Submitted:
18 August 2023
Posted:
21 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
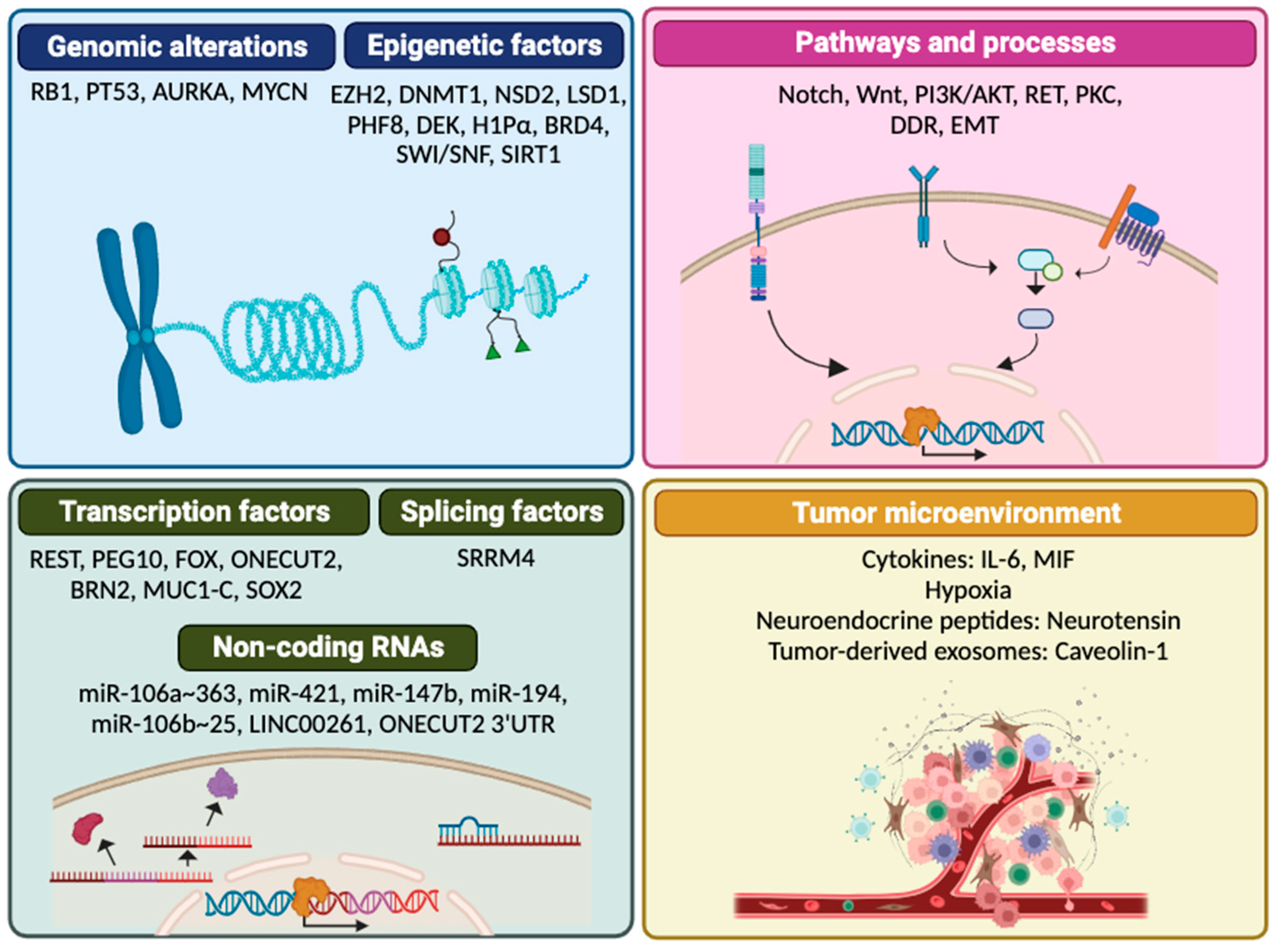
2. Molecular mechanisms underlying NEPC and key factors in neuroendocrine differentiation
2.1. Genomic alterations
2.2. Epigenomic alterations
2.3. Deregulation of transcription factors
2.4. Deregulation of splicing factors and non-coding RNAs
2.5. Altered pathways and biological processes
2.6. Tumor microenvironment
3. Potential therapeutic strategies targeting NE differentiation
3.1. Targeting genomic alterations
3.2. Targeting epigenetic factors
3.3. Targeting transcription factors
3.4. Targeting pathways and biological processes
3.5. Targeting post-transcriptional regulators
3.6. Targeting the TME
| Target | Drug | Study phase | Ref |
|---|---|---|---|
| AURKA | Danusertib (PHA-739358) Alisertib (MLN8237) CD532 VX680 7082 |
Pre-clinical (in vitro and in vivo). Clinical trial phase II completed Pre-clinical (in vitro). Clinical trial phase II completed Pre-clinical (in vitro and in vivo) Pre-clinical (in vitro) Pre-clinical (in vitro) |
[27,107,108] [28,34,110,111] [28] [112] [113] |
| MYCN | 7082 VPC-70619 |
Pre-clinical (in vitro) Pre-clinical (in vitro) |
[113] [114] |
| NK1R | Aprepitant | Pre-clinical (in vitro) | [110] |
| EZH2 | GSK343 GSK503 GSK126 DZNEP EPZ6438 (Tazemetostat) CPI-1205 |
Pre-clinical (in vitro) Pre-clinical (in vitro and in vivo) Pre-clinical (in vitro) Pre-clinical (in vitro) Pre-clinical (in vitro). Clinical trial phase Ib/II ongoing Clinical trial phase Ib/II ongoing |
[24,34] [26,34] [26,34,76,116] [116] [26], NCT04179864 NCT03480646 |
| CBX2 | SW2_152F | Pre-clinical (in vitro) | [80] |
| DNMT | Decitabine Azacytidine Guadecitabine (SGI-110) |
Pre-clinical (in vitro and in vivo). Clinical trial phase I ongoing Pre-clinical (in vitro). Clinical trial phase II completed Clinical trial phase I ongoing |
[89], NCT05037500 [117,118] NCT02998567 |
| PKA/CREB | Propranolol | Pre-clinical (in vitro and in vivo) | [116] |
| NSD2 | MCTP-39 | Pre-clinical (in vitro and in vivo) | [36] |
| LSD1 | SP-2509 SP-2577 (Seclidemstat)CC-90011 |
Pre-clinical (in vitro) Pre-clinical (in vitro and in vivo) Clinical trial phase I ongoing |
[39,120] [39] [121], NCT04628988 |
| DEK | DEK-targeted aptamers | Not tested in PC models | [44,122] |
| BET | JQ1 OTX-15 ZEN-3694 |
Pre-clinical (in vitro and in vivo) Pre-clinical (in vitro) Pre-clinical (in vitro). Clinical trial phase Ib/IIa completed and phase II ongoing |
[47,123] [123][47,124], NCT04471974, NCT04986423 |
| ONECUT2 | CSRM617 | Pre-clinical (in vitro and in vivo) | [62] |
| Hypoxia | TH-302 | Pre-clinical (in vitro and in vivo) | [61] |
| MUC1-C | GO-203, ADCs, CAR-T | Not tested in PC models | [64,125,126] |
| p38 MAPK | SB203580 | Pre-clinical (in vitro and in vivo) | [57,101] |
| SIAH2 | Menadione RLS-24 |
Pre-clinical (in vitro and in vivo) Pre-clinical (in vitro) |
[127] [128] |
| KIT | Imatinib, Sorafenib, Sunitinib Dovitinib Cabozantinib |
Pre-clinical (in vitro) Pre-clinical (in vitro and in vivo) Pre-clinical (in vitro and in vivo). Clinical trials phase II and III completed and other phase II and III ongoing |
[58] [130] [58,129,131,132,133], NCT04631744, NCT04446117, NCT05502315 |
| RET | Cabozantinib AD80 LOXO-292, BLU-667 |
Pre-clinical (in vitro and in vivo). Clinical trials phase II and III completed and other phase II and III ongoing Pre-clinical (in vitro and in vivo) Pre-clinical (in vitro) |
[58,129,131,132,133], NCT04631744, NCT04446117, NCT05502315 [88] [88] |
| SRC signalling | Dasatinib (BMS-354825) | Pre-clinical (in vitro and in vivo). Clinical trials phase II and III completed | [134,135,136,137] |
| MEK/ERK | Trametinib (TMT212) SCH772984 |
Clinical trial phase II ongoing Not tested in PC models |
NCT02881242 [139] |
| SPHK1 | FTY720, SKI-II | Pre-clinical (in vitro and in vivo) | [140] |
| PI3K/AKT/mTOR | Buparlisib (BKM-120) Dactolisib (BEZ235) PX-866 LY294002 Ipatasertib MK2206 RAD001 |
Pre-clinical in vitro. Clinical trial phase II completed Pre-clinical in vitro. Clinical trial phase I/II completed Clinical trial phase II completed Pre-clinical (in vitro) Pre-clinical (in vitro). Clinical trials phase II and III completed and other phase III ongoing Pre-clinical (in vitro). Clinical trial phase I completed Pre-clinical (in vitro) |
[34,141] [34,142] [143] [147,148] [34,145,146], NCT03072238 [34,144] [34] |
| Wnt signalling | LGK974 ICG-001 XAV-939 |
Pre-clinical (in vitro and in vivo) Pre-clinical (in vitro and in vivo) Pre-clinical (in vitro) |
[85,86] [86] [86] |
| ALK | Alectinib | Pre-clinical (in vitro and in vivo) | [86] |
| DLL3 | Rocalpituzumab tesirine (SC16LD6.5) Tarlatamab PT217 |
Pre-clinical (in vitro and in vivo). Clinical trial phase I completed Clinical trial phase I ongoing Clinical trial phase I ongoing |
[83], NCT02709889 NCT04702737 NCT05652686 |
| PTGS1 | NS-398 | Pre-clinical (in vitro and in vivo) | [151] |
| LIF | EC330 | Pre-clinical (in vitro and in vivo) | [150] |
| NGF | RO08-2750 | Pre-clinical (in vitro and in vivo) | [152] |
| CHRM4 | Ceritinib | Pre-clinical (in vitro and in vivo) | [153] |
| SNAI1 | NPI-0052 (Salinosporamide A) | Pre-clinical (in vitro) | [154,155] |
| SNAI2 | MLN4924 (Pevonedistat) | Pre-clinical (in vitro and in vivo) | [157] |
| LIN28B | Ln7, Ln15, Ln115 | Pre-clinical (in vitro) | [162] |
| PARP1 | Talazoparib Olaparib |
Pre-clinical (in vitro and in vivo) Pre-clinical (in vitro and in vivo) |
[92] [92,107,159,160] |
| PKC | Enzastaurin GF109203X |
Pre-clinical (in vitro and in vivo) Pre-clinical (in vitro) |
[90] [110] |
| SRRM4 | ASO | Pre-clinical (in vitro) | [161] |
| miR-147b | anti-miR-147b | Pre-clinical (in vitro) | [75] |
| miR-194 | miR-194 LNA inhibitor | Pre-clinical (in vitro) | [74] |
| miR-32 | miRNA32 inhibitor | Pre-clinical (in vitro) | [78] |
| ATM | Ku60019 | Pre-clinical (in vitro) | [73] |
| MIF | ISO-1 | Pre-clinical (in vitro and in vivo) | [163,164] |
| IL6/STAT3 | Siltuximab (CNTO 328) LLL12 Galiellalactone P6 |
Pre-clinical (in vitro). Clinical trial phase II completed Pre-clinical (in vitro and in vivo) Pre-clinical (in vitro and in vivo) Pre-clinical (in vitro) |
[165,167] [165] [166] [165] |
| TGF-β | Galunisertib (LY2157299) LY364947 |
Pre-clinical (in vitro) Pre-clinical (in vitro) |
[101] [101] |
4. Conclusions and future directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J Clin 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, P.A. Histological Variants of Prostatic Carcinoma and Their Significance. Histopathology 2012, 60, 59–74. [Google Scholar] [CrossRef]
- Beltran, H.; Hruszkewycz, A.; Scher, H.I.; Hildesheim, J.; Isaacs, J.; Yu, E.Y.; Kelly, K.; Lin, D.; Dicker, A.; Arnold, J.; et al. The Role of Lineage Plasticity in Prostate Cancer Therapy Resistance. Clinical Cancer Research 2019, 25, 6916–6924. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.P.; Mostaghel, E.A.; Nelson, P.S.; Montgomery, B. Androgen Deprivation Therapy: Progress in Understanding Mechanisms of Resistance and Optimizing Androgen Depletion. Nat Clin Pract Urol 2009, 6, 76–85. [Google Scholar] [CrossRef]
- Watson, P.A.; Arora, V.K.; Sawyers, C.L. Emerging Mechanisms of Resistance to Androgen Receptor Inhibitors in Prostate Cancer. Nat Rev Cancer 2015, 15, 701–711. [Google Scholar] [CrossRef]
- Yamada, Y.; Beltran, H. The Treatment Landscape of Metastatic Prostate Cancer. Cancer Lett 2021, 519, 20–29. [Google Scholar] [CrossRef]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in Metastatic Prostate Cancer before Chemotherapy. N Engl J Med 2014, 371, 424–433. [Google Scholar] [CrossRef]
- de Bono, J.S.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chi, K.N.; Jones, R.J.; Goodman, O.B.; Saad, F.; et al. Abiraterone and Increased Survival in Metastatic Prostate Cancer. N Engl J Med 2011, 364, 1995–2005. [Google Scholar] [CrossRef]
- Koivisto, P.; Kononen, J.; Palmberg, C.; Tammela, T.; Hyytinen, E.; Isola, J.; Trapman, J.; Cleutjens, K.; Noordzij, A.; Visakorpi, T.; et al. Androgen Receptor Gene Amplification: A Possible Molecular Mechanism for Androgen Deprivation Therapy Failure in Prostate Cancer. Cancer Res 1997, 57, 314–319. [Google Scholar] [CrossRef]
- Quigley, D.A.; Dang, H.X.; Zhao, S.G.; Lloyd, P.; Aggarwal, R.; Alumkal, J.J.; Foye, A.; Kothari, V.; Perry, M.D.; Bailey, A.M.; et al. Genomic Hallmarks and Structural Variation in Metastatic Prostate Cancer. Cell 2018, 174, 758–769.e9. [Google Scholar] [CrossRef] [PubMed]
- Dehm, S.M.; Tindall, D.J. Alternatively Spliced Androgen Receptor Variants. Endocr Relat Cancer 2011, 18, R183–R196. [Google Scholar] [CrossRef]
- Kraus, S.; Gioeli, D.; Vomastek, T.; Gordon, V.; Weber, M.J. Receptor for Activated C Kinase 1 (RACK1) and Src Regulate the Tyrosine Phosphorylation and Function of the Androgen Receptor. Cancer Res 2006, 66, 11047–11054. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Endoh, H.; Masuhiro, Y.; Kitamoto, T.; Uchiyama, S.; Sasaki, H.; Masushige, S.; Gotoh, Y.; Nishida, E.; Kawashima, H.; et al. Activation of the Estrogen Receptor through Phosphorylation by Mitogen-Activated Protein Kinase. Science 1995, 270, 1491–1494. [Google Scholar] [CrossRef] [PubMed]
- Arora, V.K.; Schenkein, E.; Murali, R.; Subudhi, S.K.; Wongvipat, J.; Balbas, M.D.; Shah, N.; Cai, L.; Efstathiou, E.; Logothetis, C.; et al. Glucocorticoid Receptor Confers Resistance to Antiandrogens by Bypassing Androgen Receptor Blockade. Cell 2013, 155, 1309–1322. [Google Scholar] [CrossRef]
- Yamada, Y.; Beltran, H. Clinical and Biological Features of Neuroendocrine Prostate Cancer. Curr Oncol Rep 2021, 23, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Beltran, H.; Tomlins, S.; Aparicio, A.; Arora, V.; Rickman, D.; Ayala, G.; Huang, J.; True, L.; Gleave, M.E.; Soule, H.; et al. Aggressive Variants of Castration-Resistant Prostate Cancer. Clinical Cancer Research 2014, 20, 2846–2850. [Google Scholar] [CrossRef]
- Berchuck, J.E.; Viscuse, P. V.; Beltran, H.; Aparicio, A. Clinical Considerations for the Management of Androgen Indifferent Prostate Cancer. Prostate Cancer and Prostatic Diseases 2021 24:3 2021, 24, 623–637. [Google Scholar] [CrossRef]
- Sargos, P.; Ferretti, L.; Gross-Goupil, M.; Orre, M.; Cornelis, F.; De Figueiredo, B.H.; Houédé, N.; Merino, C.; Roubaud, G.; Dallaudiére, B.; et al. Characterization of Prostate Neuroendocrine Cancers and Therapeutic Management: A Literature Review. Prostate Cancer Prostatic Dis 2014, 17, 220–226. [Google Scholar] [CrossRef]
- Zaffuto, E.; Pompe, R.; Zanaty, M.; Bondarenko, H.D.; Leyh-Bannurah, S.R.; Moschini, M.; Dell’Oglio, P.; Gandaglia, G.; Fossati, N.; Stabile, A.; et al. Contemporary Incidence and Cancer Control Outcomes of Primary Neuroendocrine Prostate Cancer: A SEER Database Analysis. Clin Genitourin Cancer 2017, 15, e793–e800. [Google Scholar] [CrossRef] [PubMed]
- Bonkhoff, H. Factors Implicated in Radiation Therapy Failure and Radiosensitization of Prostate Cancer. Prostate Cancer 2012, 2012, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Berruti, A.; Dogliotti, L.; Mosca, A.; Bellina, M.; Mari, M.; Torta, M.; Tarabuzzi, R.; Bollito, E.; Fontana, D.; Angeli, A. Circulating Neuroendocrine Markers in Patients with Prostate Carcinoma. Cancer 2000, 88, 2590–2597. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Ci, X.; Choi, S.Y.C.; Crea, F.; Lin, D.; Wang, Y. Molecular Events in Neuroendocrine Prostate Cancer Development. Nat Rev Urol 2021, 18, 581–596. [Google Scholar] [CrossRef]
- Beltran, H.; Prandi, D.; Mosquera, J.M.; Benelli, M.; Puca, L.; Cyrta, J.; Marotz, C.; Giannopoulou, E.; Chakravarthi, B.V.S.K.; Varambally, S.; et al. Divergent Clonal Evolution of Castration Resistant Neuroendocrine Prostate Cancer. Nat Med 2016, 22, 298. [Google Scholar] [CrossRef]
- Zhou, Z.; Flesken-Nikitin, A.; Corney, D.C.; Wang, W.; Goodrich, D.W.; Roy-Burman, P.; Nikitin, A.Y. Synergy of P53 and Rb Deficiency in a Conditional Mouse Model for Metastatic Prostate Cancer. Cancer Res 2006, 66, 7889–7898. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.Y.; Rosario, S.; Wang, Y.; Mu, P.; Seshadri, M.; Goodrich, Z.W.; Goodrich, M.M.; Labbé, D.P.; Gomez, E.C.; Wang, J.; et al. Rb1 and Trp53 Cooperate to Suppress Prostate Cancer Lineage Plasticity, Metastasis, and Antiandrogen Resistance. Science (1979) 2017, 355, 78–83. [Google Scholar] [CrossRef]
- Beltran, H.; Rickman, D.S.; Park, K.; Chae, S.S.; Sboner, A.; MacDonald, T.Y.; Wang, Y.; Sheikh, K.L.; Terry, S.; Tagawa, S.T.; et al. Molecular Characterization of Neuroendocrine Prostate Cancer and Identification of New Drug Targets. Cancer Discov 2011, 1, 487. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Phillips, J.W.; Smith, B.A.; Park, J.W.; Stoyanova, T.; McCaffrey, E.F.; Baertsch, R.; Sokolov, A.; Meyerowitz, J.G.; Mathis, C.; et al. N-Myc Drives Neuroendocrine Prostate Cancer Initiated from Human Prostate Epithelial Cells. Cancer Cell 2016, 29, 536–547. [Google Scholar] [CrossRef]
- Du, R.; Huang, C.; Liu, K.; Li, X.; Dong, Z. Targeting AURKA in Cancer: Molecular Mechanisms and Opportunities for Cancer Therapy. Mol Cancer 2021, 20, 15. [Google Scholar] [CrossRef]
- Cheng, W.C.; Wang, H.J. Current Advances of Targeting Epigenetic Modifications in Neuroendocrine Prostate Cancer. Tzu-Chi Medical Journal 2021, 33, 224. [Google Scholar] [CrossRef]
- Beltran, H.; Demichelis, F. Therapy Considerations in Neuroendocrine Prostate Cancer: What Next? Endocr Relat Cancer 2021, 28, T67–T78. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.; Zoubeidi, A.; Selth, L.A. The Epigenetic and Transcriptional Landscape of Neuroendocrine Prostate Cancer. Endocr Relat Cancer 2020, 27, R35–R50. [Google Scholar] [CrossRef] [PubMed]
- Clermont, P.L.; Lin, D.; Crea, F.; Wu, R.; Xue, H.; Wang, Y.; Thu, K.L.; Lam, W.L.; Collins, C.C.; Wang, Y.; et al. Polycomb-Mediated Silencing in Neuroendocrine Prostate Cancer. Clin Epigenetics 2015, 7, 1–13. [Google Scholar] [CrossRef]
- Dardenne, E.; Beltran, H.; Benelli, M.; Gayvert, K.; Berger, A.; Puca, L.; Cyrta, J.; Sboner, A.; Noorzad, Z.; MacDonald, T.; et al. N-Myc Induces an EZH2-Mediated Transcriptional Program Driving Neuroendocrine Prostate Cancer. Cancer Cell 2016, 30, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.T.; Zou, P.L.; Tang, Q.; Zheng, F.; Wu, J.J.; Chen, Z.Q.; Hann, S.S. HOTAIR-Mediated Reciprocal Regulation of EZH2 and DNMT1 Contribute to Polyphyllin I-Inhibited Growth of Castration-Resistant Prostate Cancer Cells in Vitro and in Vivo. Biochimica et Biophysica Acta (BBA) - General Subjects 2018, 1862, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Aytes, A.; Giacobbe, A.; Mitrofanova, A.; Ruggero, K.; Cyrta, J.; Arriaga, J.; Palomero, L.; Farran-Matas, S.; Rubin, M.A.; Shen, M.M.; et al. NSD2 Is a Conserved Driver of Metastatic Prostate Cancer Progression. Nat Commun 2018, 9, 5201. [Google Scholar] [CrossRef]
- Asangani, I.A.; Ateeq, B.; Cao, Q.; Dodson, L.; Pandhi, M.; Kunju, L.P.; Mehra, R.; Lonigro, R.J.; Siddiqui, J.; Palanisamy, N.; et al. Characterization of the EZH2-MMSET Histone Methyltransferase Regulatory Axis in Cancer. Mol Cell 2013, 49, 80–93. [Google Scholar] [CrossRef]
- Cai, C.; He, H.H.; Chen, S.; Coleman, I.; Wang, H.; Fang, Z.; Chen, S.; Nelson, P.S.; Liu, X.S.; Brown, M.; et al. Androgen Receptor Gene Expression in Prostate Cancer Is Directly Suppressed by the Androgen Receptor through Recruitment of Lysine-Specific Demethylase 1. Cancer Cell 2011, 20, 457–471. [Google Scholar] [CrossRef]
- Kumaraswamy, A.; Duan, Z.; Flores, D.; Zhang, C.; Sehrawat, A.; Hu, Y.-M.; Swaim, O.A.; Rodansky, E.; Storck, W.K.; Kuleape, J.A.; et al. LSD1 Promotes Prostate Cancer Cell Reprogramming by Repressing TP53 Signaling Independently of Its Demethylase Function. JCI Insight 2023, e167440. [Google Scholar] [CrossRef]
- Coleman, D.J.; Sampson, D.A.; Sehrawat, A.; Kumaraswamy, A.; Sun, D.; Wang, Y.; Schwartzman, J.; Urrutia, J.; Lee, A.R.; Coleman, I.M.; et al. Alternative Splicing of LSD1+8a in Neuroendocrine Prostate Cancer Is Mediated by SRRM4. Neoplasia 2020, 22, 253–262. [Google Scholar] [CrossRef]
- Maina, P.K.; Shao, P.; Liu, Q.; Fazli, L.; Tyler, S.; Nasir, M.; Dong, X.; Qi, H.H. C-MYC Drives Histone Demethylase PHF8 during Neuroendocrine Differentiation and in Castration-Resistant Prostate Cancer. Oncotarget 2016, 7, 75585–75602. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Pang, J.; Wang, L. ang; Huang, Z.; Xu, J.; Yang, X.; Xie, Q.; Huang, Y.; Tang, T.; Tong, D.; et al. Histone Demethylase PHF8 Drives Neuroendocrine Prostate Cancer Progression by Epigenetically Upregulating FOXA2. J Pathol 2021, 253, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Ci, X.; Hao, J.; Dong, X.; Choi, S.Y.; Xue, H.; Wu, R.; Qu, S.; Gout, P.W.; Zhang, F.; Haegert, A.M.; et al. Heterochromatin Protein 1a Mediates Development and Aggressiveness of Neuroendocrine Prostate Cancer. Cancer Res 2018, 78, 2691–2704. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Dong, X.; Wang, K.; Wyatt, A.W.; Crea, F.; Xue, H.; Wang, Y.; Wu, R.; Bell, R.H.; Haegert, A.; et al. Identification of DEK as a Potential Therapeutic Target for Neuroendocrine Prostate Cancer. Oncotarget 2015, 6, 1806–1820. [Google Scholar] [CrossRef]
- Asangani, I.A.; Dommeti, V.L.; Wang, X.; Malik, R.; Cieslik, M.; Yang, R.; Escara-Wilke, J.; Wilder-Romans, K.; Dhanireddy, S.; Engelke, C.; et al. Therapeutic Targeting of BET Bromodomain Proteins in Castration-Resistant Prostate Cancer. Nature 2014, 510, 278–282. [Google Scholar] [CrossRef]
- McNair, C.; Xu, K.; Mandigo, A.C.; Benelli, M.; Leiby, B.; Rodrigues, D.; Lindberg, J.; Gronberg, H.; Crespo, M.; De Laere, B.; et al. Differential Impact of RB Status on E2F1 Reprogramming in Human Cancer. J Clin Invest 2018, 128, 341–358. [Google Scholar] [CrossRef]
- Kim, D.H.; Sun, D.; Storck, W.K.; Leng, K.W.; Jenkins, C.; Coleman, D.J.; Sampson, D.; Guan, X.; Kumaraswamy, A.; Rodansky, E.S.; et al. BET Bromodomain Inhibition Blocks an AR-Repressed, E2F1-Activated Treatment-Emergent Neuroendocrine Prostate Cancer Lineage Plasticity Program. Clinical Cancer Research 2021, 27, 4923–4936. [Google Scholar] [CrossRef]
- Shafran, J.S.; Jafari, N.; Casey, A.N.; Győrffy, B.; Denis, G. V. BRD4 Regulates Key Transcription Factors That Drive-Mesenchymal Transition in Castration-Resistant Prostate. Prostate Cancer Prostatic Dis 2021, 24, 268–277. [Google Scholar] [CrossRef]
- Cyrta, J.; Augspach, A.; De Filippo, M.R.; Prandi, D.; Thienger, P.; Benelli, M.; Cooley, V.; Bareja, R.; Wilkes, D.; Chae, S.S.; et al. Role of Specialized Composition of SWI/SNF Complexes in Prostate Cancer Lineage Plasticity. Nat Commun 2020, 11, 5549. [Google Scholar] [CrossRef]
- Ruan, L.; Wang, L.; Wang, X.; He, M.; Yao, X. SIRT1 Contributes to Neuroendocrine Differentiation of Prostate Cancer. Oncotarget 2018, 9, 2002–2016. [Google Scholar] [CrossRef]
- Svensson, C.; Ceder, J.; Iglesias-Gato, D.; Chuan, Y.C.; Pang, S.T.; Bjartell, A.; Martinez, R.M.; Bott, L.; Helczynski, L.; Ulmert, D.; et al. REST Mediates Androgen Receptor Actions on Gene Repression and Predicts Early Recurrence of Prostate Cancer. Nucleic Acids Res 2014, 42, 999–1015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Coleman, I.M.; Brown, L.G.; True, L.D.; Kollath, L.; Lucas, J.M.; Lam, H.M.; Dumpit, R.; Corey, E.; Chéry, L.; et al. SRRM4 Expression and the Loss of REST Activity May Promote the Emergence of the Neuroendocrine Phenotype in Castration-Resistant Prostate Cancer. Clin Cancer Res 2015, 21, 4698–4708. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.T.; Lin, T.P.; Campbell, M.; Pan, C.C.; Lee, S.H.; Lee, H.C.; Yang, M.H.; Kung, H.J.; Chang, P.C. REST Is a Crucial Regulator for Acquiring EMT-like and Stemness Phenotypes in Hormone-Refractory Prostate Cancer. Sci Rep 2017, 7, 42795. [Google Scholar] [CrossRef]
- Akamatsu, S.; Wyatt, A.W.; Lin, D.; Lysakowski, S.; Zhang, F.; Kim, S.; Tse, C.; Wang, K.; Mo, F.; Haegert, A.; et al. The Placental Gene PEG10 Promotes Progression of Neuroendocrine Prostate Cancer. Cell Rep 2015, 12, 922–936. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Thaper, D.; Bidnur, S.; Toren, P.; Akamatsu, S.; Bishop, J.L.; Colins, C.; Vahid, S.; Zoubeidi, A. PEG10 Is Associated with Treatment-Induced Neuroendocrine Prostate Cancer. J Mol Endocrinol 2019, 63, 39–49. [Google Scholar] [CrossRef]
- Kim, J.; Jin, H.; Zhao, J.C.; Yang, Y.A.; Li, Y.; Yang, X.; Dong, X.; Yu, J. FOXA1 Inhibits Prostate Cancer Neuroendocrine Differentiation. Oncogene 2017 36:28 2017, 36, 4072–4080. [Google Scholar] [CrossRef]
- Paranjape, A.N.; Soundararajan, R.; Werden, S.J.; Joseph, R.; Taube, J.H.; Liu, H.; Rodriguez-Canales, J.; Sphyris, N.; Wistuba, I.; Miura, N.; et al. Inhibition of FOXC2 Restores Epithelial Phenotype and Drug Sensitivity in Prostate Cancer Cells with Stem-Cell Properties. Oncogene 2016, 35, 5963–5976. [Google Scholar] [CrossRef]
- Han, M.; Li, F.; Zhang, Y.; Dai, P.; He, J.; Li, Y.; Zhu, Y.; Zheng, J.; Huang, H.; Bai, F.; et al. FOXA2 Drives Lineage Plasticity and KIT Pathway Activation in Neuroendocrine Prostate Cancer. Cancer Cell 2022, 40, 1306–1323.e8. [Google Scholar] [CrossRef]
- Moparthi, L.; Pizzolato, G.; Koch, S. Wnt Activator FOXB2 Drives the Neuroendocrine Differentiation of Prostate Cancer. Proc Natl Acad Sci U S A 2019, 116, 22189–22195. [Google Scholar] [CrossRef]
- Qi, J.; Nakayama, K.; Cardiff, R.D.; Borowsky, A.D.; Kaul, K.; Williams, R.; Krajewski, S.; Mercola, D.; Carpenter, P.M.; Bowtell, D.; et al. Siah2-Dependent Concerted Activity of HIF and FoxA2 Regulates Formation of Neuroendocrine Phenotype and Neuroendocrine Prostate Tumors. Cancer Cell 2010, 18, 23–38. [Google Scholar] [CrossRef]
- Guo, H.; Ci, X.; Ahmed, M.; Hua, J.T.; Soares, F.; Lin, D.; Puca, L.; Vosoughi, A.; Xue, H.; Li, E.; et al. ONECUT2 Is a Driver of Neuroendocrine Prostate Cancer. Nat Commun 2019, 10, 278. [Google Scholar] [CrossRef] [PubMed]
- Rotinen, M.; You, S.; Yang, J.; Coetzee, S.G.; Reis-Sobreiro, M.; Huang, W.C.; Huang, F.; Pan, X.; Yáñez, A.; Hazelett, D.J.; et al. ONECUT2 Is a Targetable Master Regulator of Lethal Prostate Cancer That Suppresses the Androgen Axis. Nat Med 2018, 24, 1887–1898. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.L.; Thaper, D.; Vahid, S.; Davies, A.; Ketola, K.; Kuruma, H.; Jama, R.; Nip, K.M.; Angeles, A.; Johnson, F.; et al. The Master Neural Transcription Factor BRN2 Is an Androgen Receptor–Suppressed Driver of Neuroendocrine Differentiation in Prostate Cancer. Cancer Discov 2017, 7, 54–71. [Google Scholar] [CrossRef]
- Yasumizu, Y.; Rajabi, H.; Jin, C.; Hata, T.; Pitroda, S.; Long, M.D.; Hagiwara, M.; Li, W.; Hu, Q.; Liu, S.; et al. MUC1-C Regulates Lineage Plasticity Driving Progression to Neuroendocrine Prostate Cancer. Nat Commun 2020, 11, 338. [Google Scholar] [CrossRef]
- Lovnicki, J.; Gan, Y.; Feng, T.; Li, Y.; Xie, N.; Ho, C.H.; Lee, A.R.; Chen, X.; Nappi, L.; Han, B.; et al. LIN28B Promotes the Development of Neuroendocrine Prostate Cancer. J Clin Invest 2020, 130, 5338–5348. [Google Scholar] [CrossRef]
- Metz, E.P.; Wilder, P.J.; Dong, J.; Datta, K.; Rizzino, A. Elevating SOX2 in Prostate Tumor Cells Upregulates Expression of Neuroendocrine Genes, but Does Not Reduce the Inhibitory Effects of Enzalutamide. J Cell Physiol 2020, 235, 3731–3740. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.V.; Esposito, S.; Tupone, M.G.; Manzoli, L.; Airoldi, I.; Pompa, P.; Cindolo, L.; Schips, L.; Sorrentino, C.; Di Carlo, E. SOX2 Boosts Major Tumor Progression Genes in Prostate Cancer and Is a Functional Biomarker of Lymph Node Metastasis. Oncotarget 2016, 7, 12372–12385. [Google Scholar] [CrossRef]
- O’Connor, M.D.; Wederell, E.; Robertson, G.; Delaney, A.; Morozova, O.; Poon, S.S.S.; Yap, D.; Fee, J.; Zhao, Y.; McDonald, H.; et al. Retinoblastoma-Binding Proteins 4 and 9 Are Important for Human Pluripotent Stem Cell Maintenance. Exp Hematol 2011, 39, 866–879.e1. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; Li, Z.; Geng, X.; Li, M.; Tang, Q.; Wu, C.; Lu, Z. SOX2 Has Dual Functions as a Regulator in the Progression of Neuroendocrine Prostate Cancer. Lab Invest 2020, 100, 570–582. [Google Scholar] [CrossRef]
- Li, Y.; Donmez, N.; Sahinalp, C.; Xie, N.; Wang, Y.; Xue, H.; Mo, F.; Beltran, H.; Gleave, M.; Wang, Y.; et al. SRRM4 Drives Neuroendocrine Transdifferentiation of Prostate Adenocarcinoma Under Androgen Receptor Pathway Inhibition. Eur Urol 2017, 71, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.R.; Gan, Y.; Tang, Y.; Dong, X. A Novel Mechanism of SRRM4 in Promoting Neuroendocrine Prostate Cancer Development via a Pluripotency Gene Network. EBioMedicine 2018, 35, 167–177. [Google Scholar] [CrossRef]
- Bhagirath, D.; Liston, M.; Patel, N.; Akoto, T.; Lui, B.; Yang, T.L.; To, D.M.; Majid, S.; Dahiya, R.; Tabatabai, Z.L.; et al. MicroRNA Determinants of Neuroendocrine Differentiation in Metastatic Castration-Resistant Prostate Cancer. Oncogene 2020, 39, 7209–7223. [Google Scholar] [CrossRef]
- Yin, Y.; Xu, L.; Chang, Y.; Zeng, T.; Chen, X.; Wang, A.; Groth, J.; Foo, W.C.; Liang, C.; Hu, H.; et al. N-Myc Promotes Therapeutic Resistance Development of Neuroendocrine Prostate Cancer by Differentially Regulating MiR-421/ATM Pathway. Mol Cancer 2019, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.C.; Toubia, J.; Townley, S.; Hanson, A.R.; Dredge, B.K.; Pillman, K.A.; Bert, A.G.; Winter, J.M.; Iggo, R.; Das, R.; et al. Post-Transcriptional Gene Regulation by MicroRNA-194 Promotes Neuroendocrine Transdifferentiation in Prostate Cancer. Cell Rep 2021, 34, 108585. [Google Scholar] [CrossRef] [PubMed]
- Natani, S.; Ramakrishna, M.; Nallavolu, T.; Ummanni, R. MicroRNA-147b Induces Neuroendocrine Differentiation of Prostate Cancer Cells by Targeting Ribosomal Protein RPS15A. Prostate 2023, 83, 936–949. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Al-Muftah, M.A.; Al-Kowari, M.K.; Abuaqel, S.W.J.; Al-Rumaihi, K.; Al-Bozom, I.; Li, P.; Chouchane, L. Targeting Wnt/EZH2/MicroRNA-708 Signaling Pathway Inhibits Neuroendocrine Differentiation in Prostate Cancer. Cell Death Discov 2019, 5, 139. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Studach, L.; Hullinger, R.L.; Xie, J.; Andrisani, O.M. Down-Regulation of RE-1 Silencing Transcription Factor (REST) in Advanced Prostate Cancer by Hypoxia-Induced MiR-106b~25. Exp Cell Res 2014, 320, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Dang, Q.; Li, L.; Xie, H.; He, D.; Chen, J.; Song, W.; Chang, L.S.; Chang, H.C.; Yeh, S.; Chang, C. Anti-Androgen Enzalutamide Enhances Prostate Cancer Neuroendocrine (NE) Differentiation via Altering the Infiltrated Mast Cells → Androgen Receptor (AR) → MiRNA32 Signals. Mol Oncol 2015, 9, 1241–1251. [Google Scholar] [CrossRef]
- Ding, M.; Lin, B.; Li, T.; Liu, Y.; Li, Y.; Zhou, X.; Miao, M.; Gu, J.; Pan, H.; Yang, F.; et al. A Dual yet Opposite Growth-Regulating Function of MiR-204 and Its Target XRN1 in Prostate Adenocarcinoma Cells and Neuroendocrine-like Prostate Cancer Cells. Oncotarget 2015, 6, 7686–7700. [Google Scholar] [CrossRef]
- Wang, S.; Alpsoy, A.; Sood, S.; Ordonez-Rubiano, S.C.; Dhiman, A.; Sun, Y.; Jiao, G.; Krusemark, C.J.; Dykhuizen, E.C. A Potent, Selective CBX2 Chromodomain Ligand and Its Cellular Activity during Prostate Cancer Neuroendocrine Differentiation. Chembiochem 2021, 22, 2335–2344. [Google Scholar] [CrossRef] [PubMed]
- Mather, R.L.; Parolia, A.; Carson, S.E.; Venalainen, E.; Roig-Carles, D.; Jaber, M.; Chu, S.C.; Alborelli, I.; Wu, R.; Lin, D.; et al. The Evolutionarily Conserved Long Non-Coding RNA LINC00261 Drives Neuroendocrine Prostate Cancer Proliferation and Metastasis via Distinct Nuclear and Cytoplasmic Mechanisms. Mol Oncol 2021, 15, 1921–1941. [Google Scholar] [CrossRef] [PubMed]
- Steadman, K.; You, S.; Srinivas, D. V; Mouakkad, L.; Yan, Y.; Kim, M.; Venugopal, S. V; Tanaka, H.; Freeman, M.R. Autonomous Action and Cooperativity between the ONECUT2 Transcription Factor and Its 3’ Untranslated Region. Front Cell Dev Biol 2023, 11, 1206259. [Google Scholar] [CrossRef]
- Puca, L.; Gavyert, K.; Sailer, V.; Conteduca, V.; Dardenne, E.; Sigouros, M.; Isse, K.; Kearney, M.; Vosoughi, A.; Fernandez, L.; et al. Delta-like Protein 3 Expression and Therapeutic Targeting in Neuroendocrine Prostate Cancer. Sci Transl Med 2019, 11, eaav0891. [Google Scholar] [CrossRef] [PubMed]
- Henke, R.M.; Meredith, D.M.; Borromeo, M.D.; Savage, T.K.; Johnson, J.E. Ascl1 and Neurog2 Form Novel Complexes and Regulate Delta-Like3 (Dll3) Expression in the Neural Tube. Dev Biol 2009, 328, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Bland, T.; Wang, J.; Yin, L.; Pu, T.; Li, J.; Gao, J.; Lin, T.P.; Gao, A.C.; Wu, B.J. WLS-Wnt Signaling Promotes Neuroendocrine Prostate Cancer. iScience 2021, 24, 101970. [Google Scholar] [CrossRef]
- Unno, K.; Chalmers, Z.R.; Pamarthy, S.; Vatapalli, R.; Rodriguez, Y.; Lysy, B.; Mok, H.; Sagar, V.; Han, H.; Yoo, Y.A.; et al. Activated ALK Cooperates with N-Myc via Wnt/β-Catenin Signaling to Induce Neuroendocrine Prostate Cancer. Cancer Res 2021, 81, 2157. [Google Scholar] [CrossRef]
- Wu, C.; Huang, J. Phosphatidylinositol 3-Kinase-AKT-Mammalian Target of Rapamycin Pathway Is Essential for Neuroendocrine Differentiation of Prostate Cancer. J Biol Chem 2007, 282, 3571–3583. [Google Scholar] [CrossRef]
- VanDeusen, H.R.; Ramroop, J.R.; Morel, K.L.; Bae, S.Y.; Sheahan, A. V.; Sychev, Z.; Lau, N.A.; Cheng, L.C.; Tan, V.M.; Li, Z.; et al. Targeting RET Kinase in Neuroendocrine Prostate Cancer. Mol Cancer Res 2020, 18, 1176–1188. [Google Scholar] [CrossRef] [PubMed]
- Reina-Campos, M.; Linares, J.F.; Duran, A.; Cordes, T.; L’Hermitte, A.; Badur, M.G.; Bhangoo, M.S.; Thorson, P.K.; Richards, A.; Rooslid, T.; et al. Increased Serine and One-Carbon Pathway Metabolism by PKCλ/ι Deficiency Promotes Neuroendocrine Prostate Cancer. Cancer Cell 2019, 35, 385–400.e9. [Google Scholar] [CrossRef] [PubMed]
- Blanc, C.; Moktefi, A.; Jolly, A.; de la Grange, P.; Gay, D.; Nicolaiew, N.; Semprez, F.; Maillé, P.; Soyeux, P.; Firlej, V.; et al. The Neuropilin-1/PKC Axis Promotes Neuroendocrine Differentiation and Drug Resistance of Prostate Cancer. British Journal of Cancer 2022 128:5 2022, 128, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Simbulan-Rosenthal, C.M.; Rosenthal, D.S.; Luo, R.B.; Samara, R.; Espinoza, L.A.; Hassa, P.O.; Hottiger, M.O.; Smulson, M.E. PARP-1 Binds E2F-1 Independently of Its DNA Binding and Catalytic Domains, and Acts as a Novel Coactivator of E2F-1-Mediated Transcription during Re-Entry of Quiescent Cells into S Phase. Oncogene 2003, 22, 8460–8471. [Google Scholar] [CrossRef]
- Hsu, E.C.; Rice, M.A.; Bermudez, A.; Marques, F.J.G.; Aslan, M.; Liu, S.; Ghoochani, A.; Zhang, C.A.; Chen, Y.S.; Zlitni, A.; et al. Trop2 Is a Driver of Metastatic Prostate Cancer with Neuroendocrine Phenotype via PARP1. Proc Natl Acad Sci U S A 2020, 117, 2032–2042. [Google Scholar] [CrossRef]
- Davies, A.H.; Beltran, H.; Zoubeidi, A. Cellular Plasticity and the Neuroendocrine Phenotype in Prostate Cancer. Nat Rev Urol 2018, 15, 271–286. [Google Scholar] [CrossRef] [PubMed]
- McKeithen, D.; Graham, T.; Chung, L.W.K.; Odero-Marah, V. Snail Transcription Factor Regulates Neuroendocrine Differentiation in LNCaP Prostate Cancer Cells. Prostate 2010, 70, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Russo, M. V.; Airoldi, I.; Tupone, M.G.; Sorrentino, C.; Barbarito, G.; Meo, S. Di; Carlo, E. Di SNAI2/Slug Gene Is Silenced in Prostate Cancer and Regulates Neuroendocrine Differentiation, Metastasis-Suppressor and Pluripotency Gene Expression. Oncotarget 2015, 6, 17121–17134. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Tsai, Y.C.; Siu, M.K.; Yeh, H.L.; Chen, C.L.; Yin, J.J.; Huang, J.; Liu, Y.N. Inhibition of the Androgen Receptor Induces a Novel Tumor Promoter, ZBTB46, for Prostate Cancer Metastasis. Oncogene 2017, 36, 6213–6224. [Google Scholar] [CrossRef] [PubMed]
- Gururajan, M.; Cavassani, K.A.; Sievert, M.; Duan, P.; Lichterman, J.; Huang, J.M.; Smith, B.; You, S.; Nandana, S.; Chu, G.C.Y.; et al. SRC Family Kinase FYN Promotes the Neuroendocrine Phenotype and Visceral Metastasis in Advanced Prostate Cancer. Oncotarget 2015, 6, 44072–44083. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; He, Q.; Li, C.; Alsharafi, B.L.M.; Deng, L.; Long, Z.; Gan, Y. Focus on the Tumor Microenvironment: A Seedbed for Neuroendocrine Prostate Cancer. Front Cell Dev Biol 2022, 10, 955669. [Google Scholar] [CrossRef]
- Lee, G.T.; Kwon, S.J.; Lee, J.H.; Jeon, S.S.; Jang, K.T.; Choi, H.Y.; Lee, H.M.; Kim, W.J.; Lee, D.H.; Kim, I.Y. Macrophages Induce Neuroendocrine Differentiation of Prostate Cancer Cells via BMP6-IL6 Loop. Prostate 2011, 71, 1525–1537. [Google Scholar] [CrossRef] [PubMed]
- Spiotto, M.T.; Chung, T.D.K. STAT3 Mediates IL-6-Induced Neuroendocrine Differentiation in Prostate Cancer Cells. Prostate 2000, 42, 186–195. [Google Scholar] [CrossRef]
- Natani, S.; Sruthi, K.K.; Asha, S.M.; Khilar, P.; Lakshmi, P.S.V.; Ummanni, R. Activation of TGF-β - SMAD2 Signaling by IL-6 Drives Neuroendocrine Differentiation of Prostate Cancer through P38MAPK. Cell Signal 2022, 91, 110240. [Google Scholar] [CrossRef] [PubMed]
- Deeble, P.D.; Murphy, D.J.; Parsons, S.J.; Cox, M.E. Interleukin-6- and Cyclic AMP-Mediated Signaling Potentiates Neuroendocrine Differentiation of LNCaP Prostate Tumor Cells. Mol Cell Biol 2001, 21, 8471–8482. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, C.; Cui, Y.; Nadiminty, N.; Lou, W.; Gao, A.C. Interleukin-6 Induces Neuroendocrine Differentiation (NED) through Suppression of RE-1 Silencing Transcription Factor (REST). Prostate 2014, 74, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Peng, G.; Huang, H.; Liu, F.; Kong, D.P.; Dong, K.Q.; Dai, L.H.; Zhou, Z.; Wang, K.J.; Yang, J.; et al. Blocking the Feedback Loop between Neuroendocrine Differentiation and Macrophages Improves the Therapeutic Effects of Enzalutamide (MDV3100) on Prostate Cancer. Clin Cancer Res 2018, 24, 708–723. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Tian, H.; Niu, X.; Wang, J.; Li, X.; Jiang, N.; Wen, S.; Chen, X.; Ren, S.; Xu, C.; et al. Neurotensin and Its Receptors Mediate Neuroendocrine Transdifferentiation in Prostate Cancer. Oncogene 2019, 38, 4875–4884. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Yun, E.J.; Lo, U.G.; Tai, Y.L.; Deng, S.; Hernandez, E.; Dang, A.; Chen, Y.A.; Saha, D.; Mu, P.; et al. The Paracrine Induction of Prostate Cancer Progression by Caveolin-1. Cell Death & Disease 2019 10:11 2019, 10, 834. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, B.; Wu, W.; Li, L.; Broom, B.M.; Basourakos, S.P.; Korentzelos, D.; Luan, Y.; Wang, J.; Yang, G.; et al. Targeting the MYCN-PARP-DNA Damage Response Pathway in Neuroendocrine Prostate Cancer. Clin Cancer Res 2018, 24, 696–707. [Google Scholar] [CrossRef]
- Meulenbeld, H.J.; Bleuse, J.P.; Vinci, E.M.; Raymond, E.; Vitali, G.; Santoro, A.; Dogliotti, L.; Berardi, R.; Cappuzzo, F.; Tagawa, S.T.; et al. Randomized Phase II Study of Danusertib in Patients with Metastatic Castration-Resistant Prostate Cancer after Docetaxel Failure. BJU Int 2013, 111, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.W.; Burgess, S.G.; Poon, E.; Carstensen, A.; Eilers, M.; Chesler, L.; Bayliss, R. Structural Basis of N-Myc Binding by Aurora-A and Its Destabilization by Kinase Inhibitors. Proc Natl Acad Sci U S A 2016, 113, 13726–13731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-W.; Li, J.-Y.; Li, L.; Hu, W.-Q.; Tao, Y.; Gao, W.-Y.; Ye, Z.-N.; Jia, H.-Y.; Wang, J.-N.; Miao, X.-K.; et al. Neurokinin-1 Receptor Drives PKCɑ-AURKA/N-Myc Signaling to Facilitate the Neuroendocrine Progression of Prostate Cancer. Cell Death Dis 2023, 14, 384. [Google Scholar] [CrossRef]
- Beltran, H.; Oromendia, C.; Danila, D.C.; Montgomery, B.; Hoimes, C.; Szmulewitz, R.Z.; Vaishampayan, U.; Armstrong, A.J.; Stein, M.; Pinski, J.; et al. A Phase II Trial of the Aurora Kinase A Inhibitor Alisertib for Patients with Castration-Resistant and Neuroendocrine Prostate Cancer: Efficacy and Biomarkers. Clin Cancer Res 2019, 25, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.C.Y.; Frolov, A.; Li, R.; Ayala, G.; Greenberg, N.M. Targeting Aurora Kinases for the Treatment of Prostate Cancer. Cancer Res 2006, 66, 4996–5002. [Google Scholar] [CrossRef] [PubMed]
- Ton, A.T.; Singh, K.; Morin, H.; Ban, F.; Leblanc, E.; Lee, J.; Lallous, N.; Cherkasov, A. Dual-Inhibitors of N-Myc and AURKA as Potential Therapy for Neuroendocrine Prostate Cancer. Int J Mol Sci 2020, 21, 8277. [Google Scholar] [CrossRef]
- Ton, A.T.; Foo, J.; Singh, K.; Lee, J.; Kalyta, A.; Morin, H.; Perez, C.; Ban, F.; Leblanc, E.; Lallous, N.; et al. Development of VPC-70619, a Small-Molecule N-Myc Inhibitor as a Potential Therapy for Neuroendocrine Prostate Cancer. Int J Mol Sci 2022, 23, 2588. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Du, W.; Guo, W. EZH2: A Novel Target for Cancer Treatment. J Hematol Oncol 2020, 13, 104. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, D.; Zhou, T.; Song, H.; Hulsurkar, M.; Su, N.; Liu, Y.; Wang, Z.; Shao, L.; Ittmann, M.; et al. Androgen Deprivation Promotes Neuroendocrine Differentiation and Angiogenesis through CREB-EZH2-TSP1 Pathway in Prostate Cancers. Nature Communications 2018 9:1 2018, 9, 4080. [Google Scholar] [CrossRef] [PubMed]
- Gravina, G.L.; Festuccia, C.; Millimaggi, D.; Dolo, V.; Tombolini, V.; De Vito, M.; Vicentini, C.; Bologna, M. Chronic Azacitidine Treatment Results in Differentiating Effects, Sensitizes against Bicalutamide in Androgen-Independent Prostate Cancer Cells. Prostate 2008, 68, 793–801. [Google Scholar] [CrossRef]
- Sonpavde, G.; Aparicio, A.M.; Zhan, F.; North, B.; DeLaune, R.; Garbo, L.E.; Rousey, S.R.; Weinstein, R.E.; Xiao, L.; Boehm, K.A.; et al. Azacitidine Favorably Modulates PSA Kinetics Correlating with Plasma DNA LINE-1 Hypomethylation in Men with Chemonaïve Castration-Resistant Prostate Cancer. Urol Oncol 2011, 29, 682–689. [Google Scholar] [CrossRef]
- Augert, A.; Eastwood, E.; Ibrahim, A.H.; Wu, N.; Grunblatt, E.; Basom, R.; Liggitt, D.; Eaton, K.D.; Martins, R.; Poirier, J.T.; et al. Targeting NOTCH Activation in Small Cell Lung Cancer through LSD1 Inhibition. Sci Signal 2019, 12, eaau2922. [Google Scholar] [CrossRef]
- Sehrawat, A.; Gao, L.; Wang, Y.; Bankhead, A.; McWeeney, S.K.; King, C.J.; Schwartzman, J.; Urrutia, J.; Bisson, W.H.; Coleman, D.J.; et al. LSD1 Activates a Lethal Prostate Cancer Gene Network Independently of Its Demethylase Function. Proc Natl Acad Sci U S A 2018, 115, E4179–E4188. [Google Scholar] [CrossRef]
- Hollebecque, A.; Salvagni, S.; Plummer, R.; Niccoli, P.; Capdevila, J.; Curigliano, G.; Moreno, V.; de Braud, F.; de Villambrosia, S.G.; Martin-Romano, P.; et al. Clinical Activity of CC-90011, an Oral, Potent, and Reversible LSD1 Inhibitor, in Advanced Malignancies. Cancer 2022, 128, 3185–3195. [Google Scholar] [CrossRef] [PubMed]
- Mor-Vaknin, N.; Saha, A.; Legendre, M.; Carmona-Rivera, C.; Amin, M.A.; Rabquer, B.J.; Gonzales-Hernandez, M.J.; Jorns, J.; Mohan, S.; Yalavarthi, S.; et al. DEK-Targeting DNA Aptamers as Therapeutics for Inflammatory Arthritis. Nature Communications 2017, 8, 14252. [Google Scholar] [CrossRef]
- Chen, W.Y.; Thuy Dung, P.V.; Yeh, H.L.; Chen, W.H.; Jiang, K.C.; Li, H.R.; Chen, Z.Q.; Hsiao, M.; Huang, J.; Wen, Y.C.; et al. Targeting PKLR/MYCN/ROMO1 Signaling Suppresses Neuroendocrine Differentiation of Castration-Resistant Prostate Cancer. Redox Biol 2023, 62, 102686. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.R.; Schweizer, M.T.; Nanus, D.M.; Pantuck, A.J.; Heath, E.I.; Campeau, E.; Attwell, S.; Norek, K.; Snyder, M.; Bauman, L.; et al. A Phase 1b/2a Study of the Pan-BET Bromodomain Inhibitor ZEN-3694 in Combination with Enzalutamide in Patients with Metastatic Castration Resistant Prostate Cancer. Clin Cancer Res 2020, 26, 5338–5347. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Sinha, R.K.; Kumar, M.; Alam, M.; Yin, L.; Raina, D.; Kharbanda, A.; Panchamoorthy, G.; Gupta, D.; Singh, H.; et al. Intracellular Targeting of the Oncogenic MUC1-C Protein with a Novel GO-203 Nanoparticle Formulation. Clin Cancer Res 2015, 21, 2338–2347. [Google Scholar] [CrossRef] [PubMed]
- Panchamoorthy, G.; Jin, C.; Raina, D.; Bharti, A.; Yamamoto, M.; Adeebge, D.; Zhao, Q.; Bronson, R.; Jiang, S.; Li, L.; et al. Targeting the Human MUC1-C Oncoprotein with an Antibody-Drug Conjugate. JCI Insight 2018, 3, e99880. [Google Scholar] [CrossRef]
- Yan, T.; Zhou, D.; Shi, Y.; Cui, D.; Jiang, J.; Han, B.; Xia, S.; Wang, Z.; Liu, H.; Guo, W.; et al. Targeting ADT-Induced Activation of the E3 Ubiquitin Ligase Siah2 to Delay the Occurrence of Castration-Resistant Prostate Cancer. Front Oncol 2021, 11, 637040. [Google Scholar] [CrossRef]
- Feng, Y.; Sessions, E.H.; Zhang, F.; Ban, F.; Placencio-Hickok, V.; Ma, C.T.; Zeng, F.Y.; Pass, I.; Terry, D.B.; Cadwell, G.; et al. Identification and Characterization of Small Molecule Inhibitors of the Ubiquitin Ligases Siah1/2 in Melanoma and Prostate Cancer Cells. Cancer Lett 2019, 449, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Labrecque, M.P.; Brown, L.G.; Coleman, I.M.; Nguyen, H.M.; Lin, D.W.; Corey, E.; Nelson, P.S.; Morrissey, C. Cabozantinib Can Block Growth of Neuroendocrine Prostate Cancer Patient-Derived Xenografts by Disrupting Tumor Vasculature. PLoS One 2021, 16, e0245602. [Google Scholar] [CrossRef]
- Yadav, S.S.; Li, J.; Stockert, J.A.; Herzog, B.; O’Connor, J.; Garzon-Manco, L.; Parsons, R.; Tewari, A.K.; Yadav, K.K. Induction of Neuroendocrine Differentiation in Prostate Cancer Cells by Dovitinib (TKI-258) and Its Therapeutic Implications. Transl Oncol 2017, 10, 357–366. [Google Scholar] [CrossRef]
- Smith, M.R.; Sweeney, C.J.; Corn, P.G.; Rathkopf, D.E.; Smith, D.C.; Hussain, M.; George, D.J.; Higano, C.S.; Harzstark, A.L.; Sartor, A.O.; et al. Cabozantinib in Chemotherapy-Pretreated Metastatic Castration-Resistant Prostate Cancer: Results of a Phase II Nonrandomized Expansion Study. J Clin Oncol 2014, 32, 3391–3399. [Google Scholar] [CrossRef] [PubMed]
- Corn, P.G.; Zhang, M.; Nogueras-Gonzalez, G.M.; Xiao, L.; Zurita, A.J.; Subudhi, S.K.; Tu, S.M.; Aparicio, A.M.; Coarfa, C.; Rajapakshe, K.; et al. A Phase II Study of Cabozantinib and Androgen Ablation in Patients with Hormone-Naïve Metastatic Prostate Cancer. Clin Cancer Res 2020, 26, 990–999. [Google Scholar] [CrossRef]
- Sonpavde, G.P.; Pond, G.R.; Fizazi, K.; de Bono, J.S.; Basch, E.M.; Scher, H.I.; Smith, M.R. Cabozantinib for Progressive Metastatic Castration-Resistant Prostate Cancer Following Docetaxel: Combined Analysis of Two Phase 3 Trials. Eur Urol Oncol 2020, 3, 540–543. [Google Scholar] [CrossRef]
- Serk, I.P.; Zhang, J.; Phillips, K.A.; Araujo, J.C.; Najjar, A.M.; Volgin, A.Y.; Gelovani, J.G.; Kim, S.J.; Wang, Z.; Gallick, G.E. Targeting Src Family Kinases Inhibits Growth and Lymph Node Metastases of Prostate Cancer in an Orthotopic Nude Mouse Model. Cancer Res 2008, 68, 3323–3333. [Google Scholar] [CrossRef]
- Yu, E.Y.; Wilding, G.; Posadas, E.; Gross, M.; Culine, S.; Massard, C.; Morris, M.J.; Hudes, G.; Calabrò, F.; Cheng, S.; et al. Phase II Study of Dasatinib in Patients with Metastatic Castration-Resistant Prostate Cancer. Clin Cancer Res 2009, 15, 7421–7428. [Google Scholar] [CrossRef] [PubMed]
- Twardowski, P.W.; Beumer, J.H.; Chen, C.S.; Kraft, A.S.; Chatta, G.S.; Mitsuhashi, M.; Ye, W.; Christner, S.M.; Lilly, M.B. A Phase II Trial of Dasatinib in Patients with Metastatic Castration-Resistant Prostate Cancer Treated Previously with Chemotherapy. Anticancer Drugs 2013, 24, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.C.; Trudel, G.C.; Saad, F.; Armstrong, A.J.; Yu, E.Y.; Bellmunt, J.; Wilding, G.; McCaffrey, J.; Serrano, S. V.; Matveev, V.B.; et al. Docetaxel and Dasatinib or Placebo in Men with Metastatic Castration-Resistant Prostate Cancer (READY): A Randomised, Double-Blind Phase 3 Trial. Lancet Oncol 2013, 14, 1307–1316. [Google Scholar] [CrossRef]
- Yuan, T.C.; Veeramani, S.; Lin, F.F.; Kondrikou, D.; Zelivianski, S.; Igawa, T.; Karan, D.; Batra, S.K.; Lin, M.F. Androgen Deprivation Induces Human Prostate Epithelial Neuroendocrine Differentiation of Androgen-Sensitive LNCaP Cells. Endocr Relat Cancer 2006, 13, 151–167. [Google Scholar] [CrossRef]
- Jin, X.F.; Spöttl, G.; Maurer, J.; Nölting, S.; Auernhammer, C.J. Antitumoral Activity of the MEK Inhibitor Trametinib (TMT212) Alone and in Combination with the CDK4/6 Inhibitor Ribociclib (LEE011) in Neuroendocrine Tumor Cells In Vitro. Cancers (Basel) 2021, 13, 1485. [Google Scholar] [CrossRef]
- Lee, C.; Chen, Y.; Hernandez, E.; Pong, R.; Ma, S.; Hofstad, M.; Kapur, P.; Zhau, H.; Chung, L.W.; Lai, C.; et al. The Central Role of Sphingosine Kinase 1 in the Development of Neuroendocrine Prostate Cancer (NEPC): A New Targeted Therapy of NEPC. Clin Transl Med 2022, 12, e695. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Halabi, S.; Healy, P.; Alumkal, J.J.; Winters, C.; Kephart, J.; Bitting, R.L.; Hobbs, C.; Soleau, C.F.; Beer, T.M.; et al. Phase II Trial of the PI3 Kinase Inhibitor Buparlisib (BKM-120) with or without Enzalutamide in Men with Metastatic Castration Resistant Prostate Cancer. Eur J Cancer 2017, 81, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.X.; Hsieh, A.C.; Kim, W.; Friedlander, T.; Lin, A.M.; Louttit, M.; Ryan, C.J. A Phase I Study of Abiraterone Acetate Combined with BEZ235, a Dual PI3K/MTOR Inhibitor, in Metastatic Castration Resistant Prostate Cancer. Oncologist 2017, 22, 503–e43. [Google Scholar] [CrossRef]
- Hotte, S.J.; Chi, K.N.; Joshua, A.M.; Tu, D.; Macfarlane, R.J.; Gregg, R.W.; Ruether, J.D.; Basappa, N.S.; Finch, D.; Salim, M.; et al. A Phase II Study of PX-866 in Patients With Recurrent or Metastatic Castration-Resistant Prostate Cancer: Canadian Cancer Trials Group Study IND205. Clin Genitourin Cancer 2019, 17, 201–208.e1. [Google Scholar] [CrossRef] [PubMed]
- Molife, L.R.; Yan, L.; Vitfell-Rasmussen, J.; Zernhelt, A.M.; Sullivan, D.M.; Cassier, P.A.; Chen, E.; Biondo, A.; Tetteh, E.; Siu, L.L.; et al. Phase 1 Trial of the Oral AKT Inhibitor MK-2206 plus Carboplatin/ Paclitaxel, Docetaxel, or Erlotinib in Patients with Advanced Solid Tumors. J Hematol Oncol 2014, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- De Bono, J.S.; De Giorgi, U.; Rodrigues, D.N.; Massard, C.; Bracarda, S.; Font, A.; Arija, J.A.A.; Shih, K.C.; Radavoi, G.D.; Xu, N.; et al. Randomized Phase II Study Evaluating AKT Blockade with Ipatasertib, in Combination with Abiraterone, in Patients with Metastatic Prostate Cancer with and without PTEN Loss. Clin Cancer Res 2019, 25, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, C.; Bracarda, S.; Sternberg, C.N.; Chi, K.N.; Olmos, D.; Sandhu, S.; Massard, C.; Matsubara, N.; Alekseev, B.; Parnis, F.; et al. Ipatasertib plus Abiraterone and Prednisolone in Metastatic Castration-Resistant Prostate Cancer (IPATential150): A Multicentre, Randomised, Double-Blind, Phase 3 Trial. Lancet 2021, 398, 131–142. [Google Scholar] [CrossRef]
- Chen, R.; Li, Y.; Buttyan, R.; Dong, X. Implications of PI3K/AKT Inhibition on REST Protein Stability and Neuroendocrine Phenotype Acquisition in Prostate Cancer Cells. Oncotarget 2017, 8, 84863–84876. [Google Scholar] [CrossRef]
- Cortés, M.A.; Cariaga-Martinez, A.E.; Lobo, M.V.T.; Martín orozco, R.M.; Motiño, O.; Rodríguez-Ubreva, F.J.; Angulo, J.; López-Ruiz, P.; Colás, B. EGF Promotes Neuroendocrine-like Differentiation of Prostate Cancer Cells in the Presence of LY294002 through Increased ErbB2 Expression Independent of the Phosphatidylinositol 3-Kinase-AKT Pathway. Carcinogenesis 2012, 33, 1169–1177. [Google Scholar] [CrossRef]
- Yao, J.; Bergsland, E.; Aggarwal, R.; Aparicio, A.; Beltran, H.; Crabtree, J.S.; Hann, C.L.; Ibrahim, T.; Byers, L.A.; Sasano, H.; et al. DLL3 as an Emerging Target for the Treatment of Neuroendocrine Neoplasms. Oncologist 2022, 27, 940–951. [Google Scholar] [CrossRef]
- Liu, Y.N.; Niu, S.; Chen, W.Y.; Zhang, Q.; Tao, Y.; Chen, W.H.; Jiang, K.C.; Chen, X.; Shi, H.; Liu, A.; et al. Leukemia Inhibitory Factor Promotes Castration-Resistant Prostate Cancer and Neuroendocrine Differentiation by Activated ZBTB46. Clin Cancer Res 2019, 25, 4128–4140. [Google Scholar] [CrossRef]
- Chen, W.Y.; Zeng, T.; Wen, Y.C.; Yeh, H.L.; Jiang, K.C.; Chen, W.H.; Zhang, Q.; Huang, J.; Liu, Y.N. Androgen Deprivation-Induced ZBTB46-PTGS1 Signaling Promotes Neuroendocrine Differentiation of Prostate Cancer. Cancer Lett 2019, 440–441, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Wen, Y.C.; Lin, S.R.; Yeh, H.L.; Jiang, K.C.; Chen, W.H.; Lin, Y.S.; Zhang, Q.; Liew, P.L.; Hsiao, M.; et al. Nerve Growth Factor Interacts with CHRM4 and Promotes Neuroendocrine Differentiation of Prostate Cancer and Castration Resistance. Commun Biol 2021, 4, 22. [Google Scholar] [CrossRef]
- Wen, Y.C.; Tram, V.T.N.; Chen, W.H.; Li, C.H.; Yeh, H.L.; Thuy Dung, P.V.; Jiang, K.C.; Li, H.R.; Huang, J.; Hsiao, M.; et al. CHRM4/AKT/MYCN Upregulates Interferon Alpha-17 in the Tumor Microenvironment to Promote Neuroendocrine Differentiation of Prostate Cancer. Cell Death Dis 2023, 14, 304. [Google Scholar] [CrossRef]
- Baritaki, S.; Yeung, K.; Palladino, M.; Berenson, J.; Bonavida, B. Pivotal Roles of Snail Inhibition and RKIP Induction by the Proteasome Inhibitor NPI-0052 in Tumor Cell Chemoimmunosensitization. Cancer Res 2009, 69, 8376–8385. [Google Scholar] [CrossRef]
- Baritaki, S.; Chapman, A.; Yeung, K.; Spandidos, D.A.; Palladino, M.; Bonavida, B. Inhibition of Epithelial to Mesenchymal Transition in Metastatic Prostate Cancer Cells by the Novel Proteasome Inhibitor, NPI-0052: Pivotal Roles of Snail Repression and RKIP Induction. Oncogene 2009, 28, 3573–3585. [Google Scholar] [CrossRef]
- Mickova, A.; Kharaishvili, G.; Kurfurstova, D.; Gachechiladze, M.; Kral, M.; Vacek, O.; Pokryvkova, B.; Mistrik, M.; Soucek, K.; Bouchal, J. Skp2 and Slug Are Coexpressed in Aggressive Prostate Cancer and Inhibited by Neddylation Blockade. Int J Mol Sci 2021, 22, 2844. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Han, S.; Wilder-Romans, K.; Sun, G.Y.; Zhu, H.; Liu, X.; Tan, M.; Wang, G.; Feng, F.Y.; Sun, Y. Neddylation Inactivation Represses Androgen Receptor Transcription and Inhibits Growth, Survival and Invasion of Prostate Cancer Cells. Neoplasia 2020, 22, 192–202. [Google Scholar] [CrossRef]
- Yin, Y.; Xie, C.M.; Li, H.; Tan, M.; Chen, G.; Schiff, R.; Xiong, X.; Sun, Y. The FBXW2-MSX2-SOX2 Axis Regulates Stem Cell Property and Drug Resistance of Cancer Cells. Proc Natl Acad Sci U S A 2019, 116, 20528–20538. [Google Scholar] [CrossRef]
- Wu, C.; Peng, S.; Pilie, P.G.; Geng, C.; Park, S.; Manyam, G.C.; Lu, Y.; Yang, G.; Tang, Z.; Kondraganti, S.; et al. PARP and CDK4/6 Inhibitor Combination Therapy Induces Apoptosis and Suppresses Neuroendocrine Differentiation in Prostate Cancer. Mol Cancer Ther 2021, 20, 1680–1691. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, L.; Yang, G.; Geng, C.; Luo, Y.; Wu, W.; Manyam, G.C.; Korentzelos, D.; Park, S.; Tang, Z.; et al. PARP Inhibition Suppresses GR-MYCN-CDK5-RB1-E2F1 Signaling and Neuroendocrine Differentiation in Castration-Resistant Prostate Cancer. Clin Cancer Res 2019, 25, 6839–6851. [Google Scholar] [CrossRef]
- Yoshida, M.; Oda, C.; Mishima, K.; Tsuji, I.; Obika, S.; Shimojo, M. An Antisense Amido-Bridged Nucleic Acid Gapmer Oligonucleotide Targeting SRRM4 Alters REST Splicing and Exhibits Anti-Tumor Effects in Small Cell Lung Cancer and Prostate Cancer Cells. Cancer Cell Int 2023, 23, 8. [Google Scholar] [CrossRef]
- Radaeva, M.; Ho, C.H.; Xie, N.; Zhang, S.; Lee, J.; Liu, L.; Lallous, N.; Cherkasov, A.; Dong, X. Discovery of Novel Lin28 Inhibitors to Suppress Cancer Cell Stemness. Cancers (Basel) 2022, 14, 5687. [Google Scholar] [CrossRef]
- Meyer-Siegler, K.L.; Iczkowski, K.A.; Leng, L.; Bucala, R.; Vera, P.L. Inhibition of Macrophage Migration Inhibitory Factor or Its Receptor (CD74) Attenuates Growth and Invasion of DU-145 Prostate Cancer Cells. The Journal of Immunology (Baltimore, Md. : 1950) 2006, 177, 8730–8739. [Google Scholar] [CrossRef] [PubMed]
- Tawadros, T.; Alonso, F.; Jichlinski, P.; Clarke, N.; Calandra, T.; Haefliger, J.A.; Roger, T. Release of Macrophage Migration Inhibitory Factor by Neuroendocrine-Differentiated LNCaP Cells Sustains the Proliferation and Survival of Prostate Cancer Cells. Endocr Relat Cancer 2013, 20, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Kroon, P.; Berry, P.A.; Stower, M.J.; Rodrigues, G.; Mann, V.M.; Simms, M.; Bhasin, D.; Chettiar, S.; Li, C.; Li, P.K.; et al. JAK-STAT Blockade Inhibits Tumor Initiation and Clonogenic Recovery of Prostate Cancer Stem-like Cells. Cancer Res 2013, 73, 5288–5298. [Google Scholar] [CrossRef] [PubMed]
- Hellsten, R.; Johansson, M.; Dahlman, A.; Dizeyi, N.; Sterner, O.; Bjartell, A. Galiellalactone Is a Novel Therapeutic Candidate against Hormone-Refractory Prostate Cancer Expressing Activated Stat3. Prostate 2008, 68, 269–280. [Google Scholar] [CrossRef]
- Dorff, T.B.; Goldman, B.; Pinski, J.K.; Mack, P.C.; Lara, P.N.; Van Veldhuizen, P.J.; Quinn, D.I.; Vogelzang, N.J.; Thompson, I.M.; Hussain, M.H.A. Clinical and Correlative Results of SWOG S0354: A Phase II Trial of CNTO328 (Siltuximab), a Monoclonal Antibody against Interleukin-6 (IL-6), in Chemotherapy Pre-Treated Patients with Castration-Resistant Prostate Cancer (CRPC). Clin Cancer Res 2010, 16, 3028–3034. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





