trials have investigated appropriate platelet transfusion thresholds in NICU patients 5,12,13. Most recently, the Platelets for Neonatal Transfusion Study 2 (PlaNeT-2) was a randomized controlled trial that found a significantly higher risk of death or major bleeding in neonates transfused at a higher threshold (50,000 platelets/µL) than those transfused at a lower threshold (25,000 platelets/µL) 5. The higher transfusion threshold group also had an increased risk of developing bronchopulmonary dysplasia (BPD) and a lower probability of discharge home by 38 weeks corrected gestational age 5. These effects persisted regardless of patient bleeding or mortality risk stratification 6. At 2-year follow-up, those randomized to higher transfusion thresholds were at increased risk for death and neurodevelopmental impairment (NDI), as defined by cerebral palsy (CP), global developmental delay (GDD), hearing impairment, or vision impairment 14. Patients in the high threshold group also had increased risk of requiring oxygen or respiratory support at 2 years of age 14. These findings support lower platelet transfusion thresholds (~25,000 platelets/µL) for NICU patients regardless of perceived bleeding risk.
When a prophylactic platelet transfusion threshold of <25,000/µL was implemented in a tertiary and quaternary referral center NICU, platelet transfusions decreased overall 1. The biggest reduction in platelet transfusions was noted in non-bleeding, critically ill neonates. Importantly, there was no change in IVH incidence, and a significant decrease in other major bleeding complications was also observed after the lower platelet transfusion threshold was introduced.
Platelet transfusion dosing recommendations was also considered. Prior work has demonstrated no differences in efficacy between 10-15 ml/kg platelet transfusions 15–17. Several neonatal studies routinely administered 15 ml/kg platelets, including recent large clinical trials 5. However, concerns have been raised about the potential detrimental effects of this dose on neonatal physiology 8. A recent quality improvement effort demonstrated efficacy of 10 ml/kg platelet transfusions over 2 hours with no change in major bleeding occurrence 1.
In sum, there is no conclusive evidence of benefit for platelet transfusions in the prevention of major bleeding 18. Additionally, there are several pathophysiologic mechanisms by which platelet transfusions could harm NICU patients. Neonatal platelets are distinctly different from adult platelets in terms of reactivity to chemical agonists and in protein content 8,19. Thus, adult platelet transfusions may disrupt normal hemostatic balance, inflammatory mediators, and/or fluid shifts in otherwise vulnerable neonatal patients 8. These potentially detrimental effects of platelets are important considerations for clinical decision-making regarding platelet transfusion indication and dosage.
We modelled our platelet transfusion guidelines on recent landmark studies and consensus guidelines used successfully by other institutions, distinguishing between bleeding and non-bleeding neonates and including considerations for clinical situations that may warrant higher transfusion thresholds (
Table 1). For the non-bleeding neonate, we recommend platelet transfusion when the platelet count is <25,000/µL, with exceptions (
Table 1). For the bleeding neonate, we recommend platelet transfusion if the platelet count is <50,000-100,000/µL. If the platelet count is >100,000/µL, the patient should be evaluated for other potential causes of bleeding.
Table 1.
Platelet transfusion guidelines.
Table 1.
Platelet transfusion guidelines.
| PLT threshold (x103/µL) |
Non-Bleeding Neonate |
Bleeding Neonate** |
| <25 |
Transfuse (10mL/kg over 2 h)
Consider Transfusion if:
- critically ill <1000g and <1 week old*
- hemodynamically unstable
- previous major bleeding and <1 week old**
- current minor bleeding***
- concurrent coagulopathy
- within 72 h pre- or post-invasive procedure (i.e., lumbar puncture or surgery) |
Transfuse (10mL/kg over 2 h) |
| 25-50 |
Stable – No transfusion |
Transfuse |
| 50-100 |
Do not transfuse |
Transfuse |
| >100 |
Do not transfuse |
Do not transfuse |
Red Blood Cell Transfusions
Anemia of prematurity, transient erythroblastopenia of childhood, and iatrogenic blood loss are common etiologies prompting RBC transfusions in NICU patients. A majority of infants born extremely prematurely are given RBC transfusions during their initial NICU stay 20. As with platelet transfusions, packed red blood cell (RBC) transfusion practices vary widely across hospitals and even between physicians at the same institution. Indeed, such practice variations were present within our own network.
Several investigations have compared how liberal vs restrictive RBC transfusion thresholds impact clinical outcomes in NICU patients, which have varied among studies
21–24. Virtually all studies found a significant decrease in RBC transfusions in the restrictive transfusion groups, in which lower hemoglobin levels were well tolerated
21,22,24. Despite administering fewer transfusions, there were only subtle differences in blood donor exposure since patients often received RBC aliquots from the same donor unit. One study showed no difference in donor exposures
21, while another identified fewer donor exposures in the restrictive transfusion group
24. In general, these studies have found that lower hemoglobin does not adversely impact complications of prematurity, including risks of intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), retinopathy of prematurity (ROP), bronchopulmonary dysplasia (BPD), apnea of prematurity (AOP) patent ductus arteriosus (PDA), necrotizing enterocolitis (NEC), intestinal perforation, culture-proven sepsis, clinical sepsis, pneumonia or poor growth. When IVH, PVL, ROP, BPD, time on a ventilator, time on supplemental oxygen, time to regain birth weight, time to double birth weight, weight at 36 weeks postmenstrual age, and length of stay were evaluated separately, there was no significant difference in the liberal or restrictive transfusion groups
21–24. However, one study reported an increase in combined Grade 4 IVH and PVL outcome, and increased apneic events per day in the restrictive group
21. These differences were not observed in more recent studies, nor were there detectable differences in time requiring caffeine
22,23. Outcomes at 18- and 24-months corrected age did not differ with regard to death and/or neurodevelopmental impairment (NDI) between liberal and restrictive transfusion threshold groups
22,23,25. Together, these landmark studies support restrictive transfusion thresholds as being safe for NICU patients. Our transfusion guidelines are based on thresholds reported in the most recent report by Kirpalani
et al 22, and includes stratification by critical vs non-critical illness as defined by being on ≥4L HFNC for respiratory support and the week of life (
Table 2).
Table 2.
Red blood cell transfusion guidelines.
Table 2.
Red blood cell transfusion guidelines.
| |
Hgb/Hct thresholds |
| Week of life |
Critical Illness |
No Critical Illness |
| 1 |
11/32 |
10/29.5 |
| 2 |
10/29.5 |
8.5/25 |
| >/= 3 |
8.5/25 |
7/21 |
There has been variation in RBC transfusion dosing among prior studies. Most trials administered 15 mL/kg RBCs per transfusion 21,22,24, but some have provided 20 mL/kg RBCs 23. Based on these trials, our consensus guidelines recommend transfusion volumes of 15-20 mL/kg to be given over 3 hours (Table 2). Lower transfusion volumes (10-15 ml/kg) can also be recommended at the discretion of the neonatologist.
Transfusion-associated necrotizing enterocolitis
While RBC transfusions are necessary for many preterm neonates, numerous studies have investigated potential adverse consequences on neonatal physiology and disease pathogenesis. These concerns have largely centered on necrotizing enterocolitis (NEC), and clinical controversy continues to prompt prospective clinical studies in addition to publications spanning decades.
One specific clinical concern is that RBC transfusions may lead to mesenteric ischemia, putting neonates at increased risk for NEC 48 to 72 hours following a blood transfusion. While some studies have argued against changes in splanchnic oxygenation during RBC transfusions, this remains a matter of debate 26. Some clinicians have attempted to mitigate intestinal risks by withholding feeds during RBC transfusions. While studies comparing the incidence of transfusion-associated NEC before and after implementing policies to withhold feeding during and after RBC transfusions have shown a trend toward increased NEC risk, none of the studies individually reached statistical significance 27,28. Although severe anemia may predispose to NEC 29–31, there has not been clear evidence linking RBC transfusions to an increased incidence of NEC (e.g., transfusion-associated NEC). Thus, there is insufficient evidence at this time to warrant withholding feeds during RBC transfusions, although ongoing trials aim to further evaluate this subject 32.
We surveyed our neonatal network providers to assess current practices (n=102 respondents). We found that 28% of responding clinicians currently hold feeds during RBC transfusions, despite widespread opinion for a lack of strong evidence supporting this practice (88%). Of individuals who currently hold feeds during RBC transfusions, 76% reported a willingness to change practice based on updated guidelines and current literature. Of those who already continue enteral feeds during RBC transfusions, 77% offer full volume feeds or continue feeds at pre-transfusion rates. Most respondents (82%) did not consider the degree of anemia when deciding on feeding practices during RBC transfusion. Given the lack of strong evidence supporting transfusion associated NEC, our guidelines recommend continuing feeds at pre-transfusion volumes during and after RBC transfusions (Table 2).
Plasma and Cryoprecipitate Transfusions
Plasma contains coagulation factors and is typically indicated for bleeding with a coagulopathy. There have not been randomized controlled trials to directly evaluate the efficacy of plasma, coagulopathy, and bleeding risk in neonates 4. Nonetheless, neonates (particularly those born at <34 weeks gestation and critically ill) are given plasma more frequently than other pediatric patient groups 3. Neonatal plasma transfusions are often given prophylactically for abnormal laboratory values in patients without bleeding 3,4. Indeed, a retrospective analysis found that approximately half of all neonatal FFP transfusions were administered empirically for abnormal coagulation studies without hemorrhage 33. It is important to note the paucity of evidence to support prophylactic plasma transfusions for coagulopathy without active bleeding. Prophylactic plasma transfusion also does not prevent future episodes of hemorrhage 3.
Over the past 15 years, there has been a substantial decrease in neonatal plasma transfusions for abnormal coagulation laboratory tests without a concomitant change major bleeding 33. In the same time period, studies have shown no benefits for plasma administration in several clinical contexts. For example, infants receiving plasma in the setting of disseminated intravascular coagulation (DIC) had similar outcomes to patients not receiving any treatment 34. Similarly, there were no differences in short term outcomes, including immunologic responses to sepsis, following plasma administration in non-bleeding patients 35. Taken together, these findings offer reassurance that limited use of plasma transfusions in neonates is not associated with increased bleeding.
Perhaps most concerning are studies associating plasma transfusions with harm, including increased risks of pulmonary hemorrhage and venous thrombosis 3. Studies have also reported increased mortality in patients who received prophylactic plasma 36,37, while others simply demonstrated no benefit for plasma transfusions on risk of death 38. One study reported an increase in IVH in infants who received at least one plasma transfusion (although these results did not meet statistical significance) 39. Plasma transfusions in older children have been independently associated with organ dysfunction, infection, and prolonged length of hospitalization 7.
Administration of 10-15mL/kg of plasma might be expected to raise coagulation factors by 10-15% 3,4,7. However, studies have shown inconsistent effects on coagulation studies following plasma transfusion, with some reporting no change in coagulation testing 3. This may be due to differences in the hemostatic systems of neonates vs older children 40.
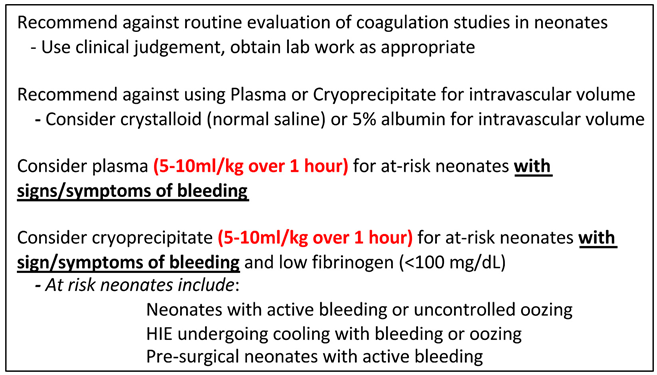
While we recommend against routine evaluation of coagulation studies in neonates, interpreting these results requires consideration of both gestational and postnatal age. Normal coagulation test values change rapidly in the neonatal period and are dependent on gestational age at delivery 41. Laboratory thresholds for coagulopathy would be PT and/or aPTT levels elevated to >2x normal references for age 41.
We developed guidelines based on judicious use of 5-10 ml/kg plasma over 1 hour that align with current expert opinion to transfuse patients with active bleeding, or prior to invasive procedures in coagulopathic patients likely to experience bleeding (
Table 3). We defined such at-risk neonates as 1) neonates with active bleeding or uncontrolled oozing, 2) neonates with hypoxic-ischemic encephalopathy undergoing therapeutic hypothermia with bleeding or oozing, and 3) presurgical neonates with active bleeding. We recommend against empiric transfusions in response to abnormal laboratory values without bleeding or coagulopathy prior to invasive procedures. If coagulation studies are obtained, we recommend basing clinical decisions to transfuse plasma for at-risk neonates in the absence of bleeding only if studies are >2x normal values for age.
Several retrospective studies also identified plasma administration in response to clinical concern for hypovolemia or hypotension in neonates, as opposed to specific concerns for coagulopathy 3. This practice is concerning, since plasma is a blood product with adverse transfusion reaction risks. Plasma is not indicated for volume expansion and we recommend against its use for this purpose (Table 3). To address volume expansion or resuscitation, we recommend isotonic crystalloid infusions. Isotonic fluids are superior to hypotonic fluids in preventing hyponatremia, with customized fluid solutions superior in preventing electrolyte disturbances in pediatric patients 42. Colloid fluids (e.g., 5% albumin – another blood derived product) have not shown superiority to crystalloids 42.
Cryoprecipitate is a concentrated plasma derivative rich in factor VIII, von Willebrand Factor, and fibrinogen. Cryoprecipitate can be used in neonates for bleeding, typically in the setting of a low fibrinogen level. Each bag of cryoprecipitate is 15 to 20 mL and contains 100 to 250 mg of fibrinogen. Pediatric dosing of cryoprecipitate is 1 to 2 bags per 10 kg of body weight to raise the fibrinogen level 60-100 mg/dL. For infants, a single unit of cryoprecipitate is a standard dose to achieve hemostasis which is equivalent to approximately 5 to 10 ml/kg 43. While there is insufficient evidence to recommend therapeutic or prophylactic cryoprecipitate transfusions in neonates and infants, our clinical guidelines outline the potential use of 5 mL/kg transfusions in actively bleeding patients with low fibrinogen or active therapeutic thrombolysis as clinically warranted 4 (Table 3).
Discussion
Neonates and infants are vulnerable patient populations and emerging studies have shown evidence for harm associated with some current blood product transfusion practices 3,5,7,12,13. Recent trials described herein have refined evidence-based practice for blood product transfusions in neonates. We have developed institutional transfusion guidelines for neonates and infants based on current evidence and implemented these network-wide to improve transfusion practice and safety. We anticipate that future trials will further clarify recommendations for specific at-risk patient populations (e.g., for extremely premature infant platelet transfusion thresholds, infants on ECMO, or those requiring massive transfusion protocols) to further refine transfusion guidelines that optimize care for neonates and infants.
Funding
This project was supported through grants from the National Institutes of Health (HL156052 to CST).
Conflicts of Interest
The authors declare no conflict of interest related to this work.
References
- Davenport PE, Chan Yuen J, Briere J, Feldman HA, Sola-Visner MC, Leeman KT.Implementation of a neonatal platelet transfusion guideline to reduce non-indicated transfusions using a quality improvement framework. Journal of Perinatology. 2021, 41, 1487–94. [CrossRef]
- Baer VL, Lambert DK, Henry E, Snow GL, Sola-Visner MC, Christensen RD. Do platelet transfusions in the NICU adversely affect survival? Analysis of 1600 thrombocytopenic neonates in a multihospital healthcare system. Journal of Perinatology [Internet]. 2007, 27, 790–796. Available online: http://www.nature.com/doifinder/10.1038/sj.jp.7211833. [CrossRef]
- Sokou R, Parastatidou S, Konstantinidi A, Tsantes AG, Iacovidou N, Doxani C, et al. Fresh frozen plasma transfusion in the neonatal population: A systematic review. Vol. 55, Blood Reviews. Churchill Livingstone; 2022.
- Steinbicker AU, Wittenmeier E, Goobie SM. Pediatric non-red cell blood product transfusion practices: What’s the evidence to guide transfusion of the “yellow” blood products? Vol. 33, Current Opinion in Anaesthesiology. Lippincott Williams and Wilkins; 2020. p. 259–267.
- Curley A, Stanworth SJ, Willoughby K, Fustolo-Gunnink SF, Venkatesh V, Hudson C, et al. Randomized Trial of Platelet-Transfusion Thresholds in Neonates. New England Journal of Medicine. 2019, 380, 242–251.
- Fustolo-Gunnink SF, Fijnvandraat K, Van Klaveren D, Stanworth SJ, Curley A, Onland W, et al. Preterm neonates benefit from low prophylactic platelet transfusion threshold despite varying risk of bleeding or death. Blood [Internet]. 2019, 134, 2354–2360. [CrossRef] [PubMed]
- Valentine SL, Cholette JM, Goobie SM. Transfusion Strategies for Hemostatic Blood Products in Critically Ill Children: A Narrative Review and Update on Expert Consensus Guidelines. Vol. 135, Anesthesia and Analgesia. Lippincott Williams and Wilkins; 2022. p. 545–557.
- Ferrer-Marín F, Sola-Visner M. Neonatal platelet physiology and implications for transfusion. Platelets. 2022, 33, 14–22. [CrossRef]
- Fernández KS, de Alarcón P. Neonatal thrombocytopenia. Neoreviews [Internet]. 2013, 14, e74–82. Available online: https://neoreviews.aappublications.org/content/14/2/e74. [CrossRef]
- Stanworth SJ, Clarke P, Watts T, Ballard S, Choo L, Morris T, et al. Prospective, observational study of outcomes in neonates with severe thrombocytopenia. Pediatrics. 2009, 124.
- Sparger KA, Assmann SF, Granger S, Winston A, Christensen RD, Widness JA, et al. Platelet transfusion practices among very-low-birth-weight infants. JAMA Pediatr. 2016, 170, 687–694. [CrossRef]
- Andrew M, Vegh P, Caco C, Kirpalani H, Jefferies A, Ohlsson A, et al. A randomized, controlled trial of platelet transfusions in thrombocytopenic premature infants. J Pediatr. 1993, 123, 285–291. Available online: http://www.ncbi.nlm.nih.gov/pubmed/8345429. [CrossRef]
- Kumar J, Dutta S, Sundaram V, Saini SS, Sharma RR, Varma N. Platelet transfusion for PDA closure in preterm infants: A randomized controlled trial. Pediatrics. 2019, 143.
- Moore CM, D’Amore A, Fustolo-Gunnink S, Hudson C, Newton A, Santamaria BL, et al. Two-year outcomes following a randomised platelet transfusion trial in preterm infants. Arch Dis Child Fetal Neonatal Ed [Internet]. 2023 Feb 21; fetalneonatal-2022-324915. 1136. Available online: https://fn.bmj.com/lookup/doi/10.1136/archdischild-2022-324915.
- Leibowitz M, Wolfe H, Flynn A, Waanders A, Burlingame C, Aumaier B, et al. Standardization of prophylactic platelet transfusion dosing in a pediatric oncology population: a quality improvement project. Transfusion (Paris). 2018, 58, 2836–2840. [CrossRef]
- Slichter SJ, Kaufman RM, Assmann SF, McCullough J, Triulzi DJ, Strauss RG, et al. Dose of Prophylactic Platelet Transfusions and Prevention of Hemorrhage. New England Journal of Medicine. 2010, 362, 600–613. [CrossRef]
- Kline A, Mackley A, Taylor SM, McKenzie SE, Paul DA. Thrombopoietin following transfusion of platelets in preterm neonates. Platelets. 2008, 19, 428–431. [CrossRef]
- Hasan R, Saifee NH. Benefits of lower neonatal platelet transfusion thresholds. Vol. 61, Transfusion. John Wiley and Sons Inc; 2021. p. 1672–5.
- Stokhuijzen E, Koornneef JM, Nota B, van den Eshof BL, van Alphen FPJ, van den Biggelaar M, et al. Differences between platelets derived from neonatal cord blood and adult peripheral blood assessed by mass spectrometry. J Proteome Res. 2017, 16, 3567–3575. Available online: https://pubs.acs.org/doi/abs/10.1021/acs.jproteome.7b00298. [CrossRef] [PubMed]
- Davenport P, Sola-Visner M. Immunologic effects of red blood cell and platelet transfusions in neonates. Curr Opin Hematol. 2022, 29, 297–305. [CrossRef]
- Bell EF, Strauss RG, Widness JA, Mahoney LT, Mock DM, Seward VJ, et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics. 2005, 115, 1685–1691. [CrossRef] [PubMed]
- Kirpalani H, Bell EF, Hintz SR, Tan S, Schmidt B, Chaudhary AS, et al. Higher or Lower Hemoglobin Transfusion Thresholds for Preterm Infants. New England Journal of Medicine. 2020, 383, 2639–2651. [CrossRef]
- Franz AR, Engel C, Bassler D, Rüdiger M, Thome UH, Maier RF, et al. Effects of liberal vs restrictive transfusion thresholds on survival and neurocognitive outcomes in extremely low-birth-weight infants: The ETTNO randomized clinical trial. JAMA. 2020, 324, 560–570. [CrossRef]
- Kirpalani H, Whyte RK, Andersen C, Asztalos E V, Heddle N, Blajchman MA, et al. The premature infants in need of transfusion (pint) study: A randomized, controlled trial of a restrictive (LOW) versus liberal (HIGH) transfusion threshold for extremely low birth weight infants. Journal of Pediatrics 2006, 149.
- Whyte RK, Kirpalani H, Asztalos E V, Andersen C, Blajchman M, Heddle N, et al. Neurodevelopmental outcome of extremely low birth weight infants randomly assigned to restrictive or liberal hemoglobin thresholds for blood transfusion. Pediatrics. 2009, 123, 207–213. [CrossRef]
- Howarth C, Banerjee J, Aladangady N. Red Blood Cell Transfusion in Preterm Infants: Current Evidence and Controversies. Vol. 114, Neonatology. S. Karger AG; 2018. p. 7–16.
- Jasani B, Rao S, Patole S. Withholding feeds and transfusion-associated necrotizing enterocolitis in preterm infants: A systematic review. Advances in Nutrition. 2017, 8, 764–769. [CrossRef]
- Bajaj M, Lulic-Botica M, Hanson A, Natarajan G. Feeding during transfusion and the risk of necrotizing enterocolitis in preterm infants. Journal of Perinatology. 2019, 39, 540–546. [CrossRef]
- Flannery DD, Foglia EE. The contributions of red blood cell transfusion and severe anemia in necrotizing enterocolitis: Causes or confounders? Vol. 37, Journal of Perinatology. Nature Publishing Group; 2017. p. 626–628.
- Patel RM, Knezevic A, Shenvi N, Hinkes M, Keene S, Roback JD, et al. Association of red blood cell transfusion, anemia, and necrotizing enterocolitis in very low-birth-weight infants. JAMA - Journal of the American Medical Association. 2016, 315, 889–897. [CrossRef]
- Faraday C, Hamad S, Jones KD, Sim K, Cherian S, James A, et al. Characteristics and incidence of transfusion-associated necrotizing enterocolitis in the UK. Journal of Maternal-Fetal and Neonatal Medicine. 2020, 33, 398–403. [CrossRef]
- Gale C, Modi N, Jawad S, Culshaw L, Dorling J, Bowler U, et al. The WHEAT pilot trial - WithHolding Enteral feeds around packed red cell Transfusion to prevent necrotising enterocolitis in preterm neonates: A multicentre, electronic patient record (EPR), randomised controlled point-of-care pilot trial. BMJ Open. 2019, 9.
- Houben NAM, Heeger LE, Stanworth SJ, New H V. , van der Bom JG, Fustolo-gunnink S, et al. Changes in the use of fresh-frozen plasma transfusions in preterm neonates: A single center experience. J Clin Med. 2020, 9, 1–9.
- Gross SJ, Filston HC, Anderson JC. Controlled study of treatment for disseminated intravascular coagulation in the neonate. Journal of Pediatrics. 1982, 100, 445–448. [CrossRef]
- Acunas BA, Peakman M, Liossis G, Davies ET, Bakoleas B, Costalos C, et al. Effect of fresh frozen plasma and gammaglobulin on humoral immunity in neonatal sepsis. Arch Dis Child. 1994, 70, F182–7. [CrossRef]
- Shukri Raban M, Harrison MC. Fresh frozen plasma use in a neonatal unit in South Africa. J Trop Pediatr. 2015, 61, 266–271. [CrossRef] [PubMed]
- Goel R, Josephson CD, Patel EU, Petersen MR, Packman Z, Gehrie E, et al. Individual- and hospital-level correlates of red blood cell, platelet, and plasma transfusions among hospitalized children and neonates: a nationally representative study in the United States. Transfusion (Paris). 2020, 60, 1700–1712.
- Tran TTH, Veldman A, Malhotra A. Does risk-based coagulation screening predict intraventricular haemorrhage in extreme premature infants? Blood Coagulation and Fibrinolysis. 2012, 23, 532–536.
- Mendicini M, Scalamandre A, Savignoni PG, Picece-Bucci S, Esuperanzi R, Bucci G. A controlled trial on therapy for newborns weighing 750-1250 g. Acta Paediatr. 1971, 60, 407–416.
- Davenport P, Sola-Visner M. Hemostatic Challenges in Neonates. Vol. 9, Frontiers in Pediatrics. Frontiers Media S.A.; 2021.
- Cantor, A. Hemostasis in the Newborn and Infant. In: Nathan and Oski’s Hematology of the Fetus and Newborn. 8th ed. 2015. p. 128–57.
- Bailey AG, Mcnaull PP, Jooste E, Tuchman JB. Perioperative crystalloid and colloid fluid management in children: Where are we and how did we get here? Vol. 110, Anesthesia and Analgesia. Lippincott Williams and Wilkins; 2010. p. 375–90.
- Wong EC, Roseff SD, Bandarenko N, editors. Pediatric Transfusion: A Handbook. 5th ed. Association for the Advancement of Blood & Biotherapies; 2020.
Table 3.
Plasma and cryoprecipitate transfusion guidelines.
Table 3.
Plasma and cryoprecipitate transfusion guidelines.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).