1. Introduction
The COVID-19 global pandemic was an unprecedented health emergency. A stable public health control measure for early containment of an emerging outbreak of directly transmitted infections involves early detection of infected persons, the isolation of infected cases as well as the tracing, testing and quarantine of their contacts. [
1] Non pharmaceutical interventions such as personal preventive actions (e.g.: hand washing, face covers), environmental cleaning, physical distancing, stay-at-home orders, school and venue closures, and workplace restrictions have been adopted nationally and internationally. However, vaccination remains the most efficient and effective control strategy against COVID-19. The implementation of widespread vaccination programs was required to curtail the pandemic, with various countries enacting vaccination programs with varying success. These responses also evolved temporally because of new variants, emerging epidemiologic data, and information from past measures and from other countries.
Global vaccine development efforts have been accelerated in response to the devastating COVID-19 pandemic, like accelerated evaluation procedures and authorization for emergency use. Several pharmaceutical companies were trying to develop an effective vaccine against SARS-CoV-2. Phase III trials reported high vaccine effectiveness (VE) against severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection with (70.4%) for Oxford-AstraZeneca (ChAdOx1 nCoV-19 vaccine),[
2] (95.0%) effectiveness with Pfizer BioNTech (BNT162b2 mRNA COVID-19 vaccine),[
3] and (94.1%) with Moderna (mRNA-1273 vaccine)[
4].
Qatar was among the first Gulf Cooperation Council (GCC) countries to procure COVID-19 vaccines and start the COVID-19 vaccination campaign nationwide for the citizens, and residents. The COVID-19 vaccine program was developed by the National Strategic Committee and implemented by the Health Protection and Communicable Diseases Division (HP-CDC) of the Ministry of Public Health (MOPH) along with Hamad Medical Corporation (HMC) and Primary Health Care Corporation (PHCC). Vaccines were provided free of cost to all nationals and residents of Qatar through the public healthcare system and mass vaccination centers such as Qatar National Convention Centre (QNCC), VCIA (Vaccination Center Industrial Area), and Qatar Vaccination Centre (QVC). The primary focus or target groups were the high-risk groups, namely frontline health care workers, those with chronic illnesses, the elderly population, and teachers; later, the workers living in close proximities and dormitories were focused through VCIA and QNCC. The mass vaccination campaigns helped to effectively increase the vaccine coverage for COVID-19 in Qatar, which helped reduce the Delta variant transmission in the community.
In Qatar, vaccination commenced on 23rd December 2020, primarily with the Pfizer BNT162b2 mRNA vaccine and later Moderna mRNA-1273 being introduced later. Four COVID-19 vaccines, namely Pfizer, Moderna, AstraZeneca and Jansen & Jansen, were approved in Qatar. A few others, like Sinopharm, Sputnik, and Sinovac, were conditionally approved. Qatari residents were mostly vaccinated with BNT162b2 (Pfizer-BioNTech) vaccine or the mRNA-1273 (Moderna) vaccine. Pfizer-BioNTech (BNT162b2) and Moderna (mRNA-1273) mRNA-based vaccines are given as two doses scheduled 3-4 weeks apart, keeping a minimum of 15 days between the two doses. As of 19th September 2021, it was estimated that over 80 percent of Qatar’s resident population had received two doses of either the BNT162b2 (Pfizer-BioNTech) vaccine or the mRNA-1273 (Moderna) vaccine. [
3,
4,
5]
Qatar has experienced five SARS-CoV-2 waves, dominated sequentially by the index virus,[
6] the B.1.1.7 (alpha) variant,[
7] the B.1.351 (beta) variant,[
2] the B.1.1.529 (omicron) subvariants BA.1 and BA.2,[
3] and the omicron subvariants BA.4 and BA.5,[
4] in addition to a prolonged low-incidence phase dominated by the B.1.617.2 (delta) variant.[
5] Community transmission of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) due to the Delta variant (B.1.617.2) was first identified in Qatar by the end of March 2021.[
6,
8,
9] Although Delta variant incidence increased and hovered at about 200 cases per day in the summer of 2021, it remained low compared to earlier variants incidences. Between 23rd of March 2021 and 7th of September 2021, 43.0% of diagnosed infections were Delta variant infections. [
2,
6] Delta variant dominance was, however, preceded by two consecutive SARS-CoV-2 Alpha (B.1.1.7) and Beta (B.1.351) waves earlier in 2021. [
2,
6,
7,
8,
9] The first Omicron variant infection in Qatar was identified on November 24, 2021. Within four weeks, it became the predominant strain.[
10]
The effectiveness against Alpha and Beta variants was high, over 75 percent in Qatar. [
8,
11,
12,
13] Whereas the effectiveness against Delta variant infection, 7 or more days after the second dose was (55.5%) (95% CI, 51.2–59.4%), irrespective of the vaccine type and 51.9 % (95% CI, 47.0–56.4%) with BNT162b2, 73.1 % (95% CI, 67.5–77.8%) with mRNA-1273 specifically. The protection is higher 14 days after the second dose of the primary series. Delta variant’s effectiveness was evaluated several months after the second dose for the residents.[
14]
While children tend to experience less symptomatic Covid-19 than adults, it's important to note that schools, youth sports, and other community events can still contribute to outbreaks and transmission. These settings can pose a significant risk even with high adult immunization rates.[
15] Vaccinating adolescents can enable them to safely return to in-person learning and reintegrate into society, addressing the debilitating mental health consequences of the Covid-19 pandemic. [
16,
17] Reducing Covid-19-related morbidity and mortality in adolescents can be achieved by administering a safe and effective vaccine. Additionally, the availability of effective vaccines for adolescents is crucial in decreasing the reservoir of SARS-CoV-2. In line with this, the BNT162b2 vaccine has received emergency use authorization for adolescents aged 12 to 15.[
18]
The World Health Organization's (WHO) Strategic Advisory Group of Experts (SAGE) on Immunization updated the roadmap for prioritizing COVID-19 vaccines on 21st January 2021. Children and adolescents with comorbidities are identified as medium-priority population groups for administering the primary series and booster doses. In contrast, healthy children and adolescents are the low-priority use group because of their low risk of severe disease, hospitalization, and fatality. European Union countries recommend primary vaccination against COVID-19 for 12-17-year-olds.[
5]
Centers for Disease Control and Prevention (CDC) recommends that adolescents aged 12-17 should receive a booster dose five months after completing the primary vaccination series. This decision was based on safety data following the administration of over 25 million vaccine doses in adolescents and data showing that booster doses strengthen protection against the multiple variants of COVID-19.[
19] The decision to expand the administration of booster doses to low-priority-use groups helped to improve the waning immunity and vaccine coverage.[
20]
Ministry of Public Health (MOPH) Qatar approved Pfizer-BioNTech (BNT162b2) COVID-19 vaccine administration to children and adolescents, based on regional and global studies showing its safety and efficacy in this age group.[
8,
9] The two-dose primary series of BNT162b2 (Pfizer-BioNTech) COVID-19 vaccine against SARS-CoV-2 infection has been approved for use in 10µg formulations among children and adolescents aged 12-17 years as of May 16, 2021 and 30µg formulations among children aged 5-11 years as of January 30, 2022. It is mandated by the MOPH that all vaccination details are entered on a real time basis in the National Vaccine Registry (SAVES) by the providers.
The primary aim of this study was to assess the real-world effectiveness of the 30μg dose of BNT162b2 COVID-19 vaccine against infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) among children and adolescents aged 12-17 years, in Qatar, before the emergence of the omicron variant.
2. Materials and Methods
Study Design: A test negative matched case-control study design. [
21,
22,
23]
Study population: Children and adolescents aged 12-17 years, residing in Qatar, who had undergone COVID-19 tests using reverse transcription-polymerase chain reaction (RT-PCR) for SARS-CoV-2 on nasopharyngeal swabs (NPS) or oropharyngeal swabs (OPS) as part of contact tracing, between June 1st and November 30th, 2021. Therefore, ensured that the first batch of children/adolescents would have received both doses of the primary series and excluded the Omicron variant-positive cases from the analysis. RT-PCR testing detects SARS-CoV-2 RNA at low levels, with analytic sensitivity of (98%) and specificity of (97%).[
24]
Cases: children/adolescents aged 12-17 years with positive test results on RT-PCR for SARS-CoV-2.
Controls: children/adolescents aged 12-17 years who had negative test results on RT-PCR for SARS-CoV-2, matched by calendar week for the RT-PCR tests.
2.1. Inclusion criteria
Any children and adolescents aged 12-17 years residing in Qatar tested for SARS-CoV-2 using RT-PCR between June 1st, 2021, and November 30th, 2021, were eligible irrespective of nationality, gender, and vaccination status.
Children and adolescents eligible to receive the Pfizer-BioNTech mRNA Covid-19 as per Ministry of Public Health (MOPH) guidelines.
2.2. Exclusion criteria
Children and adolescents who had undergone COVID-19 tests by RT-PCR for SARS-CoV-2 as part of screening or seeking healthcare, pre-travel mandate.
Children and adolescents tested for COVID-19 using a method other than RT-PCR.
Uncertainty about the COVID-19 test results, which includes ‘Inconclusive’ or if results were unavailable for any reason.
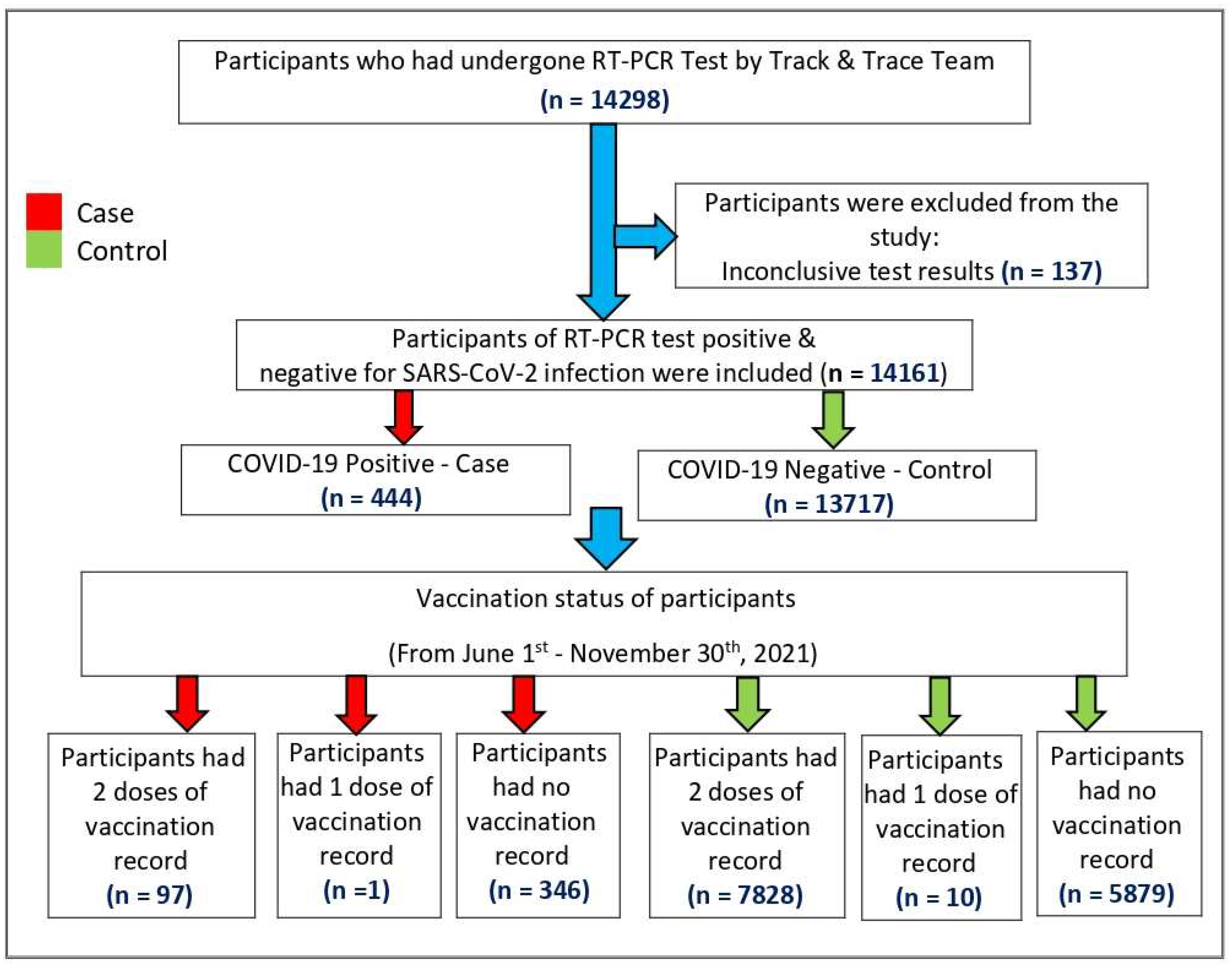
Sampling: A total of 14,298 children and adolescents aged 12-17 years who were tested for COVID-19 in the date range of the study, vaccinated or unvaccinated, were extracted from the National database. From this 137 had test result inconclusive and hence were removed, leaving us with 14,161 children and adolescents between 12 to 17 years irrespective of vaccination status, who were included in the study. The study population selection process has been illustrated in
Figure 1.
To adjust for underlying differences in the risk of exposure to different infection variants [
25,
26,
27,
28], cases and controls were matched by calendar week for the RT-PCR test. It was possible to find PCR-negative matches for most age groups due to the higher number of PCR-negative test results than PCR-positive results. Non-pharmaceutical interventions (NPI), including face masks, social distancing, hand washing, and hand hygiene, were mandated in Qatar during the study period, with varying levels of restrictions for the public as per MOPH guidelines. Cases and controls were matched in a 1:5 ratio to maximize statistical power.
Data Collection: COVID-19 case investigation teams receive a laboratory-confirmed list from various government and private sectors nationwide. Team members contact the index case by phone to obtain the necessary details and record the number and details of those he had close contact with in the last 48 hours. A close contact refers to anyone who has met someone infected with the COVID-19 virus, starting from 2 days before the onset of the infected person's illness up to 14 days after. Based on this information a swabbing dispatch list was prepared daily, which is then forwarded to the field team supervisor for action. The swab team successfully visited the home and workplaces of these confirmed cases and collected the necessary swabs from the close contacts enlisted. It is recommended to collect Nasopharyngeal and Oropharyngeal swabs in a single vial containing transport medium and only Oropharyngeal swabs for children below 14 years.
Nasopharyngeal and oropharyngeal swabs collected at Hamad Medical Corporation (HMC), Primary Health Care Corporation (PHCC), governmental & semi-governmental, or private health institutions across the country or even the field teams were placed in Universal Transport Medium (UTM), and the RT-PCR tests for SARS-CoV-2 were undertaken at the Hamad Medical Cooperation (HMC) National Virology Laboratory. The MOPH database, Surveillance and Vaccine Electronic System (SAVES), receives all real-time RT-PCR test results from the laboratory.[
10,
29] The data regarding COVID-19 laboratory testing, vaccination (which includes the types of vaccine and dates of the first and second doses of vaccine administration, place of immunization, expiry date of the vaccine, and the lot number), and associated demographic information were retrieved from the national integrated digital-health information platform, Surveillance and Vaccine Electronic System (SAVES), owned by the Ministry of Public Health (MOPH), Qatar. The vaccination details of the citizens, residents, and visitors who had been vaccinated abroad; were incorporated into the National Vaccine registry upon arrival in Qatar. [
30]
The study participants were divided into 3 categories based on vaccination status- fully vaccinated and immune (those who had completed 14 days after receiving the second dose of vaccine), fully vaccinated but not fully immune (those who had not completed 14 days after receiving the second dose of vaccine), partially vaccinated (participants who had received only one dose of vaccine) and unvaccinated (participants who had not received any dose of the vaccine).
Data Analysis: A total of 14,161 children and adolescents aged 12-17 years who were tested for COVID-19 in the date range of the study, vaccinated or unvaccinated, were included in the study. The case and control groups were described using frequency distribution. The effectiveness of the BNT162b2 COVID-19 vaccine among children and adolescents 12 to 17 years of age at least 14 days after receiving the second vaccine dose was estimated by calculating relative risk reduction (RRR). The median time gap between the first and second doses was 15 days. Subgroup analyses were conducted to investigate differences in VE of the COVID-19 vaccine according to age, gender, nationality (Qatari and non-Qatari) and vaccination status (fully vaccinated, partially vaccinated, unvaccinated). Subgroup analyses were also done to investigate the vaccine protection according to the number of days since receipt of the second dose of the vaccine.
3. Results
Table 1 shows the characterization of the 14,161 children and adolescents aged 12-17 years included in the study by age, gender, nationalities, and vaccination status. Majority (40.6%) were 12–13-year-old. The male: female proportions were nearly the same. Majority (60.9%) of the study participants were non-Qataris as expected from the population distribution of Qatar. 7925 (55.96%) were vaccinated with two doses of the BNT162b2 vaccine, and 6225 (43.96%) were unvaccinated. This higher proportion of vaccinated participants can be explained by the fact that as of May 31
st 2021, more than half of residents had received at least 1 dose and 41% had completed both doses.
Majority (65.8%) of the younger age group (12-13 years) were unvaccinated, while three-fourths (75.1%) of the 16–17-year-old were fully vaccinated. Hence,
Table 1 shows a significant (p-value <0.001) association between age and completion of the primary series of vaccines, that is as the age increases the proportion of vaccinated children increases. (
Table 1) This can be explained by the fact the older age group were enthusiastic to get vaccinated as this would give them the privilege to go out into malls and restaurants. There was no significant difference between the genders with regards to the vaccination status.
No significant difference in vaccination status was noted between the nationals and non-nationals. Similar proportion of nationals and non-nationals were vaccinated with at least one dose. This may be explained by the fact that the government provided COVID vaccines free of cost universally to both nationals and non-nationals.
According to the data presented in
Table 2, only 3.1% of the study population were infected and among the infected 44.14% were 12–13-year-old whereas only half this proportion (20.7%) of the older age group (16-17 years) were infected. The likelihood of testing positive for COVID-19 decreases with increasing age and vaccination status. This is in sync with the vaccination rates among the different age groups.
There was significant (<0.001) difference between the cases and controls with regards to nationality. More than half (53.4%) of the cases were non-Qataris in comparison to 46.6% Qataris. Similarly, a higher proportion of the control group were non-Qataris (61.2%). This can be explained by the population distribution of the residents of Qatar.
However, no significant difference between the cases and controls with regards to gender. The males: females’ proportion was similar in both case and control groups
Table 3 shows that out of 7925 fully vaccinated, only 97 (1.2%) tested positive for SARS CoV2 by RT-PCR, whereas 346 (5.6%) tested positive among the 6225 unvaccinated population. Among the case group, 21.8% were fully vaccinated while 77.9% were unvaccinated. Similarly, among the control group, 57.2% had received at least one dose of COVID vaccine, while 42.8% were unvaccinated. There is a significant difference in the proportion of fully vaccinated between the cases and controls (p<0.001).
Relative Risk Reduction (RRR) was calculated as: vaccinated among cases x unvaccinated among controls = 0.21 vaccinated among controls x unvaccinated among cases Vaccine Effectiveness (VE) = 1- RRR = 79.0 %.
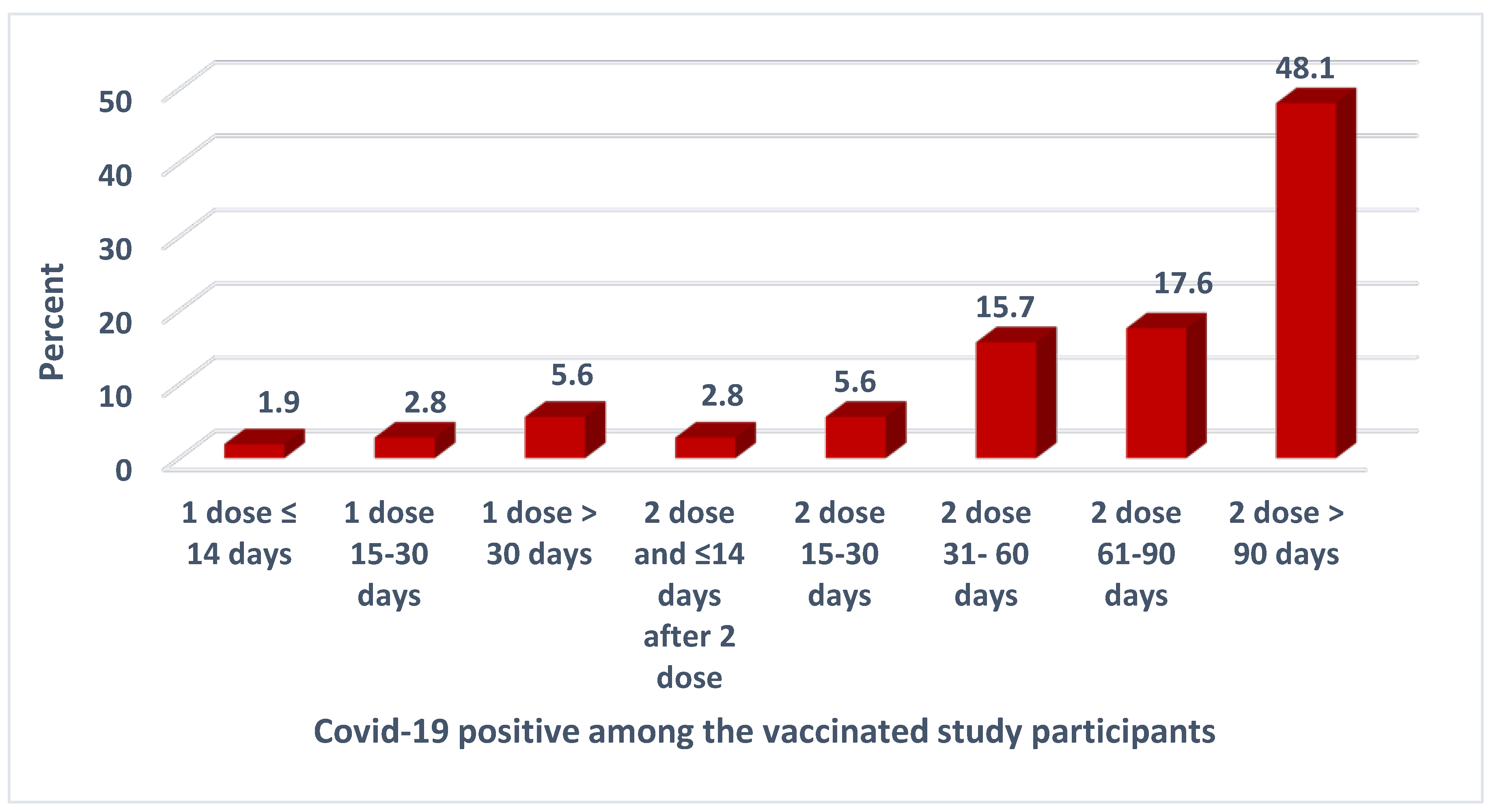
Figure 2 shows that, out of a total of 98 individuals who received the vaccine and tested SARS CoV2 positive, 2 (1.9%) tested positive within 14 days of the first dose, while 3 (2.8%) tested positive between 15-30 days after the first dose. Another 2.8% tested positive within 14 days of receiving the second dose. It’s seen that nearly half of the fully vaccinated participants (48.1%) tested positive after 90 days following the second dose while only 15.7% tested positive within 31-60 days of receiving the second dose and 17.6% were infected between 61 -90 days.
4. Discussion
The present study estimated the effectiveness of BNT162b2 (Pfizer) vaccine against COVID-19 infection, in children and adolescents aged 12-17 years, 14 or more days after the second dose to be 79% during the pre-omicron period in Qatar. Protection against SARS-CoV-2 infection among vaccinated children was higher among the older age group (p value <0.001). The VE showed a gradual decline in immunity over time following the second dose. In another study done in Qatar during the pre-omicron period, vaccine effectiveness against SARS-CoV-2 infection among adolescents was 87.6% (95% CI, 84.0 to 90.4). After receiving the second dose, the level of protection was approximately 95% and declined slowly over time but remained above 50% for at least five months.[
31]
Testing in Qatar is done at a mass scale, mostly for routine reasons, and is tracked centrally for the whole country. [
32,
33] About 75% of those diagnosed are diagnosed not because of appearance of symptoms, but because of routine testing. [
33] Given the high and durable effectiveness of natural infection [
34,
35] and mRNA primary-series vaccination [
32,
33] against COVID-19-related hospitalization and death, and the young population of Qatar,[
25] case numbers were insufficient for precise estimation of differences in protection between natural infection and vaccination against COVID-19 hospitalization and death.
The findings are consistent with evidence from other countries regarding vaccine protection among children and adolescents. [
36,
37,
38,
39,
40,
41] The vaccine demonstrated efficacy in preventing Covid-19, with an immune response similar to that observed in young adults.[
42]
The study found in Italy, fully vaccinated group had a vaccine effectiveness of 29.4% (95% CI 28.5-30.2) against SARS-CoV-2 infection and 41.1% (22.2-55.4) against severe COVID-19. Similarly, the partly vaccinated group had a vaccine effectiveness of 27.4% (26.4-28.4) against SARS-CoV-2 infection and 38.1% (20.9-51.5) against severe COVID-19. The vaccine's effectiveness in preventing disease was highest at 38.7% (with a range of 37.7-39.7%) within the first 14 days after full vaccination. However, it decreased to 21.2% (19.7-22.7%) between 43 and 84 days after full vaccination.[
36]
In a retrospective cohort study, from Singapore, the estimated vaccine effectiveness (VE) against all COVID-19 infections in the age group of 12-18 years following two doses of Comirnaty was 59 % (95% CI: 55-63%) over the period of delta variant dominance from 1st June to 20th November 2021.[
42] In a US study, the effectiveness of vaccines was 59.0 percent (95% CI 22.0–79.0) after 14–149 days following the second dose during the omicron-dominant period among adolescents aged 12–15 years. [
43]
The VISION Network analyzed 241,204 ED/UC encounters** and 93,408 hospitalizations across 10 states during August 26, 2021-January 22, 2022. VE after receipt of both 2 and 3 doses was lower during the Omicron-predominant than during the Delta-predominant period at all time points evaluated. During both periods, VE after receipt of a third dose was higher than that after a second dose; however, VE waned with increasing time since vaccination. During the Omicron period, VE against ED/UC visits was 87% during the first 2 months after a third dose and decreased to 66% among those vaccinated 4-5 months earlier; VE against hospitalizations was 91% during the first 2 months following a third dose and decreased to 78% ≥4 months after a third dose. [
44]
A study conducted in England explained that vaccination 28 days after the first dose was (76.3%) (95% CI 61.1%-85.6%) for those aged 16-17 and (83.4%) (54.0%-94.0%) for those aged 12-15. The first dose of the vaccine was most effective for 16-17-year-olds against symptomatic disease caused by the delta variant between days 14-20, with a peak effectiveness of 75.9% (95% CI 74.3-77.3). However, effectiveness gradually decreased to 29.3% (25.9-32.6) between days 84-104. Among children and adolescents aged 12-15 years and 16-17 years, the VE against Delta infection showed a peak of 68% (95% confidence interval [CI]: 64-71%) and 62% (95% CI: 57-66%) respectively, on days 21-48 after the first dose. Among those aged 16-17 years who received two doses, the effectiveness of the vaccine against Delta infection was highest at 93% (95% CI: 90-95%) between days 35-62 after vaccination but decreased to 84% (95% CI: 76-89%) after 63 days.[
23]
Out of the 991,682 children and adolescents in Denmark who underwent RT-PCR testing for SARS-CoV-2, 7.5% (74,611) tested positive. The risk of being admitted to the hospital for at least 12 hours with any variant was 0.49% (95% confidence interval 0.44% to 0.54%), while only 10 out of 73,187 participants were admitted to an ICU within 30 days of testing positive, which translates to 0.01% (0.01% to 0.03%). Compared to unvaccinated adolescents, those who received one dose of the vaccine had an estimated effectiveness of 62% (with a 95% confidence interval of 59% to 65%) after 20 days. After 60 days, the estimated effectiveness of two doses was 93% (with a 92% to 94% confidence interval) during a period when the delta variant was the most prevalent. Adolescents aged 12 to 17 years demonstrated high effectiveness of BNT162b2, with an estimated efficacy of 93% against confirmed SARS-CoV-2 infection 60 days after receiving the second dose.[
45]
5. Strengths
Testing in Qatar is done at a mass scale, mostly for routine reasons, and is tracked centrally for the whole country. The access and ability to link information on an individual level using data collected in National Vaccine registry was a great advantage and allows us to estimate the national VE in subpopulations, such as children and adolescents.
6. Limitations
Confounders like ethnicity and the presence of comorbidity have not been taken into consideration in this study. This study does not include the effectiveness of additional doses of the COVID-19 vaccine in severely immunocompromised children and adolescents, for whom additional doses should be considered as part of the primary vaccination schedule. The observational designs used previously, including test-negative case-control study design and retrospective or prospective cohort studies, are prone to unmeasured biases such as temporal trends for people who are vaccinated earlier being at sustained increased risk of infection compared with those who were vaccinated later, change in behavior after vaccination, temporal changes in testing frequency over time and differences in infection-derived immunity in the unvaccinated and possible emergence of new variants may all lead to more significant reductions in vaccine efficacy or effectiveness.[
24,
25]
7. Conclusions
The BNT162b2 vaccine was associated with high protection against SARS-CoV-2 disease in children and adolescents. At this stage, priority should be given to completing the primary vaccination course for all the eligible population. In addition, attention should be given to providing additional doses to high-risk and priority groups according to national recommendations.
Unvaccinated persons are more likely to be infected post-exposure to the virus than vaccinated individuals, leading to increased infection incidence among those unvaccinated. As Qatar continues to ease restrictions for those vaccinated, there is concern about a potentially increased risk of exposure among vaccinated individuals than unvaccinated persons. Due to their perceived lower risk, the vaccinated may have adhered less strictly to safety measures, such as masks. [
31,
43,
46]
The absence of in-person learning during the pandemic has had a detrimental impact on children. Given the vaccine's favorable safety and side-effect profile, high efficacy, and acceptable risk-to-benefit ratio in adolescents, evaluating its effectiveness in younger age groups is justified.
Author Contributions
Concept and designing the study (authors 1,4,5,11); Data collection (authors 1,2,5-10; author 1 coordinated the data collection); Data cleaning and analysis (authors 2,3,6); Manuscript writing (1, 2,3,4) Manuscript review (authors 2,4,5) guarantor (authors 4,13).
Funding
No separate funds were required for the study as it was based on secondary data. The dataset of this study is held at the Ministry of Public Health, Qatar. The researchers accessed data through a restricted-access agreement that prevents its sharing with a third party or publicly.
Acknowledgments
We thank Qatar's Ministry of Public Health staff for the diligent efforts and contributions that made this study possible.
Conflicts of Interest
The authors declare no conflict of interest.
Ethical Considerations: Ethical approval was obtained from the Health Research Governance Department at the Ministry of Public Health. The Health Research Governance Department at the Ministry of Public Health waived informed consent. All data were de-identified before sharing for analysis.
References
- Yixiang Ng, Zongbin Li, Yi Xian Chua, Wei Liang Chaw, Zheng Zhao, Benjamin Er, et al. Evaluation of the effectiveness of surveillance and containment measures for the first 100 patients with COVID-19 in Singapore—January 2–February 29, 2020. MMWR Morb Mortal Wkly Rep. 2020; 69: 307-311. 2 January. [CrossRef]
- Voysey, M., Clemens, S.A.C., Madhi, S.A., Weckx, L.Y., Folegatti, P.M., Aley, P.K., et al. (2021). Safety and effectiveness of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS– CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. The Lancet, 397(10269), 99-111.
- Skowronski, D.M., De Serres, G. (2021). Safety and Effectiveness of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med, 384(16), 1576-7.
- Baden, L.R., El Sahly, H.M., Essink, B., Kotloff, K., Frey, S., Novak, R., et al. (2021). Effectiveness and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med, 384(5), 403-16.
- European Centre for Disease Prevention and Control (ECDC). (Jan 31, 2022). Overview of the implementation of COVID-19 vaccination strategies and vaccine deployment plans in the EU/EEA. Stockholm: ECDC. https://www.ecdc.europa.eu/en/publications-data/overview-implementation-covid-19-vaccinationstrategies-and-deployment-plans.
- Ministry of Public Health of Qatar. (2022). National COVID-19 Vaccination Program Data. COVID19 National Covid-19 Vaccination Program Data (moph.gov.qa).
- World Health Organization. (Sep 29, 2021). https://www.who.int/publications/i/item/WHO-2019-nCoV-Sci_Brief-Children_and_adolescents-2021.1.
- Chantal, et.al. (2021). Multiplexed RT-qPCR to screen for SARS-COV-2 B.1.1.7, B.1.351, and P.1 variants of concern V.3. dx.doi.10.17504/protocols.io.br9vm966. https://www.protocols.io/view/multiplexed-rt-qpcr-to-screen-for-sars-cov-2-b-1-1-br9vm966.
- Abu-Raddad, L.J., Chemaitelly, H., Butt, A.A. (2021). National Study Group for Covid Vaccination. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N Engl J Med, 385, 187-9. [CrossRef]
- Butt, A.A., Dargham, S.R., Tang, P., Chemaitelly, H., Hasan, M.R., Coyle, P.V., et.al. (2022). COVID-19 disease severity in persons infected with the Omicron variant compared with the Delta variant in Qatar. J Glob Health, 12, 05032. [CrossRef]
- Abu-Raddad, L.J., Chemaitelly, H., Ayoub, H.H., AlMukdad, S., Yassine, H.M., Al-Khatib, H.A., Smatti, M.K., Tang, P. Hasan, M.R. Coyle, P., et al. (2022). Effect of MRNA Vaccine Boosters against SARS-CoV-2 Omicron Infection in Qatar. N. Engl. J. Med, 386, 1804-1816.
- Jackson, M. L., Nelson, J. C. (2013). Test-negative design for estimating influenza vaccine effectiveness. Vaccine, 31, 2165-216. [CrossRef]
- Verani, J. R., et al. (2017). Case–control vaccine effectiveness studies: preparation, design, and enrollment of cases and controls. Vaccine, 35, 3295-3302.
- Tang, P., Hasan, M.R., Chemaitelly, H., et al. (2021). BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat Med, 27, 2136–2143. [CrossRef]
- Russell FM, Greenwood B. Who should be prioritised for COVID-19 vaccination? Hum Vaccin Immunother 2021;17:1317-1321.
- Townsend E. Debate: the impact of school closures and lockdown on mental health in young people. Child Adolesc Ment Health 2020;25:265-266. [CrossRef]
- Jones EAK, Mitra AK, Bhuiyan AR. Impact of COVID-19 on mental health in adolescents: a systematic review. Int J Environ Res Public Health 2021;18:2470-2470. [CrossRef]
- Frenck RW Jr, Klein NP, Kitchin N, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med 2021;385:239-250. [CrossRef]
- Centers for Disease Control and Prevention (CDC). (Jan 5, 2022). CDC Expands Booster Shot Eligibility and Strengthens Recommendations for 12-17 Year Olds. Media Statement. Atlanta: CDC.
- World Health Organization (WHO). (2022). WHO SAGE Roadmap for prioritizing uses of COVID-19 vaccines. Geneva. https://www.who.int/publications/i/item/who-sage-roadmap-for-prioritizing-uses-ofcovid-19-vaccines.
- Chemaitelly, H., et al. (2021). mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat. Med, 27, 1614-1621. [CrossRef]
- Abu-Raddad, L. J. et al. (2021). Pfizer-BioNTech mRNA BNT162b2 Covid-19 vaccine protection against variants of concern after one versus two doses. J. Travel Med, 28(7), taab083. [CrossRef]
- Powell, A.A., Kirsebom, F., Stowe, J., et al. (2022). Effectiveness of BNT162b2 against COVID-19 in adolescents. Lancet Infect Dis, 22, 581-83. [CrossRef]
- Hanson, K.E., Caliendo, A.M., Arias, C.A., et al. (2021). IDSA Guidelines on the Diagnosis of COVID-19: Molecular Diagnostic Testing. Infectious Diseases Society of America. https://www.idsociety.org/practice-guideline/covid-19-guideline-diagnostics/.
- Abu-Raddad, L. J., et al. (2021). Characterizing the Qatar advanced-phase SARS-CoV-2 epidemic. Sci. Rep, 11, 6233.
- Jeremijenko, A., et al. (2021). Herd immunity against severe acute respiratory syndrome coronavirus 2 infection in 10 communities, Qatar. Emerg. Infect. Dis, 27, 1343-1352.
- Al- Tani, M. H., et al. (2021). SARS-CoV-2 infection is at herd immunity in the majority segment of the population of Qatar. Open Forum Infect. Dis, 8, ab221.
- Coyle, P. V., et al. (2021). SARS-CoV-2 seroprevalence in the urban population of Qatar: an analysis of antibody testing on a sample of 112,941 individuals. Iscience, 24, 102646.
- Butt, A.A., Dargham, S.R., Chemaitelly, H., Al Khal, A., Tang, P., Hasan, M.R., Coyle, P.V., Thomas, A.G., Borham, A.M., Concepcion, E.G., et al. (2022). Severity of Illness in Persons Infected With the SARS-CoV-2 Delta Variant vs Beta Variant in Qatar. JAMA Intern. Med, 182, 197.
- Bertollini, R., et al. (2021). Associations of vaccination and of prior infection with positive PCR test results for SARS-CoV-2 in airline passengers arriving in Qatar. JAMA, 326, 185-188.
- Chemaitelly, H., AlMukdad, S., Ayoub, H.H., et.al. (2022). Covid-19 Vaccine Protection among Children and Adolescents in Qatar. N Engl J Med, 387, 1865-76. [CrossRef]
- Chemaitelly, H., Tang, P., Hasan, M.R., et al. (2021). Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med, 385(24), e83.
- Altarawneh HN, Chemaitelly H, Ayoub HH et al. Effects of previous infection and vaccination on symptomatic omicron infections. N Engl J Med. 2022; 387: 21-34. [CrossRef]
- Abu-Raddad LJ, Chemaitelly H, Bertollini R. Severity of SARS-CoV-2 reinfections as compared with primary infections. N Engl J Med. 2021; 385: 2487-2489.
- Altarawneh HN, Chemaitelly H. Hasan MR. et al. Protection against the omicron variant from previous SARS-CoV-2 infection. N Engl J Med. 2022; 386: 1288-1290.
- Sacco, C., Del Manso, M., Mateo-Urdiales A, et al. (2022). Effectiveness of BNT162b2 vaccine against SARSCoV-2 infection and severe COVID-19 in children aged 5-11 years in Italy: a retrospective analysis of January-April 2022. Lancet, 400, 97-103. [CrossRef]
- Levy, M., Recher, M., Hubert, H., Javouhey, E., Flechelles, O., Leteurtre, S., et al. (2022). Multisystem Inflammatory Syndrome in Children by COVID-19 Vaccination Status of Adolescents in France. JAMA, 327(3), 281-3. [CrossRef]
- Walter, E.B., Talaat, K.R., Sabharwal, C., et al. (2022). Evaluation of the BNT162b2 Covid-19 Vaccine in Children 5 to 11 Years of Age. N Engl J Med, 386, 35-46. [CrossRef]
- Fowlkes, A.L., Yoon, S.K., Lutrick, K., et al. (2022). Effectiveness of 2-Dose BNT162b2 (Pfizer BioNTech) mRNA Vaccine in Preventing SARS-CoV-2 Infection Among Children Aged 5-11 Years and Adolescents Aged 12-15 Years - PROTECT Cohort, July 2021-February 2022. MMWR Morb Mortal Wkly Rep, 71, 422-28. 20 July.
- Cohen-Stavi, C.J., Magen, O., Barda, N., et al. (2022). BNT162b2 Vaccine Effectiveness against Omicron in Children 5 to 11 Years of Age. N Engl J Med, 387, 227-36. [CrossRef]
- Veneti, L., Berild, J.D., Watle, S.V., et al. (2022). Vaccine effectiveness with BNT162b2 (Comirnaty, Pfizer BioNTech) vaccine against reported SARS-CoV-2 Delta and Omicron infection among adolescents, Norway, August 2021 to January 2022. medRxiv, 22272854. 20 August.
- Ali. K, Berman G, Zhou H, Deng W, Faughnan V, Coronado-Voges M, Ding B, Dooley J, Girard B, Hillebrand W, Pajon R, Miller JM, Leav B, McPhee R. Evaluation of mRNA-1273 SARS-CoV-2 Vaccine in Adolescents. N Engl J Med. 2021 Dec 9;385(24):2241-2251. [CrossRef]
- Chemaitelly, H., Ayoub, H.H., AlMukdad, S., et al. (2022). Duration of mRNA vaccine protection against SARS-CoV-2 omicron BA.1 and BA.2 subvariants in Qatar. Nat Commun, 13, 3082. 3082; 13. [CrossRef]
- Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-Dose and 3-Dose Effectiveness of mRNA Vaccines Against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance - VISION Network, 10 States, August 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022 Feb 18;71(7):255-263. . PMID: 35176007; PMCID: PMC885347. [CrossRef] [PubMed] [PubMed Central]
- Helene Kildegaard, Lars Christian Lund, Mikkel Hojlund, Lone Graff Stensballe, Anton Pottegard. Risk of adverse events after covid-19 in Danish children and adolescents and effectiveness of BNT162b2 in adolescents: cohort study. BMJ.2022;377. [CrossRef]
- Usherwood, T. Usherwood, T., LaJoie, Z., Srivastava, V. (2021). A model and predictions for COVID-19 considering population behavior and vaccination. Sci. Rep, 11, 12051. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).