Submitted:
20 August 2023
Posted:
21 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. XRD Patterns of α-NixFe1-xOOH NPs
2.2. Thermogravimetric and Differential Thermal Analysis (TG-DTA) of α-NixFe1-xOOH
2.3. FTIR Spectra of α-NixFe1-xOOH NPs
2.4. XAFS Spectra of α-NixFe1-xOOH NPs
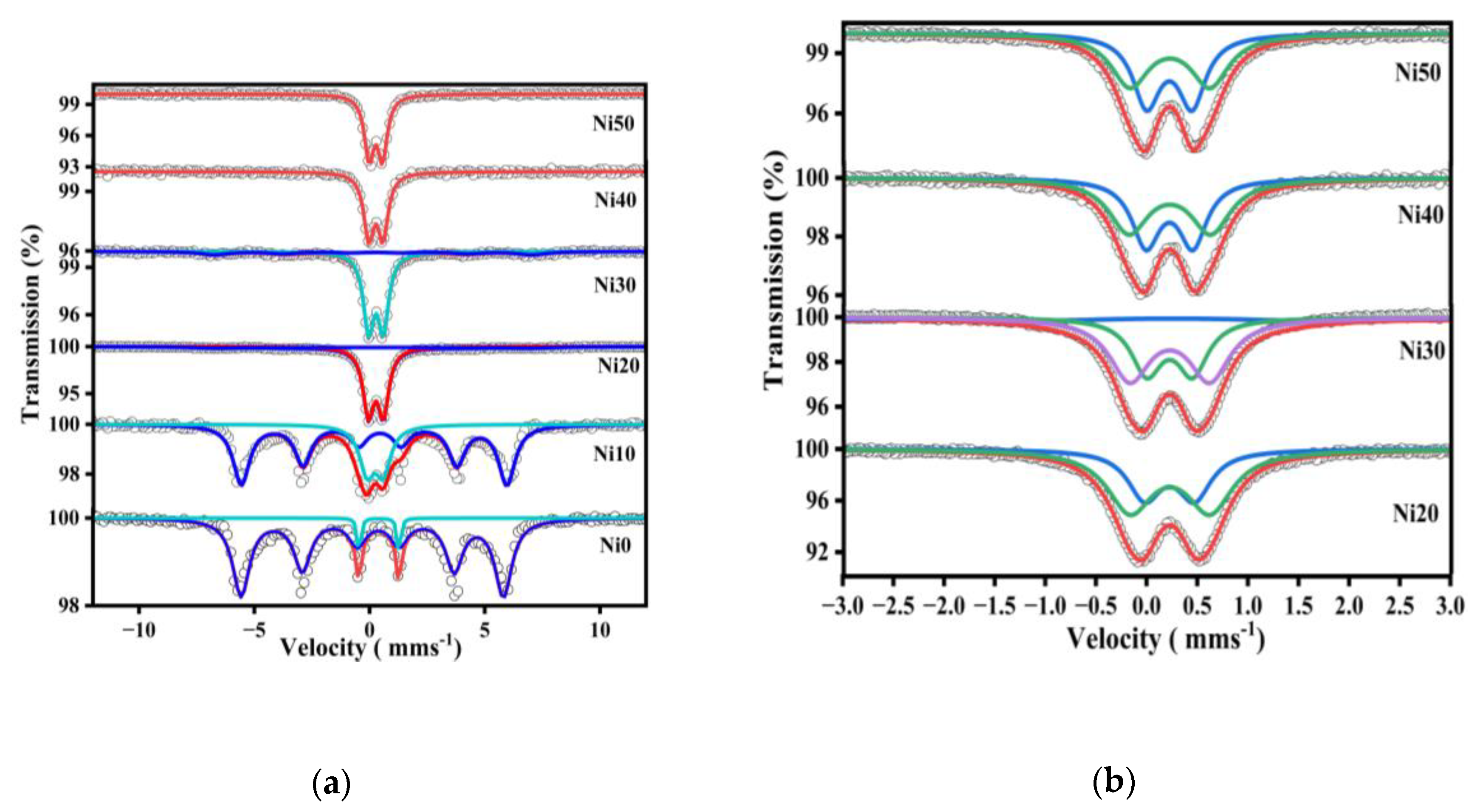
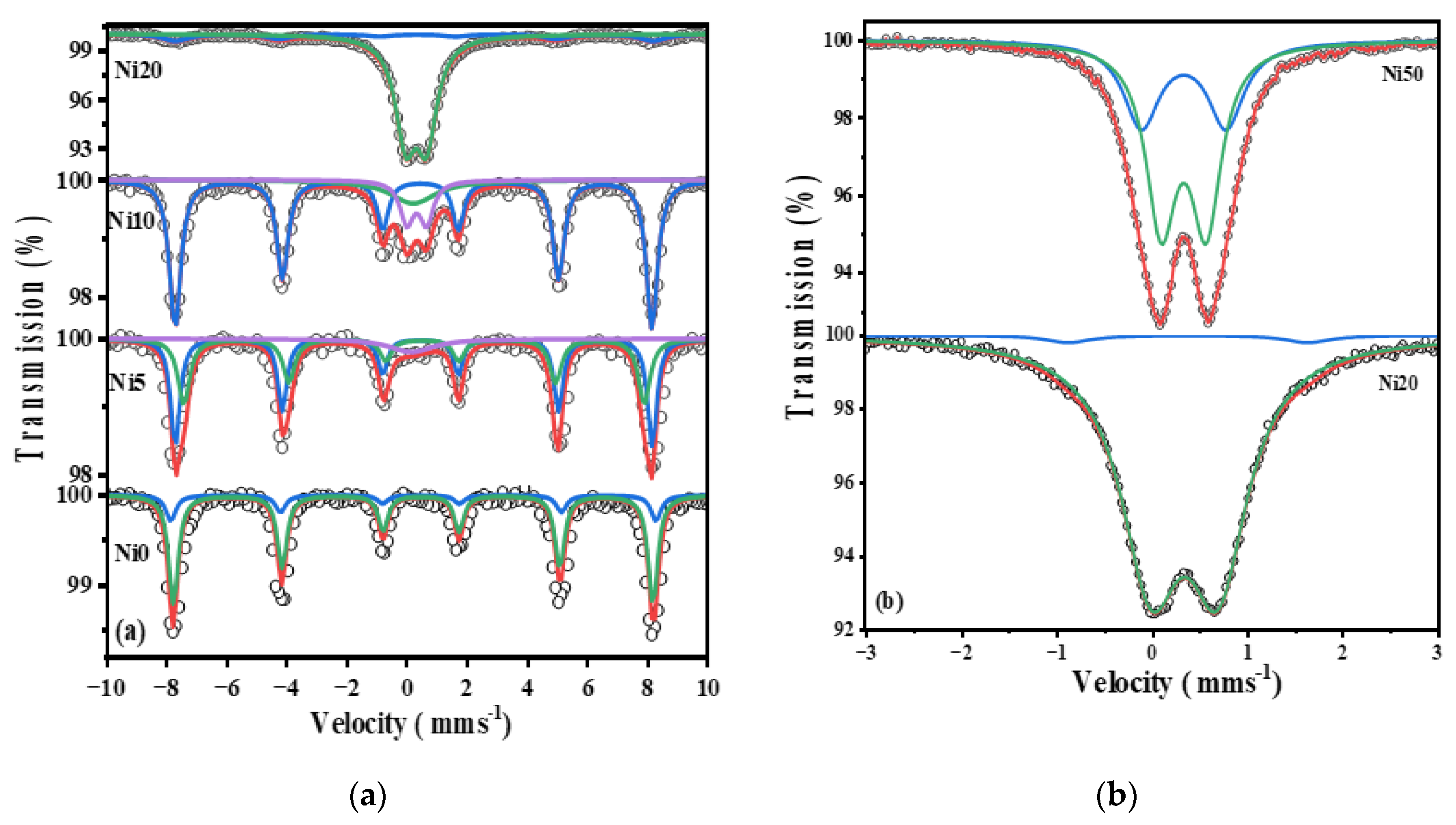
2.5. 57Fe- Mössbauer Spectra of α-NixFe1-xOOH NPs
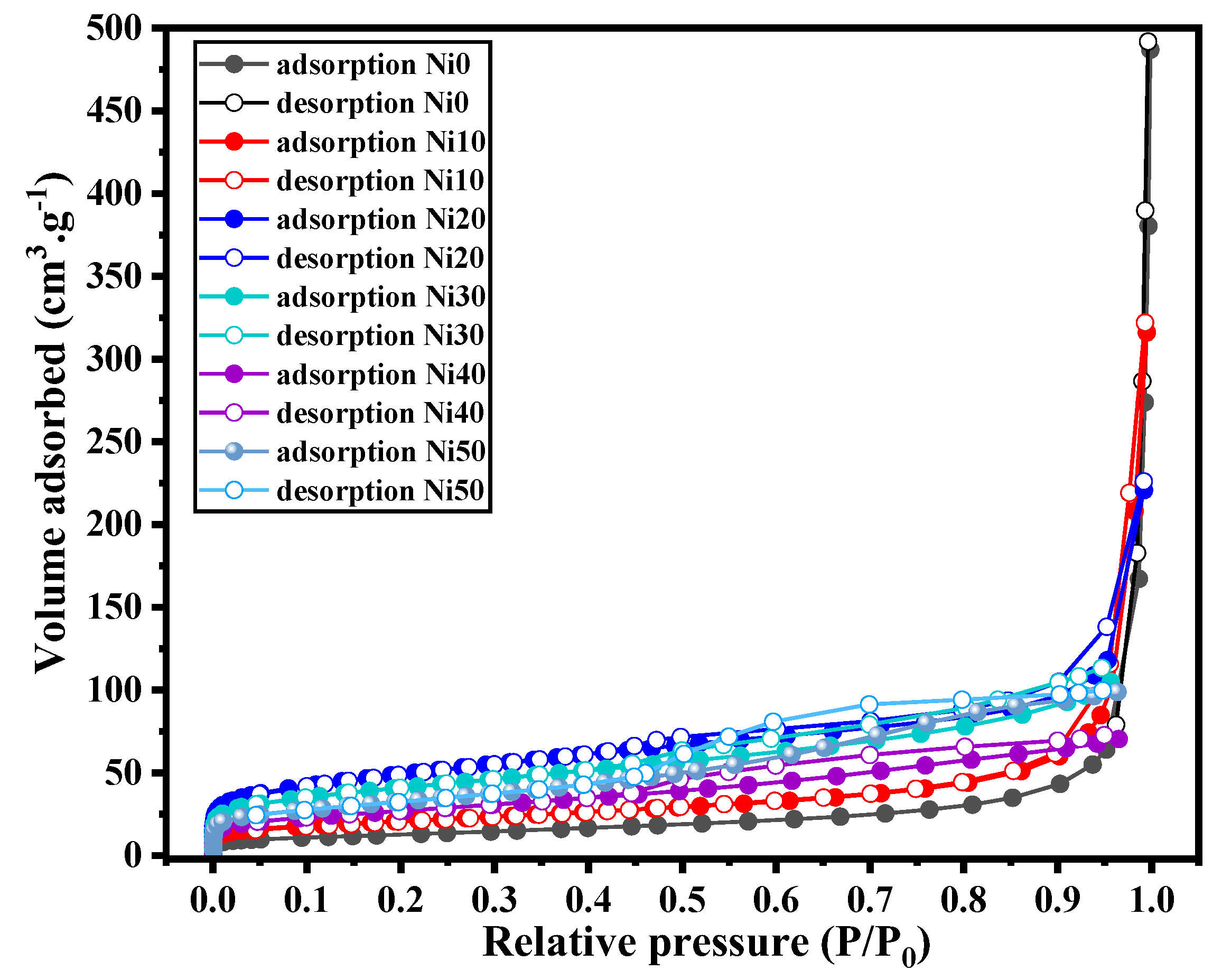
3.6. BET Analysis of α-NixFe1-xOOH NPs
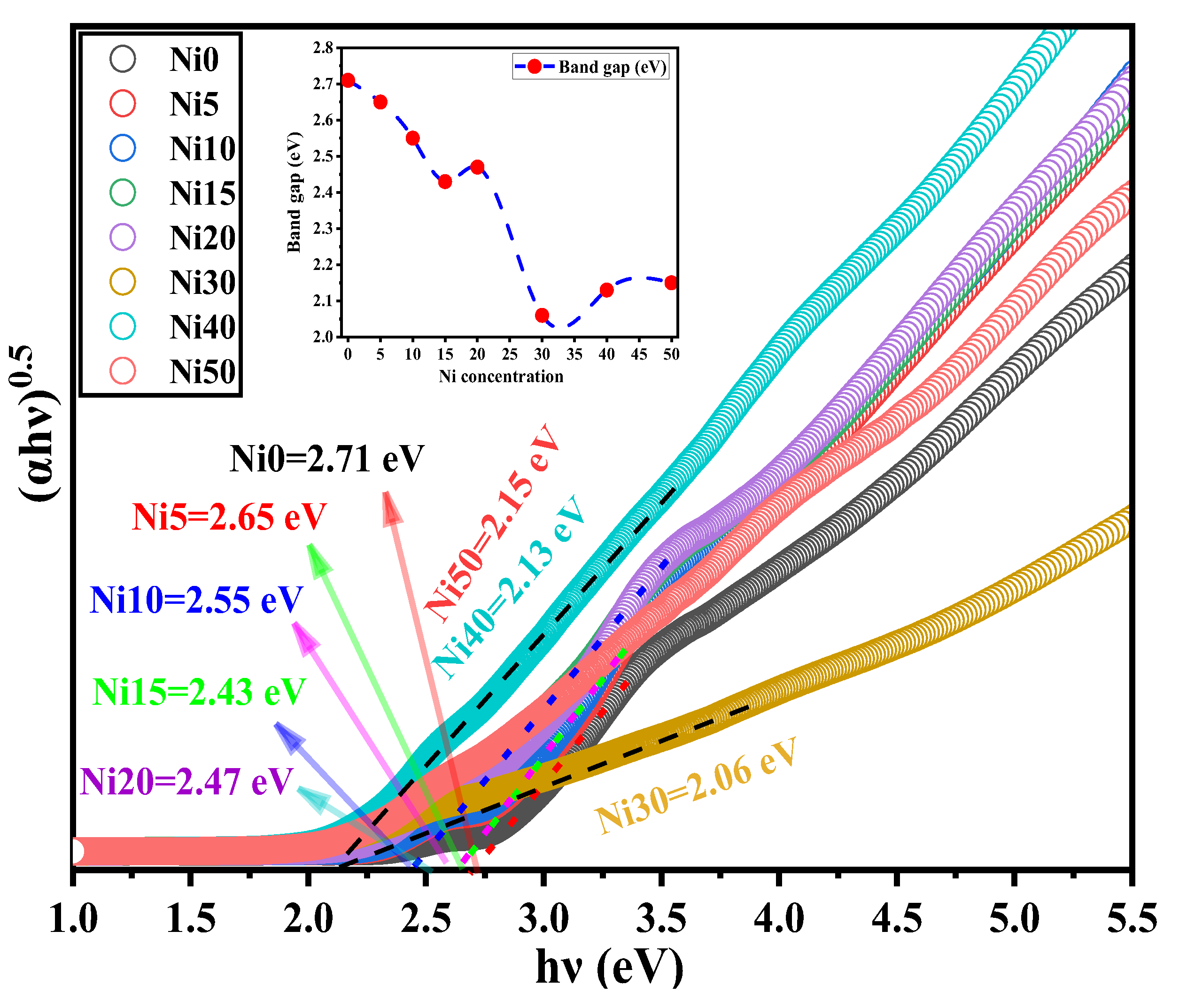
3.7. Bandgap Energy Derived from DRS of α-NixFe1-xOOH NPs
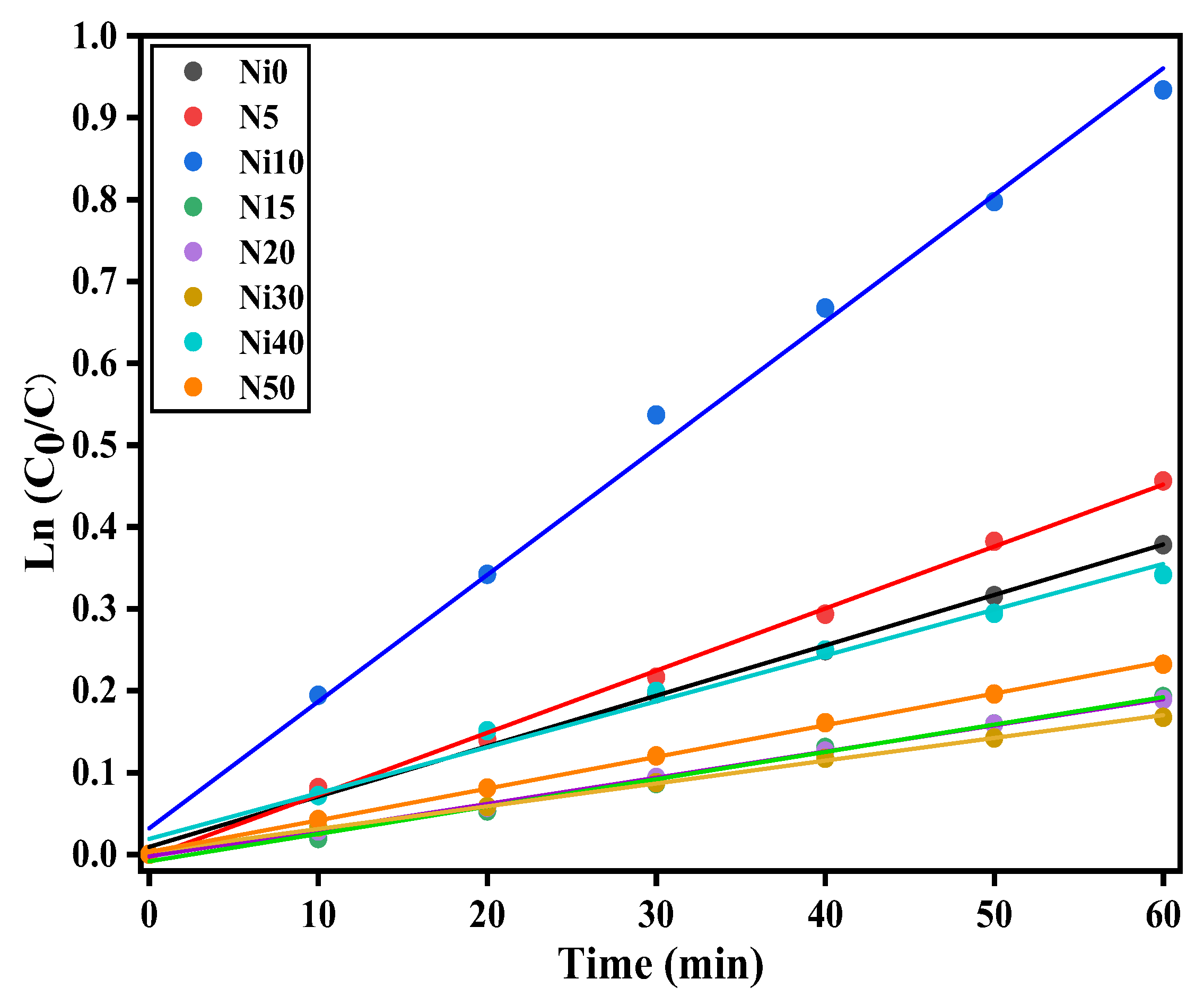
3.8. Photo-Fenton Catalytic Ability of α-NixFe1-xOOH NPs
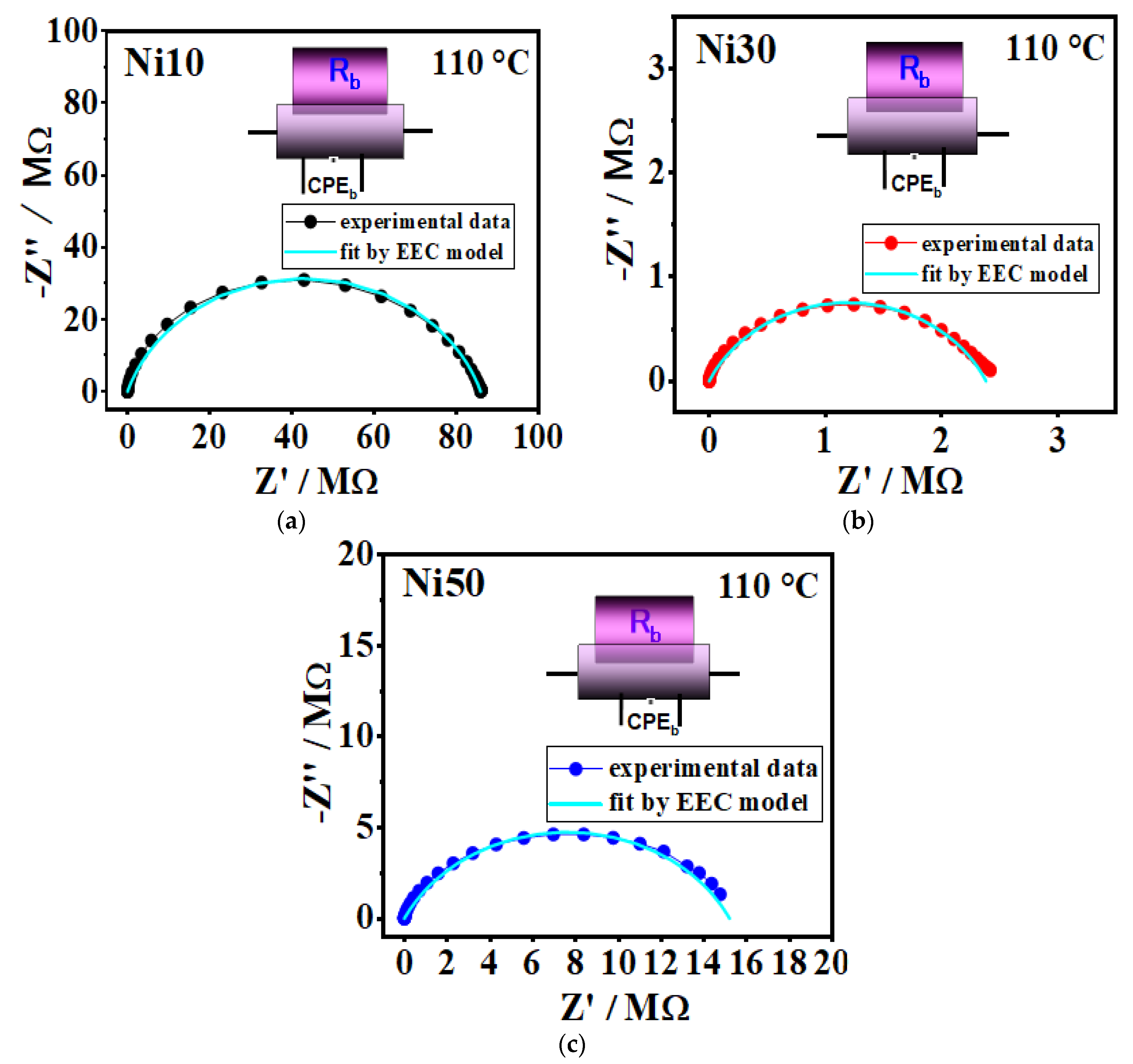
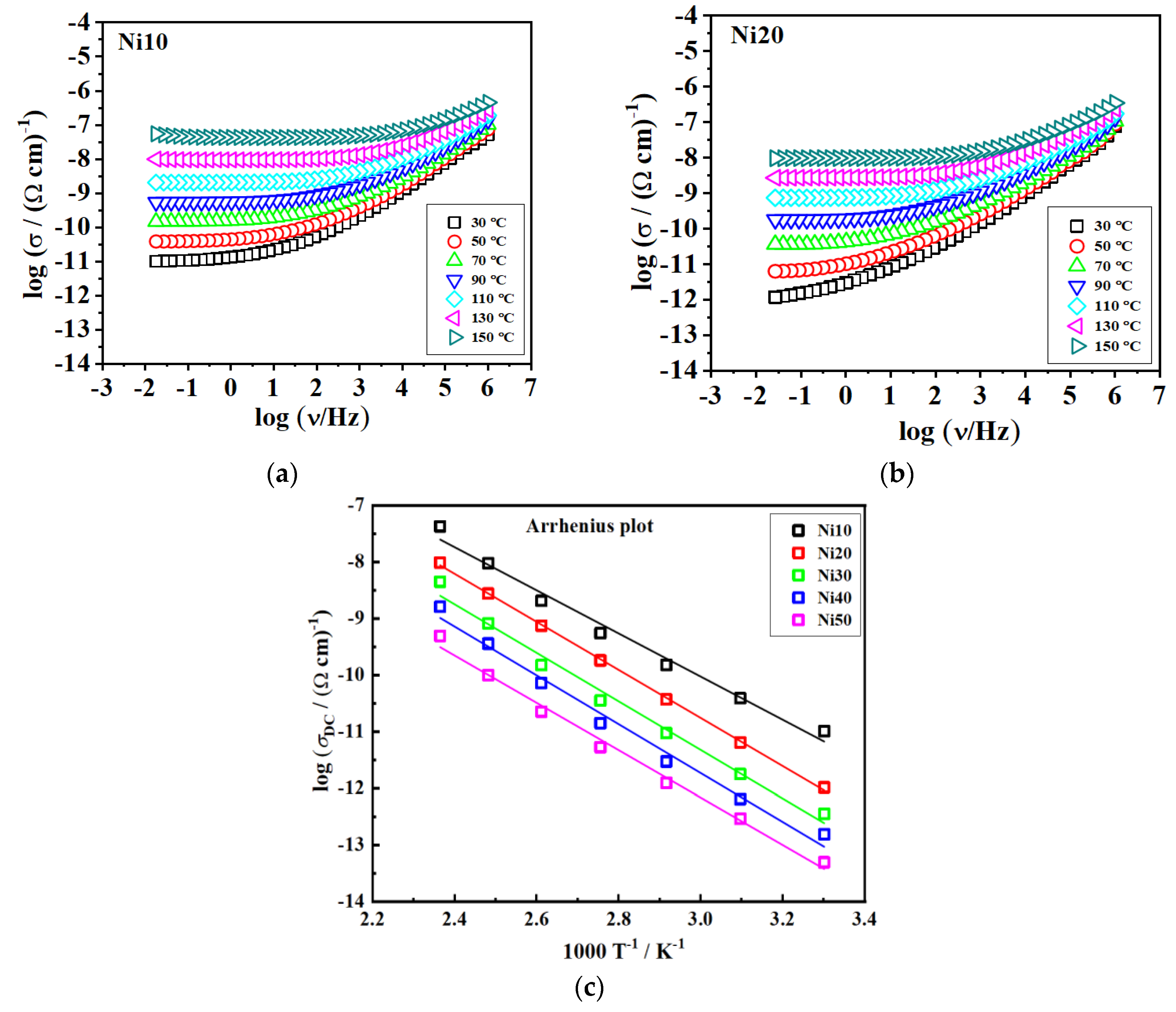
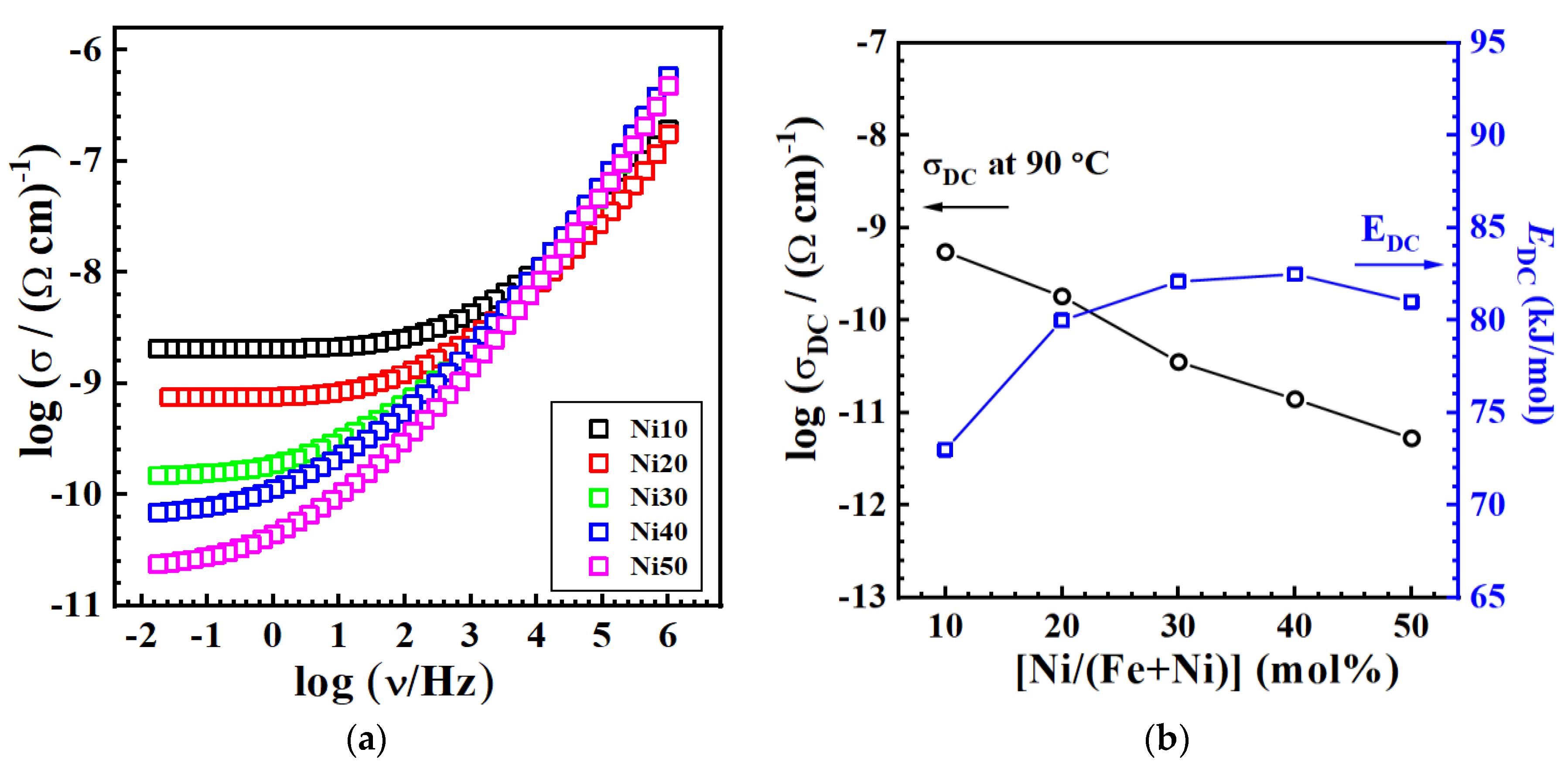
3.9. Electrical Properties - Impedance Spectra and DC Conductivity
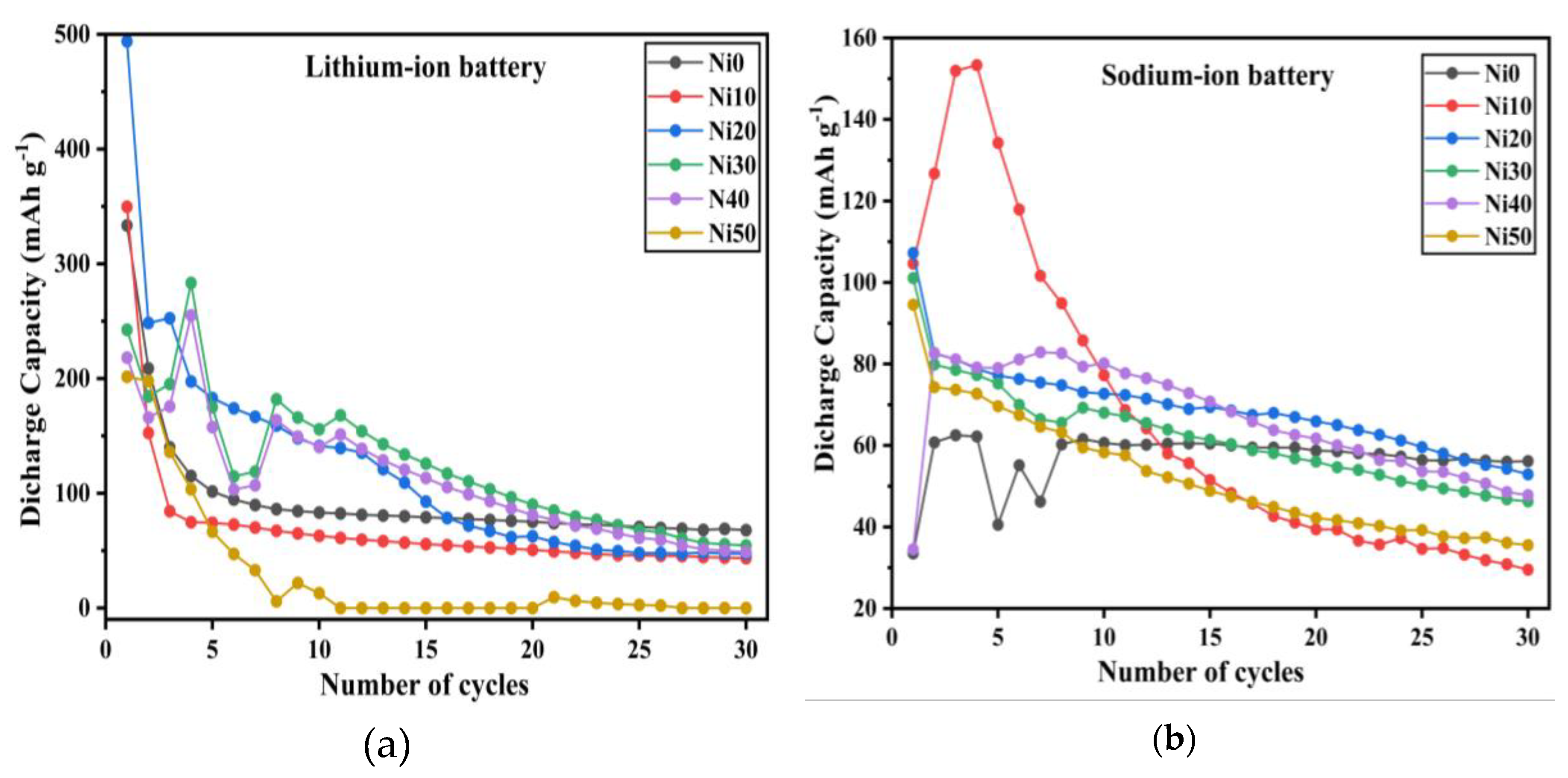
3.10. The Electrochemical Properties of α-NixFe1-xOOH NPs/Li- and Na-Ion Batteries
3. Materials and Methods
3.1. Preparation of α-NixFe1-xOOH NPs
3.2. Structural Characterization
3.3. Photocatalytic Activity
3.4. Solid-State Impedance Spectroscopy (SS-IS) of α-NixFe1-xOOH NPs
2.5. Preparation of SIB Containing α-NixFe1-xOOH NPs Cathode
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alangari, A.; Alqahtani, M. S.; Mateen, A.; Kalam, M. A.; Alshememry, A.; Ali, R.; Kazi, M.; Ghamdi, K. M. A.; Syed, R. Iron oxide nanoparticles: preparation, characterization, and assessment of antimicrobial and anticancer activity. Adsorp. Sci. Technol. 2022, 9, 1562051. [Google Scholar] [CrossRef]
- Devi, H. S.; Singh, T. D. Iron oxide nanoparticles synthesis through a benign approach and its catalytic application. Perspect. Sci. 2016, 8, 287–289. [Google Scholar] [CrossRef]
- Tharani, K.; Christy, A. J.; Sagadevan, S.; Nehru, L. C. Photocatalytic and antibacterial performance of iron oxide nanoparticles formed by the combustion method. Chem. Phys. Lett. 2021, 771, 138524. [Google Scholar] [CrossRef]
- Wu, W.; Xiao, X.; Zhang, S.; Ren, F.; Jiang, C. Facile method to synthesize magnetic iron oxides/TiO2 hybrid nanoparticles and their photodegradation application of methylene blue. Nanoscale Res. Lett. 2011, 533, 1–15. [Google Scholar] [CrossRef]
- Tao, R.; Qu, M.; Zhang, S.; Quan, F.; Zhang, M.; Shen, W.; Mei, Y. Preparation of FeOOH supported by melamine sponge and its application for efficient phosphate removal. J. Environ. Chem. Eng. 2022, 10, 108064. [Google Scholar] [CrossRef]
- Zhong, Z.; Li, R.; Lin, W.; Xu, X.; Tian, X.; Lia, X.; Chen, X.; Kang, L. One-dimensional nanocrystals of cobalt perylene diimide polymer with in situ generated FeOOH for efficient photocatalytic water oxidation. Appl. Catal. B Environ. 2020, 260, 118–135. [Google Scholar] [CrossRef]
- Wang, L.; Nhat, T. N.; Zhang, Y.; Bi, Y.; Schmuki, P. Enhanced solar water splitting by swift charge separation in Au/FeOOH sandwiched single-crystalline Fe2O3 nanoflake photoelectrodes. Chem. Sus. Chem. 2017, 10, 2720–2727. [Google Scholar] [CrossRef]
- Huang, Y.; Xie, M.; Wang, Z.; Jiang, Y.; Huang, Q.; Bai, X.; Li, L.; Wu, F.; Chen, R. All-iron sodium-ion full-cells assembled via stable porous goethite nanorods with low strain and fast kinetics. Nano Energy. 2019, 60, 294–304. [Google Scholar] [CrossRef]
- Liu, H.; Zou, J.; Ding, Y.; Liu, B.; Wang, Y. Novel α-FeOOH corner-truncated tetragonal prisms: crystal structure, growth mechanism and lithium storage properties. J. Appl. Electrochem. 2019, 49, 657–669. [Google Scholar] [CrossRef]
- Pascariu, P.; Gherasim, C.; Airinei, A. Metal oxide nanostructures (MONs) as photocatalysts for ciprofloxacin degradation, Int. J. Mol. Sci. 2023, 24, 9564. [CrossRef]
- Krehula, S.; Ristić, M.; Petrović, Ž.; Krehula, L. K.; Mitar, I.; Musić, S. Effects of Cu doping on the microstructural, thermal, optical and photocatalytic properties of α-FeOOH and α-Fe2O3 1D nanoparticles. J. Alloys Compd. 2019, 802, 290–300. [Google Scholar] [CrossRef]
- Popov, N.; Krehula, S.; Ristić, M.; Kuzmann, E.; Homonnay, Z.; Bošković, M.; Stanković, D.; Kubuki, S.; Musić, S. Influence of Cr doping on the structural, magnetic, optical and photocatalytic properties of α-Fe2O3 nanorods. J. Phys. Chem. Solids. 2021, 148, 109699. [Google Scholar] [CrossRef]
- Kobzi, B.; Watanabe, Y.; Akiyama, K.; Kuzmann, E.; Homonnay, Z.; Krehula, S.; Ristić, M.; Nishida, T.; Kubuki, S. 57Fe-Mössbauer study and methylene blue decomposing effect of nanoparticle mixtures composed of metallic iron and maghemite. J. Alloys Compd. 2017, 722, 94–100. [Google Scholar] [CrossRef]
- Zhang, B.; Khan, I.; Nagase, Y.; Ali, A. S.; Krehula, S.; Risti´c, M.; Music, S.; Kubuki, S. Highly covalent FeIII-O bonding in photo-Fenton active Sn-doped goethite nanoparticles, Mater. Chem. Phys. 2022, 287, 126247. [Google Scholar] [CrossRef]
- Darehnaranji, K.; Taghizadeh, S. M.; Mirzaei, E.; Berenjian, A.; Ebrahimi Nezhad, A. Size tuned synthesis of FeOOH nanorods toward self-assembled nanoarchitectonics. Langmuir. 2021, 37, 115–123. [Google Scholar] [CrossRef]
- Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Gerth, J. Unit-cell dimensions of pure and trace metal-associated goethites. Geochim. Cosmochim. Acta. 1990, 54, 363–371. [Google Scholar] [CrossRef]
- Ishikawa, T.; Nagashima, A.; Kandori, K. Structure of nickel-doped α-FeOOH. J. Mater. Sci. 1991, 26, 6231–6236. [Google Scholar] [CrossRef]
- Cornell, R. M.; Schneider, W.; Giovanoli, R. The effect of nickel on the conversion of amorphous iron (III) hydroxide into more crystalline iron oxides in alkaline media. J. Chem. Technol. Biot. 1992, 53, 73. [Google Scholar] [CrossRef]
- Popov, N.; Ristić, M.; Robić, M.; Gilja, V.; Krehula, L. K.; Musić, S.; Krehula, S. Synthesis and properties of Sn-doped α-FeOOH nanoparticles. Chem. Papers. 2021, 75, 6355–6366. [Google Scholar] [CrossRef]
- Cheng, W.; Lindholm, J.; Holmboe, M.; Luong, N. T.; Shchukarev, A.; Ilton, E. S.; Hanna, K.; Boily, J. F. Nanoscale Hydration in Layered Manganese Oxides. Langmuir. 2021, 37, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Krehula, S.; Ristić, M.; Mitar, I.; Wu, C.; Li, X.; Jiang, L.; Wang, J.; Sun, G.; Zhang, T.; Perović, M.; Bošković, M.; Antić, B.; Musić, S. Synthesis and Properties of Ni-doped Goethite and Ni-doped Hematite Nanorods. Croat. Chem. Acta. 2018, 91, 389–401. [Google Scholar] [CrossRef]
- Sayyed, M. I.; Abdo, M. A.; Ali, H. E.; Ahmed, H. A.; Sadeq, M.S. Influence NiO on the structure, elastic properties and buildup factor of BaO-CdO-borosilicate glasses. Phys. Scr. 2023, 98, 075937. [Google Scholar] [CrossRef]
- Ibrahim, A.; Farag, M. A.; Sadeq, M.S. Towards highly transparent tungsten zinc sodium borate glasses for radiation shielding purposes. Ceram. Int. 2022, 48, 12079–12090. [Google Scholar] [CrossRef]
- Aoyagi, T.; Fujieda, T.; Toyama, T.; Kono, K.; Takamatsu, D.; Hirano, T.; Naito, T.; Hayashi, Y.; Takizawa, H. Electrochemical properties and in-situ XAFS observation of Li2O-V2O5-P2O5-Fe2O3 quaternary-glass and crystallized-glass cathodes. J. Non-Cryst.Solids. 2016, 453, 28–35. [Google Scholar] [CrossRef]
- Ibrahim, A.; Kubo, K.; Watanabe, S.; Shiba, S.; Khan, I.; Zhang, B.; Homonnay, Z.; Kuzmann, E.; Pavić, L.; Santić, A.; Ali, A.S.; Hassaan, M. Y.; Kubuki, S. Enhancement of electrical conductivity and thermal stability of Iron- or Tin- substituted vanadate glass and glass-ceramics nanocomposite to be applied as a high-performance cathode active material in sodium-ion batteries. J. Alloys Compd. 2023, 930, 167366. [Google Scholar] [CrossRef]
- Latif, C.; Negara, V. S. I.; Wongtepa, W.; Thamatkeng, P.; Zainuri, M.; Pratapa, S. Fe K-Edge X-ray absorption near-edge spectroscopy (XANES) and X-ray diffraction (XRD) analyses of LiFePO4 and its base materials, J. Phys.: Conf. Series. 2018, 985, 12021. [Google Scholar] [CrossRef]
- Li, W.; Li, F.; Yang, H.; Wu, X.; Zhang, P.; Shan, Y.; Sun, L. A bio-inspired coordination polymer as outstanding water oxidation catalyst via second coordination sphere engineering. Nat. Commun. 2019, 10, 5074. [Google Scholar] [CrossRef]
- Ibrahim, A.; Arita, Y.; Ali, A. S.; Khan, I.; Zhang, B.; Razum, M.; Pavić, L.; Santić, A.; Homonnay, Z.; Kuzmann, E.; Hassaan, M. Y.; Wang, J.; Kubuki, S. Impact of adding Fe ions on the local structure and electrochemical performance of P2O5-V2O5 glass and glass ceramics used as a cathode in LIBs. J. Phys. Chem. Solids. 2023, 179, 111391. [Google Scholar] [CrossRef]
- M. Ismail, A. S.; Torregrosa, I. G.; Vollenbroek, J. C.; Folkertsma, L.; Bomer, J. G.; Haarman, T.; Ghiasi, M.; Schellhorn, M.; Nachtegaal, M.; Odijk, M.; Berg, A.; Wechuysen, B. M.; Groot, F. M. Detection of spontaneous FeOOH formation at the hematite/Ni (Fe)OOH interface during photoelectrochemical water splitting by operando X-ray absorption spectroscopy. ACS catalysis. 2021, 19, 12324–12335. [CrossRef]
- Chang, G.; Zhou, Y.; Wang, J.; Zhang, H.; Yan, P.; Wu, H. B.; Yu, X.Y. Dynamic reconstructed RuO2/NiFeOOH with coherent interface for efficient seawater oxidation. Small. 2023, 19, 2206768. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Liu, Q.; Liu, J.; Liu, S.; Liu, X.; Zheng, L.; Shang, J.; Yu, R.; Shui, J. Iron atom-cluster interactions increase activity and improve durability in Fe-N-C fuel cells. Nat. Commun. 2022, 13, 2963. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Zhang, B.; Matsuda, K.; Bingham, P. A.; Kitajou, A.; Inoishi, A.; Okada, S.; Yoshioka, S.; Nishida, T.; Homonnay, Z.; Kuzmann, E.; Kubuki, S. Development of electrically conductive ZrO2-CaO-Fe2O3-V2O5 glass and glass-ceramics as a new cathode active material for Na-ion batteries with high performance. J. Alloys Compd. 2022, 899, 163309. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Y.; Dong, J.; He, C. T.; Yin, H.; An, P.; Zhao, K.; Zhang, X.; Gao, C.; Zhang, L.; Lv, J.; Wang, J.; Zhang, J.; Khattak, A. M.; Khan, N. A.; Wei, Z.; Zhang, J.; Liu, S.; Zhao, H.; Tang, Z. Ultrathin metal-organic framework nanosheets for electrocatalytic oxygen evolution. Energy. 2016, 1, 16184. [Google Scholar] [CrossRef]
- Jia, C.; Zhen, C.; Yin, L.; Zhu, H.; Du, P.; Han, A.; Liu, G.; Cheng, H. M. Topologic transition-induced abundant undercoordinated Fe active sites in NiFeOOH for superior oxygen evolution. Nano Energy. 2023, 106, 108044. [Google Scholar] [CrossRef]
- Pavlenko, V.; Khosravi, S.; Żółtowska, S.; Haruna, A. B.; Zahid, M.; Mansurov, Z.; Supiyeva, Z.; Galal, A.; Ozoemena, K. I.; Abbas, Q.; Jesionowski, T. A comprehensive review of template-assisted porous carbons: Modern preparation methods and advanced applications. Mater. Sci. Eng. R Rep. 2022, 149, 1006820. [Google Scholar] [CrossRef]
- Alothman, Z. A. A Review: Fundamental aspects of silicate mesoporous materials. Materials. 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Barsotti, E.; Tan, S. P.; Piri, M.; Chen, J.H. Capillary-condensation hysteresis in naturally-occurring nanoporous media. Fuel. 2020, 263, 116441. [Google Scholar] [CrossRef]
- Blaker, C.; Muthmann, J.; Pasel, C.; Bathen, D. Characterization of activated carbon adsorbents -state of the art and novel approaches. A Chem. Bio. Eng. Rev. 2019, 6, 119–138. [Google Scholar] [CrossRef]
- Jr, S. L.; Segundo, I. R.; Freitas, E.; Vasilevskiy, M.; Carneiro, J.; Tavares, C. J. Use and misuse of the Kubelka-Munk function to obtain the band gap energy from diffuse reflectance measurements. Solid State Commu. 2022, 341, 114573. [Google Scholar] [CrossRef]
- Ibrahim, A.; Sadeq, M. S. Influence of cobalt oxide on the structure, optical transitions and ligand field parameters of lithium phosphate glasses. Ceram. Int. 2021, 47, 28536–28542. [Google Scholar] [CrossRef]
- Farag, M. A.; Ibrahim, A.; Hassaan, M. Y.; Ramadan, R. M. Enhancement of structural and optical properties of transparent sodium zinc phosphate glass-ceramics nanocomposite. J. Aust. Ceram. Soc. 2022, 58, 653–661. [Google Scholar] [CrossRef]
- Schumacher, B.; Bach, H.; Spitzer, P.; Obrzut, J. Electrical properties. In Springer Handbook of Materials Measurement Methods; Springer: Berlin, Heidelberg, 2011; pp. 431–484. [Google Scholar] [CrossRef]
- Farouk, M.; Samir, A.; Ibrahim, A.; Farag, M. A.; Solieman, A. Raman, FTIR studies and optical absorption of zinc borate glasses containing WO3. Appl. Phys. A. 2020, 126, 696. [Google Scholar] [CrossRef]
- Wang, H.; Peng, L.; Li, G.; Zhang, W.; Liang, Z.; Zhao, H.; An, T. Photocatalytic ozonation inactivation of bioaerosols by NiFeOOH nanosheets in situ grown on nickel foam. Appl. Catal. B: Environ. 2023, 324, 122273. [Google Scholar] [CrossRef]
- Guskos, N.; Papadopoulos, G. J.; Likodimos, V.; Patapis, S.; Yarmis, D.; Przepiera, A.; Przepiera, K.; Majszczyk, J.; Typek, J.; Wabia, M.; Aidinis, K.; Drazek, Z. Photoacoustic, EPR and electrical conductivity investigations of three synthetic mineral pigments: hematite, goethite and magnetite. Mater. Res. Bull. 2002, 37, 1051–1061. [Google Scholar] [CrossRef]
- Pavić, L.; Skoko, Ž.; Gajović, A.; Su, D.; Milanković, A. M. Electrical transport in iron phosphate glass-ceramics. J. Non-Cryst. Solids. 2018, 502, 44–53. [Google Scholar] [CrossRef]
- Šantić, A.; Banhatti, R. D; Pavić, L.; Ertap, H.; Yüksek, M.; Karabulut, M.; Milanković, A. M. Polaronic transport in iron phosphate glasses containing HfO2 and CeO2. Phys. Chem. Chem. Phys. 2017, 19, 3999–4009. [Google Scholar] [CrossRef]
- Milanković, A. M.; Pavić, L.; Ertap, H.; Karabulut, M. Polaronic mobility in boron doped iron phosphate glasses: Influence of structural disorder on summerfield scaling. J. Am. Ceram. Soc. 2012, 95, 2007–2014. [Google Scholar] [CrossRef]
- Pavić, L.; Sklepić, K.; Skoko, Z.; Tricot, G.; Mosner, P.; Koudelka, L.; Milanković, A. M. Ionic conductivity of lithium germanium phosphate glass-ceramics. J. Phys. Chem. C. 2019, 123, 23312–23322. [Google Scholar] [CrossRef]
- Alexandrov, V.; Rosso, K. M. Electron transport in pure and substituted iron oxyhydroxides by small-polaron migration. J. Chem. Phys. 2014, 140, 234701. [Google Scholar] [CrossRef]
- Porter, I. J.; Cushing, S. K.; Carneiro, L. M.; Lee, A.; Ondry, J. C.; Dahl, J. C.; Chang, H. T.; Alivisatos, A. P.; Leone, S. R. Photoexcited small polaron formation in goethite (α-FeOOH) nanorods probed by transient extreme ultraviolet spectroscopy. J. phys. Chem.lett. 2018, 9, 4120–4124. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Sun, M.; Wang, Q.; Jin, J.; Sui, J.; Liu, C.; Gao, C. Pressure-induced mixed protonic-electronic to pure electronic conduction transition in goethite. J. Phys. Chem. C. 2021, 125, 2713–2718. [Google Scholar] [CrossRef]
- Beda, A.; Vaulot, C.; Rabuel, F.; Morcrette, M.; Ghimbeu, C. M. The role of specific and active surface areas in optimizing hard carbon irreversible capacity loss in sodium ion batteries. Energy Adv. 2022, 1, 185–190. [Google Scholar] [CrossRef]
- Wrogemann, M.; Fromm, O.; Deckwirth, F.; Beltrop, K.; Heckmann, A.; Winter, M.; Placke, T. Impact of degree of graphitization, surface properties and particle size distribution on electrochemical performance of carbon anodes for potassium-ion batteries. Batter. Supercaps. 2022, 5, 1–12. [Google Scholar] [CrossRef]
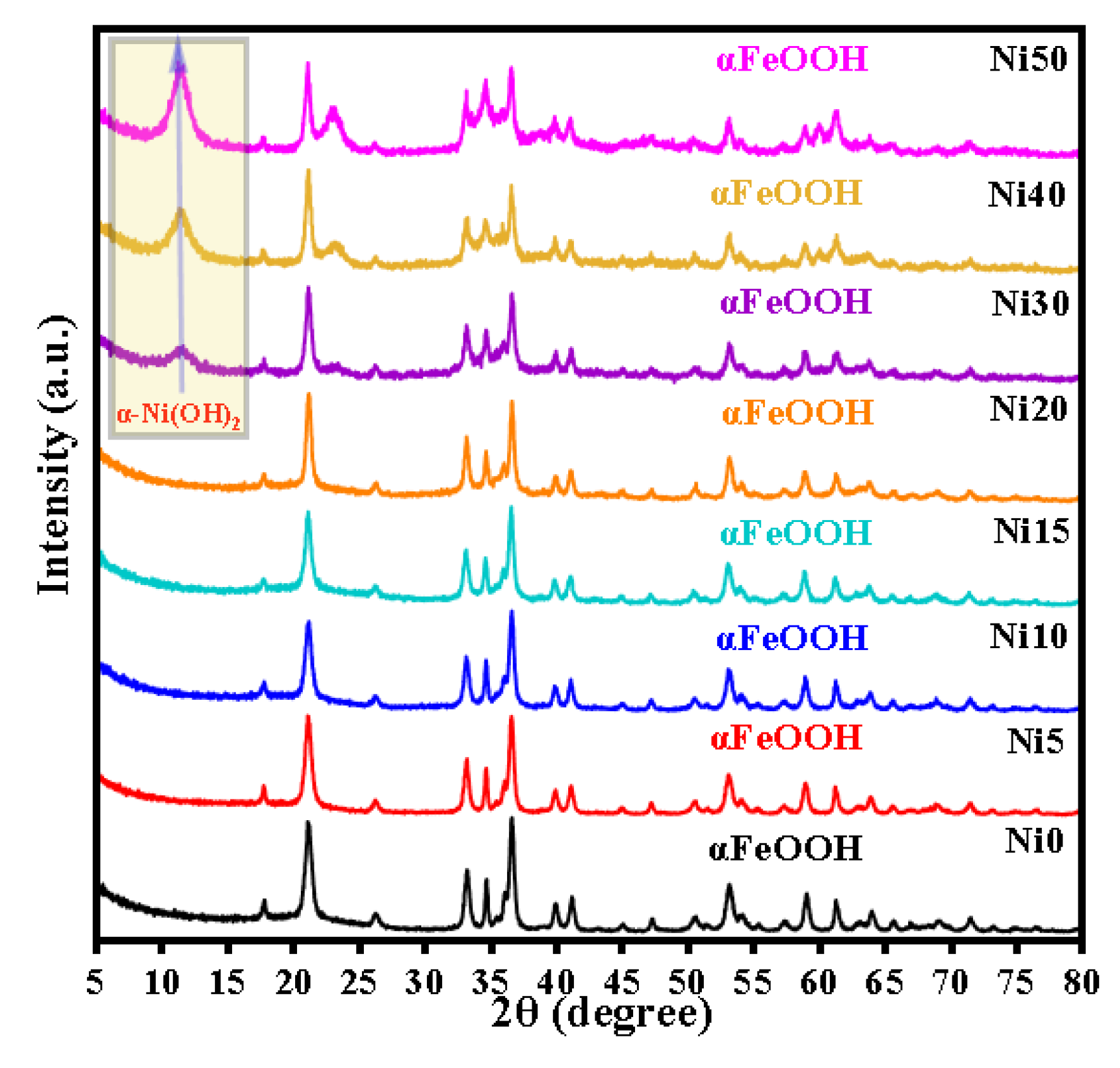
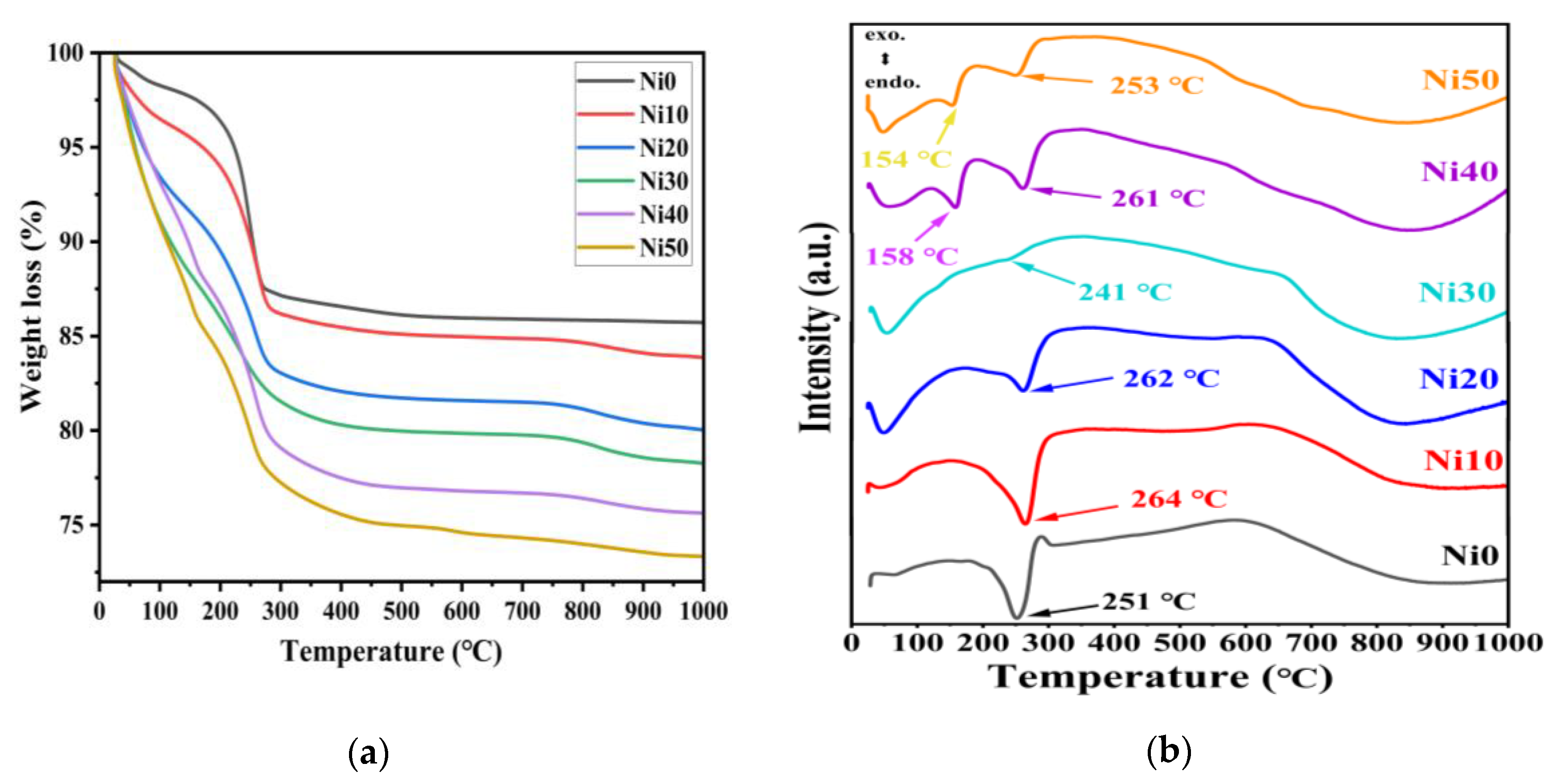
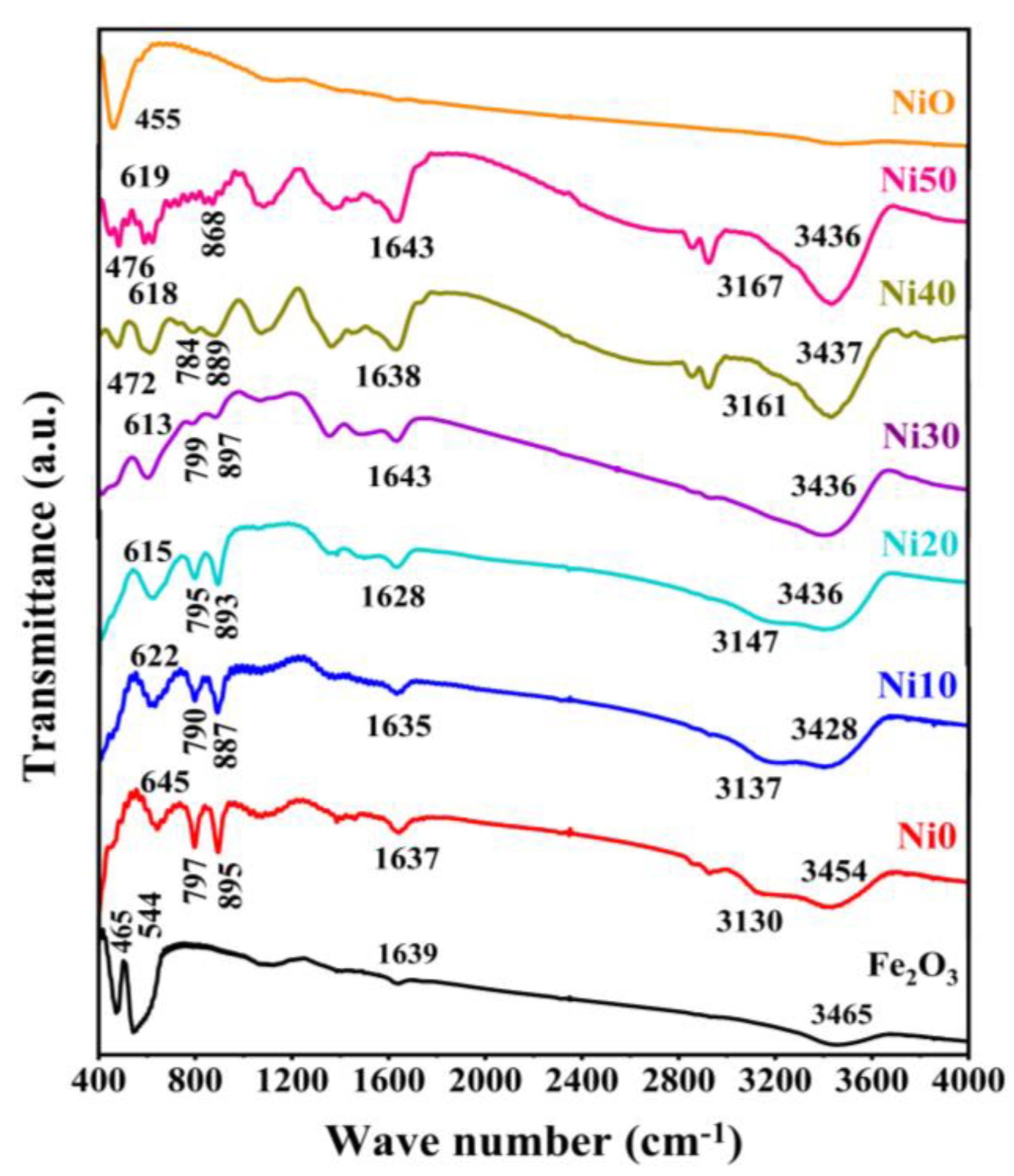
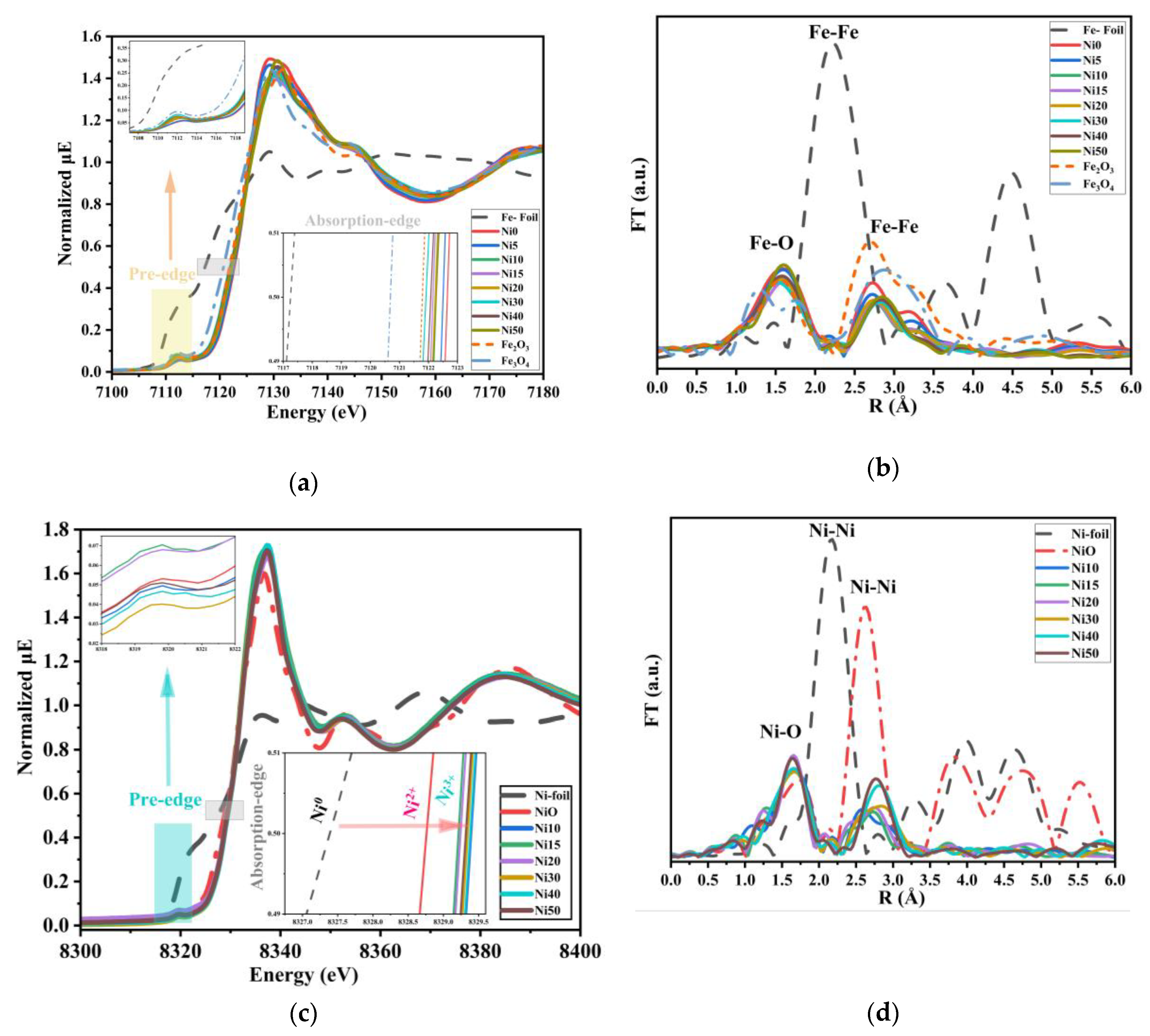
| sample | Major Crystalline Phase | Lattice parameters (Å) | V (Å3) |
FWHM (110) (deg.) |
d100 (Å) |
Space group | % of constituent phases | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Å) | (degree) | |||||||||||
| a | b | c | α | β | γ | |||||||
| Ni0 | FeOOH | 4.6215 | 9.9585 | 3.0249 | 90 | 90 | 90 | 143 (17) |
0.587 (7) |
4.1945 (4) |
Pbnm | 100 |
| Ni5 | FeOOH | 4.6223 (1) |
9.9565 (1) |
3.0260 (1) |
90 | 90 | 90 | 148 (19) |
0.570 (7) |
4.1957 (4) |
Pbnm | 100 |
| Ni10 | FeOOH | 4.6255 (1) |
9.9834 (2) |
3.0273 (1) |
90 | 90 | 90 | 148 (2) |
0.572 (8) |
4.1985 (5) |
Pbnm | 100 |
| Ni15 | FeOOH | 4.6255 (1) |
9.9999 (3) |
3.0289 (1) |
90 | 90 | 90 | 159 (4) |
0.530 (13) |
4.206 (2) |
Pnma | 100 |
| Ni20 | FeOOH | 4.6240 (1) |
9.9815 (2) |
3.0287 (1) |
90 | 90 | 90 | 194 (3) |
0.436 (7) |
4.1945 (5) |
Pnma | 100 |
| Ni30 | FeOOH | 4.6330 (1) |
9.9845 (2) |
3.0245 (1) |
90 | 90 | 90 | 168 (4) |
0.503 (12) |
4.184 (2) |
Pnma | 92.37 |
| αNi(OH)2 | 3.2006 (1) |
3.2006 (1) |
23.456 (5) |
90 | 90 | 120 | - | - | - | R3m:H | 7.63 | |
| Ni40 | FeOOH | 4.6247 (1) |
9.9792 (2) |
3.0268 (1) |
90 | 90 | 90 | 183 (4) |
0.461 (11) |
4.199 (19) |
Pnma | 91.93 |
| αNi(OH)2 | 3.1932 (1) |
3.1932 (1) |
23.445 (4) |
90 | 90 | 120 | - | - | - | R3m:H | 8.07 | |
| Ni50 | FeOOH | 4.6240 (1) |
9.9723 (2) |
3.0242 (1) |
90 | 90 | 90 | 173 (4) |
0.487 (12) |
4.199 (18) |
Pnma | 90.65 |
| αNi(OH)2 | 3.0972 (1) |
3.0972 (1) |
23.972 (7) |
90 | 90 | 120 | - | - | - | R3m:H | 9.35 | |
| Sample | Component |
A (%) |
δ (mms-1) |
Δ (mms-1) |
Γ (mms-1) |
Bhf (T) | Phase |
|---|---|---|---|---|---|---|---|
| Ni0 | sextet | 95.2 | 0.36±0.01 | -0.26±0.01 | 0.88±0.01 | 32.95 | Goethite |
| doublet | 4.8 | 0.53±0.01 | 1.73±0.01 | 0.19±0.01 | - | superparamagnetic | |
| Ni10 | sextet | 74.4 | 0.43±0.01 | -0.26±0.01 | 0.86±0.01 | 28.23 | Goethite |
| doublet | 25.6 | 0.34±0.01 | 0.71±0.01 | 0.78±0.02 | - | superparamagnetic | |
| Ni20 | sextet | 17.1 | 0.33±0.11 | -0.13±0.16 | 2.63±0.28 | 27.84 | Goethite |
| doublet | 82.9 | 0.38±0.01 | 0.65±0.01 | 0.55±0.01 | - | superparamagnetic | |
| Ni30 | sextet | 19.2 | 0.31±0.06 | -0.10±0.12 | 1.48±0.17 | 27.16 | Goethite |
| doublet | 80.8 | 0.38±0.01 | 0.64±0.01 | 0.53±0.01 | - | superparamagnetic | |
| Ni40 | doublet | 100 | 0.38±0.01 | 0.62±0.01 | 0.52±0.01 | - | superparamagnetic |
| Ni50 | doublet | 100 | 0.38±0.01 | 0.58±0.01 | 0.49±0.01 | - | superparamagnetic |
| Sample | Component |
A (%) |
δ (mms-1) |
Δ (mms-1) |
Γ (mms-1) |
Bhf (T) |
Phase |
|---|---|---|---|---|---|---|---|
| Ni20 | sextet | 2.61 | 0.25±0.01 | -0.10±0.01 | 0.33±0.26 | 27.72 | Goethite |
| doublet | 50.25 | 0.34±0.01 | 0.84±0.04 | 0.53±0.01 | - | amorphous | |
| doublet | 47.14 | 0.33±0.01 | 0.51±0.02 | 0.42±0.02 | - | Superparamagnetic | |
| Ni30 | sextet | 15.72 | 0.29±0.01 | -0.14±0.01 | 1.11±0.21 | 27.08 | Goethite |
| doublet | 51.81 | 0.33±0.01 | 0.79±0.02 | 0.48±0.01 | - | amorphous | |
| doublet | 32.46 | 0.33±0.01 | 0.45±0.01 | 0.37±0.01 | - | Superparamagnetic | |
| Ni40 | doublet | 42.27 | 0.33±0.01 | 0.85±0.03 | 0.46±0.01 | - | amorphous |
| doublet | 57.73 | 0.33±0.01 | 0.48±0.01 | 0.37±0.01 | - | Superparamagnetic | |
| Ni50 | doublet | 39.45 | 0.33±0.01 | 0.84±0.02 | 0.44±0.01 | - | amorphous |
| doublet | 60.55 | 0.33±0.01 | 0.47±0.01 | 0.35±0.01 | - | Superparamagnetic |
| sample | SSA (m2g-1) | bandgap (eV) | k / 10-3 min-1 |
|---|---|---|---|
| Ni0 | 45.1 | 2.71 | 6.2±0.1 |
| Ni5 | - | 2.65 | 7.6±0.1 |
| Ni10 | 73.9 | 2.55 | 14.6±0.6 |
| Ni15 | - | 2.43 | 3.3±0.1 |
| Ni20 | 174.0 | 2.47 | 3.2±0.1 |
| Ni30 | 145.0 | 2.06 | 2.8±0.1 |
| Ni40 | 96.3 | 2.13 | 5.6±0.1 |
| Ni50 | 117.3 | 2.15 | 3.95±0.2 |
| Glass | σDCa / (Ω cm)-1 | EDC/ kJmol-1 | σ0* / (Ω cm)-1 |
|---|---|---|---|
| Ni10 | 5.52×10-10 | 73.0 | 1.42 |
| Ni20 | 1.82×10-10 | 80.4 | 1.99 |
| Ni30 | 3.56×10-11 | 82.1 | 1.55 |
| Ni40 | 1.41×10-11 | 82.5 | 1.22 |
| Ni50 | 5.30×10-12 | 80.5 | 0.39 |
| sample | 5 mAh g-1 | 50 mA mAh g-1 | ||
|---|---|---|---|---|
| Discharge capacity (mAh g-1) | Capacity retention(%) | Discharge capacity (mAh g-1) | Capacity retention(%) | |
| Ni0 | 1069 | 0.4 | 333 | 20.3 |
| Ni10 | 363 | 7.4 | 350 | 12.4 |
| Ni20 | 271 | 0.4 | 494 | 9.6 |
| Ni30 | 250 | 0.9 | - | - |
| sample | 5 mAh g-1 | 50 mA mAh g-1 | ||
|---|---|---|---|---|
| Discharge capacity (mAhg-1) | Capacity retention(%) | Discharge capacity (mAhg-1) | Capacity retention(%) | |
| Ni0 | 110 | 31.7 | - | - |
| Ni10 | 116 | 21.6 | - | - |
| Ni20 | 223 | 12.7 | 107 | 49.4 |
| Ni30 | 189 | 21.3 | 101 | 45.8 |
| Ni50 | 202 | 27.7 | 95 | 37.6 |
| Sample code | [Fe] (mol. L-1) |
[Ni] (mol. L-1) |
|
|---|---|---|---|
| Ni0 | 0.100 | 0 | 0 |
| Ni5 | 0.095 | 0.005 | 5 |
| Ni10 | 0.090 | 0.010 | 10 |
| Ni15 | 0.085 | 0.015 | 15 |
| Ni20 | 0.080 | 0.020 | 20 |
| Ni30 | 0.070 | 0.030 | 30 |
| Ni40 | 0.060 | 0.040 | 40 |
| Ni50 | 0.050 | 0.050 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





