Introduction
Ultra-processed foods, as defined using the Nova food classification system, encompass a broad range of ready-to-eat products often packaged in single-use plastics 1. Packaged snacks, carbonated soft drinks, instant noodles, ready-made meals and milk formulas are all examples of ultra-processed foods 1. These products are characterised as industrial formulations primarily composed of chemically modified substances extracted from foods, along with additives to enhance taste, texture, appearance, and durability, with minimal to no inclusion of whole foods 2. Analyses of worldwide ultra-processed food sales data and consumption patterns indicate a shift towards an increasingly ultra-processed global diet 3,4, although considerable diversity exists within and between countries and regions 5,6. Across high-income countries, the share of dietary energy derived from ultra-processed foods ranges from 42% and 58% in Australia and the United States respectively, to as low as 10% and 25% in Italy and South Korea 5,6. In low and middle-income countries such as Colombia and Mexico, for example, these figures range from 16% to 30% of total energy intake, respectively 5. Notably, across recent decades, there has been a substantial and rapid elevation in the availability and variety of ultra-processed products sold in countries across diverse economic development levels, but especially in many highly-populated low and middle-income nations 3.
The shift from unprocessed and minimally processed foods to ultra-processed foods and their subsequent increasing contribution to global dietary patterns in recent years have been attributed to key drivers including behavioural mechanisms, food environments, and commercial influences on food choices 7–11. These factors, combined with the specific features of ultra-processed foods, raise concerns regarding overall diet quality and the health of populations more broadly. For example, some characteristics of ultra-processed foods include alterations to food matrices and textures, potential contaminants from packaging material and processing, the presence of food additives and other industrial ingredients, as well as nutrient-poor profiles (e.g., higher energy, salt, sugar, and saturated fat, with lower levels of dietary fibre, micronutrients, and vitamins) 6,12. While mechanistic research is still in its infancy, emerging evidence suggests that such properties may pose synergistic or compounded consequences for chronic inflammatory diseases and may act through known or plausible physiological mechanisms including changes to the gut microbiome and increased inflammation 12–16. Researchers, public health experts, and the general public have shown considerable interest in ultra-processed dietary patterns, foods, and their constituent parts given their potential role as modifiable risk factors for chronic diseases and mortality.
Although several meta-analyses have made efforts to consolidate the many individual original research articles that have investigated the associations between exposure to ultra-processed foods and the risk of adverse health outcomes in the past decade 17,18, no comprehensive umbrella review has offered a broad overview and assessment of the existing meta-analytic evidence. Undertaking such a comprehensive review has the potential to enhance our understanding of these associations and provide valuable insights for better informing public health policies and strategies. This is particularly pertinent as the global debate continues regarding the need for public health measures to tackle exposure to ultra-processed foods in general populations 19,20. To bridge this gap in evidence and contribute to the ongoing discussion on the role of ultra-processed food exposure in chronic diseases, we undertook an umbrella review to evaluate the evidence provided by meta-analyses of observational epidemiological studies exploring the associations between ultra-processed food exposure and the risk of adverse health outcomes.
Methods
We conducted and reported this systematic umbrella review of meta-analyses (herein referred to as ‘meta-analysis studies’) in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines 21. Our review was prospectively registered with the International Prospective Register of Systematic Reviews, better known as PROSPERO (ID: CRD42023412732).
Inclusion Criteria and Searches
We found no existing pooled analyses of randomised controlled trials during the pilot phase of this review. Consequently, we refined our search approach and scope to focus on observational epidemiological studies. Thus, inclusion criteria were outlined in accordance with the population, exposure, comparisons, outcomes, and study design (PECOS) reporting structure 22. Eligible meta-analysis studies comprised both healthy and diseased human populations across the life course (population) and examined associations of dietary intake of ultra-processed foods, as defined by the Nova food classification system (exposure), comparing both categorical and/or continuous exposure to ultra-processed foods (non-dose-response) or focusing solely on continuous exposure (dose-response) (comparison), with any adverse health endpoint (outcome). Included in our review were observational epidemiological study designs (e.g., prospective cohort, case-control, and/or cross-sectional) that pooled categorical or continuous outcome data using meta-analysis (study design).
We searched the MEDLINE, PsycINFO, EMBASE, and Cochrane databases, for studies from 2009 to June 2023, since this was when the details and principles of the Nova food classification system, along with its concept of ultra-processed foods, were first published 23. No language limitations were applied.
To identify relevant meta-analysis studies, we used key search terms related to ultra-processed food, Nova, and meta-analysis study design (
Table S1). We used Covidence systematic review software
24 to undertake duplicate primary screening based on titles and abstracts (MML and EG) and duplicate secondary screening based on full-text articles (MML and WM). We screened references cited within the eligible meta-analysis studies to identify any additional relevant meta-analysis studies (EG). Any disagreements between those conducting eligibility screening were resolved through consensus. We included the most recent and/or largest meta-analysis study when multiple pooled analyses were available for the same adverse health outcome. This is consistent with the methods used in previous umbrella reviews
25–27. In cases where the most recent meta-analysis study examined non-dose-response and dose-response exposure to ultra-processed foods, we included both meta-analysed effect estimates.
Data Extraction, Outcomes, and Data Synthesis
Characteristics of the original research articles included in the retained meta-analysis studies were extracted in duplicate using a pre-piloted custom Microsoft Excel spreadsheet (MML, EG, SD, DNA, AJM, and SG). These data included details such as study design, sample size, number of cases versus non-cases, types of outcomes, risk of bias assessments, and effect estimates. Furthermore, we extracted data related to competing interests and funding disclosures of meta-analysis study authors. We prioritised pooled estimates with the largest number of prospective cohorts given prospective studies guarantee temporality in epidemiological associations and strongly limit reverse causality bias 28. Additionally, we extracted pooled estimates for related health outcomes that were meta-analysed together and separately, as long as the separate subgroup analyses were considered clinically informative (e.g., metabolic syndrome and its individual components including low high-density lipoprotein cholesterol and hypertriglyceridaemia). If information was missing or unclear in the meta-analysis studies, we obtained the data from the original research articles or directly requested it from the corresponding author(s) of those meta-analysis studies. If there were discrepancies between the data reported in the original research articles and the meta-analyses, we prioritised extracting data from the original research article.
Data Analysis
We expected that extracted data were highly heterogeneous and subsequently used a random-effects meta-analysis model to reanalyse the effect estimates for each outcome 29, such as hazard ratios (HR), odds ratios (OR), and risk ratios (RR). We computed 95% prediction intervals to estimate the potential range of effect estimates expected in future studies for pooled analyses with more than three original research articles. In an umbrella review, if the 95% prediction intervals exclude the null, it indicates a statistically significant range of effect estimates 30.
The
I2 statistic was used to assess the proportion of variability in a pooled analysis that was explained by between-study heterogeneity, rather than by sampling error, and to reflect the extent to which 95% confidence intervals from the different original research articles overlap with each other
31. A value of 50% or more was considered moderate heterogeneity and a value of 75% or more was considered high heterogeneity
31. Egger’s regression asymmetry test was used to detect potential “small-study effects”, whereby smaller studies sometimes show different, often larger, effect estimates than large studies
32. A
P below 0.10 was considered indicative of small-study effects
33. A test for excess significance was conducted for all outcomes. This test determines whether the number of studies with nominally significant results (
P < 0.05) was higher than expected, based on statistical power
34. Furthermore, we assessed whether effect estimates from the largest original research article (i.e., the study with the highest participant count) included in the pooled analyses exhibited a
P below 0.05. This evaluation is expected to provide the most reliable and precise estimation considering the statistical power involved
35. The data analyses were performed using the online version of the R statistical package called
metaumbrella (
https://metaumbrella.org)
36. Finally, for visually comparative purposes, forest plots were developed whereby pooled effect estimates were harmonised to equivalent odds ratios (eOR) using methods proposed by Fusar-Poli et al. (2018)
37. In this instance, an eOR greater than 1 indicates higher odds, while an eOR less than 1 indicates lower odds, of an outcome.
The terms “direct” and “inverse” were used to describe the direction of observed associations between ultra-processed food exposure and adverse health outcomes, with “direct” referring to a higher risk associated with greater exposure, and “inverse” referring to a lower risk. These terms were chosen over “positive” or “negative” associations to avoid ambiguous interpretations.
Credibility Assessment of Each Pooled Analysis Assessing Associations between Ultra-Processed Food Exposure and Adverse Health Outcomes
We categorised each re-meta-analysed result of our umbrella review as convincing, highly suggestive, suggestive, weak, or no evidence in accordance with the “evidence classification criteria”
38 and previous umbrella reviews
25–27. These classifications were determined based on the criteria outlined in
Table 1 38.
Quality Assessment of Each Pooled Analysis Assessing Associations between Ultra-Processed Food Exposure and Adverse Health Outcomes
We used the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system to evaluate the quality of evidence for each unique pooled analysis, and categorised them as either “High”, “Moderate”, “Low”, or “Very Low” 39. It is worth noting that the GRADE approach initially considers all observational studies as evidence of low quality 39. Out of the eight criteria put forth in the GRADE method, five have the potential to diminish confidence in the accuracy of effect estimates, leading to downgrading: 1) risk of bias, 2) inconsistency of results across studies, 3) indirectness of evidence, 4) imprecision, and 5) publication bias 39. Additionally, three criteria are proposed to enhance confidence, or upgrade it: 1) a substantial effect size with no plausible confounders, 2) a dose-response relationship, and 3) a conclusion that all plausible residual confounding would further support inferences regarding exposure effect 39.
Patient and Public Involvement
Patients and the public were not involved in the conduct of this study or the development of the manuscript, as it relied on secondary data.
Results
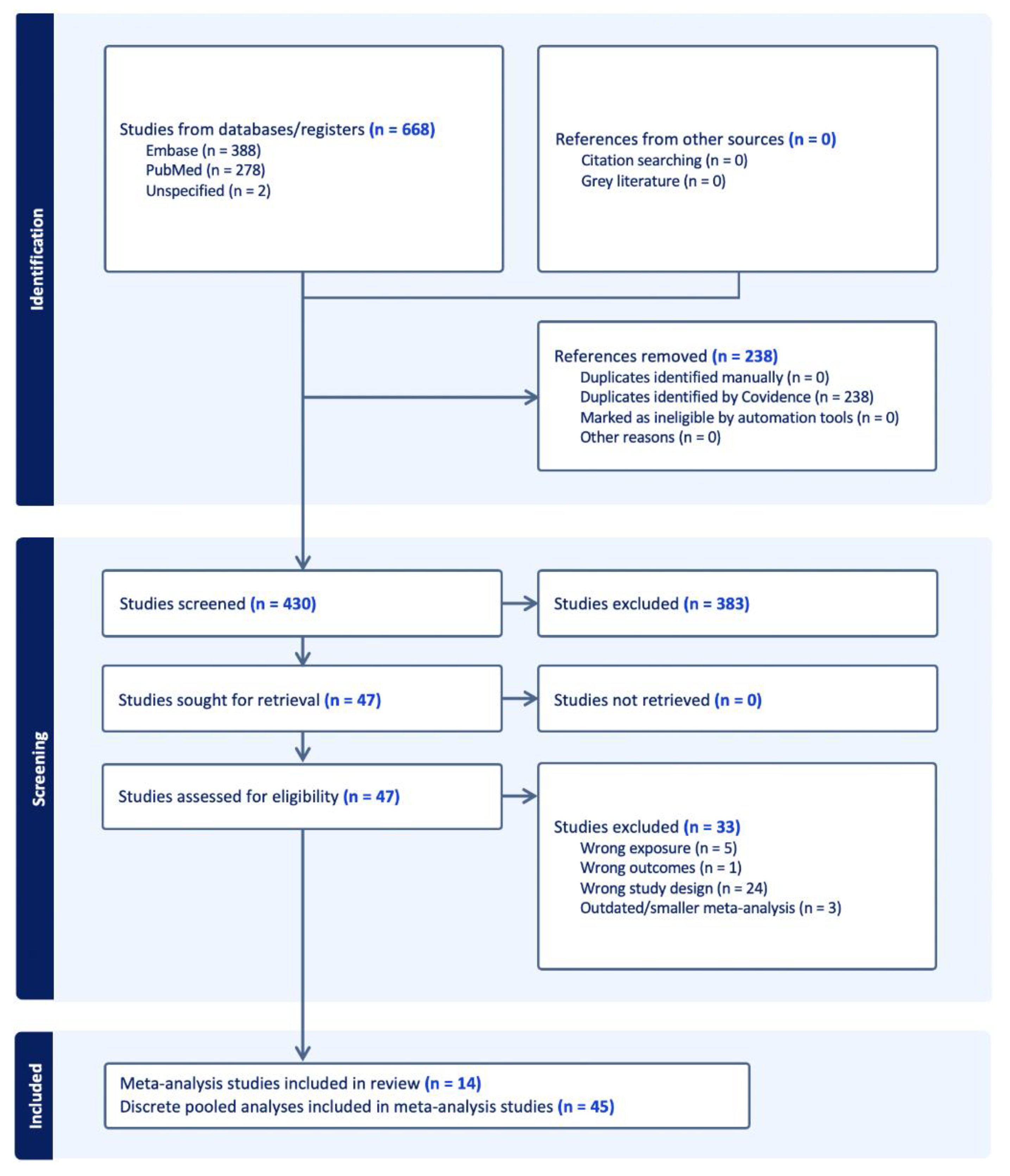
The systematic search identified 430 deduplicated articles (
Figure 1). After applying the eligibility criteria, 14 meta-analysis studies with 45 distinct pooled analyses were included
18,41–53.
Study Characteristics
Table 2 shows the range of adverse health outcomes reviewed across the 45 discrete pooled analyses. All meta-analysis studies were published in the last three years and none were funded by companies involved in the production of ultra-processed foods. The average number of original research articles included in the pooled analyses was four and ranged from two to nine. The sum total number of participants included across the pooled analyses was 9,888,373 (ranging from 1,113
17 to 962,593
50).
Table S2 details the characteristics of the original research articles included in each of the pooled analyses, such as study design, population and exposure measurement. Pooled analyses included estimates from original research articles that comprised either prospective cohorts (
n=18), mixed study designs (
n=15), or cross-sectional (
n=12) designs. Most pooled analyses included adults as the main population, except for five, which included children and adolescents in examining mental health outcomes
51,52 and respiratory conditions
18. In 87% of pooled analyses, estimates of exposure to ultra-processed food were obtained from a combination of tools, including food frequency questionnaires, twenty-four-hour dietary recalls, and dietary history, as reported in the meta-analysis studies. Six pooled analyses pertaining to respiratory conditions
18, non-alcohol fatty liver disease
48, heart disease-related mortality
53, and cancer-cause mortality
53 included estimates of exposure from food frequency questionnaires alone.
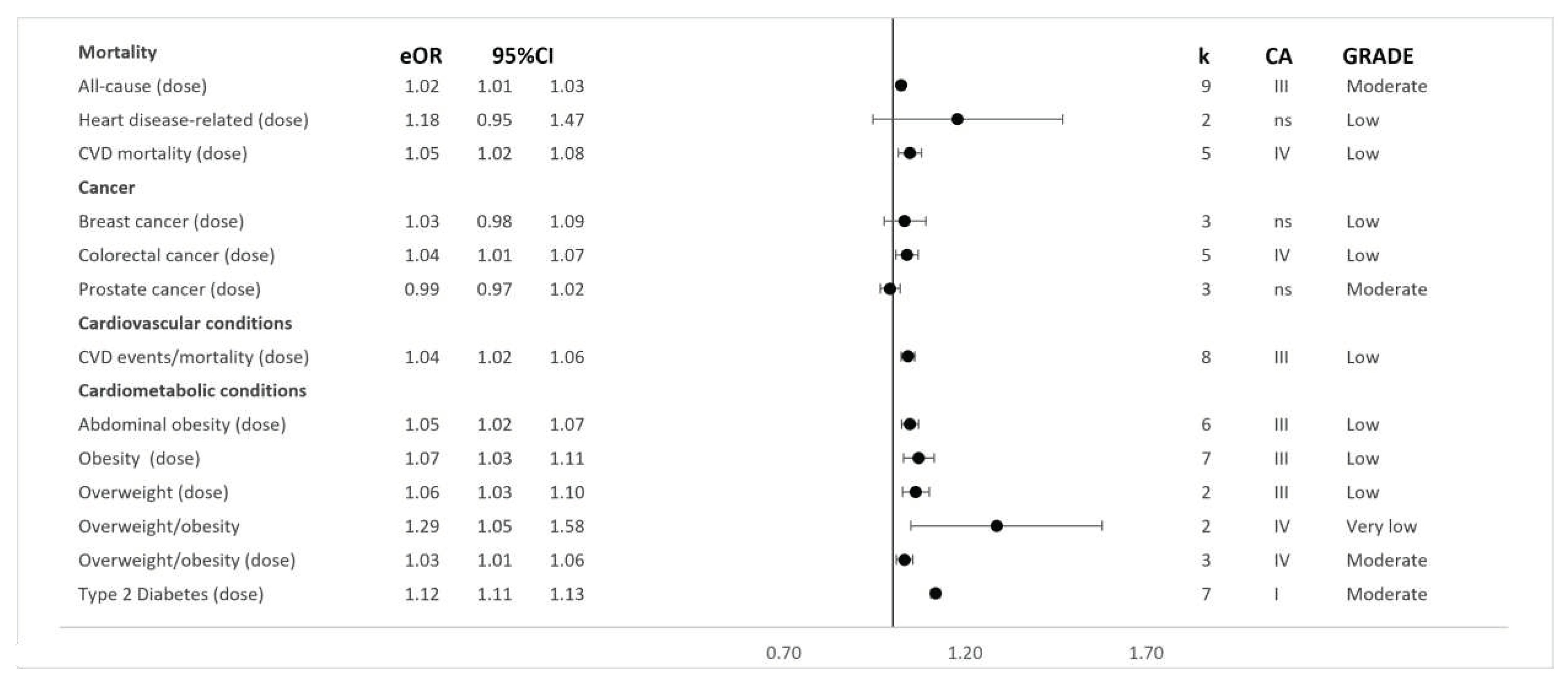
Each of the meta-analysis studies examined the non-dose-response associations between exposure to ultra-processed foods and adverse health outcomes. However, an additional analysis involving dose-response modelling of the ultra-processed food exposure variable was conducted in 13 pooled analyses across five meta-analysis studies 41,43,44,49,53. Using this approach, the outcomes considered included all-cause mortality 44 and cardiovascular disease events 44, such as cardiovascular disease morbidity and mortality, associated with each daily serve increase in ultra-processed food 44. One meta-analysis study specifically pooled heart disease-related deaths 53, such as ischaemic heart disease mortality and cerebrovascular disease mortality, with each 10% increase in total ultra-processed food exposure 53. Additionally, associations for other outcomes, such as type two diabetes 49, abdominal obesity 43, overweight and obesity 43, and colorectal 41, breast 41, and prostate 41 cancers, were modelled based on each 10% increase in ultra-processed food exposure.
Results of syntheses
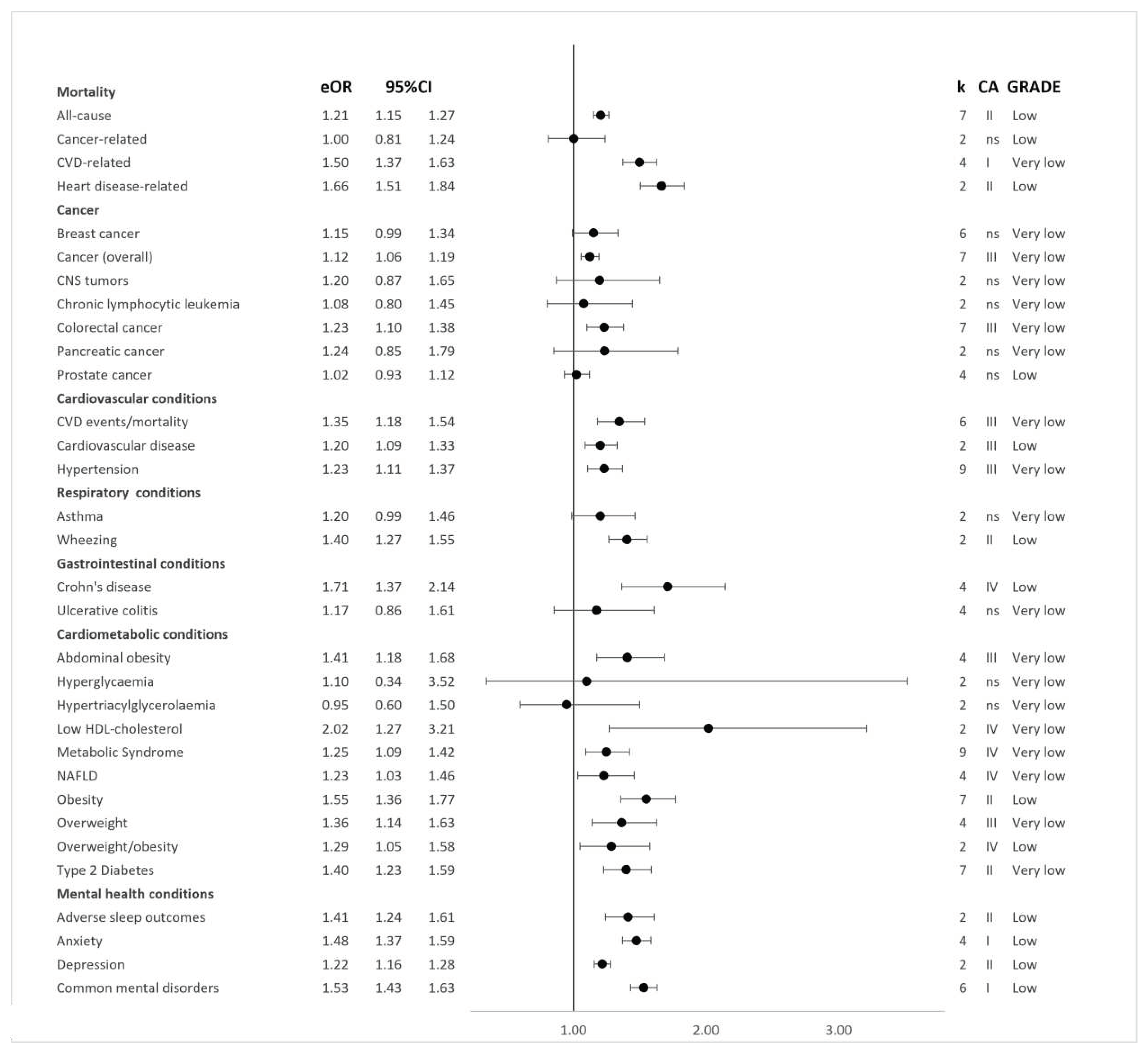
Figure 2 and
Figure 3 show the direction and sizes of effect estimates for both the non-dose-response and dose-response relations between exposure to ultra-processed foods and each adverse health outcome, respectively.
Based on the random-effects model, 32 (71%) distinct pooled analyses showed direct associations between greater ultra-processed food exposure and a higher risk of adverse health outcomes at the significance level of
P ≤ 0.05 (
Table 2). Additionally, out of these combined analyses, a total of 11 (34%) demonstrated continued statistical significance when a more stringent threshold was applied (
P < 0.000001). These included the incidence of all-cause mortality
44, cardiovascular disease mortality
44, heart disease-related cause mortality
53, type two diabetes (dose- and non-dose-response)
49, and depressive outcomes
52, as well as the prevalence of anxiety
52 and combined common mental disorder outcomes
52, adverse sleep-related outcomes
51, and wheezing
18.
There was evidence of moderate (I2 ≥ 50–74.9%) to high (I2 ≥ 75%) heterogeneity in 13 (29%) and seven (16%) of the 45 discrete pooled analyses, respectively. In addition, there was evidence of small-study effects across 5 (11%) pooled analyses. This small-study effect was found for associations of greater ultra-processed food exposure with higher risks of all-cause mortality (dose- and non-dose-response) 44, breast cancer 41, metabolic syndrome 47, and obesity (dose-response) 43.
The 95% prediction intervals were statistically significant for seven (25%) of the 28 pooled analyses with more than three original research articles, including direct associations of greater ultra-processed food exposure with higher risks of all-cause mortality 44, common mental disorder outcomes 52, Crohn’s disease 50, cardiovascular disease mortality 44, obesity 43, and type two diabetes (dose-response) 49.
Effect estimates from the largest original research article were nominally statistically significant for 21 (47%) pooled analyses and pertained to associations of greater ultra-processed food exposure with higher risks of abdominal obesity (dose- and non-dose-response) 43, adverse sleep outcomes 51, all-cause mortality (dose- and non-dose-response) 44, common mental disorder outcomes 52, asthma 18, central nervous system tumours 41, cardiovascular disease events (dose- and non-dose-response) 44, heart disease-related mortality (dose- and non-dose-response) 53, metabolic syndrome 47, low high-density lipoprotein concentrations 17, hyperglycaemia 17, non-alcoholic fatty liver disease 48, obesity and overweight (dose-response) 43, type two diabetes (dose- and non-dose-response) 49, and wheezing 18.
Credibility and Quality Assessments
Cancer
Pooled analyses from seven cohort studies showed direct associations between greater exposure to ultra-processed foods and higher risks of incident cancer overall (HR: 1.12; 95%CIs: 1.06 to 1.19
Credibility assessment: Class III; GRADE Assessment: Very Low)
42 (
Figure 4 and
Table S3). Synthesised analyses including mixed cohort and case-control study designs additionally showed direct associations with a risk of colorectal cancer (dose-response OR: 1.04; 95%CIs: 1.01 to 1.07;
Class IV; Low and non-dose-response OR: 1.23; 95%CIs: 1.10 to 1.38;
Class III; Very Low)
41.
Limited evidence was found for pooled analyses, including mixed cohort and case-control study designs, of the association between greater ultra-processed food exposure and higher risks of breast cancer (dose-response OR: 1.03; 95%CIs: 0.98 to 1.09; Class V; Low and non-dose-response OR: 1.15; 95%CIs: 0.99 to 1.34; Class V; Very Low) 41, prostate cancer (dose-response OR: 0.99; 95%CIs: 0.97 to 1.02; Class V; Moderate and non-dose-response OR: 1.02; 95%CIs: 0.93 to 1.22; Class V; Low), central nervous system tumours (OR: 1.20; 95%CIs: 0.87 to 1.65; Class V; Very Low), chronic lymphocytic leukaemia (OR: 1.08; 95%CIs: 0.80 to 1.45; Class V; Very Low), and pancreatic cancer (OR: 1.24; 95%CIs: 0.85 to 1.79; Class V; Very Low).
Gastrointestinal Conditions
Weak or no evidence was found for pooled analyses incorporating data from four cohorts on associations between greater exposure to ultra-processed foods and higher risks of incident Crohn’s disease (HR: 1.71; 95%CIs: 1.37 to 2.14; Class IV; Low) 50 and ulcerative colitis (HR: 1.17; 95%CIs: 0.86 to 1.61; Class V; Very Low) 50.
Mental Health
Using data from six cross-sectional designs, there was evidence for direct associations between greater exposure to ultra-processed foods and higher risks of the prevalence of common mental disorder outcomes (OR: 1.53; 95%CIs: 1.43 to 1.63; Class I; Low). Similar associations were observed in separate assessments of incident depressive outcomes across two cohorts (OR: 1.22; 95%CIs: 1.16 to 1.28; Class I; Low) and prevalent anxiety outcomes across four cross-sectional designs (OR: 1.45; 95%CIs: 1.37 to 1.59; Class I; Low) 52. A direct association was also observed when pooling data from two cross-sectional designs for greater exposure to ultra-processed foods and a higher risk of the prevalence of adverse sleep-related outcomes (OR: 1.41; 95%CIs: 1.24 to 1.61; Class II; Low) 51.
Mortality
Pooled effect estimates from nine dose-response and seven non-dose-response cohorts showed direct associations between greater exposure to ultra-processed foods and higher risks of incident all-cause mortality (dose-response RR: 1.02; 95%CIs: 1.01 to 1.03; Class III; Moderate and non-dose-response RR: 1.21; 95%CIs: 1.15 to 1.27; Class II; Low) 44. Four dose-response and five non-dose-response cohorts informed the synthesis of associations between greater exposure to ultra-processed foods and higher risks of incident cardiovascular disease mortality (dose-response RR: 1.05; 95%CIs: 1.02 to 1.08; Class IV; Low and non-dose-response RR: 1.50; 95%CIs: 1.37 to 1.63; Class I; Very Low) 44. Effect estimates from two cohorts were pooled and showed limited evidence supporting direct associations between greater ultra-processed food exposure and a higher risk of incident heart disease-related mortality (dose-response HR: 1.18; 95%CIs: 0.95 to 1.47; Class V; Low and non-dose-response HR: 1.66; 95%CIs: 1.51 to 1.84; Class II; Low) 53. Further limited evidence was found for an association between greater ultra-processed food exposure and incident cancer-related mortality (HR: 1.00; 95%CIs: 0.81 to 1.24; Class V; Low) 53.
Respiratory Conditions
Pooled analyses that included two cross-sectional studies provided limited evidence of an association between higher exposure to ultra-processed foods and risks of prevalent wheezing (RR: 1.40; 95%CIs: 1.27 to 1.55; Class II; Low) 18 and asthma 18 (RR: 1.20; 95%CIs: 0.99 to 1.46; Class V; Very Low).
Discussion
Statement of Principal Findings
Our umbrella review provides a comprehensive overview and evaluation of the evidence of associations between dietary exposure to ultra-processed foods and various adverse health outcomes. Our review included 45 distinct pooled analyses, encompassing a total population of 9,888,373 participants, and spanning six outcome domains related to cardiometabolic, gastrointestinal, and respiratory conditions, cancer, mental disorders, and mortality. Across the pooled analyses, higher exposure to ultra-processed foods, whether measured as higher versus lower consumption, additional servings per day, or a 10% increment, was consistently associated with an increased risk of adverse health outcomes (71% of outcomes).
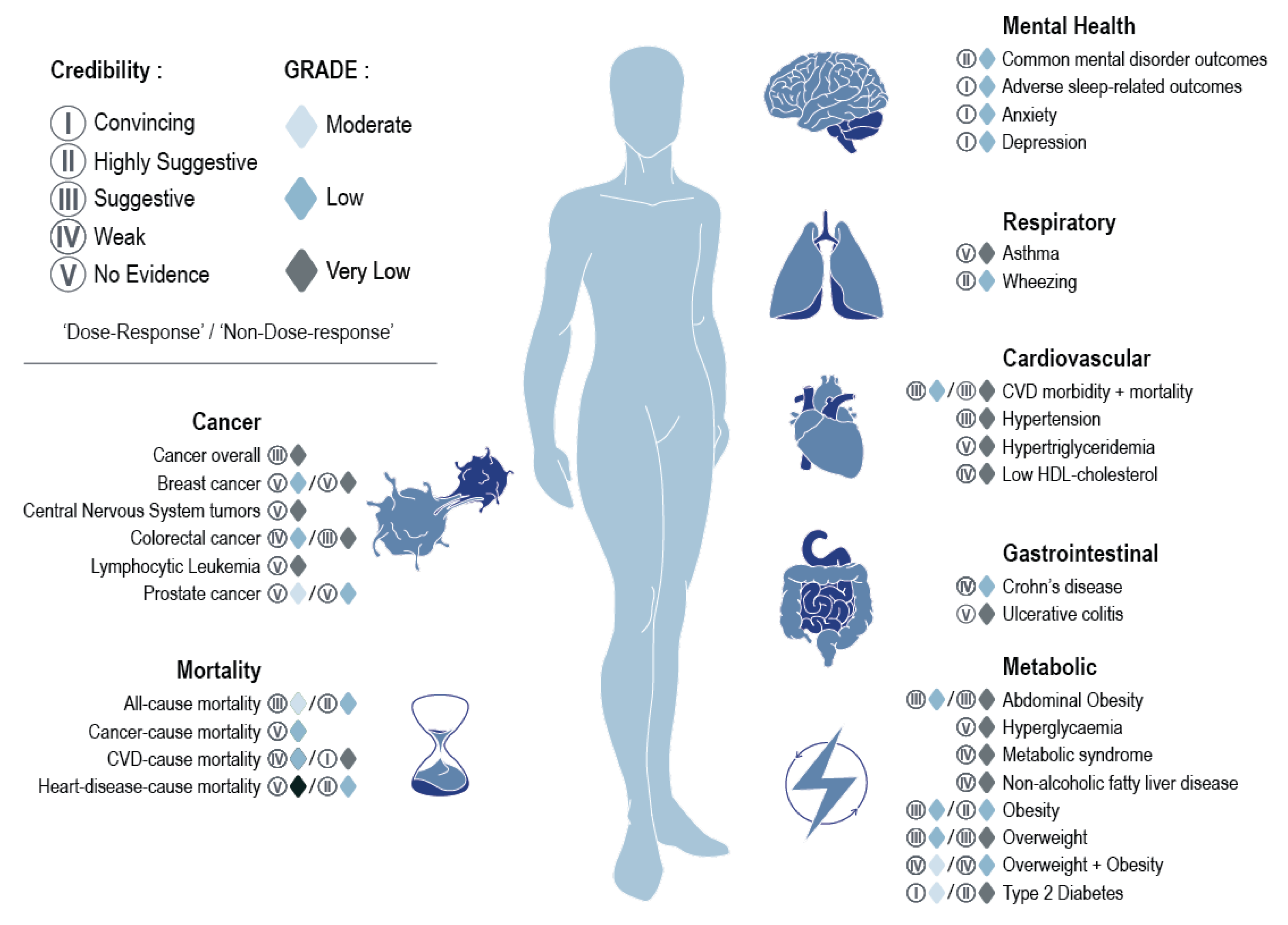
When considering the evidence classification criteria assessments, 11% of the pooled analyses were graded as providing convincing evidence (Class I), including those measuring risks of cardiovascular disease mortality, common mental disorder outcomes, and type two diabetes (dose-response) (
Figure 4). Thirteen per cent of pooled analyses (all non-dose-response) were graded as providing highly suggestive evidence (Class II) and encompassed risks of adverse sleep-related outcomes, all-cause mortality, heart disease-related mortality, obesity, type two diabetes, and wheezing. Approximately 29% of the pooled analyses were graded as suggestive evidence (Class III), covering a range of conditions from risks of overweight to abdominal obesity, with 18% graded as weak evidence (Class IV), encompassing outcomes such as risks of colorectal cancer and overweight and obesity (evaluated together as single outcome). The remaining 29% of pooled analyses were graded as lacking evidence (Class V) and spanned conditions from asthma to ulcerative colitis. As previously noted, moderate to high levels of heterogeneity were observed across 45% of pooled analyses. Based on GRADE assessments, which initially assign observational epidemiological studies as “Low” quality evidence
39, approximately 29% of the pooled analyses remained unchanged, indicating that there were no additional concerns identified based on GRADE criteria, with a further 9% upgraded to a “Moderate” rating owing to a dose-response gradient (
Figure 1). Dose-response pooled analyses upgraded to “Moderate” quality evidence related to all-cause mortality, overweight and obesity (assessed together), type two diabetes, and prostate cancer. Associations were downgraded largely due to inconsistencies or heterogeneity in the effect estimates found across the original research articles or due to imprecision (i.e., wide confidence intervals).
The heterogeneity and imprecision noted across a number of the pooled analyses, as demonstrated by both the evidence classification criteria and GRADE assessments, may be partly explained by the treatment of different effect estimates derived from original research articles (HR, OR, and RR) as approximately equivalent in various meta-analysis studies
41,43,44,47,49. Such variations in scales may introduce heterogeneity and reduce precision in pooled estimates, even if the original research articles share conceptual similarities in exposures and outcomes
54. Moreover, the synthesis of results based on three or fewer original research articles may contribute to heterogeneity and imprecision
55, impacting outcomes assessed in our review such as asthma, certain cancers, and intermediate cardiometabolic risk factors. While the pooled analyses relating to these outcomes were rated as having no or low-quality evidence based on the evidence classification criteria and GRADE assessments, this does not necessarily negate the potential for an association, particularly as more data may become available in the future. Furthermore, when analysing the overall body of evidence, 93% of pooled analyses indicated point estimates in the same direction (greater than one) (
Figure 2 and
Figure 3). The presence of 95% confidence intervals that included the null value in 24% of these pooled analyses signifies some uncertainty in the data, which may be partly due to insufficient sample size, particularly in analyses with a small number of original research articles and results showing wide confidence intervals
56. This underscores the importance of conducting additional original research and subsequent meta-analyses in the respective disease areas.
Potential Mechanisms of Action
Understanding the aspects of ultra-processed dietary patterns that link them to poor health and early death requires more research 12,57. The available evidence indicates that ultra-processed foods differ from unprocessed and minimally processed foods in several aspects, potentially explaining their plausible links with adverse health outcomes. These differences include poorer nutrient profiles, the displacement of non-ultra-processed foods from the diet, and alterations to the physical structure of consumables through intensive ultra-processing. More specifically, diets rich in ultra-processed foods are associated with markers of poor diet quality, with higher levels of added sugars, saturated fat and sodium, and higher energy density, with lower fibre, protein, and micronutrients 6,58. Ultra-processed foods displace more nutritious foods in diets, such as fruits, vegetables, legumes, nuts, and seeds, and breastmilk in infants and children, resulting in reduced intakes of beneficial bioactive compounds that are present in these foods, including polyphenols 59. Such nutrient-poor dietary profiles have been implicated in the prevalence and incidence of chronic diseases through various pathways, including inflammatory mechanisms 13,14,16.
The adverse health outcomes associated with ultra-processed foods may not be fully explained by their nutrient composition and energy density alone, but also by physical and chemical properties associated with industrial processing methods, ingredients, and by-products. Firstly, alterations in the food matrix during intensive processing, also known as dietary reconstitution, may affect digestion, nutrient absorption, and feelings of satiety 60. Secondly, emerging evidence in humans demonstrates links between exposure to additives, including non-sugar sweeteners, emulsifiers, colourants, and nitrates/nitrites, and detrimental health outcomes 61–66. A recent review of experimental research revealed that ultra-processed weight loss formulations composed of ostensibly balanced nutrient profiles but containing different additives, including non-sugar sweeteners, may have adverse effects on the gut microbiome – which is thought to play an important function in many of the diseases studied here – and related inflammation 67. Indeed, the World Health Organization recently warned against the ongoing use of sugar substitutes for weight control and non-communicable illnesses 68, and according to their new report, non-sugar sweeteners may also elevate the risk of cardiometabolic diseases and mortality 68. In addition, citing “limited evidence” in humans, the International Agency for Research on Cancer recently classified the non-sugar sweetener aspartame as “possibly carcinogenic to humans” (Group 2B) 69. There is also a growing body of data revealing instances of exposure to combinations of multiple additives, which may have potential “cocktail effects” with greater implications for human health than exposure to a single additive 70. Thirdly, the intensive industrial processing of food may produce potentially harmful substances that have been linked to higher risks of chronic inflammatory diseases, including acrolein, acrylamide, advanced glycation end products, furans, heterocyclic amines, industrial trans-fatty acids, and polycyclic aromatic hydrocarbons 12. Finally, ultra-processed foods can contain contaminants with health implications that migrate from packaging materials, such as bisphenols, microplastics, mineral oils, and phthalates 12.
Experimental evidence indicates a robust causal relationship between ultra-processed diets and increased energy intake and weight gain (approximately 500 kilocalories per day and 0.9 kilograms during the ultra-processed diet) 71. Other experimental evidence has also shown that using the Nova food classification system for nutritional counselling and adjunctive to physical activity effectively prevents excessive weight gain in pregnant women with high body mass index 72. The mechanisms contributing to the excess consumption effect of ultra-processed diets appear to involve the nature of the energy source, specifically whether it comes from solid foods or beverages 71. Furthermore, the greater energy density, faster eating rate, and hyper-palatability attributed to ultra-processed diets are regarded as important factors influencing this effect 73. The extensive marketing strategies employed by ultra-processed food manufacturers, which involve visually captivating packaging with eye-catching designs and health-related assertions, have also been suggested as a potential contributing factor to excessive consumption 74.
Strengths and Weaknesses in Relation to other Studies
Recognising the importance of establishing causality, it is essential to acknowledge that further randomised controlled trials are required, particularly for outcomes where there is strong meta-analytic epidemiological evidence, such as cardiometabolic and common mental disorder outcomes. However, only short-term trials testing the impact of ultra-processed food exposure on intermediate outcomes (such as alterations to body weight, insulin resistance, depressive and anxiety symptoms, gut microbiome, and inflammation) would be feasible. It will not be possible to set up trials testing the effect of long-term exposure to interventions with suspected deleterious properties (i.e., ultra-processed diets) on hard disease endpoints such as cardiovascular disease or cancer, for obvious ethical reasons. In this context, our umbrella review of extant observational epidemiological research provides complementary insights and has implications for public health, especially in light of the current debate about addressing (or not) exposure to ultra-processed foods through public health measures. It stands as the first comprehensive synthesis of current evidence derived from meta-analyses of epidemiological studies, exploring the associations between dietary exposure to ultra-processed foods and various adverse health outcomes. We employed rigorous systematic methods, including duplicate study selection and data extraction, alongside the evidence classification criteria and GRADE assessments, to evaluate the credibility and quality of the pooled analyses. An additional strength of our review is that we reviewed the competing interests and funding disclosures of the included meta-analysis studies, with none funded by companies involved in the production of ultra-processed foods.
One limitation of umbrella reviews, in general, is their high-level overview. As a result, specific confounder or mediator adjustments and sensitivity analyses were not considered as part of our review but may be important factors, particularly in the context of ultra-processed foods. The consumption of ultra-processed foods is linked to a lower intake of unprocessed or minimally processed fruits, vegetables, legumes, and seafood 6. This raises the question of whether the associations between exposure to ultra-processed foods and poorer health are due to an overall unhealthy dietary pattern. While not addressed in our review, a recent meta-analysis found that adjusting for diet quality or patterns does not change the consistent evidence for direct associations between greater ultra-processed food exposure and a higher risk of adverse health outcomes (as per inference criteria and sizes of effect estimates) 75. Furthermore, the inclusion of original research articles with different methods of assessing ultra-processed food intake, such as dietary history, food frequency questionnaires, food records, and 24-hour dietary recalls, introduces an inevitable measurement bias regardless of whether validated methods were applied 76. It is also important to consider that observational epidemiological studies have inherent limitations, with residual confounding perhaps most pertinent 77. However, the consistent findings across most pooled analyses in our review support the notion that residual confounding does not fully explain the observed associations.
While our umbrella review provides a systematic synthesis of the role of ultra-processed dietary patterns in chronic disease outcomes, a related consideration is the possible heterogeneity of associations between subgroups and subcategories of ultra-processed foods and chronic disease outcomes. A meta-analysis by Chen et al. (2023), included in our review, established a clear link between overall consumption of ultra-processed foods and a higher risk of type two diabetes, consistently observed across multiple cohorts 49. However, while certain subcategories of ultra-processed foods further demonstrated higher risk, others were inversely associated, such as ultra-processed cereals, dark/whole grain bread, packaged sweet and savoury snacks, fruit-based products and yoghurt, and dairy-based desserts 49. These findings underscore the complexity of the relationship between ultra-processed foods and adverse health. Nonetheless, while some subcategories of ultra-processed items may have better nutrient and ingredient profiles, the overall consumption of ultra-processed foods remains consistently associated with a higher risk of chronic diseases, as evidenced by our review. Some have argued that understanding the differences within subcategories of ultra-processed foods may aid consumers in adopting a healthier dietary pattern compared to maximally reducing their consumption on the whole 78. However, others propose that the focus should be on the overall quality of the diet, including all ultra-processed foods, and its link to higher disease risk, rather than specific subcategories or individual products 79.
When considering the former and examining subcategories of ultra-processed foods, composite interactions between various consumables within broader dietary patterns are unaccounted for. This limitation may partially account for differences in the strength of evidence observed in our review compared to another recent umbrella review focusing on dietary sugar consumption, including sugar-sweetened beverages as a commonly consumed subcategory of ultra-processed foods 26. In that review, while highly suggestive (Class II) evidence supported links between greater exposure to sugar-sweetened beverages and higher risks of obesity, coronary heart disease, hypertension, and type two diabetes 26, there was no convincing (Class I) evidence for adverse health outcomes linked to sugar consumption 26. Our umbrella review reveals compelling evidence (Class I and/or “Moderate” quality) that supports direct associations between greater dietary exposure to ultra-processed foods and higher risks of poorer health spanning cardiometabolic, common mental disorder, and mortality outcomes. These findings support recommendations to consider overall diet quality in nutritional epidemiology 79 and suggest that higher consumption of ultra-processed foods within broader dietary patterns may have synergistic or compounded consequences compared to lower intakes, as hypothesised elsewhere 12–15.
Meaning of the Study: Possible Explanations and Implications for Clinicians and Policymakers
Organisations such as the American Heart Association have cautiously advised to choose unprocessed and minimally processed foods over ultra-processed foods, noting the absence of a widely accepted definition for ultra-processed foods 80. Although various food classification systems have been developed to classify foods based on processing-related criteria 81–85, the most commonly used classification system worldwide is the Nova food classification system 86. Furthermore, Nova has received recognition from authoritative reports by the Food and Agricultural Organization of the United Nations 87–90 and the Pan American Health Organization of the World Health Organization 91. A recent statement from the United Kingdom’s Scientific Advisory Committee on Nutrition (SACN) evaluated the Nova classification system among others and concluded that Nova is the only suitable classification for potential use in the country 92. However, SACN expressed concerns regarding three key points. Firstly, they noted that the classification of certain foods using Nova may not align with existing nutritional and other food-based classifications 92. Secondly, they highlighted that the available studies are primarily epidemiological in nature, and there may be a lack of adequate consideration for confounding factors or covariates 92. Lastly, SACN indicated that existing national dietary recommendations may already address the adverse associations between ultra-processed foods and poor health outcomes 92. Indeed, other criticisms of Nova exist regarding its possible imprecision 93–95 and inconsistency among evaluators 96. In contrast, more recent assessments demonstrate acceptable construct validity and strong agreement among coders 97,98, with the definitions and examples provided by the Nova system deemed adequate in classifying over 70% of the food items reported in food frequency questionnaires from various cohorts from the United States of America 99, as well as over 90% of the food items reported in 24-hour dietary recalls from participants of a national Brazilian dietary survey 100. Recent efforts including best practice guidelines have further focused on improving the efficiency and transparency of the categorisation process for Nova food groups, which ultimately aim to enhance the accuracy of effect estimates 101.
Public health measures promoting a reduction or avoidance of ultra-processed products have already been implemented in Latin America 102,103, France 104, Israel 105, and Malaysia 106, with warning labels on food packaging in Latin American countries also recently assisting in identifying ultra-processed foods 58. Organisations including the United Kingdom’s First Steps Nutrition Trust and The Lancet, in partnership with The European Association for the Study of the Liver, emphasise similar strategies for paediatric development and preventing liver disease, respectively 107,108. It is also worth noting that the World Health Organization and the International Agency for Research on Cancer endorse public health strategies to limit the intake of components commonly present in ultra-processed foods, including high levels of added sugar and non-sugar sweeteners 68,69,109. When considering sugar intake in particular, several measures have been proposed and implemented, including taxation, marketing restrictions, labelling, and education campaigns 110–114. Acknowledging the importance of being responsive and sensitive to factors that influence access to fresh produce and food choices, including the relatively greater time, effort, and (in some contexts) cost of preparing non-ultra-processed food 95, the existing strategies targeting sugar intake may serve as models for similar initiatives aimed at minimising exposure to ultra-processed foods.
Conclusions and Recommendations
This umbrella review reports a higher risk of adverse health outcomes associated with ultra-processed food exposure. The strongest available evidence pertained to direct associations between greater exposure to ultra-processed foods and higher risks of all-cause mortality, cardiovascular disease-related mortality, common mental disorder outcomes, overweight and obesity, and type two diabetes. Evidence for the associations of ultra-processed food exposure with asthma, ulcerative colitis, some cancers, and intermediate cardiometabolic risk factors remains limited and warrants further investigation. Coupled with existing population-based strategies, we recommend the urgent evaluation and development of comprehensive population-based strategies, including government-led policy frameworks and dietary guidelines, to determine whether targeting and reducing dietary exposure to ultra-processed foods can minimise their harm to human health.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Author Contributions
MML: Conceptualisation, Literature search, Data curation, Project administration, Resources, Writing—original draft, Writing—review & editing, and Guarantor; EG, SD, DNA, AJM, SG: Conceptualisation, Data curation, and Writing—review & editing; TS: Visualisation and Writing—review & editing; PB, ML, CMR, BS, MT, FNJ, AO: Conceptualisation and Writing—review & editing; WM: Conceptualisation, Data curation, Formal analysis, Supervision, Visualisation and Writing—review & editing.
Funding
This work was not funded.
Patient and Public Involvement
No patients or members of the general public were involved.
Conflicts of Interest
The Food & Mood Centre has received Grant/Research support from Fernwood Foundation, Wilson Foundation, the A2 Milk Company, and Be Fit Foods.
References
- Monteiro, C.A.; Cannon, G.; Lawrence, M.; Costa Louzada, M.L.; Machado, P.P. Ultra-processed foods, diet quality, and health using the NOVA classification system; Food and Agriculture Organization of the United Nations (FAO): Rome, 2019. [Google Scholar]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.C.; Louzada, M.L.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr 2019, 22, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.; Machado, P.; Santos, T.; Sievert, K.; Backholer, K.; Hadjikakou, M.; Russell, C.; Huse, O.; Bell, C.; Scrinis, G.; et al. Ultra-processed foods and the nutrition transition: Global, regional and national trends, food systems transformations and political economy drivers. Obesity Reviews 2020, 21, e13126. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Moubarac, J.C.; Cannon, G.; Ng, S.W.; Popkin, B. Ultra-processed products are becoming dominant in the global food system. Obes Rev 2013, 14 Suppl 2, 21–28. [Google Scholar] [CrossRef]
- Marino, M.; Puppo, F.; Del Bo’, C.; Vinelli, V.; Riso, P.; Porrini, M.; Martini, D. A Systematic Review of Worldwide Consumption of Ultra-Processed Foods: Findings and Criticisms. Nutrients 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.; Godos, J.; Bonaccio, M.; Vitaglione, P.; Grosso, G. Ultra-Processed Foods and Nutritional Dietary Profile: A Meta-Analysis of Nationally Representative Samples. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.; Miranda, L.; Claro, R.; Horta, P. Food marketing in supermarket circulars in Brazil: An obstacle to healthy eating. Prev Med Rep 2021, 21, 101304. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Hawk, T.; Aggarwal, A.; Drewnowski, A. Characterizing Ultra-Processed Foods by Energy Density, Nutrient Density, and Cost. Front Nutr 2019, 6, 70. [Google Scholar] [CrossRef]
- Luiten, C.M.; Steenhuis, I.H.M.; Eyles, H.; Ni Mhurchu, C.; Waterlander, W.E. Ultra-processed foods have the worst nutrient profile, yet they are the most available packaged products in a sample of New Zealand supermarkets. Public Health Nutrition 2016, 19, 530–538. [Google Scholar] [CrossRef]
- Poti, J.M.; Braga, B.; Qin, B. Ultra-processed Food Intake and Obesity: What Really Matters for Health-Processing or Nutrient Content? Curr Obes Rep 2017, 6, 420–431. [Google Scholar] [CrossRef]
- Srour, B.; Kordahi, M.C.; Bonazzi, E.; Deschasaux-Tanguy, M.; Touvier, M.; Chassaing, B. Ultra-processed foods and human health: From epidemiological evidence to mechanistic insights. The Lancet Gastroenterology & Hepatology 2022. [Google Scholar] [CrossRef]
- Fardet, A. Characterization of the Degree of Food Processing in Relation With Its Health Potential and Effects. Adv Food Nutr Res 2018, 85, 79–129. [Google Scholar] [CrossRef] [PubMed]
- Martínez Leo, E.E.; Peñafiel, A.M.; Hernández Escalante, V.M.; Cabrera Araujo, Z.M. Ultra-processed diet, systemic oxidative stress, and breach of immunologic tolerance. Nutrition 2021, 91-92, 111419. [Google Scholar] [CrossRef] [PubMed]
- Tristan Asensi, M.; Napoletano, A.; Sofi, F.; Dinu, M. Low-Grade Inflammation and Ultra-Processed Foods Consumption: A Review. Nutrients 2023, 15. [Google Scholar] [CrossRef]
- Marx, W.; Lane, M.; Hockey, M.; Aslam, H.; Berk, M.; Walder, K.; Borsini, A.; Firth, J.; Pariante, C.M.; Berding, K.; et al. Diet and depression: Exploring the biological mechanisms of action. Molecular Psychiatry 2021, 26, 134–150. [Google Scholar] [CrossRef]
- Pagliai, G.; Dinu, M.; Madarena, M.P.; Bonaccio, M.; Iacoviello, L.; Sofi, F. Consumption of ultra-processed foods and health status: A systematic review and meta-analysis. British Journal of Nutrition 2020. [Google Scholar] [CrossRef]
- Lane, M.M.; Davis, J.A.; Beattie, S.; Gómez-Donoso, C.; Loughman, A.; O'Neil, A.; Jacka, F.; Berk, M.; Page, R.; Marx, W.; et al. Ultraprocessed food and chronic noncommunicable diseases: A systematic review and meta-analysis of 43 observational studies. Obes Rev 2021, 22, e13146. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Astrup, A. Does the concept of “ultra-processed foods” help inform dietary guidelines, beyond conventional classification systems? YES. The American Journal of Clinical Nutrition 2022. [Google Scholar] [CrossRef]
- Astrup, A.; Monteiro, C.A. Does the concept of “ultra-processed foods” help inform dietary guidelines, beyond conventional classification systems? NO. The American Journal of Clinical Nutrition 2022. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Morgan, R.L.; Whaley, P.; Thayer, K.A.; Schünemann, H.J. Identifying the PECO: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int 2018, 121, 1027–1031. [Google Scholar] [CrossRef]
- Monteiro, C.A. Nutrition and health. The issue is not food, nor nutrients, so much as processing. Public Health Nutr 2009, 12, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Veritas Health Innovation. Covidence systematic review software, Melbourne, Australia. Available online: www.covidence.org.
- Veronese, N.; Demurtas, J.; Thompson, T.; Solmi, M.; Pesolillo, G.; Celotto, S.; Barnini, T.; Stubbs, B.; Maggi, S.; Pilotto, A.; et al. Effect of low-dose aspirin on health outcomes: An umbrella review of systematic reviews and meta-analyses. Br J Clin Pharmacol 2020, 86, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, Z.; Chen, B.; Li, J.; Yuan, X.; Li, J.; Wang, W.; Dai, T.; Chen, H.; Wang, Y.; et al. Dietary sugar consumption and health: Umbrella review. BMJ 2023, 381, e071609. [Google Scholar] [CrossRef] [PubMed]
- Belbasis, L.; Bellou, V.; Evangelou, E.; Ioannidis, J.P.; Tzoulaki, I. Environmental risk factors and multiple sclerosis: An umbrella review of systematic reviews and meta-analyses. Lancet Neurol 2015, 14, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Mann, C.J. Observational research methods. Research design II: Cohort, cross sectional, and case-control studies. Emergency Medicine Journal 2003, 20, 54–60. [Google Scholar] [CrossRef]
- Dettori, J.R.; Norvell, D.C.; Chapman, J.R. Fixed-Effect vs Random-Effects Models for Meta-Analysis: 3 Points to Consider. Global Spine J 2022, 12, 1624–1626. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Spiegelhalter, D.J. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc 2009, 172, 137–159. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed.) 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Sterne, J.A.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J Clin Epidemiol 2001, 54, 1046–1055. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. Bmj 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.; Trikalinos, T.A. An exploratory test for an excess of significant findings. Clin Trials 2007, 4, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Markozannes, G.; Aretouli, E.; Rintou, E.; Dragioti, E.; Damigos, D.; Ntzani, E.; Evangelou, E.; Tsilidis, K.K. An umbrella review of the literature on the effectiveness of psychological interventions for pain reduction. BMC Psychol 2017, 5, 31. [Google Scholar] [CrossRef]
- Gosling, C.J.; Solanes, A.; Fusar-Poli, P.; Radua, J. metaumbrella: The first comprehensive suite to perform data analysis in umbrella reviews with stratification of the evidence. BMJ Ment Health 2023, 26. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Radua, J. Ten simple rules for conducting umbrella reviews. Evid Based Ment Health 2018, 21, 95–100. [Google Scholar] [CrossRef]
- Ioannidis, J.P. Integration of evidence from multiple meta-analyses: A primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. Cmaj 2009, 181, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; deBeer, H.; et al. GRADE guidelines: 1. 1. Introduction—GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. Bmj 2017, 358, j4008. [Google Scholar] [CrossRef]
- Lian, Y.; Wang, G.-P.; Chen, G.-Q.; Chen, H.-N.; Zhang, G.-Y. Association between ultra-processed foods and risk of cancer: A systematic review and meta-analysis. Frontiers in Nutrition 2023, 10. [Google Scholar] [CrossRef]
- Isaksen, I.M.; Dankel, S.N. Ultra-processed food consumption and cancer risk: A systematic review and meta-analysis. Clin Nutr 2023, 42, 919–928. [Google Scholar] [CrossRef]
- Moradi, S.; Entezari, M.H.; Mohammadi, H.; Jayedi, A.; Lazaridi, A.V.; Kermani, M.A.H.; Miraghajani, M. Ultra-processed food consumption and adult obesity risk: A systematic review and dose-response meta-analysis. Crit Rev Food Sci Nutr 2023, 63, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Hu, H.; Li, T.; Zhang, J.; Feng, Y.; Yang, X.; Li, Y.; Wu, Y.; Li, X.; Huang, H.; et al. Dose-response meta-analysis of ultra-processed food with the risk of cardiovascular events and all-cause mortality: Evidence from prospective cohort studies. Food Funct 2023, 14, 2586–2596. [Google Scholar] [CrossRef] [PubMed]
- Pagliai, G.; Dinu, M.; Madarena, M.P.; Bonaccio, M.; Iacoviello, L.; Sofi, F. Consumption of ultra-processed foods and health status: A systematic review and meta-analysis. Br J Nutr 2021, 125, 308–318. [Google Scholar] [CrossRef]
- Wang, M.; Du, X.; Huang, W.; Xu, Y. Ultra-processed Foods Consumption Increases the Risk of Hypertension in Adults: A Systematic Review and Meta-analysis. Am J Hypertens 2022, 35, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Zhang, X.; Zhou, J.; Zhu, Q.; Si, C. Ultra-processed food consumption and increased risk of metabolic syndrome: A systematic review and meta-analysis of observational studies. Frontiers in Nutrition 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Henney, A.E.; Gillespie, C.S.; Alam, U.; Hydes, T.J.; Cuthbertson, D.J. Ultra-Processed Food Intake Is Associated with Non-Alcoholic Fatty Liver Disease in Adults: A Systematic Review and Meta-Analysis. Nutrients 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Khandpur, N.; Desjardins, C.; Wang, L.; Monteiro, C.A.; Rossato, S.L.; Fung, T.T.; Manson, J.E.; Willett, W.C.; Rimm, E.B.; et al. Ultra-Processed Food Consumption and Risk of Type 2 Diabetes: Three Large Prospective U.S. Cohort Studies. Diabetes Care 2023. [Google Scholar] [CrossRef]
- Narula, N.; Chang, N.H.; Mohammad, D.; Wong, E.C.L.; Ananthakrishnan, A.N.; Chan, S.S.M.; Carbonnel, F.; Meyer, A. Food Processing and Risk of Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol 2023. [Google Scholar] [CrossRef]
- Delpino, F.M.; Figueiredo, L.M.; Flores, T.R.; Silveira, E.A.; Silva Dos Santos, F.; Werneck, A.O.; Louzada, M.; Arcêncio, R.A.; Nunes, B.P. Intake of ultra-processed foods and sleep-related outcomes: A systematic review and meta-analysis. Nutrition 2023, 106, 111908. [Google Scholar] [CrossRef]
- Lane, M.M.; Gamage, E.; Travica, N.; Dissanayaka, T.; Ashtree, D.N.; Gauci, S.; Lotfaliany, M.; O’Neil, A.; Jacka, F.N.; Marx, W. Ultra-Processed Food Consumption and Mental Health: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2022, 14. [Google Scholar] [CrossRef]
- Suksatan, W.; Moradi, S.; Naeini, F.; Bagheri, R.; Mohammadi, H.; Talebi, S.; Mehrabani, S.; Hojjati Kermani, M.A.; Suzuki, K. Ultra-Processed Food Consumption and Adult Mortality Risk: A Systematic Review and Dose-Response Meta-Analysis of 207,291 Participants. Nutrients 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Li, T.; Deeks, J. Chapter 6: Choosing effect measures and computing estimates of effect. In Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated 22); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane, 20 February 2022. [Google Scholar]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. When Does it Make Sense to Perform a Meta-Analysis? In Introduction to Meta-Analysis; John Wiley & Sons, 2009. [Google Scholar]
- Jones, S.R.; Carley, S.; Harrison, M. An introduction to power and sample size estimation. Emergency Medicine Journal 2003, 20, 453. [Google Scholar] [CrossRef] [PubMed]
- Tobias, D.K.; Hall, K.D. Eliminate or reformulate ultra-processed foods? Biological mechanisms matter. Cell Metabolism 2021. [Google Scholar] [CrossRef] [PubMed]
- Machado, P.; Cediel, G.; Woods, J.; Baker, P.; Dickie, S.; Gomes, F.S.; Scrinis, G.; Lawrence, M. Evaluating intake levels of nutrients linked to non-communicable diseases in Australia using the novel combination of food processing and nutrient profiling metrics of the PAHO Nutrient Profile Model. European Journal of Nutrition 2022, 61, 1801–1812. [Google Scholar] [CrossRef]
- Coletro, H.N.; Bressan, J.; Diniz, A.P.; Hermsdorff, H.H.M.; Pimenta, A.M.; Meireles, A.L.; Mendonça, R.D.; Carraro, J.C.C. Habitual polyphenol intake of foods according to NOVA classification: Implications of ultra-processed foods intake (CUME study). Int J Food Sci Nutr 2023, 74, 338–349. [Google Scholar] [CrossRef]
- Fardet, A. Minimally processed foods are more satiating and less hyperglycemic than ultra-processed foods: A preliminary study with 98 ready-to-eat foods. Food Funct 2016, 7, 2338–2346. [Google Scholar] [CrossRef]
- Debras, C.; Chazelas, E.; Srour, B.; Druesne-Pecollo, N.; Esseddik, Y.; Szabo de Edelenyi, F.; Agaësse, C.; De Sa, A.; Lutchia, R.; Gigandet, S.; et al. Artificial sweeteners and cancer risk: Results from the NutriNet-Santé population-based cohort study. PLOS Medicine 2022, 19, e1003950. [Google Scholar] [CrossRef]
- Debras, C.; Chazelas, E.; Sellem, L.; Porcher, R.; Druesne-Pecollo, N.; Esseddik, Y.; de Edelenyi, F.S.; Agaësse, C.; De Sa, A.; Lutchia, R.; et al. Artificial sweeteners and risk of cardiovascular diseases: Results from the prospective NutriNet-Santé cohort. BMJ 2022, 378, e071204. [Google Scholar] [CrossRef]
- Suez, J.; Cohen, Y.; Valdés-Mas, R.; Mor, U.; Dori-Bachash, M.; Federici, S.; Zmora, N.; Leshem, A.; Heinemann, M.; Linevsky, R.; et al. Personalized microbiome-driven effects of non-nutritive sweeteners on human glucose tolerance. Cell 2022, 185, 3307–3328. [Google Scholar] [CrossRef]
- Bettini, S.; Boutet-Robinet, E.; Cartier, C.; Coméra, C.; Gaultier, E.; Dupuy, J.; Naud, N.; Taché, S.; Grysan, P.; Reguer, S.; et al. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Scientific Reports 2017, 7, 40373. [Google Scholar] [CrossRef]
- Srour, B.; Chazelas, E.; Druesne-Pecollo, N.; Esseddik, Y.; de Edelenyi, F.S.; Agaësse, C.; De Sa, A.; Lutchia, R.; Debras, C.; Sellem, L.; et al. Dietary exposure to nitrites and nitrates in association with type 2 diabetes risk: Results from the NutriNet-Santé population-based cohort study. PLoS Med 2023, 20, e1004149. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Compher, C.; Bonhomme, B.; Liu, Q.; Tian, Y.; Walters, W.; Nessel, L.; Delaroque, C.; Hao, F.; Gershuni, V.; et al. Randomized controlled-feeding study of dietary emulsifier carboxymethylcellulose reveals detrimental impacts on the gut microbiota and metabolome. Gastroenterology 2021. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.; Howland, G.; West, M.; Hockey, M.; Marx, W.; Loughman, A.; O’Hely, M.; Jacka, F.; Rocks, T. The effect of ultra-processed very low-energy diets on gut microbiota and metabolic outcomes in individuals with obesity: A systematic literature review. Obesity Research & Clinical Practice 2020, 14, 197–204. [Google Scholar] [CrossRef]
- World Health Organization (WHO) - Guidelines Review Committee - Nutrition and Food Safety. Use of non-sugar sweeteners: WHO guideline; ISBN: 978-92-4-007361-6. Access date: July 7th, 2023; 2023.
- Riboli, E.; Beland, F.A.; Lachenmeier, D.W.; Marques, M.M.; Phillips, D.H.; Schernhammer, E.; Afghan, A.; Assunção, R.; Caderni, G.; Corton, J.C.; et al. Carcinogenicity of aspartame, methyleugenol, and isoeugenol. The Lancet Oncology. [CrossRef]
- Chazelas, E.; Druesne-Pecollo, N.; Esseddik, Y.; de Edelenyi, F.S.; Agaesse, C.; De Sa, A.; Lutchia, R.; Rebouillat, P.; Srour, B.; Debras, C.; et al. Exposure to food additive mixtures in 106,000 French adults from the NutriNet-Santé cohort. Scientific Reports 2021, 11, 19680. [Google Scholar] [CrossRef]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab 2019, 30, 67–77. [Google Scholar] [CrossRef]
- Sartorelli, D.S.; Crivellenti, L.C.; Baroni, N.F.; de Andrade Miranda, D.E.G.; da Silva Santos, I.; Carvalho, M.R.; de Lima, M.C.; Carreira, N.P.; Chaves, A.V.L.; Manochio-Pina, M.G.; et al. Effectiveness of a minimally processed food-based nutritional counselling intervention on weight gain in overweight pregnant women: A randomized controlled trial. Eur J Nutr 2023, 62, 443–454. [Google Scholar] [CrossRef]
- Fazzino, T.L.; Courville, A.B.; Guo, J.; Hall, K.D. Ad libitum meal energy intake is positively influenced by energy density, eating rate and hyper-palatable food across four dietary patterns. Nature Food 2023, 4, 144–147. [Google Scholar] [CrossRef]
- Adams, J.; Hofman, K.; Moubarac, J.-C.; Thow, A.M. Public health response to ultra-processed food and drinks. BMJ 2020, 369, m2391. [Google Scholar] [CrossRef]
- Dicken, S.J.; Batterham, R.L. The Role of Diet Quality in Mediating the Association between Ultra-Processed Food Intake, Obesity and Health-Related Outcomes: A Review of Prospective Cohort Studies. Nutrients 2021, 14. [Google Scholar] [CrossRef]
- Kipnis, V.; Midthune, D.; Freedman, L.; Bingham, S.; Day, N.E.; Riboli, E.; Ferrari, P.; Carroll, R.J. Bias in dietary-report instruments and its implications for nutritional epidemiology. Public Health Nutr 2002, 5, 915–923. [Google Scholar] [CrossRef]
- Boyko, E.J. Observational research--opportunities and limitations. J Diabetes Complications 2013, 27, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Vadiveloo, M.K.; Gardner, C.D. Not All Ultra-Processed Foods Are Created Equal: A Case for Advancing Research and Policy That Balances Health and Nutrition Security. Diabetes Care 2023, 46, 1327–1329. [Google Scholar] [CrossRef]
- Scrinis, G.; Monteiro, C. From ultra-processed foods to ultra-processed dietary patterns. Nature Food 2022, 3, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.H.; Appel, L.J.; Vadiveloo, M.; Hu, F.B.; Kris-Etherton, P.M.; Rebholz, C.M.; Sacks, F.M.; Thorndike, A.N.; Horn, L.V.; Wylie-Rosett, J. 2021 Dietary Guidance to Improve Cardiovascular Health: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e472–e487. [Google Scholar] [CrossRef] [PubMed]
- Slimani, N.; Deharveng, G.; Southgate, D.A.; Biessy, C.; Chajès, V.; van Bakel, M.M.; Boutron-Ruault, M.C.; McTaggart, A.; Grioni, S.; Verkaik-Kloosterman, J.; et al. Contribution of highly industrially processed foods to the nutrient intakes and patterns of middle-aged populations in the European Prospective Investigation into Cancer and Nutrition study. Eur J Clin Nutr 2009, 63 Suppl 4, S206–225. [Google Scholar] [CrossRef]
- Eicher-Miller, H.A.; Fulgoni, V.L., 3rd; Keast, D.R. Contributions of processed foods to dietary intake in the US from 2003-2008: A report of the Food and Nutrition Science Solutions Joint Task Force of the Academy of Nutrition and Dietetics, American Society for Nutrition, Institute of Food Technologists, and International Food Information Council. J Nutr 2012, 142, 2065s–2072s. [Google Scholar] [CrossRef]
- Asfaw, A. Does consumption of processed foods explain disparities in the body weight of individuals? The case of Guatemala. Health Econ 2011, 20, 184–195. [Google Scholar] [CrossRef]
- Poti, J.M.; Mendez, M.A.; Shu Wen, N.; Popkin, B.M. Is the degree of food processing and convenience linked with the nutritional quality of foods purchased by US households? American Journal of Clinical Nutrition 2015, 101, 1251–1262. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Levy, R.B.; Claro, R.M.; Castro, I.R.R.d.; Cannon, G. A new classification of foods based on the extent and purpose of their processing. Cadernos de Saúde Pública 2010, 26, 2039–2049. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Moubarac, J.-C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutrition 2018, 21, 5–17. [Google Scholar] [CrossRef]
- Food Agriculture Organization (FAO) of the United Nations. Guidelines on the collection of information on food processing through food consumption surveys; Rome, 2015. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). Minimum dietary diversity for women; Rome, 2021. [Google Scholar]
- United Nations Children's Fund (UNICEF); for every child. Policy Brief: Sugar-Sweetened Beverage Taxation; 2021. [Google Scholar]
- AO; IFAD; UNICEF; WFP; and WHO. The State of Food Security and Nutrition in the World 2023. Urbanization, agrifood systems transformation and healthy diets across the rural–urban continuum.; FAO: Rome, 2023. [Google Scholar]
- Moubarac, J.C. Ultra-processed food and drink products in Latin America: Trends, impact on obesity, policy implications; Pan American Health Organization World Health Organization: Washington, DC, USA, 2015. [Google Scholar]
- Scientific Advisory Committee on Nutrition (SACN). SACN statement on processed foods and health, 2023.
- Gibney, M.J. Ultra-Processed Foods: Definitions and Policy Issues. Current Developments in Nutrition 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Gibney, M.J.; Forde, C.G.; Mullally, D.; Gibney, E.R. Ultra-processed foods in human health: A critical appraisal. American Journal of Clinical Nutrition 2017, 106, 712–724. [Google Scholar] [CrossRef]
- Jones, J.M. Food processing: Criteria for dietary guidance and public health? Proc Nutr Soc 2019, 78, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Braesco, V.; Souchon, I.; Sauvant, P.; Haurogné, T.; Maillot, M.; Féart, C.; Darmon, N. Ultra-processed foods: How functional is the NOVA system? European Journal of Clinical Nutrition 2022. [Google Scholar] [CrossRef]
- Sneed, N.M.; Ukwuani, S.; Sommer, E.C.; Samuels, L.R.; Truesdale, K.P.; Matheson, D.; Noerper, T.E.; Barkin, S.L.; Heerman, W.J. Reliability and validity of assigning ultraprocessed food categories to 24-h dietary recall data. The American Journal of Clinical Nutrition 2023, 117, 182–190. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem Med (Zagreb) 2012, 22, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Khandpur, N.; Rossato, S.; Drouin-Chartier, J.-P.; Du, M.; Martinez, E.; Sampson, L.; Monteiro, C.; Zhang, F.F.; Willett, W.; Fung, T.T.; et al. Categorizing ultra-processed food intake in large-scale cohort studies: Evidence from the Nurses’ Health Studies, the Health Professionals Follow-up Study, and the Growing Up Today Study. medRxiv 2021. [Google Scholar] [CrossRef]
- Louzada, M.; Cruz, G.L.D.; Silva, K.; Grassi, A.G.F.; Andrade, G.C.; Rauber, F.; Levy, R.B.; Monteiro, C.A. Consumption of ultra-processed foods in Brazil: Distribution and temporal evolution 2008-2018. Rev Saude Publica 2023, 57, 12. [Google Scholar] [CrossRef]
- Martinez-Steele, E.; Khandpur, N.; Batis, C.; Bes-Rastrollo, M.; Bonaccio, M.; Cediel, G.; Huybrechts, I.; Juul, F.; Levy, R.B.; da Costa Louzada, M.L.; et al. Best practices for applying the Nova food classification system. Nature Food 2023, 4, 445–448. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Food-based dietary guidelines. Available online: https://www.fao.org/nutrition/education/food-dietary-guidelines/regions/en/.
- Ministry of Health of Brazil. Dietary guidelines for the Brazilian population. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/dietary_guidelines_brazilian_population.pdf.
- Le Haut Conseil de la santé publique (The High Council for Public Health). Relatif Aux Objectifs de Santé Publique Quantifiés Pour La Politique Nutritionnelle de Santé Publique (PNNS) 2018–2022 (Quantified Public Health Objectives for Public Health Nutrition Policy (PNNS) 2018–2022). Available online: https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=648.
- The Israeli Ministry of Health. Nutritional recommendations. Available online: https://www.health.gov.il/PublicationsFiles/dietary%20guidelines%20EN.pdf.
- Ministry of Health Malaysia. Malaysian dietary guidelines. Available online: https://nutrition.moh.gov.my/wp-content/uploads/2021/07/Web%20MDG.pdf.
- Karlsen, T.H.; Sheron, N.; Zelber-Sagi, S.; Carrieri, P.; Dusheiko, G.; Bugianesi, E.; Pryke, R.; Hutchinson, S.J.; Sangro, B.; Martin, N.K.; et al. The EASL-Lancet Liver Commission: Protecting the next generation of Europeans against liver disease complications and premature mortality. The Lancet 2022, 399, 61–116. [Google Scholar] [CrossRef] [PubMed]
- Childs, R.; Sibson, V. Ultra-processed foods (UPF) in the diets of infants and young children in the UK: What they are, how they harm health, and what needs to be done to reduce intakes; 2023; pp 1-96.
- World Health Organization (WHO). Guideline: Sugars intake for adults and children. Available online: https://www.who.int/publications/i/item/9789241549028.
- Silver, L.D.; Ng, S.W.; Ryan-Ibarra, S.; Taillie, L.S.; Induni, M.; Miles, D.R.; Poti, J.M.; Popkin, B.M. Changes in prices, sales, consumer spending, and beverage consumption one year after a tax on sugar-sweetened beverages in Berkeley, California, US: A before-and-after study. PLoS medicine 2017, 14, e1002283. [Google Scholar] [CrossRef] [PubMed]
- Pell, D.; Mytton, O.; Penney, T.L.; Briggs, A.; Cummins, S.; Penn-Jones, C.; Rayner, M.; Rutter, H.; Scarborough, P.; Sharp, S.J. Changes in soft drinks purchased by British households associated with the UK soft drinks industry levy: Controlled interrupted time series analysis. bmj 2021, 372. [Google Scholar] [CrossRef] [PubMed]
- Colchero, M.A.; Rivera-Dommarco, J.; Popkin, B.M.; Ng, S.W. In Mexico, evidence of sustained consumer response two years after implementing a sugar-sweetened beverage tax. Health Affairs 2017, 36, 564–571. [Google Scholar] [CrossRef]
- Popkin, B.M.; Hawkes, C. Sweetening of the global diet, particularly beverages: Patterns, trends, and policy responses. Lancet Diabetes Endocrinol 2016, 4, 174–186. [Google Scholar] [CrossRef]
- Muth, N.D.; Dietz, W.H.; Magge, S.N.; Johnson, R.K. Public Policies to Reduce Sugary Drink Consumption in Children and Adolescents. Pediatrics 2019, 143. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).