1. Introduction
Nowadays breast conserving surgery followed by radiotherapy is considered the standard of care for breast cancer treatment. Although the intensive mammographic screening has led to early identification of breast cancer, since the late 1990′s there is an increase of mastectomy rate, accounting for 11.3% at the year 2020, 33% in women aged 31-40 years and 39.9% in women younger than 30 years old [
1]. The last decades the implementation of Magnetic Resonance Imaging (MRI) in the preoperative evaluation of breast cancer patients has outlined a more precise estimation of the extent of the disease that on one hand has led to an 11% increase in mastectomy rate but on the other hand it is counterbalanced by a 3% lower reoperation rate [
2].
Mastectomy is not only performed for curative purposes but also prophylactically in women at high risk for developing breast cancer [
3]. The rate of prophylactic mastectomy has increased to reach 35.7% for bilateral mastectomy and 22.9% for contralateral mastectomy [
4].
Immediate breast reconstruction has positive effects on body image and psychosocial well-being and current guidelines recommend clinicians to offer immediate breast reconstruction to every patient with an indication for mastectomy, with similar oncological outcomes to delayed reconstruction [
5,
6,
7]. Breast reconstruction should be available and proposed to all women (who are fit for this more major surgery) requiring mastectomy. There is a high degree of satisfaction and psychosocial well-being in the long term compared with mastectomy and no immediate breast reconstruction [
8]. During the last years numerous methods have been employed for breast reconstruction tailored to each patient’s needs and aiming to a lower complication rate with improved aesthetic outcome. The many reconstructive options available and the changing aspects of the field make this a complex area of plastic surgery, requiring knowledge and expertise in many different reconstructive options but also familiarity with the technical improvements influencing patient care.
Ideally one should strive for a treatment roadmap, even before mastectomy. An algorithm should be developed to include any eventuality and discuss options with the patient. This process starts with the evaluation of the patient’s health and body, assessing donor sites, the availability of fat deposits, the scars and possible radiation treatment of the other breast. The patient’s age and family history will also be very crucial. Comorbidities such as uncontrolled diabetes mellitus, obesity, cardiac disease, hypercoagulation, smoking are related with increased rate of complications that could compromise autologous reconstructive surgery, whereas stage T4 tumors invading chest wall have a negative profile for breast reconstruction. The possibility of a hereditary breast cancer needs to be suspected facing a significant family history or a young age at diagnosis [
9,
10].
Issues to be discussed with the breast caring team and the patient should include the type of surgery performed, the type of incision to be used, whether one should opt for a delayed or an immediate reconstruction as well as the timing of each intervention. At the same time the possibility of a bilateral surgery, either reconstructive or even prophylactic, needs to be taken into account and steer the reconstructive team towards choosing options that will be able to accomplish that [
11].
Autologous tissue reconstruction is preferred in patients undergoing postmastectomy radiotherapy, due to the lower risk of complications compared to implant reconstruction. Since postsurgical radiotherapy is not an absolute contraindication for implant-based reconstruction, these patients opt for the placement of a tissue expander only, whether in the pre- or retro- pectoral position. In this fashion the patient has the experience of the prosthetic reconstruction and decides to proceed or switch to autologous reconstruction after all oncological treatment is delivered, or if prosthetic reconstruction fails. At the same time, all treatment is completed with the expander in place, sparing the potential autologous tissue from being exposed to radiation therapy [
12,
13].
The question of immediate or delayed reconstruction is also very significant. There are very few absolute contraindications for immediate breast reconstruction, such as inflammatory breast cancer. Even in the setting of metastatic disease one should discuss this with the patient. A possible reason to avoid breast reconstruction is the possibility that a complication might delay the delivery of chemotherapy or radiation treatment.
Care should be taken to address the expectations of the woman and try to inform her regarding limitations and shortcomings of each method of reconstruction. The issues regarding breast implants including Breast Implant Associated Atypical Large Cell Lymphoma and Squamous Cell Carcinoma (BIA-ALCL and BIA-SCC) should be presented in their true proportions to answer patients’ concerns, including what should be done to thwart such an outcome.
The breast reconstruction options are summarized in three major categories: the autologous reconstruction, the alloplastic reconstruction, and the pure fat transfer reconstruction. Autologous reconstruction and alloplastic reconstruction could be complemented by fat transfer or by the use of meshes, resorbable or non-resorbable, biologic or synthetic.
It is important to understand the different surgical techniques for breast reconstruction in order to familiarize with the normal postoperative imaging appearance of the reconstructed breast and also recognize common benign complications associated with each reconstruction method. The identification of breast cancer recurrence and malignant disease associated with breast implants is another issue that is discussed in this review.
2. Surgical Techniques for Breast Reconstruction
2.1. Autologous Reconstruction
Although the autologous breast reconstruction is considered the best available option, since the neobreast has a more natural ptotic appearance, feel and texture, yet about 80% of reconstructions are performed differently. Autologous reconstruction is most often used in the case of previous exposure to radiation treatment. The poor vascularity and elasticity tissues make the use of expanders and implants more difficult, something reflected in the higher percentages of failure in such scenarios. The added vascularity of the autologous tissues provides significant improvement in the healing potential of such tissues leading to uneventful healing and successful reconstructions that will tolerate the test of time. Another scenario where autologous tissues present their advantages is that of delayed reconstruction. There, especially after a horizontal scar mastectomy with removal of all excess skin, the deficit of coverage is the most significant factor for a successful reconstruction. Depending on tissue elasticity, one could consider alloplastic reconstruction, but in the setting of significant radiation changes and extensive scarring or tight tissues, the ample tissue provided by autologous reconstruction excels. Unilateral reconstruction is also an area where autologous reconstruction offers significant advantages. One should keep in mind though that this means adequate donor areas and that there will be some amount of transplanted skin that will be visible, creating a patchwork appearance on the breast of a quality difficult to predict.
2.1.1. Transverse Abdominal Myocutaneous Pedicle (TRAM) Flap
The donor site is the lower abdomen and TRAM flaps are harvested with two techniques, either as a pedicle flap with blood supply from the superior epigastric vascular system, or as a free flap based on the inferior epigastric vasculature. The TRAM flaps in both cases contain fatty tissue, muscle, fascia and vessels. The pedicled TRAM flap is rotated on its vascular pedicle from the lower abdomen to the mastectomy site, whereas the free flap is completely separated from its abdominal blood supply and positioned at the mastectomy site where it is anastomosed to thoracodorsal or internal mammary vessels [
14].
2.1.2. Latissimus Dorsi Myocutaneous (LD) Flap
The latissimus dorsi is harvested from the middle back and after identification of the thoracodorsal vessels the myocutaneous or myofascial flap is transferred to the mastectomy site though a subcutaneous tunnel from the axilla [
15]. Most often the combined use of an implant is required to augment its size, or more recently the use of fat grafting to achieve the same goal [
16]. The LD flap has slowly fallen out of favor, especially as a primary reconstruction method. It is still favored as a salvage solution, particularly in radiation induced complications after reconstruction, and as a first line method for patients that are not eligible for TRAM flap reconstruction due to prior abdominoplasty, slim patients with insufficient abdominal tissue and patients with comorbidities such as diabetes, obesity or tobacco use.
More innovative surgical techniques such as the extended LD flap [
17], the scarless approach [
18] and the muscle sparing LD flap [
19] provide excellent aesthetic outcome with lower flap-related complications.
2.1.3. Muscle Sparing Free Flaps
With the workhorse TRAM flap, reconstructive surgery has moved on to other options namely the muscle sparing flaps, where a large part of the rectus muscle is preserved (free TRAM flap), and then to the free alternatives of the same flaps, as the Deep Inferior Epigastric Perforator (DIEP) flap, where the skin and fat are harvested together with their vessels but with no muscle [
9]. At the Superficial Inferior Epigastric Artery (SIEA) flap skin and subcutaneous tissue are excised from the lower abdomen, with vascular supply from superficial subdermal vascular plexus arising from the superficial inferior epigastric vessels, while the rectus muscle and fat are spared [
20]. These flaps will require the use of Microsurgery and prolonged operation time but because the muscle is spared there is reduced donor site morbidity and better patient recovery [
20,
21,
22].
Many more flaps have been described, in case the abdominal tissues are not available (as after an abdominoplasty) or not sufficient. These include upper thigh flaps (Transverse Upper Gracilis - TUG flap), gluteal flaps (Gluteal Artery Perforator - GAP flap) and even lumbar tissue flaps [
23]. All these options are free flaps, requiring Microsurgery and careful dissection during harvesting. Another option if a little more exotic in case of tissue paucity is the use of more than one flaps in one breast in the stacked flaps option. In this scenario one would combine two flaps to achieve the reconstruction of one breast of larger size [
24].
2.2. Alloplastic Reconstruction
2.2.1. Implants
Implants containing saline or silicone have been the mainstay of breast reconstruction for the last decades. With the evolution of 3
rd and 4
th generation of silicone gel implants the use of saline implants is limited, accounting for only 4% of cases worldwide in 2016 [
25]. There is a wide variance in silicone implant filler, shell, texture and shape and ongoing development in the pursuit of an optimal device targeting towards limitation of complications with an improved aesthetic outcome. The 6
th generation of implants was introduced in 2011 with a smooth and uniform surface design that reduces chronic inflammatory foreign body response [
26]. Silicone implants may consist of a single lumen or a double lumen with a silicone filled inner chamber surrounded by a saline filled outer chamber that can be expanded through a valve [
27].
Almost any postmastectomy patient is a candidate for a prosthetic implant reconstruction, but there are limitations in patients that will receive radiation therapy or have already been irradiated, since the irradiated breast is less elastic and there is an increased risk of complications [
13].
Breast reconstruction can be performed at a single stage or in two stages, in any case immediately or delayed after mastectomy. The double stage reconstruction is indicated for women with small breasts or insufficient postoperative skin flaps. An implant with an expander is inserted initially that gradually inflates to create a pocket and is replaced with a permanent implant at a second operation. [
28]. In addition to the well-known breast tissue expanders, with an incorporated magnet in their filling port, making them incompatible with MRI, now there are available tissue expanders with non-magnetic ports, incorporated in their anterior wall, compatible with MRI [
29]. There are also temporary expanders with remote ports, which are usually compatible with MRI and double lumen permanent tissue expanders, with remote filling ports that possess an outer silicone gel layer and an inner expansile cavity, connected to the filling port [
30].
Since the advent of BIA-ALCL cases and their clustering in patients having aggressively textured surface devices, a classification of the types of breast implant surfaces has emerged, separating them into macro-, micro-, nano- and smooth surfaces. Based on surface area characteristics and measurements, the textures are separated into: smooth/nanotexture (80–100 mm2), microtexture (100–200 mm2), macrotexture (200–300 mm2), and macrotexture-plus (> 300 mm2). Most if not all cases have been diagnosed on women exposed to macro- (mostly) or micro- textures. Nano and smooth are very rare if at all present. This is another factor that has been impacting on the choice of implants when approaching breast reconstruction patients.
2.2.2. Acellular Dermal Matrices (ADMs)
Since the development of ADMs, grafts prepared from human or pig skin or bovine pericardium pretreated to remove the cells, the options for breast reconstruction have multiplied. For about a little more than a decade, the traditional reconstruction with the implants placed mostly or totally under a muscle coverage has been challenged [
31]. To begin with, the placement moved to the dual plane position, meaning still under the cover of the pectoralis major muscle cephalad, but now the lower pole was under the coverage of the mesh, most often an ADM, an allo- or xenograft. At the same time synthetic meshes appeared, starting to assume the same role as above. These could be resorbable or not, but they were man-made except for one which was out of silk fibers. The benefits of such an approach include the decreased amount of pain and tightness of the lower pole of the reconstructed breast, while at the same time there is a more natural shape of the inframammary fold. In the case of ADMs there has been evidence of better tolerance of radiation treatments as well as less capsular contracture than without them [
32]. Once more experience was accumulated with ADMs, the next step was to combine many sheets and prepare a circumferential “ravioli” type coverage of the expander or implant and place them in the subcutaneous or prepectoral level. Despite all their noteworthy characteristics, which may actually turn out to include protection against BIA-ALCL, one of the obvious drawbacks of ADMs is their cost and the higher chance of the patient developing a seroma or infection.
2.3. Autologous Fat Reconstruction
Autologous fat grafting, also referred as lipofilling or lipotransfer is a novel approach in breast reconstruction, alone or usually in combination with breast implants to improve breast contour and volume. Other indications include the treatment of postmastectomy pain syndrome, capsular contracture pain and post-irradiation fibrosis [
33]. Fat is harvested with liposuction usually from the flanks and abdomen. In the USA over 30,000 cases of autologous fat grafting were reported in 2018. Common mammographic findings are fat necrosis (0%-50%), calcifications (0%-45%), and scar (1.5%-28.5%) [
34].
2.3. Nipple Areola Complex (NAC) Reconstruction
Many attempts are made to preserve the NAC when certain criteria apply. It seems that this attempt at preservation is employed more often with tumors closer to the NAC, provided imaging and pathology confirm the negative margins, with good results. In the cases where preservation will seem unwise, one proceeds with NAC reconstruction. The simplest approach is 3-D tattooing of the NAC, which provides a good or even excellent visual effect. On MRI the tattoo can produce a blooming artifact with mild heterogenous enhancement [
35].
3. Preoperative Imaging
To prepare for an autologous tissue reconstruction, all members of the treating team should be thoroughly familiar with the procedure. Particularly in the use of a perforator flap, preoperative imaging, although probably not always required, will certainly speed up the procedure by allowing the surgical team to focus on the best candidate vessels rather than explore all possibilities. CT and MR scans provide exceptional images with detailed coordinate location of the vessels, allowing dissection to proceed in a speedy fashion [
36,
37]. CT angiography and MR angiography can create a preoperative vascular roadmap outlining potential anatomical variances that will optimize the decision making and flap excision [
38,
39,
40]. Color Doppler and/or Duplex ultrasound (US) will provide some help in the absence of the above modalities [
41,
42]. Recent advances in preoperative imaging report that laser-assisted indocyanine green fluorescence angiography (LA-ICGFA) and dynamic infrared thermography (DIRT) can successfully identify the dominant vessels in autologous reconstruction [
38,
43].
The use of clinical pathways can assist delivery of excellent patient care in all complex surgical procedures, and this is a case in point. Steps to minimize patient discomfort such as local anesthetic infusion pumps, blocks or use of long-acting local anesthetics can go a long way towards prompt mobilization and discharge of the patient.
4. Normal Imaging Appearance of the Reconstructed Breast
4.1. Autologous Reconstruction
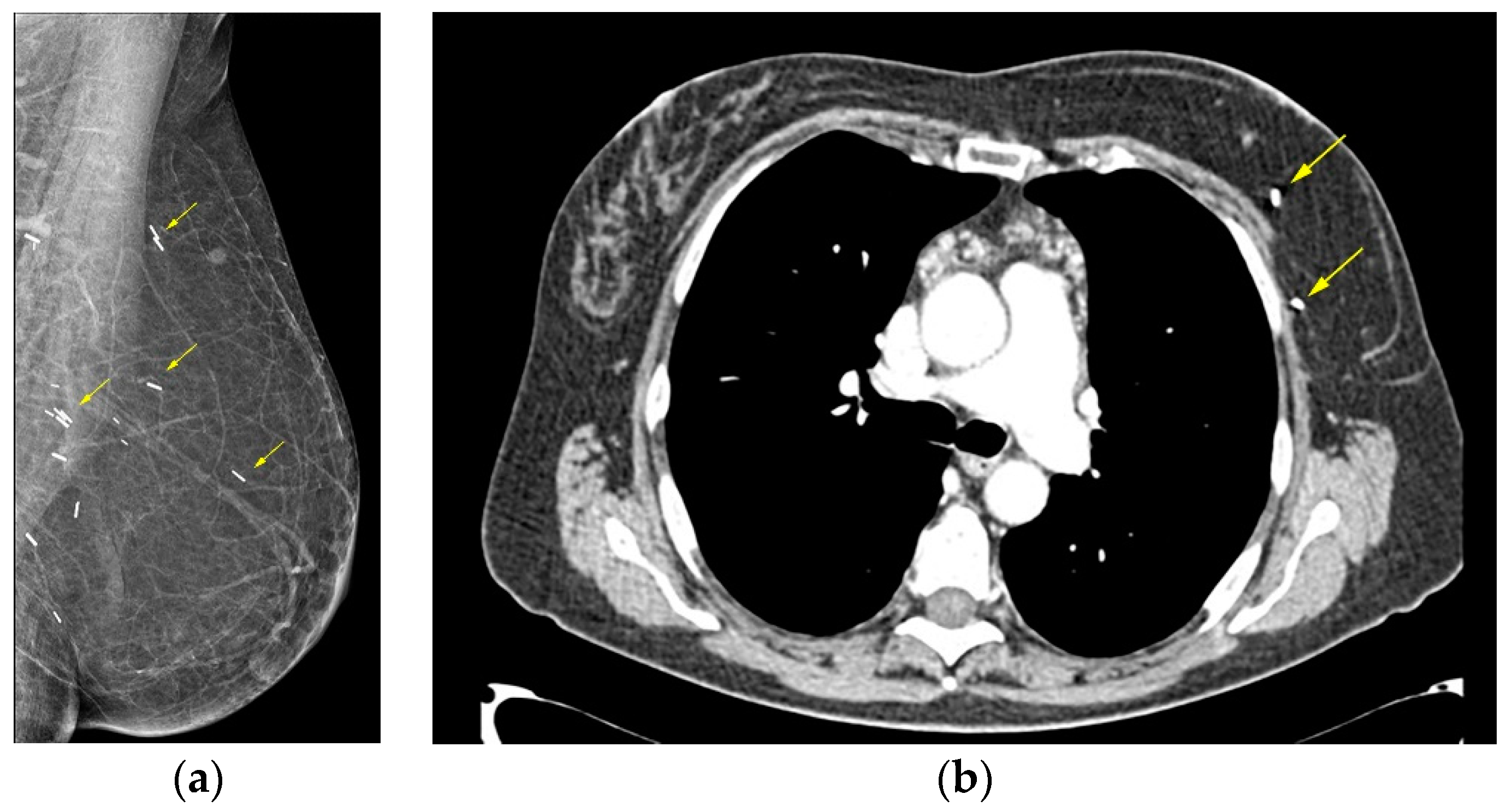
On mammography the reconstructed breast is consisted predominantly by fat and in case of muscle baring flaps muscle strands can be visible. Postoperative scarring and clips are common findings (
Figure 1) [
44].
The high-resolution MRI images allow excellent imaging of the reconstructed breast providing anatomical details that can differentiate the various surgical techniques used for autologous reconstruction. The MRI protocol should include unenhanced fat saturated T1 weighted sequences, non-fat saturated T2 weighted sequences, dynamic contrast enhanced fat saturated T1 weighted sequences, and delayed sagittal fat saturated T1 weighted sequences. The homogeneous fat suppression is of paramount importance that should be considered a prerequisite for MR imaging of the reconstructed breast.
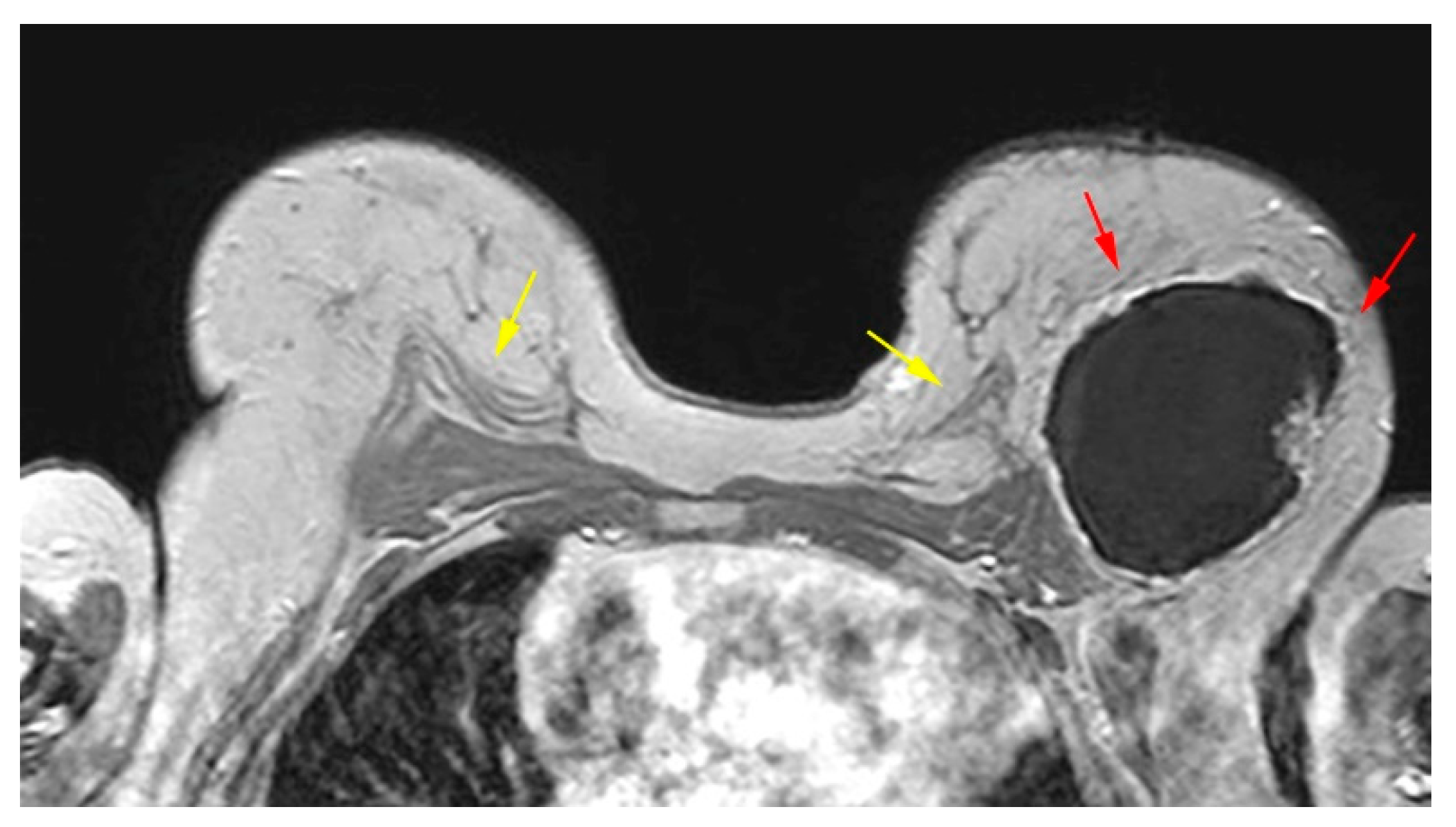
The pedicled or free TRAM flap and the LD flap consist of the rectus abdominis and latissimus dorsi muscle respectively and also the overlying skin and subcutaneous fat. In the TRAM reconstruction the flap is recognized centrally along the anterior chest wall in axial images. In the LD reconstruction the flap is more eccentric with a tailed appearance of the muscle in the lateral breast as a result of the flipping and tunneling of the flap harvested from the back, differentiating it from a TRAM flap (
Figure 2) [
35]. In both cases the muscle atrophies over time. A thin low signal intensity curvilinear line parallel to the breast contour is often visible, best appreciated on sagittal images, representing the dermal layer of the lower abdominal or the dorsal wall [
35,
45]. The contact zone of the TRAM flap to the mastectomy site, that corresponds to the musculovascular pedicle may exhibit contrast enhancement [
46].
At the muscle sparing free reconstruction flaps (DIEP, SIEA, GAP, TUG) the breast is replaced by fatty tissue from the lower abdomen, gluteal region and thighs and on MRI the reconstructed breast consists of fat and a thin vascular pedicle anastomosed with the internal mammary artery [
35]. The absence of muscular component can differentiate them from TRAM and LD flaps.
4.2. Implant Reconstruction
First and foremost, it is important to have knowledge of the type of implant that has been used for breast reconstruction and identify the different normal imaging findings of each implant type.
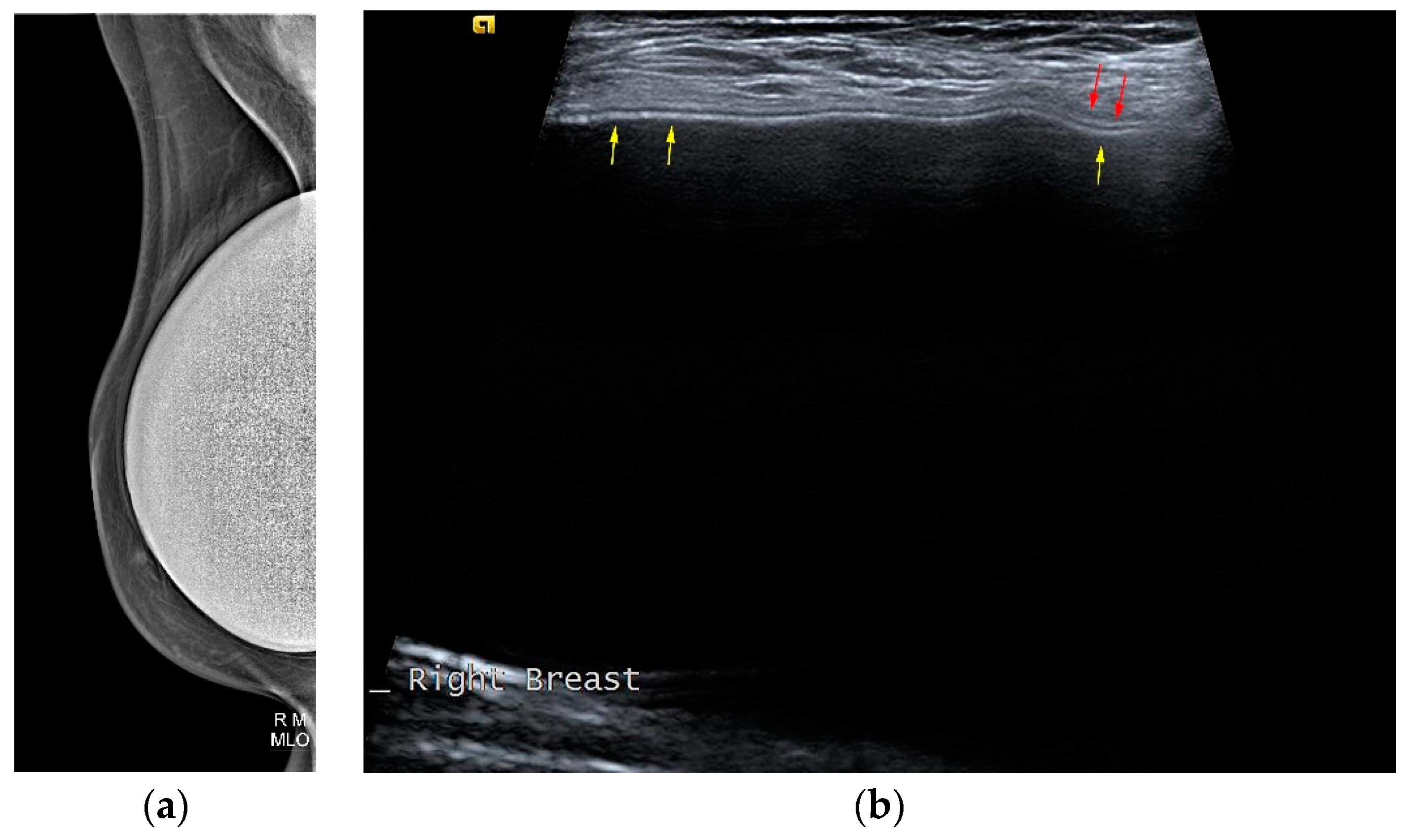
The typical mammographic appearance of an implant is a radiopaque oval mass with smooth margins whose density varies depending on the filling material (
Figure 3a). A band of soft density tissue surrounding the implant, with or without calcifications represents the thick fibrous capsule that is formulated after the implant insertion as a result of a foreign body reaction. Folds within the implant and the valve may be visible with the appropriate mammographic technique [
47].
Ultrasonographically both saline and silicone implants are anechoic and the shell appears either as one echogenic line or as parallel echogenic lines. Internal folds may be recognized as wavy lines without disruption. The fibrous capsule is visible as an echogenic line parallel to the implant’s shell, sometimes with calcifications producing focal acoustic shadowing (
Figure 3b). A small peri-implant fluid effusion is a normal finding. At implants with expanders the valve is visible and caution should be taken so that partially expanded implants should not be mistaken for ruptured implants [
47].
MRI has a high spatial resolution and is the most accurate modality for evaluation of implants. Another advantage of MRI over conventional imaging modalities is the ability to enhance or suppress the signal of water, silicone and fat. The most frequently used MR sequences are a fast T1-weighted multiplanar sequence, a T2-weighted fast spin-echo sequence, silicone-only sequences (silicone high signal, water low signal intensity) and silicone saturated sequences (silicone low signal, water high signal) [
48,
49].
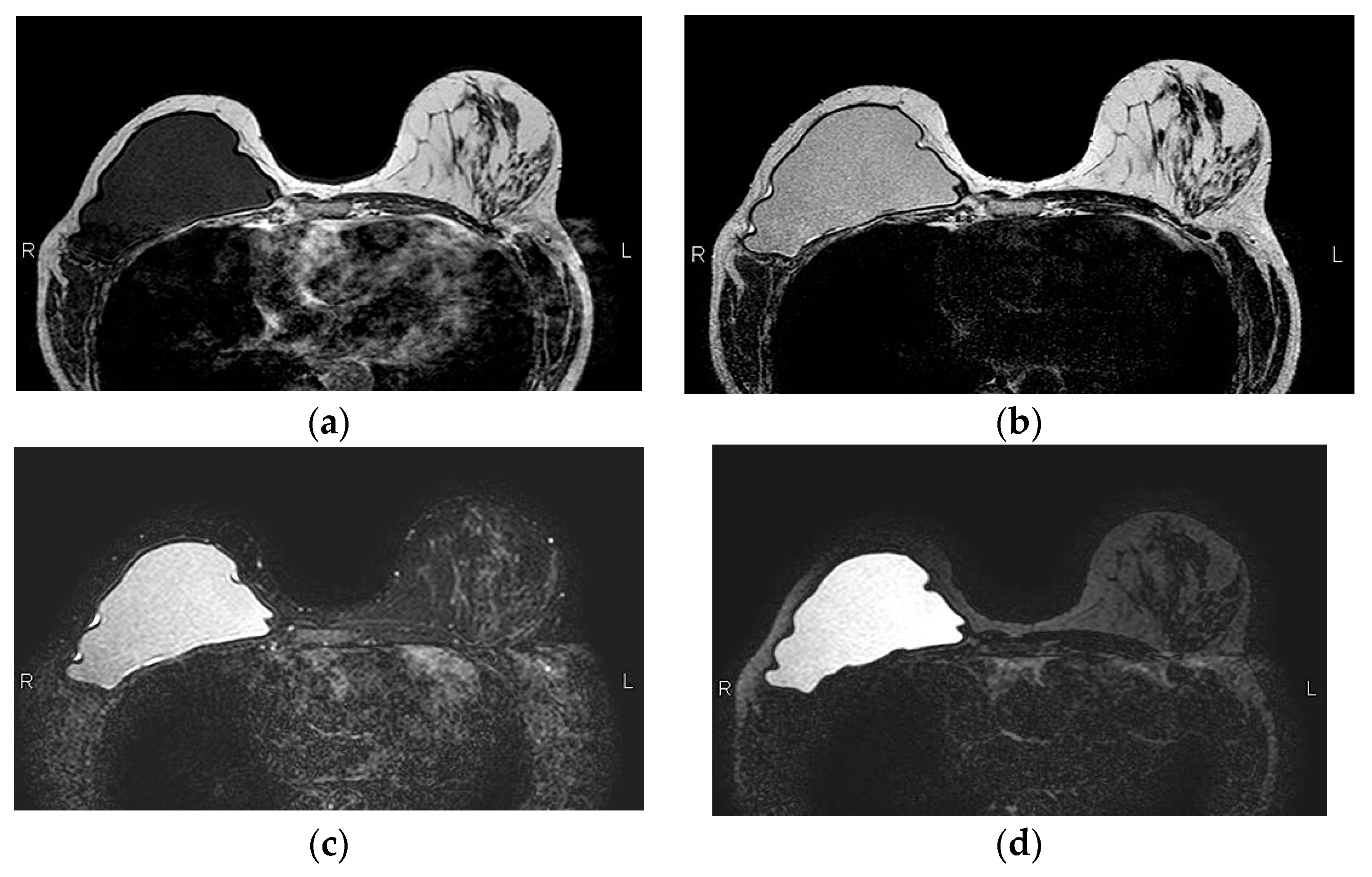
A single lumen implant has an intact shell and is surrounded by a thin fibrous capsule with low signal intensity on all sequences. Saline implants appear with water signal intensity on all sequences, and a valve is recognized within the lumen with low signal intensity, while silicone implants show high signal on T2-weighted and silicone-selective sequences and low signal on T1-weighted sequences (
Figure 4). A double lumen implant appears with an inner chamber of silicone with high signal intensity and an outer chamber of saline with water signal intensity (
Figure 5) [
47,
49].
Internal radial folds and a small amount of peri-implant reactive fluid are normal findings [
27,
48]. Radial folds are infoldings of the shell extending from the periphery of the implant, usually as a result of implant contracture and are characterized as simple when they are short and straight, and complex that are longer and curved that may mimic an intracapsular rupture (
Figure 6). Radial folds have a sheetlike appearance and a more perpendicular orientation to the imaging axis, while the rupture is more parallel. It is important to review multiple sequential images on all planes in order to recognize the folds communicating with the shell and differentiate them from intracapsular rupture [
50].
5. Complications
5.1. Autologous Reconstruction
The complications in autologous reconstruction are divided into two categories; complications of the flap and complications of the donor site. The most common benign complications of autologous flaps are edema, seroma, hematoma, skin thickening, fibrosis and fat necrosis.
5.1.1. Seromas and Hematomas
Seromas and hematomas are common early postoperative complications that usually resolve gradually although they may persist for months or even years after surgery [
45]. Seromas are typical multilocular fluid collections with high T2 signal on MRI, while hematomas present with variable appearance depending on the age of the blood products.
5.1.2. Skin Thickening and Fibrosis
Skin thickening is usually present within 6 months after radiation therapy, as a result of impaired venous and lymphatic drainage, that resolves after 2 to 3 years [
51]. At MRI the thickened skin appears as a band with low T1 signal and high T2 signal that is uniform and not very intense [
45].
Fibrosis occurs gradually after 1 year after radiation therapy. The differentiation of irregular fibrotic masses from recurrent breast cancer is challenging and although they share common imaging characteristics on mammography, MRI can be helpful. Fibrosis has a low signal intensity without enhancement or minimal gradual enhancement, whereas a recurrent tumor is isointense or slightly hyperintense exhibiting strong contrast enhancement with washout kinetics [
35,
45]. On should keep in mind, however, that granulation tissue can enhance for up to 1 year or even longer after surgery causing diagnostic dilemmas and the need for histological confirmation after biopsy [
52].
5.1.3. Fat Necrosis
The initially dominant reconstructive technique the pedicle TRAM flap was associated with increased donor site morbidity, leading to adoption of less invasive techniques without muscle excision like the free TRAM flap and the DIEP flap. However, the preservation of the rectus abdominis muscle produces flaps with fewer perforator vessels, thus compromising the flap vascularity and increasing the risk of perfusion related complications. Fat necrosis is a result of ischemia due to insufficient arterial flow and poor venous drainage, with an incidence reaching up to 35% [
45,
51,
53]. Free TRAM flaps and DIEP have a more robust vascular supply than pedicled TRAM, but anastomotic thrombosis has been reported in 2.4-6.3% in microsurgery that can lead to the development of fat necrosis [
54]. A recent meta-analysis of flap perfusion of 1891 pedicled versus 866 free TRAM and 1211 DIEP flaps showed that free TRAM flap demonstrated lower risk of fat necrosis than pedicled TRAM, although there was no difference between DIEP and pedicled flaps [
21]. Flaps with increased weight have a greater risk for fat necrosis with odds increasing by 1.5 for every 100 gr increase of flap weight, that can be counterbalanced by an increased number of perforators [
55]. Surgeons should be cautious in technique selection taking into account the patient’s body mass index, comorbidities and tobacco use that can contribute in the development of fat necrosis.
Fat necrosis exhibits a wide spectrum of imaging appearances evolving over time, that in some cases mimic local recurrence. At mammography a well circumscribed radiolucent mass is the most frequent finding, but fat necrosis can present with suspicious pleiomorphic microcalcifications accompanied by a mass with irregular or spiculated margins due to pronounced fibrosis [
56]. Ultrasonographically it can appear as a cyst, a complex cystic lesion or a mass with indistinct margins. The absence of vasculature at Color Doppler helps in the differentiation of fat necrosis from tumor recurrence [
57].
The most common appearance of fat necrosis on MRI is an oval mass isointense to fat in all sequences, with low T1 signal on fat saturated images, usually non-enhancing. The most challenging feature is the enhancing granulation tissue with focal or irregular configuration. The degree of enhancement is variable depending on the severity of inflammation and the kinetic analysis may be confusing. Rapid initial uptake and even washout of the contrast media have been reported similar to malignancy [
56,
58]. A reliable feature that differentiates rim enhancing fat necrosis from necrotic cancer is that the central non-enhancing area of cancer has high T2 signal, while the central area of fat necrosis is fat that can be confirmed from the review of the non-contrast images. Fat has an equal signal intensity to fat at non-fat suppressed T1 images and a characteristic markedly low signal at the STIR sequence, a fast spin echo sequence with inversion recovery that allows fat suppression. This is characterized as “black hole” sign and is pathognomonic of fat necrosis [
59].
5.1.4. Donor Site Complications
The incidence of donor site complications ranges from 7.7% to 38% for pedicled TRAM flaps and 17.9-24.7% for free TRAM flaps [
60]. The muscle excision at the pedicled TRAM flap contributes to abdominal wall weakness leading to abdominal wall hernia in 16% of patients [
51]. At the muscle sparing flaps although the muscle is preserved and the abdominal wall weakness is minimized nevertheless abdominal budge is developed in 11,25% of free TRAM flaps and 8.07% of DIEP [
61]. Factors related to abdominal wall bulging include patient age, comorbidities, previous abdominal surgery, operative time and chemotherapy. BMI greater than 23kg/m
2, and US measured thickness ratio of rectus abdominis muscle evaluated at exercise between donor and normal site less than 49% are reported as factors related to asymptomatic exercised abdominal wall bulging [
62].
5.2. Implants and Alloplastic Reconstruction
Complications may manifest early or delayed after implant reconstruction. Seromas, hematomas and infections are the most common early complications. The late complications include capsule contracture, bulging and herniation, implant rupture, gel bleed, fat necrosis and malignant conditions such as BIA-ALCL and others.
5.2.1. Seromas
Seromas are the most common early postoperative complications and although they are usually absorbed within 4-5 months after surgery, they can persist for up to 1 year after. Seromas are recognized on US as peri-implant fluid collections with high signal intensity on T2-weighted images and low signal intensity on T1-weighted images on MRI. Although ADMs have been accused for seroma formation such a connection has not been established [
63].
5.2.2. Hematomas
The incidence of hematomas after breast augmentation ranges from 0.2 to 5.7% [
64]. The appearance of hematomas on MRI is variable depending on their age, with acute and subacute hematomas appearing with high signal intensity on T1-weighted images without contrast enhancement [
48].
5.2.3. Infection
Infection is a significant complication reported in 5.8-28% of implant reconstructions that may contribute to reconstruction failure [
65,
66]. Acute infections occur immediately after surgery and have the clinical signs of cellulitis [
67], while subacute and late infections occur months to years after surgery and are usually clinically occult [
68]. An increased infection rate is reported with the auxilliary use of ADMs that is even higher in respect to ADM burden [
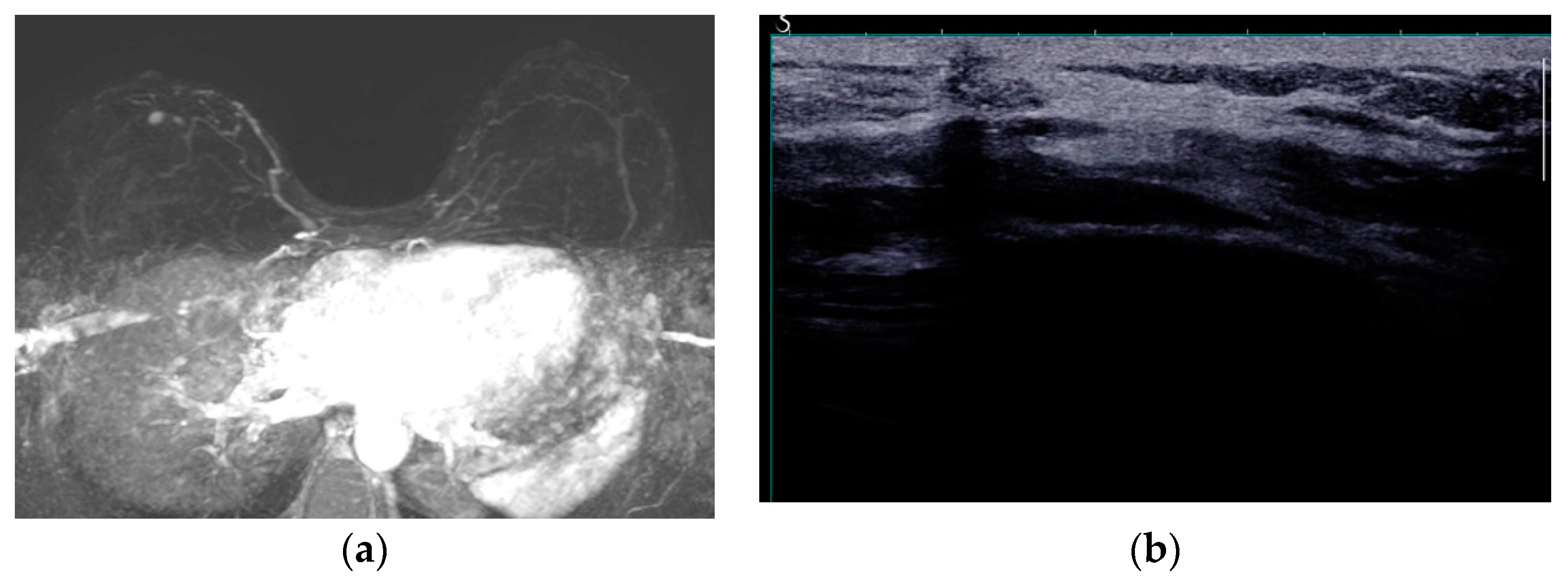
69]. Infection is usually manifested with a peri-implant effusion, while on MRI the capsule may present thickening and contrast enhancement (
Figure 7).
5.2.4. Capsular Contracture
Capsular contracture is one of the most common complications occurring in 5-19% of patients after reconstruction [
70]. After placement of the implant a band of fibrous tissue is formulated as a result of a foreign body reaction. Capsular contracture occurs when the fibrous capsule becomes excessive and irregular that causes abnormal contraction [
71]. The 3
rd and 4
th generation of implants, and implants with smooth surface were more prone to capsular contracture [
72]. A higher incidence is reported for implants with retropectoral positioning [
73]. Radiation therapy may also induce capsular contracture [
74]. The diagnosis is mostly clinical. Imaging findings suggestive of capsular contracture are a thickened irregular fibrous capsule, occasionally enhancing on MRI, spherical shape of the implant and increased radial folds [
49]. Clinical examination remains the gold standard for the estimation of capsular contracture and the Baker classification provides a standardized scoring system:
grade I: normal soft tissues implant texture,
grade II: firm texture with normal contour,
grade III: firm texture with altered contour,
grade IV: firm texture with altered contour with concomitant pain [
75].
5.2.5. Implant Rupture
Implant rupture is a well-known potential complication occurring in both saline and silicone implants. The rupture of saline implants is clinically dramatic since the implant loses volume and the breast is deformed, and it is best described by the term “deflate” rather than rupture. Because the saline is absorbed from the body, the absence of imaging findings is quite frequent [
76]. On the other hand, silicone implant rupture is often asymptomatic especially the intracapsular rupture that is evident only on imaging. The incidence of implant rupture is related with the age of the implant with a 12-fold increased prevalence odds ratio for rupture of implants between 16 and 20 years of age, compared with implants between 3 and 5 years of age [
77]. A minimum of 15% of modern implants are expected to rupture 3 to 10 years after implantation and for implants intact 3 years after implantation there is an estimated rupture-free survival of 98% at 5 years and 83% to 85% at 10 years. Double lumen implants have a lower prevalence of rupture compared with single lumen implants [
78].
There are two types of silicone implant rupture. The intracapsular rupture where the implant’s shell is ruptured and silicone is leaking outside the shell but remains within the intact fibrous capsule (up to 78% of reported ruptures), and the extracapsular rupture where silicone is leaking from a breach at the fibrous capsule outside of the implant to the surrounding breast tissue (up to 22%of reported ruptures). [
79].
Mammography is the least sensitive method for detection of implant rupture with a reported sensitivity of 11-70% [
80,
81]. Mammography is unable to depict an intracapsular rupture due the lack of visualization of the internal structure of the implant. Subtle signs of extracapsular rupture are the deformity of the implant, while radiopaque silicone inside the breast can be easily detected making a definite diagnosis of extracapsular rupture with an increased specificity of up to 89% [
82].
Ultrasound is a widely available, cost-effective method for implant evaluation [
83], with a reported sensitivity of 30-75% [
80,
84,
85]. The “stepladder” sign is the most reliable sign of intracapsular rupture representing a series of echogenic lines coursing parallel to the probe in the anterior of the implant, produced by the ruptured shell [
80,
86]. Other signs such as the “keyhole” sign and the “subcapsular line” sign are early signs of intracapsular rupture but the differentiation from radial folds is challenging [
79]. The “snowstorm” artifact recognized outside the implant is a sign of free silicone within the breast after extracapsular rupture. A snowstorm appearance can be also encountered at the axillary lymph nodes (
Figure 8). The sole presence of silicone at the lymph nodes is not a solid evidence of extracapsular implant rupture since it may be a result of “gel bleed”. “Gel bleed” is actually a misnomer that describes the leakage of silicone at a microscopic level though a weakened but intact polymer shell [
27,
86,
87].
MRI is considered the gold standard for the evaluation of silicone implant integrity, with a perfect sensitivity of nearly 100% but with lower specificity of 63% to 97% [
48,
49,
81,
82]. Several signs of intracapsular rupture have been reported and the “linguine” sign is most reliable with a sensitivity of 96% and a specificity of 94% [
88]. The collapsed elastomer shell of the implant floats inside the silicone and it is depicted as curvy lines of low signal intensity within the silicone (
Figure 9). Other definitive signs of intracapsular rupture are the subcapsular lines, low signal intensity lines parallel the fibrous capsule surrounded by silicone. The “keyhole or teardrop” sign, the “salad-oil” sign and the “rat-tail” sign are possible signs of intracapsular rupture. The “keyhole and teardrop” signs are focal silicone invaginations between the implant shell and the fibrous capsule caused by a focal tear of the shell (
Figure 9c). Although they are considered as an early sign of uncollapsed intracapsular rupture they are non-specific [
27,
47,
49,
79,
89]. The “salad-oil” or “droplet” sign describes the silicone gel mixing with droplets of peri-implant fluid (
Figure 9d). Contour irregularities and deformities such as the “rat-tail” sign of silicone extending along the chest wall are also non-specific signs of rupture [
81].
The extracapsular rupture involves the rupture of both the implant shell and the fibrous capsule with leakage of silicone to the surrounding tissues [
27,
79]. The free silicone is best recognized at the silicone-selective MR sequences as areas of high signal intensity outside the fibrous capsule (
Figure 10a). Silicone may also migrate through the lymphatics to the axillary lymph nodes that can be perceived at the silicone-selective sequences (
Figure 10b).
The presence of silicone outside the implant may induce an inflammatory reaction leading to the formation of a silicone granuloma that can present contrast enhancement thus causing diagnostic problems (
Figure 11) [
90,
91]. Not infrequently, percutaneous biopsy is warranted to differentiate between silicone granuloma and local relapse.
Although MRI has a high negative predictive value of 98%, it has a low positive predictive value of 77% [
92] and one should be aware that there are pitfalls in image interpretation, like overestimation of contour abnormalities or long and complex radial folds and the diagnosis of rupture should not be supported by only a single imaging finding.
5.2.6. Breast Implant Associated Atypical Large Cell Lymphoma (BIA-ALCL)
BIA-ALCL was first described in 1997 [
93] but it was recognized in 2016 by the World Health Organization (WHO) as a unique T-cell ALK-negative ALCL, with similar morphologic and immunophenotypic characteristics with systemic and cutaneous ALK-negative BIA-ALCL [
94]. The estimated incidence is 1 to 3 cases per million women and it presents on average 11 years after implantation with higher incidence reported in textured implants [
95,
96].
The pathogenesis is not fully elucidated, yet two hypotheses have been described. The first presents the Gram-negative Ralstonia bacterium as a causative factor, that is frequently encountered adjacent to the textured breast implants in patients with BIA-ALCL, while the second theory implicates silicone bleed and leakage of microparticles are the trigger factor. In both cases chronic inflammation is induced with repetitive T-cell activation [
97].
The most common clinical presentation is unilateral breast edema and occasionally a palpable mass adjacent to the implant. Two different types of BIA-ALCL are described: peri-implant effusion and peri-implant mass. MRI and US are the most accurate imaging modalities for detection of effusion and mass (US 84% and 46%, MRI 82 and 50% respectively) On US the most common finding is a homogeneous peri-implant effusion and occasionally a solid oval mass, with well-defined margins. [
98].
MRI is the second in line modality for BIA-ALCL diagnosis with the standard protocol before and after contrast administration. The main findings are the presence of fluid (with hyperintense in T2 images, homogeneous or heterogeneous) between the capsule and the implant, and peri-implant masses, round or irregular with heterogeneous enhancement. Commonly the capsule presents with irregular thickness and contrast enhancement [
99].
BIA-ALCL is a rare entity that radiologists should suspect in case of delayed peri-implant effusion and cytology should be performed or tissue sampling in case of suspicious peri-implant mass.
5.2.6. Others
Breast Implant Associated Squamous Cell Carcinoma (BIA-SCC) is a very rare but potentially aggressive epithelial tumor emanating from the breast implant capsule, with sheets of squamous cells varying from normal to dysplasia, metaplasia and carcinoma. The clinical presentation of BIA-SCC is very similar to BIA-ALCL and BIA-SCC should be also considered in case of late onset seroma and US and MRI should be performed [
100].
Desmoid tumors of the breast are extremely rare accounting for 0.2% of all breast tumors and 4% of all extra-abdominal desmoid tumors. The present of silicone prostheses is reported as risk factor for developing breast desmoid tumors arising from the fibrous capsule as a result of postsurgical trauma [
101]. They are best evaluated on MRI with two distinct features: chest wall tumors presenting as oval and lobulated masses, that are locally aggressive invading the intercostal muscles and pleura, and breast tumors presenting as spiculated masses. The masses are isointense to muscle at T1 images with variable T2 signal intensity and heterogeneous enhancement [
102].
6. Recurrence of Breast Cancer
Mastectomy significantly decreases the risk for breast cancer but it does not eliminate it, since residual glandular tissue can remain even at the microscopic level. Recurrence rates after mastectomy with or without reconstruction are 1-2% annually for the first 5 years, and overall recurrence rate is 2-15% [
103]. The incidence of local recurrence after mastectomy without reconstruction is higher than in women with breast reconstruction (2-7.5% vs 2-4%) with comparable rates for delayed and immediate reconstruction [
104,
105]. Reported risk factors for local recurrence are the patients age younger than 50 years old at the time of diagnosis, large tumor size, and aggressive molecular subtypes [
104,
106].
The recurrences are commonly located superficially in the skin and the subcutaneous tissue (60%) and are easily detected with clinical examination, while in 32.5% the location is deep adjacent to the pectoralis muscle and it can be occult, masked by the autologous tissue or implant [
104]. The European Society for Radiotherapy and Oncology (ESTRO) recently issued guidelines for the clinical target volume for postmastectomy radiation therapy after immediate implant reconstruction that encompasses the location of most local recurrences [
107,
108].
The clinical benefit of imaging surveillance of women with breast reconstruction is under intense debate with conflicting suggestions at the literature. The National Comprehensive Cancer Network (NCCN) advises against imaging for asymptomatic patients with breast cancer treated with mastectomy with or without reconstruction, while the American College of Radiology (ACR) recommends surveillance with mammography or digital tomosynthesis for women with mastectomy and autologous reconstruction with or without prosthesis [
103]. On the other hand Adrada et al [
104] and Pinel-Giroux et al [
51] support the use of MRI in women with high recurrence risk. Although the pooled overall cancer detection rate per 1000 examinations of MRI is higher than mammography and US (5.17, 1.86 and 2.66 respectively) [
109] the level of evidence at the literature is low to dictate a surveillance strategy in this context, and further studies are required with special focus on prognosis and cost-effectiveness.
The imaging appearance of tumor recurrence is similar to breast cancer as an irregular mass on all imaging modalities, but the radiologists should be aware that in 50% of cases the mass has pseudo-benign appearance [
104]. US is used as the first line method for evaluation of palpable masses and the majority of lesions are hypoechoic and 8.6% are complex cystic masses [
110]. MRI is the most sensitive method to detect tumor recurrence, irrespective of tumor location (deep or superficial) and type of reconstruction (implant or autologous) (
Figure 12) [
104].
7. Male Breast
Breast cancer is rare in men accounting for less than 1% of all breast cancers. Due to the typical central retroareolar location and the frequent involvement of the nipple the standard surgical treatment of male breast cancer is a modified radical mastectomy with excision of the nipple and axillary node dissection [
111]. Although scarce, there are reports at the literature regarding postmastectomy breast reconstruction in men. Autologous reconstruction is the most common method with local flaps followed by the TRAM flap and there is a single report of fat grafting for chest symmetry [
112,
113].
8. Conclusions
Breast reconstruction has evolved tremendously the past years based on either autologous or alloplastic techniques, offering a variety of options tailored to the specific needs of each patient. Plastic surgery in this field aims to provide an improved aesthetic outcome while minimizing the postoperative complications, always within the context of oncological safety. Although there is no consensus and guidelines regarding the imaging surveillance of post-mastectomy patients that underwent reconstructive surgery, it is important to be familiar with the normal imaging appearance of different reconstructive techniques and to be able to recognize associated complications, either benign or malignant. One must always keep in mind that even though the risk of recurrent breast cancer is radically minimized after mastectomy, it is not eliminated and can be clinically occult.
Author Contributions
“Conceptualization, T.K. and A.A.; writing—original draft preparation, T.K., D.P.M., P.D.M., E.C.F.; writing—review and editing T.K., A.A; supervision, A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fefferman, M.; Nicholson, K.; Kuchta, K.; Pesce, C.; Kopkash, K.; Yao, K. Rates of Bilateral Mastectomy in Patients With Early-Stage Breast Cancer. JAMA Netw Open 2023, 6, e2251348. [Google Scholar] [CrossRef] [PubMed]
- Sardanelli, F.; Trimboli, R.M.; Houssami, N.; Gilbert, F.J.; Helbich, T.H.; Alvarez Benito, M.; Balleyguier, C.; Bazzocchi, M.; Bult, P.; Calabrese, M.; et al. Magnetic resonance imaging before breast cancer surgery: Results of an observational multicenter international prospective analysis (MIPA). Eur Radiol 2022, 32, 1611–1623. [Google Scholar] [CrossRef]
- Taylor, A.; Tischkowitz, M. Informed decision-making is the key in women at high risk of breast cancer. Eur J Surg Oncol 2014, 40, 667–669. [Google Scholar] [CrossRef]
- Alaofi, R.K.; Nassif, M.O.; Al-Hajeili, M.R. Prophylactic mastectomy for the prevention of breast cancer: Review of the literature. Avicenna J Med 2018, 8, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Kaidar-Person, O.; Poortmans, P.; Offersen, B.V.; Boersma, L.J.; de Ruysscher, D.; Noy, V.; Hermann, N.; Kuhn, T. What are the guidelines for immediate breast reconstruction? Eur J Surg Oncol 2021, 47, 1214–1215. [Google Scholar] [CrossRef]
- Popowich, B.; Kostaras, X.; Temple-Oberle, C. Breast reconstruction after therapeutic or prophylactic mastectomy for breast cancer: A comparison of guideline recommendations. Eur J Surg Oncol 2020, 46, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Mureau, M.A.M. Breast Reconstruction Guideline Working, G. Dutch breast reconstruction guideline. J Plast Reconstr Aesthet Surg 2018, 71, 290–304. [Google Scholar] [CrossRef]
- Zehra, S.; Doyle, F.; Barry, M.; Walsh, S.; Kell, M.R. Health-related quality of life following breast reconstruction compared to total mastectomy and breast-conserving surgery among breast cancer survivors: A systematic review and meta-analysis. Breast Cancer 2020, 27, 534–566. [Google Scholar] [CrossRef]
- Nahabedian, M.Y.; Momen, B.; Galdino, G.; Manson, P.N. Breast Reconstruction with the free TRAM or DIEP flap: Patient selection, choice of flap, and outcome. Plast Reconstr Surg 2002, 110, 466–475, discussion 476–467. [Google Scholar] [CrossRef]
- Nahabedian, M.Y.; Patel, K. Autologous flap breast reconstruction: Surgical algorithm and patient selection. J Surg Oncol 2016, 113, 865–874. [Google Scholar] [CrossRef]
- Savalia, N.B.; Silverstein, M.J. Oncoplastic breast reconstruction: Patient selection and surgical techniques. J Surg Oncol 2016, 113, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.H.; Diaz, R.; Orman, A.G. Breast Reconstruction and Radiation Therapy. Cancer Control 2018, 25, 1073274818795489. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.P.; Shaw, J.; Pusic, A.; Wyld, L.; Morrow, M.; King, T.; Matrai, Z.; Heil, J.; Fitzal, F.; Potter, S.; et al. Oncoplastic breast consortium recommendations for mastectomy and whole breast reconstruction in the setting of post-mastectomy radiation therapy. Breast 2022, 63, 123–139. [Google Scholar] [CrossRef]
- Serletti, J.M. Breast reconstruction with the TRAM flap: Pedicled and free. J Surg Oncol 2006, 94, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Sood, R.; Easow, J.M.; Konopka, G.; Panthaki, Z.J. Latissimus Dorsi Flap in Breast Reconstruction: Recent Innovations in the Workhorse Flap. Cancer Control 2018, 25, 1073274817744638. [Google Scholar] [CrossRef] [PubMed]
- Escandon, J.M.; Escandon, L.; Ahmed, A.; Weiss, A.; Nazerali, R.; Ciudad, P.; Langstein, H.N.; Manrique, O.J. Breast reconstruction using the Latissimus Dorsi Flap and Immediate Fat Transfer (LIFT): A systematic review and meta-analysis. J Plast Reconstr Aesthet Surg 2022, 75, 4106–4116. [Google Scholar] [CrossRef]
- Chang, D.W.; Youssef, A.; Cha, S.; Reece, G.P. Autologous breast reconstruction with the extended latissimus dorsi flap. Plast Reconstr Surg 2002, 110, 751–759, discussion 760–751. [Google Scholar] [CrossRef]
- Elliott, L.F.; Ghazi, B.H.; Otterburn, D.M. The scarless latissimus dorsi flap for full muscle coverage in device-based immediate breast reconstruction: An autologous alternative to acellular dermal matrix. Plast Reconstr Surg 2011, 128, 71–79. [Google Scholar] [CrossRef]
- Cook, J.; Waughtel, J.; Brooks, C.; Hardin, D.; Hwee, Y.K.; Barnavon, Y. The Muscle-Sparing Latissimus Dorsi Flap for Breast Reconstruction: A Retrospective Review of 126 Consecutive Flaps. Ann Plast Surg 2017, 78 (Suppl. S5), S263–S268. [Google Scholar] [CrossRef]
- Chevray, P.M. Breast reconstruction with superficial inferior epigastric artery flaps: A prospective comparison with TRAM and DIEP flaps. Plast Reconstr Surg 2004, 114, 1077–1083, discussion 1084–1075. [Google Scholar] [CrossRef]
- Jeong, W.; Lee, S.; Kim, J. Meta-analysis of flap perfusion and donor site complications for breast reconstruction using pedicled versus free TRAM and DIEP flaps. Breast 2018, 38, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.T.; Mun, G.H. Effects of Obesity on Postoperative Complications After Breast Reconstruction Using Free Muscle-Sparing Transverse Rectus Abdominis Myocutaneous, Deep Inferior Epigastric Perforator, and Superficial Inferior Epigastric Artery Flap: A Systematic Review and Meta-analysis. Ann Plast Surg 2016, 76, 576–584. [Google Scholar] [PubMed]
- Healy, C.; Allen, R.J., Sr. The evolution of perforator flap breast reconstruction: Twenty years after the first DIEP flap. J Reconstr Microsurg 2014, 30, 121–125. [Google Scholar] [PubMed]
- DellaCroce, F.J.; Sullivan, S.K.; Trahan, C. Stacked deep inferior epigastric perforator flap breast reconstruction: A review of 110 flaps in 55 cases over 3 years. Plast Reconstr Surg 2011, 127, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Kaoutzanis, C.; Winocour, J.; Unger, J.; Gabriel, A.; Maxwell, G.P. The Evolution of Breast Implants. Semin Plast Surg 2019, 33, 217–223. [Google Scholar] [CrossRef]
- Sforza, M.; Hammond, D.C.; Botti, G.; Heden, P.; Chacon Quiros, M.; Munhoz, A.M.; Kinney, B.M.; Corduff, N. Expert Consensus on the Use of a New Bioengineered, Cell-Friendly, Smooth Surface Breast Implant. Aesthet Surg J 2019, 39 (Suppl. S3), S95–S102. [Google Scholar] [CrossRef]
- Juanpere, S.; Perez, E.; Huc, O.; Motos, N.; Pont, J.; Pedraza, S. Imaging of breast implants-a pictorial review. Insights Imaging 2011, 2, 653–670. [Google Scholar] [CrossRef]
- Spear, S.L.; Spittler, C.J. Breast reconstruction with implants and expanders. Plast Reconstr Surg 2001, 107, 177–187, quiz 188. [Google Scholar] [CrossRef]
- Schiaffino, S.; Cozzi, A.; Pompei, B.; Scarano, A.L.; Catanese, C.; Catic, A.; Rossi, L.; Del Grande, F.; Harder, Y. MRI-Conditional Breast Tissue Expander: First In-Human Multi-Case Assessment of MRI-Related Complications and Image Quality. J Clin Med 2023, 12. [Google Scholar] [CrossRef]
- Pacella, S.J. Evolution in Tissue Expander Design for Breast Reconstruction: Technological Innovation to Optimize Patient Outcomes. Plast Reconstr Surg 2018, 142, 21S–30S. [Google Scholar] [CrossRef]
- Masia, J. The largest multicentre data collection on prepectoral breast reconstruction: The iBAG study. J Surg Oncol 2020, 122, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Preminger, B.A.; McCarthy, C.M.; Hu, Q.Y.; Mehrara, B.J.; Disa, J.J. The influence of AlloDerm on expander dynamics and complications in the setting of immediate tissue expander/implant reconstruction: A matched-cohort study. Ann Plast Surg 2008, 60, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Khouri, R.K., Jr.; Khouri, R.K. Current Clinical Applications of Fat Grafting. Plast Reconstr Surg 2017, 140, 466e–486e. [Google Scholar] [CrossRef] [PubMed]
- Shamoun, F.; Asaad, M.; Hanson, S.E. Oncologic Safety of Autologous Fat Grafting in Breast Reconstruction. Clin Breast Cancer 2021, 21, 271–277. [Google Scholar] [CrossRef]
- Dialani, V.; Lai, K.C.; Slanetz, P.J. MR imaging of the reconstructed breast: What the radiologist needs to know. Insights Imaging 2012, 3, 201–213. [Google Scholar] [CrossRef]
- Rozen, W.M.; Phillips, T.J.; Ashton, M.W.; Stella, D.L.; Taylor, G.I. A new preoperative imaging modality for free flaps in breast reconstruction: Computed tomographic angiography. Plast Reconstr Surg 2008, 122, 38e–40e. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.D.; Thimmappa, N.D.; Vasile, J.V.; Levine, J.L.; Allen, R.J.; Greenspun, D.T.; Ahn, C.Y.; Chen, C.M.; Hedgire, S.S.; Prince, M.R. Autologous breast reconstruction: Preoperative magnetic resonance angiography for perforator flap vessel mapping. J Reconstr Microsurg 2015, 31, 1–11. [Google Scholar]
- Mohan, A.T.; Saint-Cyr, M. Advances in imaging technologies for planning breast reconstruction. Gland Surg 2016, 5, 242–254. [Google Scholar]
- Zhang, X.; Mu, D.; Yang, Y.; Li, W.; Lin, Y.; Li, H.; Luan, J. Predicting the Feasibility of Utilizing SIEA Flap for Breast Reconstruction with Preoperative BMI and Computed Tomography Angiography (CTA) Data. Aesthetic Plast Surg 2021, 45, 100–107. [Google Scholar] [CrossRef]
- Vasile, J.V.; Levine, J.L. Magnetic resonance angiography in perforator flap breast reconstruction. Gland Surg 2016, 5, 197–211. [Google Scholar]
- Rozen, W.M.; Phillips, T.J.; Ashton, M.W.; Stella, D.L.; Gibson, R.N.; Taylor, G.I. Preoperative imaging for DIEA perforator flaps: A comparative study of computed tomographic angiography and Doppler ultrasound. Plast Reconstr Surg 2008, 121, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Salgarello, M.; Visconti, G. Designing Lateral Thoracic Wall Perforator Flaps for Breast Reconstruction Using Ultrasound. J Reconstr Microsurg 2022, 38, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Thiessen, F.E.F.; Tondu, T.; Cloostermans, B.; Dirkx, Y.A.L.; Auman, D.; Cox, S.; Verhoeven, V.; Hubens, G.; Steenackers, G.; Tjalma, W.A.A. Dynamic InfraRed Thermography (DIRT) in DIEP-flap breast reconstruction: A review of the literature. Eur J Obstet Gynecol Reprod Biol 2019, 242, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Hogge, J.P.; Zuurbier, R.A.; de Paredes, E.S. Mammography of autologous myocutaneous flaps. Radiographics 1999, 19, S63–S72. [Google Scholar] [CrossRef] [PubMed]
- Devon, R.K.; Rosen, M.A.; Mies, C.; Orel, S.G. Breast reconstruction with a transverse rectus abdominis myocutaneous flap: Spectrum of normal and abnormal MR imaging findings. Radiographics 2004, 24, 1287–1299. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.J.; Jung, J.I.; Park, C.; Park, W.C.; Jeon, H.M.; Hahn, S.T.; Lee, J.M. Breast MRI findings after modified radical mastectomy and transverse rectus abdominis myocutaneous flap in patients with breast cancer. J Magn Reson Imaging 2005, 21, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, S.; Hines, N.; Slanetz, P.J. Challenges in mammography: Part 2, multimodality review of breast augmentation--imaging findings and complications. AJR Am J Roentgenol 2011, 197, W1031–W1045. [Google Scholar] [CrossRef]
- Wong, T.; Lo, L.W.; Fung, P.Y.; Lai, H.Y.; She, H.L.; Ng, W.K.; Kwok, K.M.; Lee, C.M. Magnetic resonance imaging of breast augmentation: A pictorial review. Insights Imaging 2016, 7, 399–410. [Google Scholar] [CrossRef]
- Norena-Rengifo, B.D.; Sanin-Ramirez, M.P.; Adrada, B.E.; Luengas, A.B.; Martinez de Vega, V.; Guirguis, M.S.; Saldarriaga-Uribe, C. MRI for Evaluation of Complications of Breast Augmentation. Radiographics 2022, 42, 929–946. [Google Scholar] [CrossRef] [PubMed]
- Soo, M.S.; Kornguth, P.J.; Walsh, R.; Elenberger, C.D.; Georgiade, G.S. Complex radial folds versus subtle signs of intracapsular rupture of breast implants: MR findings with surgical correlation. AJR Am J Roentgenol 1996, 166, 1421–1427. [Google Scholar] [CrossRef]
- Pinel-Giroux, F.M.; El Khoury, M.M.; Trop, I.; Bernier, C.; David, J.; Lalonde, L. Breast reconstruction: Review of surgical methods and spectrum of imaging findings. Radiographics 2013, 33, 435–453. [Google Scholar] [CrossRef]
- Losken, A.; Nicholas, C.S.; Pinell, X.A.; Carlson, G.W. Outcomes evaluation following bilateral breast reconstruction using latissimus dorsi myocutaneous flaps. Ann Plast Surg 2010, 65, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Andrades, P.; Fix, R.J.; Danilla, S.; Howell, R.E., 3rd; Campbell, W.J.; De la Torre, J.; Vasconez, L.O. Ischemic complications in pedicle, free, and muscle sparing transverse rectus abdominis myocutaneous flaps for breast reconstruction. Ann Plast Surg 2008, 60, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, A.K.; Chevray, P.M.; Chang, D.W. Comparison of donor-site complications and functional outcomes in free muscle-sparing TRAM flap and free DIEP flap breast reconstruction. Plast Reconstr Surg 2006, 117, 737–746, discussion 747–750. [Google Scholar] [CrossRef] [PubMed]
- Mulvey, C.L.; Cooney, C.M.; Daily, F.F.; Colantuoni, E.; Ogbuago, O.U.; Cooney, D.S.; Rad, A.N.; Manahan, M.A.; Rosson, G.D.; Sacks, J.M. Increased Flap Weight and Decreased Perforator Number Predict Fat Necrosis in DIEP Breast Reconstruction. Plast Reconstr Surg Glob Open 2013, 1, 1–7. [Google Scholar] [CrossRef]
- Kerridge, W.D.; Kryvenko, O.N.; Thompson, A.; Shah, B.A. Fat Necrosis of the Breast: A Pictorial Review of the Mammographic, Ultrasound, CT, and MRI Findings with Histopathologic Correlation. Radiol Res Pract 2015, 2015, 613139. [Google Scholar] [CrossRef]
- Taboada, J.L.; Stephens, T.W.; Krishnamurthy, S.; Brandt, K.R.; Whitman, G.J. The many faces of fat necrosis in the breast. AJR Am J Roentgenol 2009, 192, 815–825. [Google Scholar] [CrossRef]
- Daly, C.P.; Jaeger, B.; Sill, D.S. Variable appearances of fat necrosis on breast MRI. AJR Am J Roentgenol 2008, 191, 1374–1380. [Google Scholar] [CrossRef]
- Trimboli, R.M.; Carbonaro, L.A.; Cartia, F.; Di Leo, G.; Sardanelli, F. MRI of fat necrosis of the breast: The “black hole” sign at short tau inversion recovery. Eur J Radiol 2012, 81, e573–e579. [Google Scholar] [CrossRef]
- Chirappapha, P.; Somintara, O.; Lertsithichai, P.; Kongdan, Y.; Supsamutchai, C.; Sukpanich, R. Complications and oncologic outcomes of pedicled transverse rectus abdominis myocutaneous flap in breast cancer patients. Gland Surg 2016, 5, 405–415. [Google Scholar] [CrossRef]
- Sailon, A.M.; Schachar, J.S.; Levine, J.P. Free transverse rectus abdominis myocutaneous and deep inferior epigastric perforator flaps for breast reconstruction: A systematic review of flap complication rates and donor-site morbidity. Ann Plast Surg 2009, 62, 560–563. [Google Scholar] [PubMed]
- Chirappapha, P.; Trikunagonvong, N.; Prapruttam, D.; Rongthong, S.; Lertsithichai, P.; Sukarayothin, T.; Leesombatpaiboon, M.; Panawattanakul, R.; Thaweepworadej, P. Donor-Site Complications and Remnant of Rectus Abdominis Muscle Status after Transverse Rectus Abdominis Myocutaneous Flap Reconstruction. Plast Reconstr Surg Glob Open 2017, 5, e1387. [Google Scholar] [CrossRef] [PubMed]
- Caputo, G.G.; Mura, S.; Albanese, R.; Zingaretti, N.; Pier Camillo, P. Seroma Formation in Pre-pectoral Implant-Based ADM Assisted Breast Reconstruction: A Comprehensive Review of Current Literature. Chirurgia (Bucur) 2021, 116 (Suppl. S2), 16–23. [Google Scholar] [CrossRef]
- Daar, D.A.; Bekisz, J.M.; Chiodo, M.V.; DeMitchell-Rodriguez, E.M.; Saadeh, P.B. Hematoma After Non-Oncologic Breast Procedures: A Comprehensive Review of the Evidence. Aesthetic Plast Surg 2021, 45, 2602–2617. [Google Scholar] [PubMed]
- Dassoulas, K.R.; Wang, J.; Thuman, J.; Ndem, I.; Schaeffer, C.; Stovall, M.; Tilt, A.; Lee, A.; Lin, K.Y.; Campbell, C.A. Reducing Infection Rates in Implant-Based Breast Reconstruction: Impact of an Evidence-based Protocol. Ann Plast Surg 2018, 80, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Barr, S.P.; Topps, A.R.; Barnes, N.L.; Henderson, J.; Hignett, S.; Teasdale, R.L.; McKenna, A.; Harvey, J.R.; Kirwan, C.C.; Northwest Breast Surgical Research, C. Infection prevention in breast implant surgery - A review of the surgical evidence, guidelines and a checklist. Eur J Surg Oncol 2016, 42, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Singh, D.; Parsa, F.D. Review of Early Signs of Breast Implant Infection. Aesthetic Plast Surg 2022, 46, 2152–2158. [Google Scholar] [CrossRef]
- Dobke, M.; Hauch, A.; Crowley, J. Subclinical Infection of the Silicone Breast Implant Surface as a Possible Cause of Capsular Contracture: A Follow-Up. Aesthetic Plast Surg 2020, 44, 1148–1150. [Google Scholar] [CrossRef]
- Driscoll, C.R.; Prabhu, S.S.; Davidson, A.L.; Katz, A.J. Quantity of Acellular Dermal Matrix in Immediate Breast Reconstruction and Outcomes. Ann Plast Surg 2022, 88 (Suppl. S5), S410–S413. [Google Scholar] [CrossRef]
- Marques, M.; Brown, S.A.; Oliveira, I.; Cordeiro, M.; Morales-Helguera, A.; Rodrigues, A.; Amarante, J. Long-term follow-up of breast capsule contracture rates in cosmetic and reconstructive cases. Plast Reconstr Surg 2010, 126, 769–778. [Google Scholar] [CrossRef]
- Bachour, Y. Capsular Contracture in Breast Implant Surgery: Where Are We Now and Where Are We Going? Aesthetic Plast Surg 2021, 45, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Prantl, L.; Angele, P.; Schreml, S.; Ulrich, D.; Poppl, N.; Eisenmann-Klein, M. Determination of serum fibrosis indexes in patients with capsular contracture after augmentation with smooth silicone gel implants. Plast Reconstr Surg 2006, 118, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Graziano, F.D.; Henderson, P.W.; Jacobs, J.; Salzberg, C.A.; Sbitany, H. How to Optimize Prepectoral Breast Reconstruction. Aesthet Surg J 2020, 40 (Suppl. S2), S22–S28. [Google Scholar] [CrossRef] [PubMed]
- Haran, O.; Bracha, G.; Tiosano, A.; Menes, T.; Madah, E.; Gur, E.; Barnea, Y.; Arad, E. Postirradiation Capsular Contracture in Implant-Based Breast Reconstruction: Management and Outcome. Plast Reconstr Surg 2021, 147, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Spear, S.L.; Baker, J.L., Jr. Classification of capsular contracture after prosthetic breast reconstruction. Plast Reconstr Surg 1995, 96, 1119–1123, discussion 1124. [Google Scholar] [CrossRef]
- Green, L.A.; Karow, J.A.; Toman, J.E.; Lostumbo, A.; Xie, K. Review of breast augmentation and reconstruction for the radiologist with emphasis on MRI. Clin Imaging 2018, 47, 101–117. [Google Scholar] [CrossRef]
- Holmich, L.R.; Kjoller, K.; Vejborg, I.; Conrad, C.; Sletting, S.; McLaughlin, J.K.; Fryzek, J.; Breiting, V.; Jorgensen, A.; Olsen, J.H. Prevalence of silicone breast implant rupture among Danish women. Plast Reconstr Surg 2001, 108, 848–858, discussion 859–863. [Google Scholar] [CrossRef]
- Holmich, L.R.; Friis, S.; Fryzek, J.P.; Vejborg, I.M.; Conrad, C.; Sletting, S.; Kjoller, K.; McLaughlin, J.K.; Olsen, J.H. Incidence of silicone breast implant rupture. Arch Surg 2003, 138, 801–806. [Google Scholar] [CrossRef]
- Seiler, S.J.; Sharma, P.B.; Hayes, J.C.; Ganti, R.; Mootz, A.R.; Eads, E.D.; Teotia, S.S.; Evans, W.P. Multimodality Imaging-based Evaluation of Single-Lumen Silicone Breast Implants for Rupture. Radiographics 2017, 37, 366–382. [Google Scholar] [CrossRef]
- Ahn, C.Y.; DeBruhl, N.D.; Gorczyca, D.P.; Shaw, W.W.; Bassett, L.W. Comparative silicone breast implant evaluation using mammography, sonography, and magnetic resonance imaging: Experience with 59 implants. Plast Reconstr Surg 1994, 94, 620–627. [Google Scholar] [CrossRef]
- Ikeda, D.M.; Borofsky, H.B.; Herfkens, R.J.; Sawyer-Glover, A.M.; Birdwell, R.L.; Glover, G.H. Silicone breast implant rupture: Pitfalls of magnetic resonance imaging and relative efficacies of magnetic resonance, mammography, and ultrasound. Plast Reconstr Surg 1999, 104, 2054–2062. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, M.; Caskey, C.I. Imaging spectrum of breast implant complications: Mammography, ultrasound, and magnetic resonance imaging. Semin Ultrasound CT MR 2000, 21, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.C.; Malay, S.; Shauver, M.J.; Kim, H.M. Economic analysis of screening strategies for rupture of silicone gel breast implants. Plast Reconstr Surg 2012, 130, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Salzman, M.J. Silent Rupture of Silicone Gel Breast Implants: High-Resolution Ultrasound Scans and Surveys of 584 Women. Plast Reconstr Surg 2022, 149, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Goldammer, F.; Pinsolle, V.; Dissaux, C.; Pelissier, P. Accuracy of mammography, sonography and magnetic resonance imaging for detecting silicone breast implant ruptures: A retrospective observational study of 367 cases. Ann Chir Plast Esthet 2021, 66, 25–41. [Google Scholar] [CrossRef]
- Rochira, D.; Cavalcanti, P.; Ottaviani, A.; Tambasco, D. Longitudinal Ultrasound Study of Breast Implant Rupture Over a Six-Year Interval. Ann Plast Surg 2016, 76, 150–154. [Google Scholar] [CrossRef]
- Salemis, N.S. Axillary silicone lymphadenopathy. An important differential in patients with post-mastectomy reconstruction with silicone gel implants. Breast J 2020, 26, 1821–1822. [Google Scholar] [CrossRef]
- Safvi, A. Linguine sign. Radiology 2000, 216, 838–839. [Google Scholar] [CrossRef]
- Soo, M.S.; Kornguth, P.J.; Walsh, R.; Elenberger, C.; Georgiade, G.S.; DeLong, D.; Spritzer, C.E. Intracapsular implant rupture: MR findings of incomplete shell collapse. J Magn Reson Imaging 1997, 7, 724–730. [Google Scholar] [CrossRef]
- Fleury, E.F.; Rego, M.M.; Ramalho, L.C.; Ayres, V.J.; Seleti, R.O.; Ferreira, C.A.; Roveda, D., Jr. Silicone-induced granuloma of breast implant capsule (SIGBIC): Similarities and differences with anaplastic large cell lymphoma (ALCL) and their differential diagnosis. Breast Cancer (Dove Med Press) 2017, 9, 133–140. [Google Scholar] [CrossRef]
- Castro, C.; Fernandes, D.; Mendonca, M.; Roveda Junior, D.; Badan, G.; Fleury, E.F.C. Silicone-induced granuloma of breast implant capsule mimicking anaplastic large cell lymphoma. Breast J 2020, 26, 1028–1030. [Google Scholar] [CrossRef] [PubMed]
- Maijers, M.C.; Niessen, F.B.; Veldhuizen, J.F.; Ritt, M.J.; Manoliu, R.A. MRI screening for silicone breast implant rupture: Accuracy, inter- and intraobserver variability using explantation results as reference standard. Eur Radiol 2014, 24, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Keech, J.A., Jr.; Creech, B.J. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg 1997, 100, 554–555. [Google Scholar] [CrossRef] [PubMed]
- Siaghani, P.J.; Song, J.Y. Updates of Peripheral T Cell Lymphomas Based on the 2017 WHO Classification. Curr Hematol Malig Rep 2018, 13, 25–36. [Google Scholar] [CrossRef]
- Marra, A.; Viale, G.; Pileri, S.A.; Pravettoni, G.; Viale, G.; De Lorenzi, F.; Nole, F.; Veronesi, P.; Curigliano, G. Breast implant-associated anaplastic large cell lymphoma: A comprehensive review. Cancer Treat Rev 2020, 84, 101963. [Google Scholar] [CrossRef]
- Doren, E.L.; Miranda, R.N.; Selber, J.C.; Garvey, P.B.; Liu, J.; Medeiros, L.J.; Butler, C.E.; Clemens, M.W. U.S. Epidemiology of Breast Implant-Associated Anaplastic Large Cell Lymphoma. Plast Reconstr Surg 2017, 139, 1042–1050. [Google Scholar] [CrossRef]
- Sharma, B.; Jurgensen-Rauch, A.; Pace, E.; Attygalle, A.D.; Sharma, R.; Bommier, C.; Wotherspoon, A.C.; Sharma, S.; Iyengar, S.; El-Sharkawi, D. Breast Implant-associated Anaplastic Large Cell Lymphoma: Review and Multiparametric Imaging Paradigms. Radiographics 2020, 40, 609–628. [Google Scholar] [CrossRef]
- Adrada, B.E.; Miranda, R.N.; Rauch, G.M.; Arribas, E.; Kanagal-Shamanna, R.; Clemens, M.W.; Fanale, M.; Haideri, N.; Mustafa, E.; Larrinaga, J.; Reisman, N.R.; Jaso, J.; You, M.J.; Young, K.H.; Medeiros, L.J.; Yang, W. Breast implant-associated anaplastic large cell lymphoma: Sensitivity, specificity, and findings of imaging studies in 44 patients. Breast Cancer Res Treat 2014, 147, 1–14. [Google Scholar] [CrossRef]
- Rotili, A.; Ferrari, F.; Nicosia, L.; Pesapane, F.; Tabanelli, V.; Fiori, S.; Vanazzi, A.; Meneghetti, L.; Abbate, F.; Latronico, A.; Cassano, E. MRI features of breast implant-associated anaplastic large cell lymphoma. Br J Radiol 2021, 94, 20210093. [Google Scholar] [CrossRef]
- Glasberg, S.B.; Sommers, C.A.; McClure, G.T. Breast Implant-associated Squamous Cell Carcinoma: Initial Review and Early Recommendations. Plast Reconstr Surg Glob Open 2023, 11, e5072. [Google Scholar] [CrossRef]
- Tzur, R.; Silberstein, E.; Krieger, Y.; Shoham, Y.; Rafaeli, Y.; Bogdanov-Berezovsky, A. Desmoid Tumor and Silicone Breast Implant Surgery: Is There Really a Connection? A Literature Review. Aesthetic Plast Surg 2018, 42, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Grubstein, A.; Rapson, Y.; Zer, A.; Gadiel, I.; Atar, E.; Morgenstern, S.; Gutman, H. MRI diagnosis and follow-up of chest wall and breast desmoid tumours in patients with a history of oncologic breast surgery and silicone implants: A pictorial report. J Med Imaging Radiat Oncol 2019, 63, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Breast, I.; Heller, S.L.; Lourenco, A.P.; Niell, B.L.; Ajkay, N.; Brown, A.; Dibble, E.H.; Didwania, A.D.; Jochelson, M.S.; Klein, K.A.; Mehta, T.S.; Pass, H.A.; Stuckey, A.R.; Swain, M.E.; Tuscano, D.S.; Moy, L. ACR Appropriateness Criteria(R) Imaging After Mastectomy and Breast Reconstruction. J Am Coll Radiol 2020, 17 (Suppl. S11), S403–S414. [Google Scholar]
- Adrada, B.E.; Karbasian, N.; Huang, M.; Rauch, G.M.; Woodtichartpreecha, P.; Whitman, G. Imaging Surveillance of the Reconstructed Breast in a Subset of Patients May Aid in Early Detection of Breast Cancer Recurrence. J Clin Imaging Sci 2021, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Bargon, C.A.; Young-Afat, D.A.; Ikinci, M.; Braakenburg, A.; Rakhorst, H.A.; Mureau, M.A.M.; Verkooijen, H.M.; Doeksen, A. Breast cancer recurrence after immediate and delayed postmastectomy breast reconstruction-A systematic review and meta-analysis. Cancer 2022, 128, 3449–3469. [Google Scholar] [CrossRef]
- Casey, W.J., 3rd; Rebecca, A.M.; Silverman, A.; Macias, L.H.; Kreymerman, P.A.; Pockaj, B.A.; Gray, R.J.; Chang, Y.H.; Smith, A.A. Etiology of breast masses after autologous breast reconstruction. Ann Surg Oncol 2013, 20, 607–614. [Google Scholar] [CrossRef]
- Kaidar-Person, O.; Vrou Offersen, B.; Hol, S.; Arenas, M.; Aristei, C.; Bourgier, C.; Cardoso, M.J.; Chua, B.; Coles, C.E.; Engberg Damsgaard, T.; Gabrys, D.; Jagsi, R.; Jimenez, R.; Kirby, A.M.; Kirkove, C.; Kirova, Y.; Kouloulias, V.; Marinko, T.; Meattini, I.; Mjaaland, I.; Nader Marta, G.; Witt Nystrom, P.; Senkus, E.; Skytta, T.; Tvedskov, T.F.; Verhoeven, K.; Poortmans, P. ESTRO ACROP consensus guideline for target volume delineation in the setting of postmastectomy radiation therapy after implant-based immediate reconstruction for early stage breast cancer. Radiother Oncol 2019, 137, 159–166. [Google Scholar] [CrossRef]
- Joo, J.H.; Yang, J.D.; Park, H.Y.; Park, J.; Wu, Z.Y.; Ko, B.; Park, J.; Kim, S.S. The patterns and spatial locations of local recurrence in breast cancer with implant-based reconstruction after mastectomy. Radiother Oncol 2022, 170, 111–117. [Google Scholar] [CrossRef]
- Smith, D.; Sepehr, S.; Karakatsanis, A.; Strand, F.; Valachis, A. Yield of Surveillance Imaging After Mastectomy With or Without Reconstruction for Patients With Prior Breast Cancer: A Systematic Review and Meta-analysis. JAMA Netw Open 2022, 5, e2244212. [Google Scholar] [CrossRef]
- Edeiken, B.S.; Fornage, B.D.; Bedi, D.G.; Sneige, N.; Parulekar, S.G.; Pleasure, J. Recurrence in autogenous myocutaneous flap reconstruction after mastectomy for primary breast cancer: US diagnosis. Radiology 2003, 227, 542–548. [Google Scholar] [CrossRef]
- Fentiman, I.S. Surgical options for male breast cancer. Breast Cancer Res Treat 2018, 172, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Al-Kalla, T.; Komorowska-Timek, E. Breast total male breast reconstruction with fat grafting. Plast Reconstr Surg Glob Open 2014, 2, e257. [Google Scholar] [CrossRef] [PubMed]
- Deldar, R.; Sayyed, A.A.; Towfighi, P.; Aminpour, N.; Sogunro, O.; Son, J.D.; Fan, K.L.; Song, D.H. Postmastectomy Reconstruction in Male Breast Cancer. Breast J 2022, 2022, 5482261. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Deep Inferior Epigastric Perforator (DIEP) reconstruction. (a) Mediolateral Oblique mammographic (MLO) view of the reconstructed breast depicts essentially fatty tissue and surgical clips (arrows); (b) Axial thoracic CT scan confirms the presence of a viable DIEP reconstruction with no complications.
Figure 1.
Deep Inferior Epigastric Perforator (DIEP) reconstruction. (a) Mediolateral Oblique mammographic (MLO) view of the reconstructed breast depicts essentially fatty tissue and surgical clips (arrows); (b) Axial thoracic CT scan confirms the presence of a viable DIEP reconstruction with no complications.
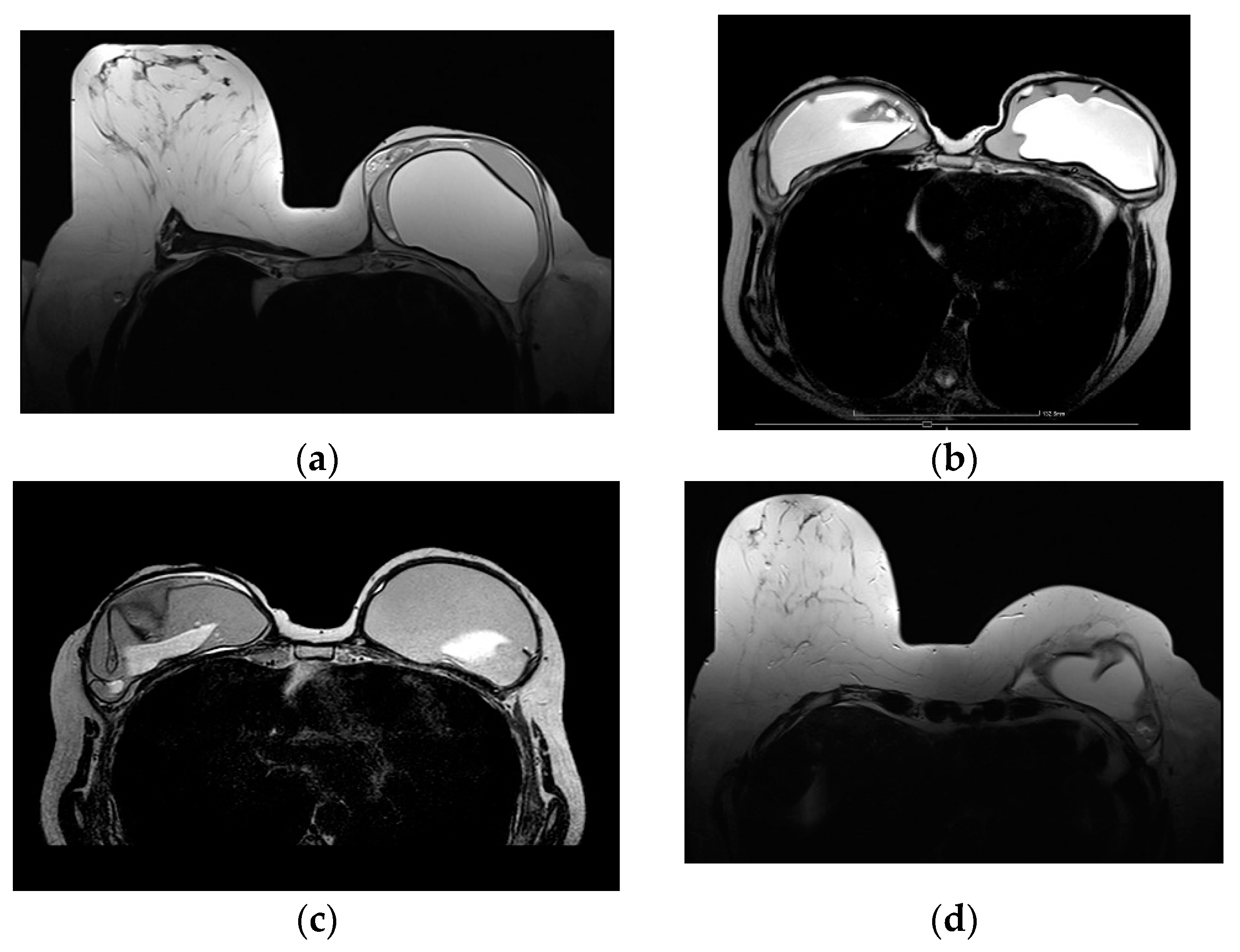
Figure 2.
Bilateral autologous Latissimus Dorsi (LD) reconstruction. Yellow arrows point the characteristic tailed aspect of the muscle. Red arrows depict a hypointense circumscribed area with irregular wall enhancement and associated solid enhancing nodule corresponding to a local relapse of high grade invasive ductal carcinoma, triple negative, with marked central necrosis.
Figure 2.
Bilateral autologous Latissimus Dorsi (LD) reconstruction. Yellow arrows point the characteristic tailed aspect of the muscle. Red arrows depict a hypointense circumscribed area with irregular wall enhancement and associated solid enhancing nodule corresponding to a local relapse of high grade invasive ductal carcinoma, triple negative, with marked central necrosis.
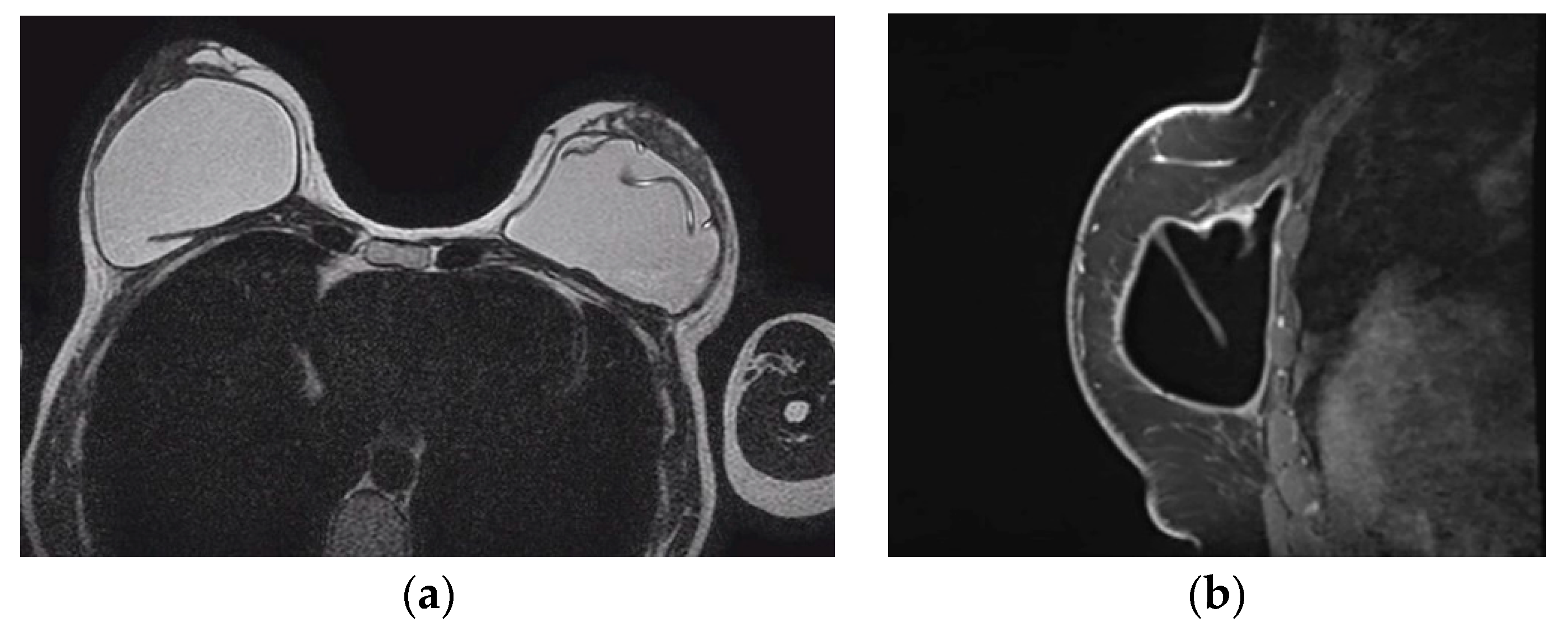
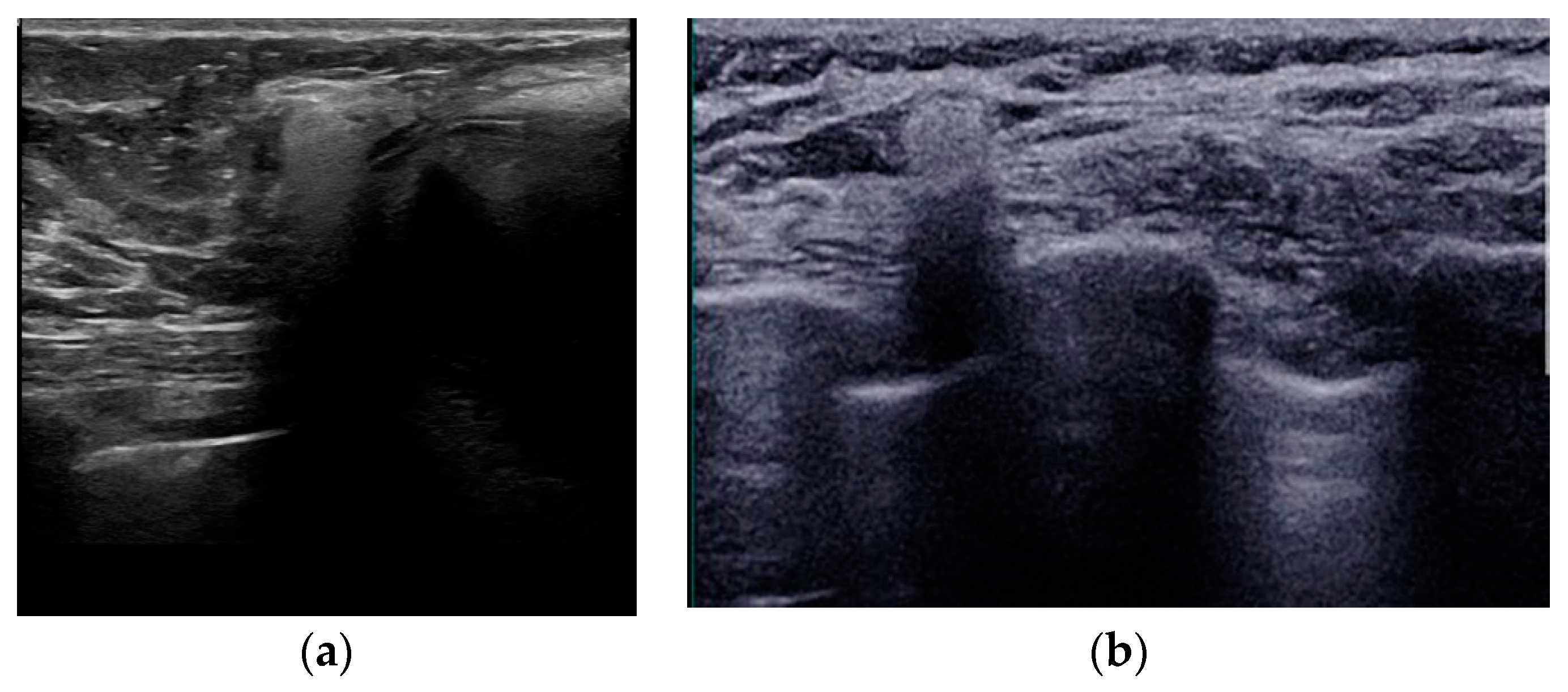
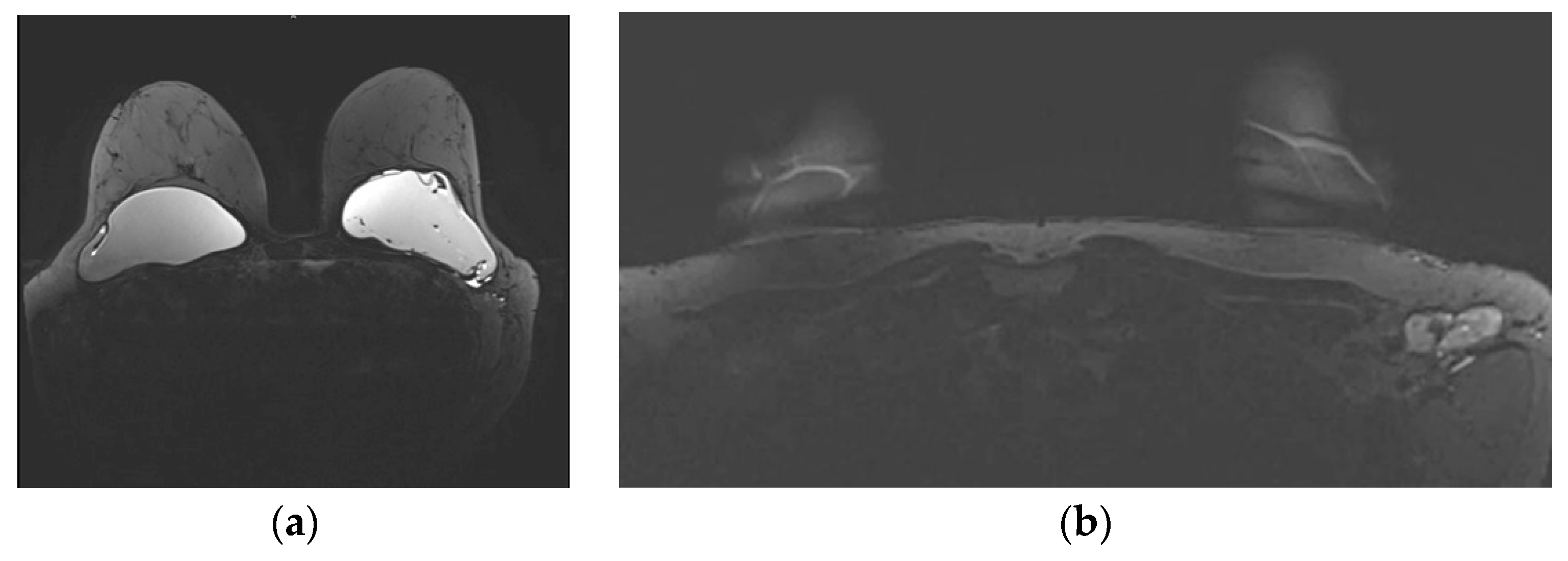
Figure 3.
Mediolateral oblique MLO mammography view and ultrasound (US) of the right breast after mastectomy and reconstruction with a single lumen silicone implant (a) At the MLO view the implant appears as a homogenous radiopaque oval mass with smooth borders; (b) On US the implant is anechoic. The intact shell appears as echogenic line (yellow arrows) and the fibrous capsule is seen as a parallel echogenic line (red arrows).
Figure 3.
Mediolateral oblique MLO mammography view and ultrasound (US) of the right breast after mastectomy and reconstruction with a single lumen silicone implant (a) At the MLO view the implant appears as a homogenous radiopaque oval mass with smooth borders; (b) On US the implant is anechoic. The intact shell appears as echogenic line (yellow arrows) and the fibrous capsule is seen as a parallel echogenic line (red arrows).
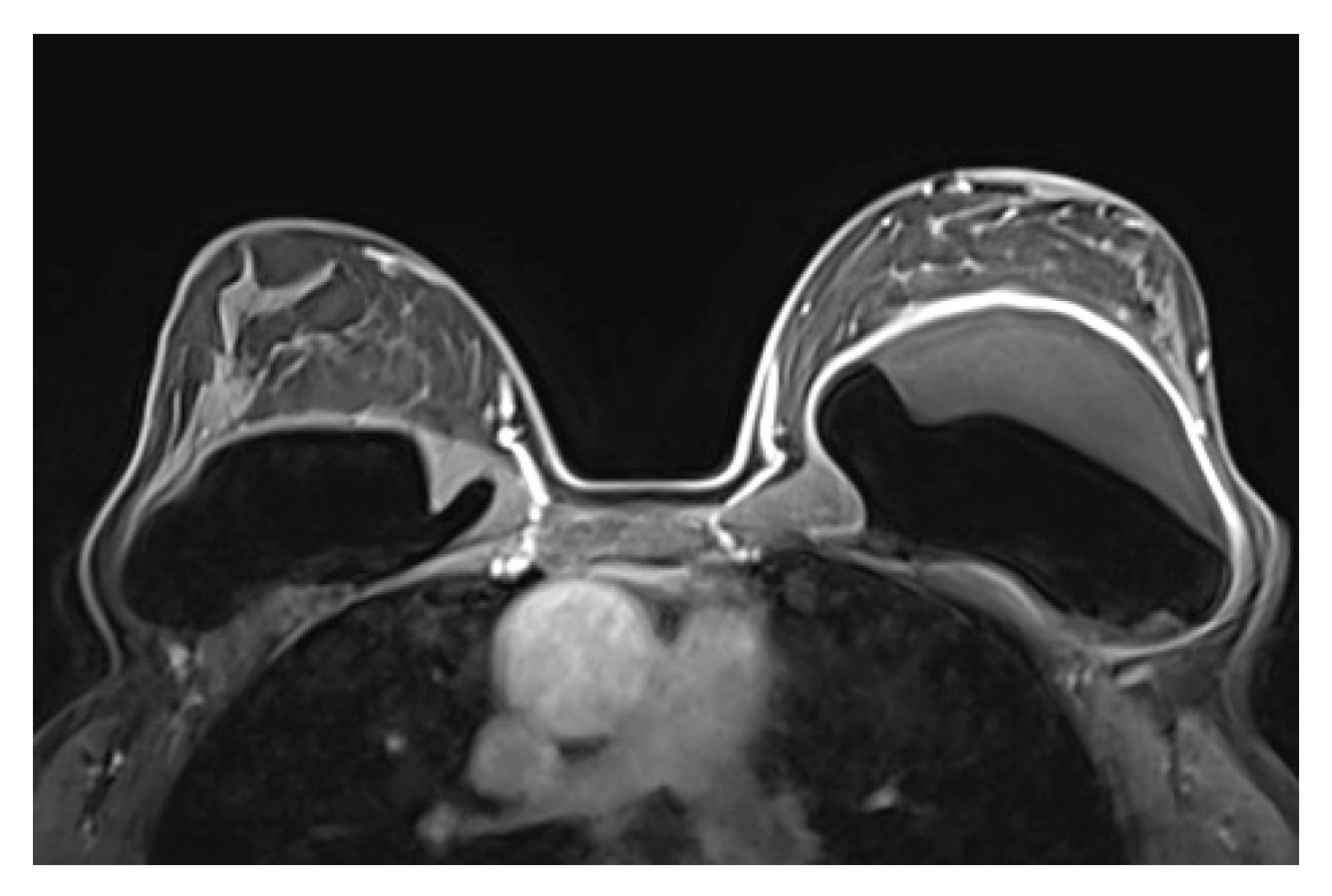
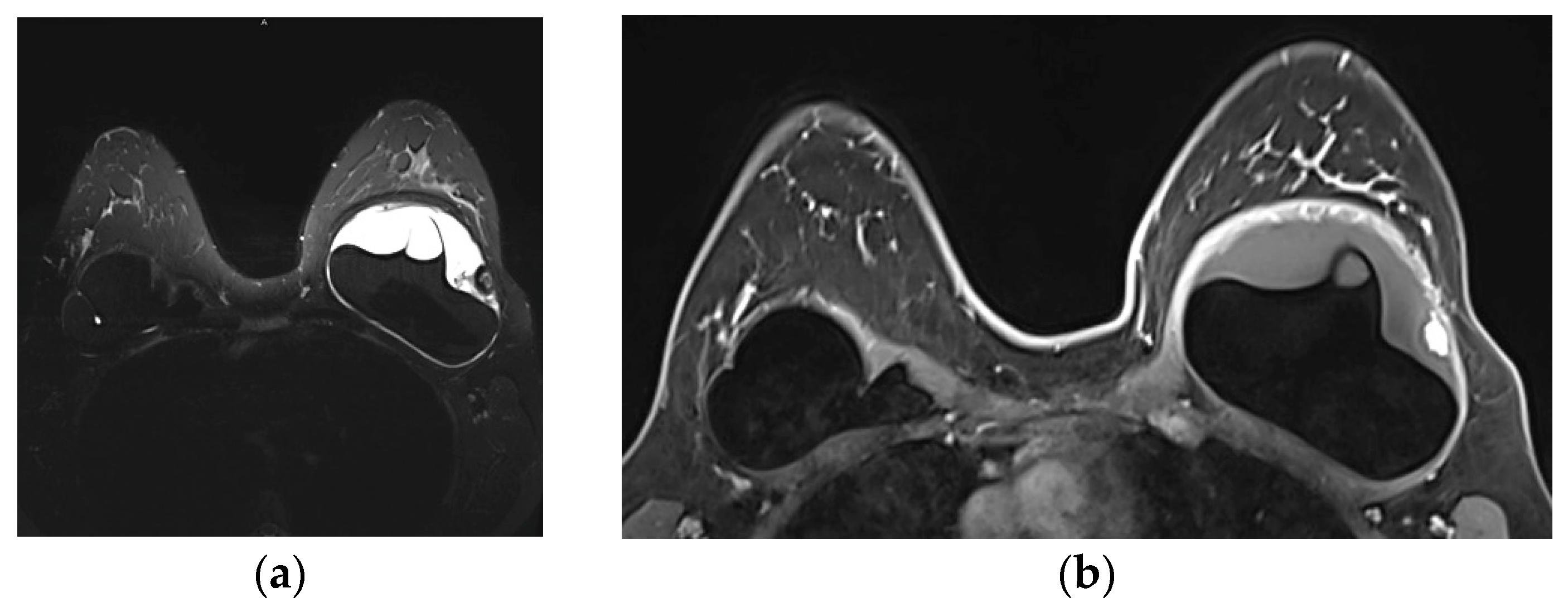
Figure 4.
Normal MRI appearance of an intact single lumen silicone implant of the reconstructed right breast; (a) At the T1 non-fat saturated sequence the silicone has low signal intensity; On T2-weighted images, non-fat saturated (b) and fat-saturated (c) the silicone has high signal intensity. Minimal peri-implant fluid is a common normal finding with high signal intensity; (d) At the silicone-selective sequence the silicone appears with high signal while the peri-implant fluid has low signal intensity.
Figure 4.
Normal MRI appearance of an intact single lumen silicone implant of the reconstructed right breast; (a) At the T1 non-fat saturated sequence the silicone has low signal intensity; On T2-weighted images, non-fat saturated (b) and fat-saturated (c) the silicone has high signal intensity. Minimal peri-implant fluid is a common normal finding with high signal intensity; (d) At the silicone-selective sequence the silicone appears with high signal while the peri-implant fluid has low signal intensity.
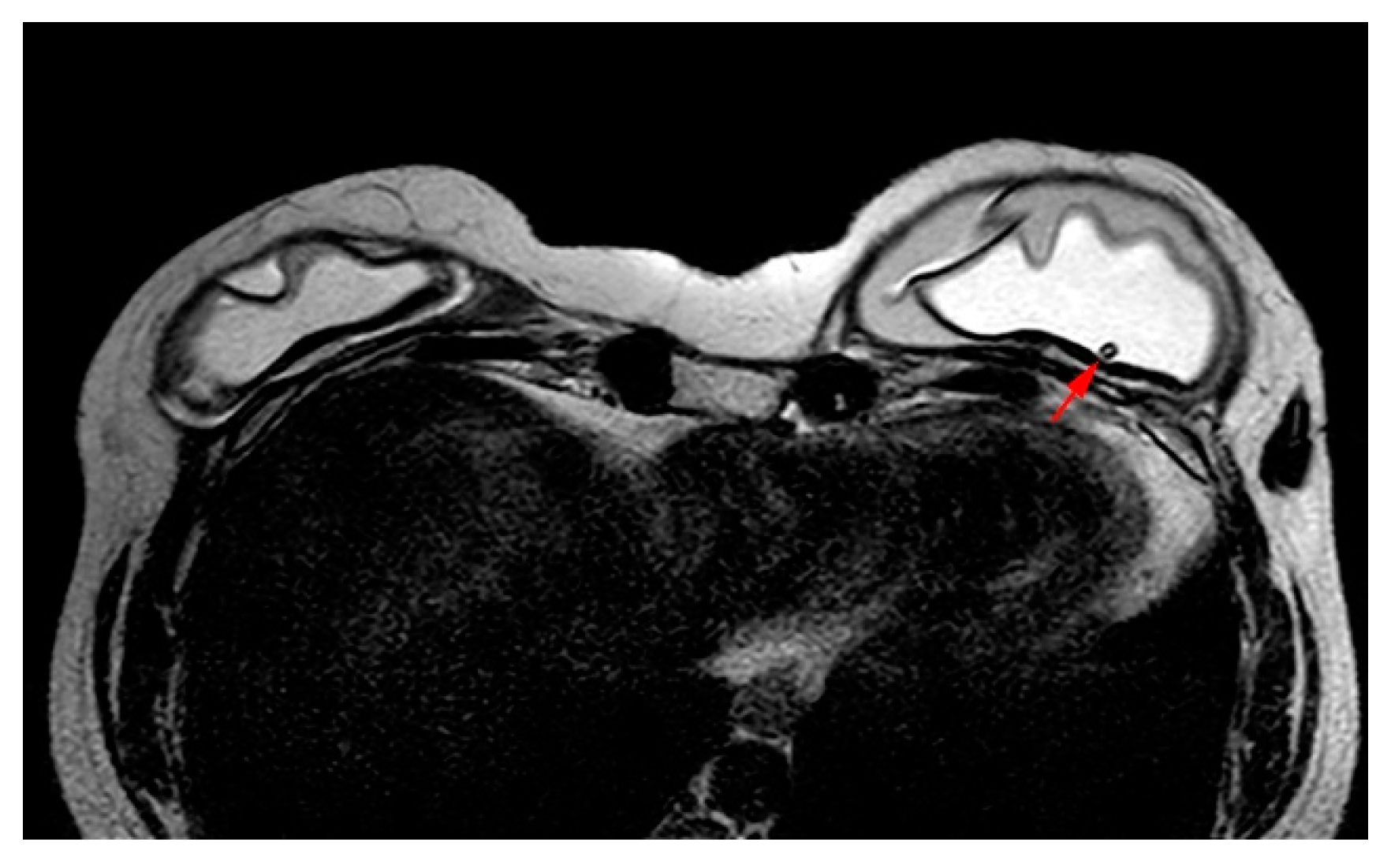
Figure 5.
MRI appearance of double lumen implant (silicone in the outer lumen, saline in the inner lumen). Red arrow points at the valve used to inflate the inner saline part.
Figure 5.
MRI appearance of double lumen implant (silicone in the outer lumen, saline in the inner lumen). Red arrow points at the valve used to inflate the inner saline part.
Figure 6.
MRI appearance of single lumen saline implants with radial fold of the right implant and intracapsular rupture of the left implant; (a) Axial T2 sequence depicts a perpendicular to the implant shell line corresponding to a radial fold whereas the curvilinear, parallel to the shell, line indicates a subcapsular line, in concordance with an intracapsular rupture; (b) Sagittal view confirms the “perpendicular to implant-shell” character of the radial fold of the right implant. Sagittal views can be of help in differentiating radial folds from true intracapsular rupture.
Figure 6.
MRI appearance of single lumen saline implants with radial fold of the right implant and intracapsular rupture of the left implant; (a) Axial T2 sequence depicts a perpendicular to the implant shell line corresponding to a radial fold whereas the curvilinear, parallel to the shell, line indicates a subcapsular line, in concordance with an intracapsular rupture; (b) Sagittal view confirms the “perpendicular to implant-shell” character of the radial fold of the right implant. Sagittal views can be of help in differentiating radial folds from true intracapsular rupture.
Figure 7.
MRI appearance of left implant infection. Axial T1-weighted fat-saturation post-contrast view of a left peri-implant inflammatory infusion with peripheral smooth contrast uptake.
Figure 7.
MRI appearance of left implant infection. Axial T1-weighted fat-saturation post-contrast view of a left peri-implant inflammatory infusion with peripheral smooth contrast uptake.
Figure 8.
Ultrasound appearance of extracapsular rupture. (a) Free silicone in the breast along the upper outer part of the implant, indicating extracapsular rupture. Typical appearance of a marked hyperechoic area with posterior shadowing, referred to as “snowstorm appearance”. (b) “Snowstorm” appearance of an axillary lymph node in another patient. This was an isolated finding, and no rupture was documented on MRI. This was related to silicone gel bleed, i.e.microscopic diffusion of silicone molecules through the semipermeable weakened but intact implant elastomer shell.
Figure 8.
Ultrasound appearance of extracapsular rupture. (a) Free silicone in the breast along the upper outer part of the implant, indicating extracapsular rupture. Typical appearance of a marked hyperechoic area with posterior shadowing, referred to as “snowstorm appearance”. (b) “Snowstorm” appearance of an axillary lymph node in another patient. This was an isolated finding, and no rupture was documented on MRI. This was related to silicone gel bleed, i.e.microscopic diffusion of silicone molecules through the semipermeable weakened but intact implant elastomer shell.
Figure 9.
MRI appearance of various signs indicative of intracapsular and extracapsular rupture. All images are axial T2-weigthed images from different patients. (a) Salad oil and subcapsular line sign, evocating intracapsular rupture. (b) Isolated salad-oil sign in both implants. This sign is not sufficient per se to validate an intracapsular rupture. (c) key-hole sign, lasso sign and linguni sign, indicating intracapsular rupture of the right implant. (d) extracapsular rupture can be safely indicated on this characteristic aspect of extra-implant presence of silicone.
Figure 9.
MRI appearance of various signs indicative of intracapsular and extracapsular rupture. All images are axial T2-weigthed images from different patients. (a) Salad oil and subcapsular line sign, evocating intracapsular rupture. (b) Isolated salad-oil sign in both implants. This sign is not sufficient per se to validate an intracapsular rupture. (c) key-hole sign, lasso sign and linguni sign, indicating intracapsular rupture of the right implant. (d) extracapsular rupture can be safely indicated on this characteristic aspect of extra-implant presence of silicone.
Figure 10.
MRI appearance of extracapsular rupture. (a) Axial silicone-only sequence depicts hyperintense free droplets of silicone outside the left implant capsule. (b) Same patient, axial silicone-only sequence through the axillary level depicts marked hyperintense ipsilateral axillary lymph nodes, evocating silicone deposits.
Figure 10.
MRI appearance of extracapsular rupture. (a) Axial silicone-only sequence depicts hyperintense free droplets of silicone outside the left implant capsule. (b) Same patient, axial silicone-only sequence through the axillary level depicts marked hyperintense ipsilateral axillary lymph nodes, evocating silicone deposits.
Figure 11.
Silicone granuloma associated with peri-implant effusion and mild inflammation. (a) Axial Short Tau Inversion Recovery (STIR) sequence depicts hyperintense peri-implant effusion associated with a hypointense nodular component. (b) Axial T1 fat-saturated post contrast sequence readily depicts the strong enhancement of the nodular component and the mild enhancement of the peri-implant effusion collection. This aspect could evocate a silicone granuloma, nevertheless a core biopsy was performed to validate this diagnosis.
Figure 11.
Silicone granuloma associated with peri-implant effusion and mild inflammation. (a) Axial Short Tau Inversion Recovery (STIR) sequence depicts hyperintense peri-implant effusion associated with a hypointense nodular component. (b) Axial T1 fat-saturated post contrast sequence readily depicts the strong enhancement of the nodular component and the mild enhancement of the peri-implant effusion collection. This aspect could evocate a silicone granuloma, nevertheless a core biopsy was performed to validate this diagnosis.
Figure 12.
BRCA1 carrier. Right mastectomy due to extensive comedo-DCIS and autologous reconstruction. Prophylactic left mastectomy and reconstruction. (a) Maximum Intensity Projection (MIP) images depict a small enhancing nodule, with a millimetric satellite lesion, both suspicious in this context. (b) Second-look post MRI ultrasound depicts a hypoechoic, taller-than-wider solid nodule with irregular margins. Core biopsy confirmed local relapse of high-grade DCIS.
Figure 12.
BRCA1 carrier. Right mastectomy due to extensive comedo-DCIS and autologous reconstruction. Prophylactic left mastectomy and reconstruction. (a) Maximum Intensity Projection (MIP) images depict a small enhancing nodule, with a millimetric satellite lesion, both suspicious in this context. (b) Second-look post MRI ultrasound depicts a hypoechoic, taller-than-wider solid nodule with irregular margins. Core biopsy confirmed local relapse of high-grade DCIS.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).