1. Introduction
Advanced heart failure (AHF) is a clinical syndrome characterized by signs and symptoms of volume overload and inadequate blood perfusion due to a severe cardiac dysfunction, persisting despite maximal therapy, and causing recurrent hospitalizations and high mortality (1). The prevalence of AHF is increasing due to ageing of population, better treatment, and survival from HF (2).
Despite this, the prognosis of these patients is still poor with a 1-year mortality ranging from 25% to 75% (1). Moreover, they suffer from recurrent episodes of pulmonary or systemic congestion, low output and/or malignant arrhythmias causing at least one unplanned visit or hospitalization/year (3).
Usually, AHF patients have a severe impairment of exercise capacity (6-minute walking test (6MWT) distance (<300 m) or pVO2 <12 ml/kg/min or <50% predicted value), and severely depressed left ventricular (LV) function (4).
At this stage, patients respond poorly to conventional therapies including optimal medical therapy, implantable cardioverter defibrillator (ICD), cardiac resynchronization therapy (CRT), percutaneous and surgical approaches to the valves and coronary arteries with a wide clinical scenario ranging from progressive refractory deterioration to cardiogenic shock (1).
Although inotropic agents are helpful, they can be used for a short period of time, and they can cause myocardial ischemia and/or tachyarrhythmias (5). According to the latest European Society of cardiology (ESC) heart failure guidelines, sacubritil-valsartan or sodium-glucose co-transporter-2 (SGLT2) inhibitors can be added, the dose of loop diuretics can be doubled, or they can be combined with thiazide-type diuretics or metolazone.in patients who are refractory to diuretic treatment continuous renal replacement therapy should be considered (1).
The INTERMACS classification is a key classification system establishing different degrees of HF severity, starting from class III NYHA up to critical cardiogenic shock despite escalating support (6). Short-term mechanical cardiac support (MCS) devices are the system of choice in INTERMACS 1 or 2 patients (7). Therefore, patients that are not stabilized by medical therapies alone are eligible for MCS devices either in acute or in the chronic setting, and clinicians have at their disposal both short- and long-term MCS devices (7, 8).
The gold standard of care for eligible patients with advanced, refractory HF is represented by orthotopic cardiac transplant (OCT), which demonstrated to increase quality of life and survival (9). However, the limited availability of donors and the presence of numerous contraindications make this option applicable in a limited number of cases (1).
MCS can be used as bridge to decision (BTD), bridge to recovery (BTR), bridge to other bridge therapies (BTB) such as long-term MCS, or to urgent cardiac transplant (BTT) (8). They include intra-aortic balloon pump (IABP), Impella and veno-arterial extracorporeal membrane oxygenation (ECMO) and left ventricular assist devices (LVAD).
ESC guidelines identify as potential LVAD candidates those patients with advanced HF, severe symptoms despite optimal medical or device therapy, without severe right ventricular dysfunction or tricuspid regurgitation, and without major contraindications, who have at least one of the following:
1. LVEF <25% or peak Vo2 < 12 ml/kg/min and/or <50% of predicted value measured by cardiopulmonary exercise testing or patients unable of performing the test.
2. At least 3 hospitalizations in the last year without a clear precipitating cause.
3. Dependence of intravenous inotropic agents or short-term MCS. Since outcomes in INTERMACS 3 are better than in class 1-2, this class has been advocated as the optimal group for implantation.
4. Progressive end-organ dysfunction due to reduced perfusion and inadequate ventricular filling pressure (1).
LVAD can be used as BTT, bridge to candidacy (BTC), or as permanent treatment, “destination therapy” (DT) (refractory HF, no transplant candidate) in order to overcome the shortage of heart donors (10). In this setting, the right ventricle (RV) assessment is crucial since the RV supports the cardiac output and RV failure occurs in up to 50% cases following LVAD implantation and resulting in high perioperative mortality and morbidity rates (11, 12). A biventricular assist device (BiVAD) is an implantable pump designed to help heart function better when both the right and left pumping chambers of heart are failing. However, BiVAD recipients have greater mortality and morbidity than LVAD recipients (13). Therefore, MCS with BiVAD and total artificial heart (TAH) options remain challenging.
The purpose of this review is to provide a multimodality imaging approach to the patient with AHF. Clinical decisions about AHF management are frequently based on measurements of LV function, relying mainly on echocardiographic (TTE and TEE) measurements. These tools are almost always available in primary care, this means that AHF clinical diagnosis and decision making can take weeks, even months of in hospital stay of costly frequent visit to HF outpatient clinic. As a result, the opportunity for early detection of AHF is often lost. In the current, the availability in tertiary centers of other imaging modalities, such as cardiac computed tomography (CCT) and cardiac magnetic resonance imaging (CMR) are of utmost importance in the assessment of complex scenarios, and this may have an impact in survival of AHF patients.
2. Multimodal approach to advanced heart failure
2.1.1. Transthoracic echocardiography in AHF
The echocardiographic parameters to evaluate in patients with AHF are:
1. Left ventricular ejection fraction (LV EF). Usually in these patients, LVEF is less than 30% (1), (
Table 1). It must be obtained by biplane method or 3D, and in case of poor acoustic window the ultrasound enhancing agents (UEAs) should be used to better visualize the endocardial borders (14).
2. Presence of regional wall motion abnormalities (RWMA).
3. Ventricular diameters and volumes: LV end-diastolic diameter (EDD) upper cut off normal values are > 52.2 mm in females and >58.4 mm in males. LV end-diastolic volume (EDV) upper cut off values are> 61 mm/m2 in the female sex and >74 mm/m2 in the male sex. Three-dimensional (3D) echocardiography is currently the most accurate technique in determining LV volume and function. It correlates with cardiac magnetic resonance, reducing the need for geometric assumptions. However, it has some limitations, such as lower spatial and temporal resolution (15).
4. Stroke volume (SV): the SV through the aortic valve is calculated as the product of the cross-sectional area times the integral of the velocity / time curve of flow through that area. The cut off for indexed SV value in these patients is< 35 ml/m2 (16).
5. LV global longitudinal strain (GLS). The normal value is strictly variable depending on sex and age, with a mean normal value of -22.5 ± 2.7 and a confidence interval = -17.2 to - 27.7 [PMID: 28637227]. In patients with HF with reduced LVEF, GLS is an accurate non-invasive measure of myocardial fibrosis and a better predictor of all causes of mortality than other echocardiographic parameters, especially in males and in sinus rhythm (17).
6. Mitral and tricuspidal regurgitation. Transthoracic echocardiography using quantitative parameters allows the quantification of the seriousness of these valvular heart diseases, being able to also give indications on the need for a possible percutaneous treatment. There are qualitative, semiquantitative and quantitative parameters (Pisa radium, regurgitant Volume (RV) and effective regurgitant orifice area (EROA). For mitral regurgitation, the presence of EROA > 40 mm2 and Rvol > 60 ml is indicative of severity, while the severity cut-off for tricuspid regurgitation are: EROA > 40 mm, Rvol> 45 ml and Pisa Radium > 9 mm (18);
7. Diastolic function. E/E' ratio > 14 and average e’ velocity < 9 cm/s identify an increase in LV filling pressure. During diastole, blood flows through the mitral valve when the LV relaxes, causing an early diastolic mitral velocity (E), and then additional blood is pumped through the valve when the left atrium contracts during late diastole (A). The E/A ratio can be altered as diastolic dysfunction progresses (with an initial decline (E/A < 1), then a pseudonormalization (E/A ≥ 1) and, finally, the appearance of a restrictive filling pattern (E/A ≥ 2) [PMID: 31617033]. Tissue Doppler imaging is an echocardiographic technique that measures the velocity of the mitral annulus. This velocity has been shown to be an important marker of early myocardial dysfunction. With abnormal active relaxation, mitral annulus velocity during early diastole (e’) is decreased while mitral annulus velocity during late diastole (a’) is increased, resulting in a lowered e’/a’ ratio (19) and higher E/e’ ratio.
8. LV mass and wall thickness. The quantification of the myocardial mass and the measurement of the thickness allows the identification of pathological hypertrophy. An example is hypertrophic cardiomyopathy in which there is asymmetric LV hypertrophy with septal thicknesses above 15 mm. There are two main patterns of hypertrophy: concentric and eccentric. Concentric hypertrophy occurs in cases of chronic pressure overload such as in aortic stenosis or poorly controlled arterial hypertension. Eccentric hypertrophy is typical of volume overload, typical for example of aortic insufficiency or cases of dilated heart disease; the latter type of hypertrophy belongs to usually dysfunctional ventricles and therefore is a negative prognostic marker (18).
9. Left atrial (LA) function. LA enlargement predicts cardiovascular risk and alteration of left atrial deformation property (strain) is a marker of negative outcome such as cardiovascular morbidity and mortality (20).
10. Advanced echocardiography. RV global longitudinal strain (RVGLS) and free wall right ventricular longitudinal strain (RVFWS) are two important parameters to evaluate RV function. The normal values are >17.5% for RVGLS and >15.3 % for RVFWS. RVFWS is a more sensitive indicator of RV function since RVGLS, involving interventricular septum deformation analysis, can be influenced by LV dysfunction (21).
2.1.2. Role of Cardiac Magnetic Resonance Imaging in AHF
CMR imaging plays a fundamental role in AHF due to its high sensitivity in identifying the underlying etiology (22). Late gadolinium enhancement (LGE) patterns help distinguishing between ischaemic cardiomyopathy (ICM) and non-ischaemic cardiomyopathy (NICM) (23).
In ICM, LGE is transmural or subendocardial, while in a certain proportion of NICM, the presence of intramural or subepicardial LGE is detected. Notably, the absence of LGE does not completely exclude ICM in the case of hibernating myocardium (24). In ICM, an important role of CMR is the assessment of myocardial viability. The presence of scars extending more than 75% of the myocardial wall indicates a low probability of recovery after revascularization. On the other hand, the presence of scars affecting less than 25% of the myocardial wall indicates a good chance of recovery (24). In NICM, LGE has important prognostic implications in terms of site and distribution as its extent correlates to a major number of cardiovascular events. Examples of CMR findings are shown in
Figure 1.
2.1.3. Role of Cardiac Computed Tomography in AHF
In patients with AHF, CCT can be used to assess ventricular function when echocardiographic windows are suboptimal and CMR is contraindicated (i.e., for the presence of devices, which are particularly frequent in such patients). CCT provides a true volumetric method to assess both LV and RV size and systolic function at high spatial resolution. It can also identify typical characteristics of non-compact LV, hypertrophic cardiopathy, and arrhythmogenic RV cardiomyopathy (RV dilation and dysfunction, adipose infiltration) (25, 26).
2.2. Short-term mechanical support
2.2.1. The intra-aortic balloon pump
The intra-aortic balloon pump (IABP) consists in a percutaneously placed device that inflates in diastole, thus increasing blood flow to the coronary arteries, and deflates in systole, thus decreasing afterload. The two actions combined reduce myocardial oxygen demand and increase myocardial oxygen supply (27).
The IABP is typically placed in the cardiac catheterization laboratory under fluoroscopic guidance. However, TEE can be used to in the guide its positioning intubated patient in the intra-operative setting. Ideal positioning of the balloon tip is 1–2 cm distal to the left subclavian artery. This position can be confirmed by visualizing the descending aorta and then withdrawing the TEE probe until the left subclavian artery and aortic arch are visualized. Once the balloon pump is activated, the gas filled balloon will cause shadowing and reverberation artefacts, that can be used as confirmation of device functioning properly. In the absence of these artefacts or if bubbles are visualized in the aorta, rupture of the IABP should be suspected. After IABP placement, TTE can be used to monitor LV function and guide the weaning of IABP support. It can also visualize any new or worsening aortic regurgitation (28).
Coronary computed tomography may play a role in detecting possible complications of IABP. First it can highlight a fearful complication that is aortic dissection. Moreover, it can show the displacement of the aortic balloon or arterial embolization and organ parenchyma. CMR is not indicated in monitoring possible complications.
Table 2 depicts indications and timing of echocardiographic and CCT evaluation.
2.2.2. The Impella
The Impella is a rotary micro axial pump with insertion into the femoral artery and retrograde advancement up to the LV across the aortic valve: blood is aspirated from the LV and pushed into the descending aorta. This system allows a reduction in LV preload and an improvement in cardiac output (29). TTE evaluation is crucial to determine if a patient is eligible for the Impella placement. The presence of severe aortic stenosis and mechanical aortic valves represent contraindications to Impella placement, whereas the presence of aortic regurgitation does not contraindicate the Impella positioning, but it should be known that regurgitation can worsen after its placement (30). The presence of LV thrombosis is another contraindication as systemic embolization can occur. The presence of patent foramen ovalis, atrial and interventricular defects must be reported because the positioning of the Impella can increase a possible right-to-left shunt (31).
As for IABP, Impella devices are commonly placed under fluoroscopic guidance, but in patients with refractory shock, preventing transportation of the patient to the cardiac catheterization laboratory, TEE can help bedside positioning of the device (32). One single centre study demonstrated no difference in Impella-related complications when comparing TEE alone guided placement with the fluoroscopic guided cohort (33). The mid-esophageal long-axis and 4-chamber views can be used to visualize the guidewire crossing the aortic valve. The catheter should be oriented towards the ventricular apex. TEE can also confirm the absence of iatrogenic aortic dissection from the procedure (32). Both TTE and TEE are helpful in identifying correct positioning of the Impella device (
Figure 2).
The distance from the aortic valve to the Impella inlet should be 3.5-5 cm, while the Impella outlet should be 1.5-2 cm above the sinuses of Valsalva (32). Color flow Doppler shows a mosaic pattern at the Impella inlet and outlet confirming further its proper position. To note, the Impella devices can migrate: in this case the mosaic pattern will be visualized on the same side of the aortic valve (32). Three-dimensional echocardiography can help visualization of Impella positioning in comparison to other anatomical structures (34). After placement, additional complication of the Impella placement such as damage to the mitral or aortic valve, pericardial effusion, and rupture of LV free wall must be excluded. The ideal position of the septum is median during the diastole, and displacements may indicate the presence of a right dysfunction or the need to change the speed of the Impella device. Finally, echocardiographic data can be used in conjunction with haemodynamic ones to guide the weaning of the Impella by evaluating the response of the LV to progressive reduction in the support provided by the Impella (the P level). CCT plays an important role in confirming endoventricular thrombi before Impella implantation (35). We know that the most common complications after Impella placement are hemolysis, vascular complications, bleeding, and limb ischemia. CCT scan can identify complications such as damage to the mitral and aortic valve systems and positioning of the device. CMR is not indicated in monitoring after Impella implantation (see
Table 3).
2.2.3. The veno-arterial extracorporeal membrane oxygenation
The veno-arterial extracorporeal membrane oxygenation (V-A ECMO) system is a percutaneous system that takes over the heart and lungs. It consists of a system of inflow and outflow cannulas, a centrifugal pump, and an oxygenating membrane (36). The ECMO provides a blood flow rate greater than 4.5 L. The effect is a noticeable reduction in LV preload without reducing the afterload (37).
An echocardiographic evaluation should be performed prior to ECMO cannulation (38). First, reversible causes of cardiovascular collapse, such as cardiac tamponade and acute valve pathology, must be excluded. The presence of an aortic dissection is a relative contraindication to ECMO positioning as it can cause extension of the dissecting flap. The presence of aortic stenosis or mitral regurgitation should be also evaluated as they may worsen due to increased afterload due to ECMO (39). The ECMO can be placed under fluoroscopic, TTE or TEE guidance. Usually, the venous cannula is placed in the right atrium. The mid-oesophageal bicaval view at the TEE can easily show complications such as the passage of the cannula through the atrial septum. The arterial cannula is typically positioned within the descending aorta; TEE can confirm this location. Moreover, TEE can prevent atheromatous plaque embolization from this procedural step by referring their presence in the aorta to the operator (40).
Echocardiography also plays an essential role in assessing cardiac function when supported by the ECMO system (40) is important to ascertain that the aortic valve opens during systole, since the high afterload due to the arterial cannula can reduce the valve opening frequency increasing the risk of LV and aortic valve thrombosis.
Finally, echocardiography can guide the ECMO weaning (41). Echocardiographic parameters that are predictors of successful weaning are LVEF >20–25%, aortic velocity time integral (VTI) >10 cm, and lateral mitral annular systolic wave velocity (S’) >6 cm/sec (41).
The CCT scan plays a role in the identification of complications related to the placement of the ECMO. The presence of opacification defects of the arterial system is indicative of pseudo-lesion with emergent surgical indication. CCT can also be used to evaluate other complications such as cannula malposition, hematoma formation, and haemothorax (
Figure 3).
An important complication of ECMO is thrombosis of the arterial system, in particular of the ascending aorta proximal to the insertion of the arterial cannula; this is mainly linked to the low flow which determines blood stasis and therefore leads to the formation of thrombi (42).
In patients with impaired RV function there is also a predisposition to the development of pulmonary embolism (42). Pulmonary circulation evaluation in these patients can be difficult since the contrast injected at the venous level is captured by the venous cannula before an adequate opacification of the pulmonary circulation. As a solution, the revs of the ECMO can be reduced to 500 / min for 15-25 seconds during contrast injection (42).
Table 4 shows the timing and role of different imaging modalities.
2.3. Long-term mechanical circulatory support
LVAD consists of a pump that holds the LV by receiving blood from it by means of an inflow cannula and pushing it to the level of the aorta by means of an outflow cannula. The device is placed in the mediastinum and is powered by a cable that extends abdominally to connect to a controller and a power source. There are two main types of FDA approved LVADs: pulsatile and non-pulsatile. Those of the older generation were characterized by a pulsatile flow with a high risk of device malfunction and low survival. Heartware and Heart Mate III are characterized by a centrifugal flow, the pump is intrapericardial. Heart Mate II is characterized by an axial flow, the pump is in a pocket (43).
2.3.1. Selection of LVAD potential candidates
TTE has a central role in the selection of the optimal candidate for LVAD implant, since it allows evaluating (see also table 5) (43):
1. LVEF (particularly the demonstration of a LVEF < 25%), ventricular size and cardiac output. It may be difficult to implant patients with small LV size, especially with increased LV trabeculation.
2. Presence of intracardiac thrombi; this is not an absolute contraindication to LVAD implant, but it increases the risk of stroke during the LV cannulation procedure.
3. RV function. It is essential to evaluate the presence of signs of RV dysfunction (such as TAPSE < 18mm, s’<9.5cm/s, FAC <35%), RV dilation, dilation of inferior vena cava, and moderate or greater tricuspid regurgitation. The presence of preoperative severe RV dysfunction may suggest the use of a biventricular MCS.
4. Valve Diseases. Before LVAD implant it is important to detect and quantify valvular regurgitation, valvular stenosis, and prosthetic valve dysfunction. The presence of moderate to severe mitral stenosis can prevent LV cannula inflow. The presence of aortic stenosis of any severity does not affect LVAD function, in fact LVAD bypass the native LVOT. Is important to exclude significant aortic regurgitation (AR) before LVAD implant because it can create a vicious cycle in which blood pumped into the aorta regurgitates into the LV. To note that in patient with advanced HF and severe stroke volume reduction it may be difficult to quantify aortic regurgitation. The presence of pre-operatory severe mitral regurgitation is often markedly improved after initiation of LVAD support because of reduced LV size, reduced filling pressure and improved coaptation of MV leaflets; for these reasons any grade of mitral regurgitation is not a contraindication to LVAD implant. Conversely, the presence of pre-operative moderate or severe tricuspid regurgitation may indicate RV dysfunction. In patients with AV mechanical valve prostheses, reduced blood flow through the prosthesis after an LVAD implant may increase the risk of thrombosis, therefore biological valve replacement may be considered. Finally, it is also important to exclude moderate or severe pulmonary regurgitation and pulmonary stenosis.
5. Congenital heart diseases. Some congenital common anomalies require correction before LVAD implantation. The presence of ventricular septal defects should be also excluded (43).
2.3.2. LVAD Surveillance Echocardiography
Periodic standard TTE exams are recommended after LVAD implant (43). The first one is performed 2 weeks after the implant, and then at 1, 3, 6, 12-month post-implant and every 6 to 12 months thereafter. During the standard echocardiographic exam is important to evaluate and report (43):
1. LV size and function. The most reproducible is the LV internal diameter end diastole (LVIDd) from the 2D parasternal long axis image. The LVIDd may be paradoxically smaller than the Left ventricular internal diameter end systole (LVIDs), and this is an important finding, as it is associated with excessive LVAD unloading and/or severe RV dysfunction. The evaluation of LVEF can demonstrate possible LV worsening or recovery. A possible complication to evaluate is LV suction with induced ventricular ectopy; this condition is due to LV underfilling that causes impact of inflow cannula with LV endocardium, and the solution is speed turndown.
2. Position of interventricular septum (IVS) and cannulas. The end-diastolic IVS position should be neutral, leftward-shifted or rightward-shifted. A leftward shift can be due to elevated RV end-diastolic pressures, reduced LV preload, or LV over-decompression resulting from excessive LVAD speed. A rightward IVS shift is generally due to elevated LV end-diastolic pressures resulting from an inadequate LVAD speed setting, pump dysfunction, severe AR, or an increased LV afterload. The inflow cannula can be evaluated in the parasternal or apical TTE views. Is important to reveal the cannula’s location and orientation in relation to IVS and other LV structures. The colour Doppler interrogation should demonstrate a one directional, laminar flow from LV to inflow cannula without turbulence or regurgitation. At continuous Doppler interrogation, the flow should have peak velocity between 1 and 2 m/sec; a higher velocity may suggest inflow obstruction.
3. Aortic valve (AV) opening and AR severity. It is important to evaluate the presence e the degree of AV opening because it is determined by different parameters like LVAD speed, LV native function, volume status and peripheral vascular resistance. LVAD types differ in aortic valve opening pattern, especially for intermittent low speed phase (es. 9 seconds for Jarvick). It is recommended LVAD speed to be set to allow at least one intermittent opening of the AV. The AV opening is assessed with M-Mode. In patient with very depressed LVEF, AV opening may not occur. When AV remains closed, aortic root thrombus should also be excluded. Another risk in LVAD patients is the development of AR, which is not uncommon after LVAD implantation. Assessment of its severity is partly based on careful colour Doppler analysis in the parasternal long-axis view.
4. RV size and function. During TTE follow-up, RV function must be carefully evaluated. The shift of the IVS to the left side by LVAD may reduce the IVS contribution to the RV contraction. Furthermore, increased venous return created by increased cardiac output from the LVAD may worsen the RV function. This increased workload is a concern for worsening RV function that LVAD patients may already have. The classical criteria for RV dysfunction included the following parameters: TAPSE < 17 mm, tricuspid annulus systolic peak velocity (S') velocity < 10 cm/s and RVFAC < 35% (16). Nevertheless, the evaluation of RV function is challenging also because correlation between RV systolic function and TAPSE and/or S' should be considered weaker after cardiothoracic surgery.
5. Evidence of intracardiac thrombi. Recent studies on patients implanted with new generation LVAD suggest that the LV may be a relevant site of local thrombosis and cardioembolism. Pump speed, AV opening, cannula location, and orientation are important determinants of LV flow that are drastically disrupted in LVAD patients, leading to blood stasis or abnormally large shear stresses (
Figure 4) (43).
2.3.3. Advanced echocardiography in LVAD patients
Some patients with LVAD have very difficult acoustic access in traditional transthoracic view. Several factors influence poor image quality in LVAD patients. First, LVAD, inflow and outflow cannula limit the acoustic window. Furthermore, the device may cause artefacts and due to the device, the probe positioning during the examination may not be optimal. In such cases, ultrasound enhancing agents UEAs are a good alternative, feasible, safe, and reproducible (44). UEAs allow a better definition of endocardial borders; this is useful for better quantification of the LV end-diastolic diameter and residual function. It also increases the possibility to detect intracavitary thrombi. Moreover, it permits a better visualization of the RV and helps to identify RV dysfunction and to recognize patients at higher risk of RV failure. Finally, during the follow up, UEAs can reveal the presence of pseudoaneurysms demonstrating a bidirectional flow between the pseudoaneurysm and the LV (43).
2.3.4. Role of cardiac computed tomography in LVAD patients
CMR is contraindicated in patients with LVAD; therefore, CCT scan represents an opportunity for non-invasive evaluation of the function of the device and its complications (45-47). A limitation of echocardiography in patients with LVAD is the incomplete visualization of the outflow cannula; the latter is well seen with the help of the CCT scan. During the follow-up, CCT can reveal complications such as compression of the right ventricle (due to pericardial clots), thrombosis, malposition and kinking of the outflow cannula. Indications for CCT in LVAD patients include suspicion of:
- 1)
inflow-cannula malposition (i.e. in case of unexplained frequent LVAD suction events, recurring ventricular dysrhythmias, or residual HF due to only partial LV unloading);
- 2)
pump thrombosis involving the inflow cannula or outflow tract with evidence of haemolysis;
- 3)
LVAD malfunction due to outflow-graft kinking; exclusion of an intracardiac and/or aortic root clot in patients with an unexplained transient ischemic attack or stroke.
Finally, whenever poor acoustic windows prevent appropriate assessment of ventricular size and function, it is possible to use either multiple-gated acquisition equilibrium radionuclide angiography or electrocardiographically gated CCT as a second-line alternative test (47).
2.4. Imaging in orthotopic cardiac transplant (OTC)
TTE is essential in the follow-up of OTC patients. It has a role both in the immediate post-operative period and in the surveillance of short and long-term complications (48).
During the first 3 months, ventricular thicknesses and mass are increased due to infiltration of inflammatory cells and graft oedema. The persistence of ventricular hypertrophy after this period can be related to immunosuppressive therapy or to repeated episodes of acute rejection. In most cases, LV function and regional wall motion are preserved during the first 10-15 years. An early reduction in LVEF may indicate allograft rejection or vasculopathy (48).
Diastolic function can be difficult to evaluate since cardiac denervation and the subsequent high cardiac frequency can cause E and A waves fusion. E’ and a’ waves are of smaller amplitude than in the normal population. A restrictive filling pattern may be present in the early post-transplant stages. Its persistence can be linked to inflammation, fibrosis and vasculopathy of the allograft (20).
After cardiac surgery, the longitudinal parameters are abnormal; therefore, they are not considered sensitive parameters (including TAPSE and RV TVI). As for the atrial morphology, in the historical bi-atrial technique, an atrial enlargement, and the presence of a ridge at the anastomosis are visualized. In the more recent bi-caval technique, the atrial shape and size do not differ significantly from those of normal subjects. Atrial reservoir function is markedly reduced in OCT recipients related to elevated PCWP and LA-enlargement in the LA and in the RA impaired longitudinal right ventricular function (20).
Normally, valve morphology and function of transplanted hearts are normal. There may be mild tricuspid and mitral regurgitation. Mitral regurgitation can be linked to papillary muscle oedema and tends to decrease over time. Tricuspid regurgitation can be detected during the first phase due to the increased pulmonary pressures, while in more advanced stages it can be linked to valve damage due to frequent biopsies or dilatation of right chambers (48).
The presence of severe pericardial effusion leading to cardiac tamponade is rare and may be related to the presence of hearts that are smaller compared to the body surface. When a pericardial effusion is found it is important to perform serial echocardiographic examinations (every 1-3 months) to evaluate the size, extent, and haemodynamic impact of the effusion (49).
2.4.2. Advanced echocardiography
STE may help identifying acute cell rejection (ACR) (50). It has been shown that the reduction of LV torsion by at least 25% predicts with high specificity and high negative predictive value ACR of at least a second degree.
Stress echocardiography (SE), mainly with dobutamine, is recommend in patients whit prohibitive risk for invasive coronary angiography, according to the ISHLTV guidelines. A recent meta-analysis demonstrated that SE has a very low sensitivity (about 60%) in the detection of cardiac allograft vasculopathy (CAV) and mostly SE cannot detect mild and moderated CAV degree (51).
2.4.3. Cardiac Magnetic Resonance
CMR allows an early identification of rejection and CAV in OCT patients (52-54). Moreover, the presence of late gadolinium enhancement was found to be prognostically relevant, although its sensitive is low in OCT patients (53). The new mapping techniques may have an emerging role in the diagnosis of OCT rejection (54). T1-mapping showed to decrease after successful treatment and to display excellent negative predictive value for the non-invasive detection of rejection (52). Using a multiparametric sequential approach by combining T2 mapping with extracellular volume fraction (ECV), diagnostic accuracy of CMR for detecting ACR improves (53) (
Figure 5). Finally, stress perfusion CMR can be useful in assessing the microvascular disease through the estimation of myocardial perfusion reserve (MPR) which has a high sensitivity in detecting CAV (54).
2.4.3. Cardiac computed tomography angiography
CCT has increasingly been used to detect CAV in OTC patients (55-57). Wever-Pinzon et al. in a meta-analysis of 13 studies evaluated 615 HTx patients, demonstrating a high diagnostic specificity, sensibility, and accuracy of CCT in comparison with invasive coronary angiography (ICA) for the detection of any CAV and significant CAV using 16 and 64-slice CCT (55). In addition, CAC<0 was associated with an increased risk of MACE, death, graft loss and showed a good correlation with International Society for Heart and Lung Transplantation (ISHLT) CAV grade (58).
Newer CCT technologies, such as dual source CT and multidetector CT, increasing temporal and spatial resolution, allow a better acquisition even at higher rate as in the denervated transplanted heart (55-57). More recently, Nous et al. in a prospective observational study on 129 OTC patients demonstrated that CCT (using 2° and 3° generation dual source CT) could be a safe and accurate alternative to ICA in CAV evaluation (
Figure 6).
Interestingly, in small retrospective studies, quantitative coronary wall assessment and plaque analysis allowed an early detection of CAV not detected by ICA (57). Finally, Budde et al. demonstrated that 25% of OTC patients with a focal stenosis >30% showed low value of FFR-CT; even without a focal stenosis, FFR-CT values were found often abnormal in Htx patients (59).
Table 6 describes the timing and role of different imaging modalities in OCT.
3. Conclusions (take home messages) and future perspectives
This paper aims to highlight the steady advances in multi-modality imaging techniques in AHF, which offer a unique opportunity for a comprehensive evaluation of such complex scenarios. In this literature review, we aim to suggest a practical, stepwise algorithm with integrative multimodality imaging approach for better assessment of underlying mechanisms, patterns of progression and possible complication in patients with end stage HF and supported with short- or long-term MSD. Finally, we did not include in the present review BiVAD and TAH, aiming to provide some reflections towards this future direction. Moreover, the role of new imaging markers such as pFAI in predicting cardiovascular outcomes and CAV in TCO patients should be investigated in perspective studies.
Author Contributions
Conceptualization, V.P.. and G.P..; methodology, V.P., M.P.M. and G.M..; validation, S.I., and P.P.F; writing—original draft preparation, F.A., C.M.D, M.C.P. and G.M.; writing—review and editing, S.M.., M.C., A.I.G. and R.M..; visualization, S.N.; supervision, S.D.G, A.D and R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-3726. Erratum in: Eur Heart J. 2021 Oct 14. [CrossRef] [PubMed]
- Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev 2017 Apr;3(1):7-11. [CrossRef] [PubMed]
- Xanthakis V, Enserro DM, Larson MG, Wollert KC, Januzzi JL, Levy D, Aragam J, Benjamin EJ, Cheng S, Wang TJ, Mitchell GF, Vasan RS. Prevalence, neurohormonal correlates, and prognosis of heart failure stages in the community. JACC Heart Fail 2016,Oct;4(10):808 815. [CrossRef] [PubMed] [PubMed Central]
- Giannitsi S, Bougiakli M, Bechlioulis A, Kotsia A, Michalis LK, Naka KK. 6-minute walking test: a useful tool in the management of heart failure patients. Ther Adv Cardiovasc Dis. 2019 Jan-Dec;13:1753944719870084. [CrossRef] [PubMed] [PubMed Central]
- Francis GS, Bartos JA, Adatya S. Inotropes. J Am Coll Cardiol. 2014 May 27;63(20):2069-2078. Epub 2014 Feb 12. 27 May. [CrossRef] [PubMed] [PubMed Central]
- Kittleson MM, Shah P, Lala A, McLean RC, Pamboukian S, Horstmanshof DA, Thibodeau J, Shah K, Teuteberg J, Gilotra NA, Taddei-Peters WC, Cascino TM, Richards B, Khalatbari S, Jeffries N, Stevenson LW, Mann D, Aaronson KD, Stewart GC. INTERMACS profiles and outcomes of ambulatory advanced heart failure patients: a report from the REVIVAL Registry, J Heart Lung Transplant 2020;39:1626. [CrossRef] [PubMed]
- Barge-Caballero E, Almenar-Bonet L, Gonzalez-Vilchez F, Lambert-Rodriguez JL, Gonzalez-Costello J, Segovia-Cubero J, Castel-Lavilla MA, Delgado-Jimenez J, Garrido-Bravo IP, Rangel-Sousa D, Martinez-Selles M, De la Fuente-Galan L, Rabago-AracilJuan- G, Sanz-Julve M, Hervas-Sotomayor D, Mirabet-Perez S, Muniz J, Crespo-Leiro MG. Clinical outcomes of temporary mechanical circulatory support as a direct bridge to heart transplantation: a nationwide Spanish registry. Eur J Heart Fail 2018, 20(1):178-186. [CrossRef] [PubMed]
- Mehra M, Cleveland J Jr., Uriel N, Cowger J, Hall S, Horstmanshof D, et al. Primary results of long-term outcomes in the momentum 3 pivotal trial and continued access protocol study phase: A study of 2200 heartmate 3 left ventricular assist device implants. Eur J Heart Fail. (2021) 23:1392–400. [CrossRef]
- Mehra MR, Canter CE, Hannan MM, Semigran MJ, Uber PA, Baran DA, Danziger-Isakov L, Kirklin JK, Kirk R, Kushwaha SS, Lund LH, Potena L, Ross HJ, Taylor DO, Verschuuren EAM, Zuckermann A. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10-year update. J Heart Lung Transplant 2016 Jan;35(1):1-23. [CrossRef] [PubMed] [PubMed Central]
- Cameli M, Pastore MC, Campora A, Lisi M, Mandoli GE. Donor shortage in heart transplantation: How can we overcome this challenge? Front Cardiovasc Med. 2022 Oct 17;9:1001002. [CrossRef] [PubMed]
- Dandel M, Javier MFDM, Javier Delmo EMD, Hetzer R. Accurate assessment of right heart function before and after long-term left ventricular assist device implantation. Expert Rev Cardiovasc Ther. 2020 May;18(5):289-308. [CrossRef]
- Kapur NK, Esposito ML, Bader Y, Morine KJ, Kiernan MS, Pham DT, Burkhoff D. Mechanical Circulatory Support Devices for Acute Right Ventricular Failure. Circulation. 2017 Jul 18;136(3):314-326. [CrossRef] [PubMed]
- Shehab S, Hayward CS. Choosing Between Left Ventricular Assist Devices and Biventricular Assist Devices. Card Fail Rev. 2019 Feb;5(1):19-23. [CrossRef] [PubMed Central]
- Feng Xie. Contrast echocardiography: latest developments and clinical utility. Curr Cardiol Rep 2015 Mar;17(3):569. [CrossRef]
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015 Mar;16(3):233-70. Erratum in: Eur Heart J Cardiovasc Imaging. 2016 Apr;17(4):412. Erratum in: Eur Heart J Cardiovasc Imaging. 2016 Sep;17 (9):969. [CrossRef]
- Mandoli GE, Benfari G, Baggiano A, Florea R, Cameli M. Editorial: Advances in cardiac imaging and heart failure management. Front Cardiovasc Med. 2023 Jan 9;9:1095829. [CrossRef] [PubMed Central]
- Karlsen S, Dahlslett T, Grenne B, Sjøli B, Smiseth O, Edvardsen T, Brunvand H. Global longitudinal strain is a more reproducible measure of left ventricular function than ejection fraction regardless of echocardiographic training. Cardiovasc Ultrasound. 2019 Sep 2;17(1):18. [CrossRef] [PubMed] [PubMed Central]
- Galderisi M, Cosyns B, Edvardsen T, Cardim N, Delgado V, Di Salvo G, Donal E, Sade LE, Ernande L, Garbi M, Grapsa J, Hagendorff A, Kamp O, Magne J, Santoro C, Stefanidis A, Lancellotti P, Popescu B, Habib G; 2016–2018 EACVI Scientific Documents Committee; 2016–2018 EACVI Scientific Documents Committee. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2017 Dec 1;18(12):1301-1310. [CrossRef] [PubMed]
- Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016 Apr;29(4):277-314. [CrossRef] [PubMed]
- Bech-Hanssen O, Pergola V, Al-Admawi M, Fadel BM, Di Salvo G. Atrial function in heart transplant recipients operated with the bicaval technique. Scand Cardiovasc J. 2016;50(1):42-51. Epub 2015 Oct 14. [CrossRef]
- Carluccio E, Biagioli P, Lauciello R, Zuchi C, Mengoni A, Bardelli G, Alunni G, Gronda EG, Ambrosio G. Superior Prognostic Value of Right Ventricular Free Wall Compared to Global Longitudinal Strain in Patients With Heart Failure. J Am Soc Echocardiogr. 2019 Jul;32(7):836-844.e1. Epub 2019 Apr 9. [CrossRef]
- Contaldi C, Dellegrottaglie S, Mauro C, Ferrara F, Romano L, Marra AM, Ranieri B, Salzano A, Rega S, Scatteia A, Cittadini A, Cademartiri F, Bossone E. Role of Cardiac Magnetic Resonance Imaging in Heart Failure. Heart Fail Clin. 2021 Apr;17(2):207-221. Epub 2021 Feb 3. [CrossRef]
- Vöhringer M, Mahrholdt H, Yilmaz A, Sechtem U. Significance of late gadolinium enhancement in cardiovascular magnetic resonance imaging (CMR). Herz. 2007 Mar;32(2):129-37. [CrossRef] [PubMed]
- Memon S, Ganga HV, Kluger J. Late Gadolinium Enhancement in Patients with Nonischemic Dilated Cardiomyopathy. Pacing Clin Electrophysiol. 2016 Jul;39(7):731-47. Epub 2016 May 19. [CrossRef]
- Aziz W, Claridge S, Ntalas I, Gould J, de Vecchi A, Razeghi O, Toth D, Mountney P, Preston R, Rinaldi CA, Razavi R, Niederer S, Rajani R. Emerging role of cardiac computed tomography in heart failure. ESC Heart Fail. 2019 Oct;6(5):909-920. Epub 2019 Aug 10. [CrossRef] [PubMed Central]
- Ramanathan R, Anumandla AK, Haramati LB, Spevack DM, Godelman A, Jain VR, Kazam J, Burton WB, Levsky JM. Evaluation of the cardiac chambers on axial CT: comparison with echocardiography. J Comput Assist Tomogr. 2014 Jan-Feb;38(1):53-60. [CrossRef] [PubMed]
- Agdamag AC, Riad S, Maharaj V, Jackson S, Fraser M, Charpentier V, Nzemenoh B, Martin CM, Alexy T. Temporary Mechanical Circulatory Support Use and Clinical Outcomes of Simultaneous Heart/Kidney Transplant Recipients in the Pre- and Post-Heart Allocation Policy Change Eras. Transplantation. 2023 Jan 19. Epub ahead of print. [CrossRef] [PubMed]
- Stainback RF, Estep JD, Agler DA, Birks EJ, Bremer M, Hung J, Kirkpatrick JN, Rogers JG, Shah NR; American Society of Echocardiography. Echocardiography in the Management of Patients with Left Ventricular Assist Devices: Recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2015 Aug;28(8):853-909. [CrossRef] [PubMed] [PubMed Central]
- Zein R, Patel C, Mercado-Alamo A, Schreiber T, Kaki A. A Review of the Impella Devices. Interv Cardiol. 2022 Apr 8;17:e05. [CrossRef] [PubMed]
- Sef D, Kabir T, Lees NJ, Stock U. Valvular complications following the Impella device implantation. J Card Surg. 2021 Mar;36(3):1062-1066. Epub 2021 Jan 6. [CrossRef] [PubMed]
- Senthil Anand, Timothy Barry, Reza Arsanjani, Lisa LeMond. Echocardiography in Cardiac Assist Devices. Rev. Cardiovasc. Med. 2022, 23(7), 253. 7.
- Crowley J, Cronin B, Essandoh M, D'Alessandro D, Shelton K, Dalia AA. Transesophageal Echocardiography for Impella Placement and Management. J Cardiothorac Vasc Anesth. 2019 Oct;33(10):2663-2668. Epub 2019 Jan 23. [CrossRef] [PubMed]
- Pieri M, Pappalardo F. Bedside insertion of impella percutaneous ventricular assist device in patients with cardiogenic shock. Int J Cardiol 2020 Oct 1;316:26-30. [CrossRef] [PubMed]
- Yastrebov K, Brunel L, Paterson HS, Williams ZA, Wise IK, Burrows CS, Bannon PG. Implantation of Impella CP left ventricular assist device under the guidance of three-dimensional intracardiac echocardiography. Sci Rep. 2020 Oct 15;10(1):17485. Erratum in: Sci Rep. 2021 Feb 25;11(1):5091. [CrossRef] [PubMed] [PubMed Central]
- Nakao Y, Aono J, Namiguchi K, Nishimura T, Izutani H, Higashi H, Inaba S, Nishimura K, Inoue K, Ikeda S, Yamaguchi O. Usefulness of contrast computed tomography for diagnosing left ventricular thrombus before impella insertion. J Cardiol Cases. 2020 Aug 22;22(6):291-293. [CrossRef] [PubMed] [PubMed Central]
- Swedzky F, Barbagelata A, Perrone S, Kaplinsky E, Ducharme A. Emerging concepts in heart failure management and treatment: circulatory support with extracorporeal membrane oxygenation (ECMO). Drugs Context. 2023 Jan 4;12:2022-7-7. [CrossRef] [PubMed] [PubMed Central]
- Hockstein MA, Singam NS, Papolos AI, Kenigsberg BB. The Role of Echocardiography in Extracorporeal Membrane Oxygenation. Curr Cardiol Rep. 2023 Jan;25(1):9-16. [CrossRef] [PubMed]
- Tian L, Zhang S, Xu J, Han X. Extracorporeal Membrane Oxygenation as a Bridge between Transfer and Perioperative Periods in Refractory Cardiogenic Shock Secondary to a Large Left Atrial Myxoma. Heart Surg Forum. 2021 Mar 3;24(2):E215-E216. [CrossRef] [PubMed]
- Ostadal P, Vondrakova D, Popkova M, Hrachovina M, Kruger A, Janotka M, Naar J, Kittnar O, Neuzil P, Mlcek M. Aortic stenosis and mitral regurgitation modify the effect of venoarterial extracorporeal membrane oxygenation on left ventricular function in cardiogenic shock. Sci Rep. 2022 Oct 12;12(1):17076. [CrossRef] [PubMed] [PubMed Central]
- Platts DG, Sedgwick JF, Burstow DJ, Mullany DV, Fraser JF. The role of echocardiography in the management of patients supported by extracorporeal membrane oxygenation. J Am Soc Echocardiogr. 2012 Feb;25(2):131-41. Epub 2011 Dec 9. Erratum in: J Am Soc Echocardiogr. 2012 Apr;25(4):427. [CrossRef] [PubMed]
- Kim D, Jang WJ, Park TK, Cho YH, Choi JO, Jeon ES, Yang JH. Echocardiographic Predictors of Successful Extracorporeal Membrane Oxygenation Weaning After Refractory Cardiogenic Shock. J Am Soc Echocardiogr. 2021 Apr;34(4):414-422.e4. Epub 2020 Dec 13. [CrossRef] [PubMed]
- Lee S, Chaturvedi A. Imaging adults on extracorporeal membrane oxygenation (ECMO). Insights Imaging. 2014 Dec;5(6):731-42 Epub 2014 Oct 9. [CrossRef] [PubMed Central]
- Stainback RF, Estep JD, Agler DA, Birks EJ, Bremer M, Hung J, Kirkpatrick JN, Rogers JG, Shah NR; American Society of Echocardiography. Echocardiography in the Management of Patients with Left Ventricular Assist Devices: Recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2015 Aug;28(8):853-909. [CrossRef] [PubMed]
- Pergola V, Tarzia V, Baroni G, Iliceto S, Gerosa G, Mele D. Utilization of an Ultrasound-Enhancing Agent Improves the Evaluation of the Right Ventricle in Patients With Left Ventricular Assist Device. J Am Soc Echocardiogr. 2022 Nov 29:S0894-7317(22)00634-4. Epub ahead of print. [CrossRef] [PubMed]
- Agdamag, A.C., Velangi, P.S., Salavati, A. et al. CT Imaging of Left Ventricular Assist Devices and Associated Complications. Curr Cardiovasc Imaging Rep 13, 26 (2020). [CrossRef]
- Pontone G, Rossi A, Guglielmo M, Dweck MR, Gaemperli O, Nieman K, Pugliese F, Maurovich-Horvat P, Gimelli A, Cosyns B, Achenbach S. Clinical applications of cardiac computed tomography: a consensus paper of the European Association of Cardiovascular Imaging-part I. Eur Heart J Cardiovasc Imaging. 2022 Feb 22;23(3):299-314 Erratum in: Eur Heart J Cardiovasc Imaging. 2022 Jun 1;23(6):e274. [CrossRef]
- Agdamag, A.C., Velangi, P.S., Salavati, A. et al. CT Imaging of Left Ventricular Assist Devices and Associated Complications. Curr Cardiovasc Imaging Rep 13, 26 (2020). [CrossRef]
- Masarone D, Kittleson M, Gravino R, Valente F, Petraio A, Pacileo G. The Role of Echocardiography in the Management of Heart Transplant Recipients. Diagnostics (Basel). 2021 Dec 11;11(12):2338. [CrossRef] [PubMed] [PubMed Central]
- Badano LP, Miglioranza MH, Edvardsen T, Colafranceschi AS, Muraru D, Bacal F, Nieman K, Zoppellaro G, Marcondes Braga FG, Binder T, Habib G, Lancellotti P; Document reviewers. European Association of Cardiovascular Imaging/Cardiovascular Imaging Department of the Brazilian Society of Cardiology recommendations for the use of cardiac imaging to assess and follow patients after heart transplantation. Eur Heart J Cardiovasc Imaging. 2015 Sep;16(9):919-48. Epub 2015 Jul 2. [CrossRef] [PubMed]
- Narang A, Blair JE, Patel MB, Mor-Avi V, Fedson SE, Uriel N, Lang RM, Patel AR. Myocardial perfusion reserve and global longitudinal strain as potential markers of coronary allograft vasculopathy in late-stage orthotopic heart transplantation. Int J Cardiovasc Imaging. 2018 Oct;34(10):1607-1617. Epub 2018 May 4. [CrossRef]
- Elkaryoni A, Abu-Sheasha G, Altibi AM, Hassan A, Ellakany K, Nanda NC. Diagnostic accuracy of dobutamine stress echocardiography in the detection of cardiac allograft vasculopathy in heart transplant recipients: A systematic review and meta-analysis study. Echocardiography. 2019 Mar;36(3):528-536. Epub 2019 Feb 6. [CrossRef]
- Sade LE, Hazirolan T, Kozan H, Ozdemir H, Hayran M, Eroglu S, Pirat B, Sezgin A, Muderrisoglu H (2019) T1 mapping by cardiac magnetic resonance and multidimensional speckle-tracking strain by echocardiography for the detection of acute cellular rejection in cardiac allograft recipients. JACC Cardiovasc Imaging 12(8 Pt 2):1601–1614.
- Vermes E, Pantaléon C, Auvet A, Cazeneuve N, Machet MC, Delhommais A, Bourguignon T, Aupart M, Brunereau L. Cardiovascular magnetic resonance in heart transplant patients: diagnostic value of quantitative tissue markers: T2 mapping and extracellular volume fraction, for acute rejection diagnosis. J Cardiovasc Magn Reson. 2018 Aug 27;20(1):59. [CrossRef]
- Erbel C, Mukhammadaminova N, Gleissner CA, Osman NF, Hofmann NP, Steuer C, Akhavanpoor M, Wangler S, Celik S, Doesch AO, Voss A, Buss SJ, Schnabel PA, Katus HA, Korosoglou G. Myocardial Perfusion Reserve and Strain-Encoded CMR for Evaluation of Cardiac Allograft Microvasculopathy. JACC Cardiovasc Imaging. 2016 Mar;9(3):255-66. [CrossRef] [PubMed]
- Shah NR, Blankstein R, Villines T, Imran H, Morrison AR, Cheezum MK. Coronary CTA for Surveillance of Cardiac Allograft Vasculopathy. Curr Cardiovasc Imaging Rep. 2018;11(11):26. Epub 2018 Sep 24. Erratum in: Curr Cardiovasc Imaging Rep. 2019;12(1):1. [CrossRef]
- Wever-Pinzon O, Romero J, Kelesidis I, Wever-Pinzon J, Manrique C, Budge D, Drakos SG, Piña IL, Kfoury AG, Garcia MJ, Stehlik J. Coronary computed tomography angiography for the detection of cardiac allograft vasculopathy: a meta-analysis of prospective trials. J Am Coll Cardiol. 2014 ;63(19):1992-2004. Epub 2014 Mar 26. 20 May. [CrossRef]
- Nous FMA, Roest S, van Dijkman ED, Attrach M, Caliskan K, Brugts JJ, Nieman K, Hirsch A, Constantinescu AA, Manintveld OC, Budde RPJ. Clinical implementation of coronary computed tomography angiography for routine detection of cardiac allograft vasculopathy in heart transplant patients. Transpl Int. 2021 Oct;34(10):1886-1894. Epub 2021 Sep 19. [CrossRef]
- Günther A, Andersen R, Gude E, Jakobsen J, Edvardsen T, Sandvik L, Abildgaard A, Aaberge L, Gullestad L. The predictive value of coronary artery calcium detected by computed tomography in a prospective study on cardiac allograft vasculopathy in heart transplant patients. Transpl Int. 2018 Jan;31(1):82-91. Epub 2017 Sep 21. [CrossRef]
- Budde RPJ, Nous FMA, Roest S, Constantinescu AA, Nieman K, Brugts JJ, Koweek LM, Hirsch A, Leipsic J, Manintveld OC. CT-derived fractional flow reserve (FFRct) for functional coronary artery evaluation in the follow-up of patients after heart transplantation. Eur Radiol. 2022 Mar;32(3):1843-1852. Epub 2021 Sep 15. [CrossRef]
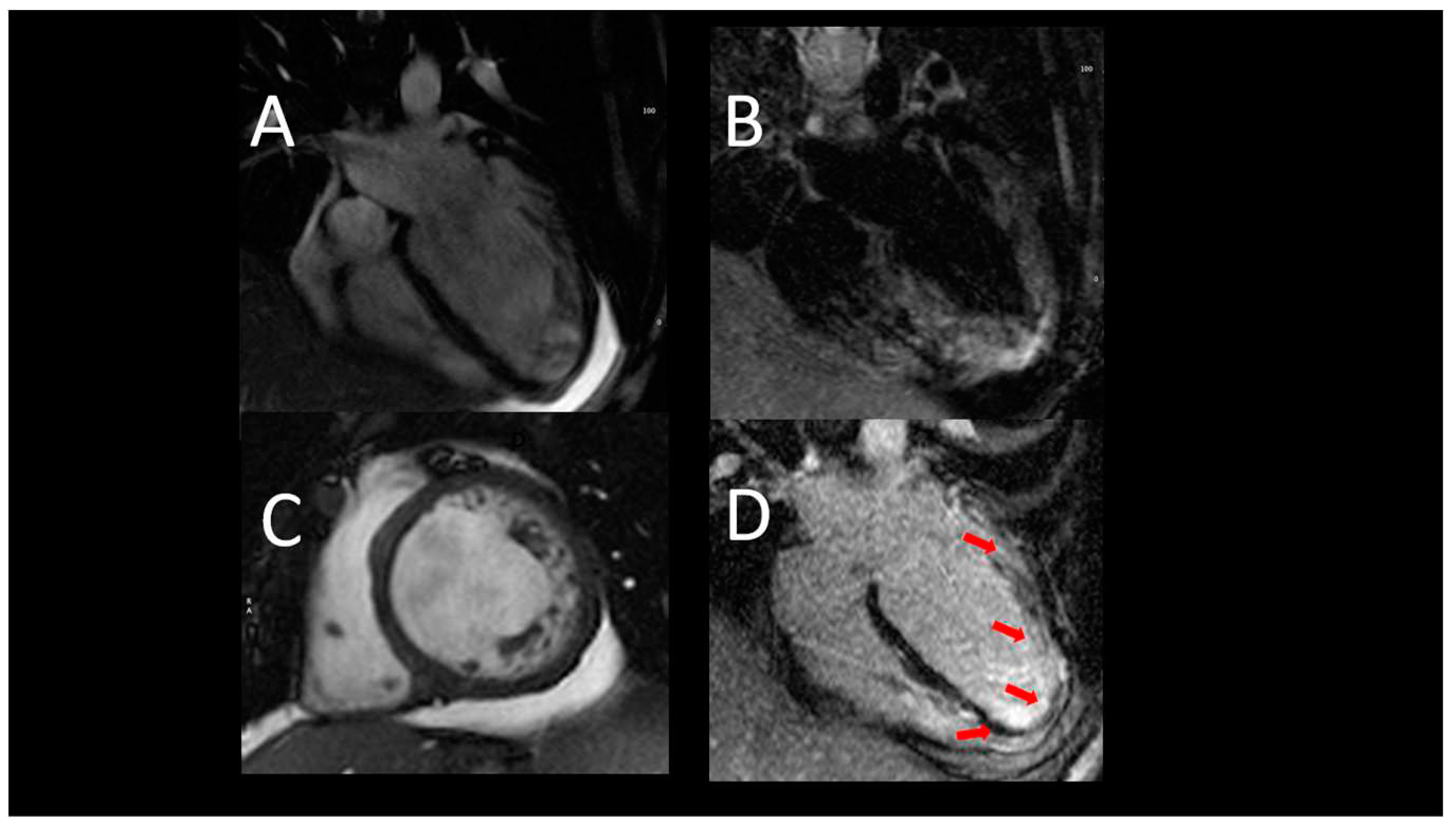
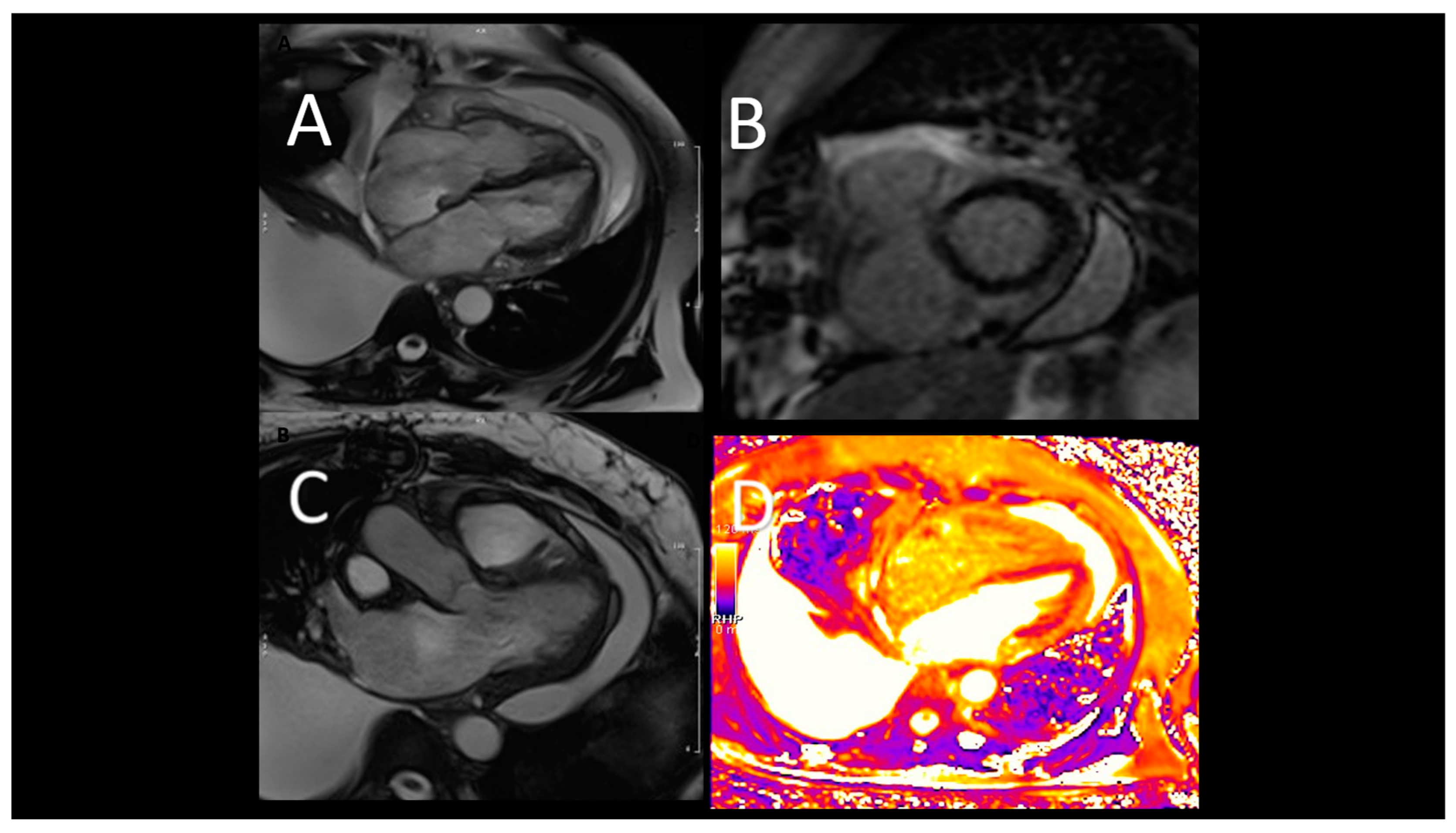
Figure 1.
Representative case of use of CMR inf first acute heart failure. A, B) acute heart failure, CMR images showing severe LV dilatation, associated with hypertrabeculation. C) T2-weighet images exclude oedema; D- a diffuse endocardial late enhancement (red arrows) was detected, compatible with endomyoardial disease.
Figure 1.
Representative case of use of CMR inf first acute heart failure. A, B) acute heart failure, CMR images showing severe LV dilatation, associated with hypertrabeculation. C) T2-weighet images exclude oedema; D- a diffuse endocardial late enhancement (red arrows) was detected, compatible with endomyoardial disease.
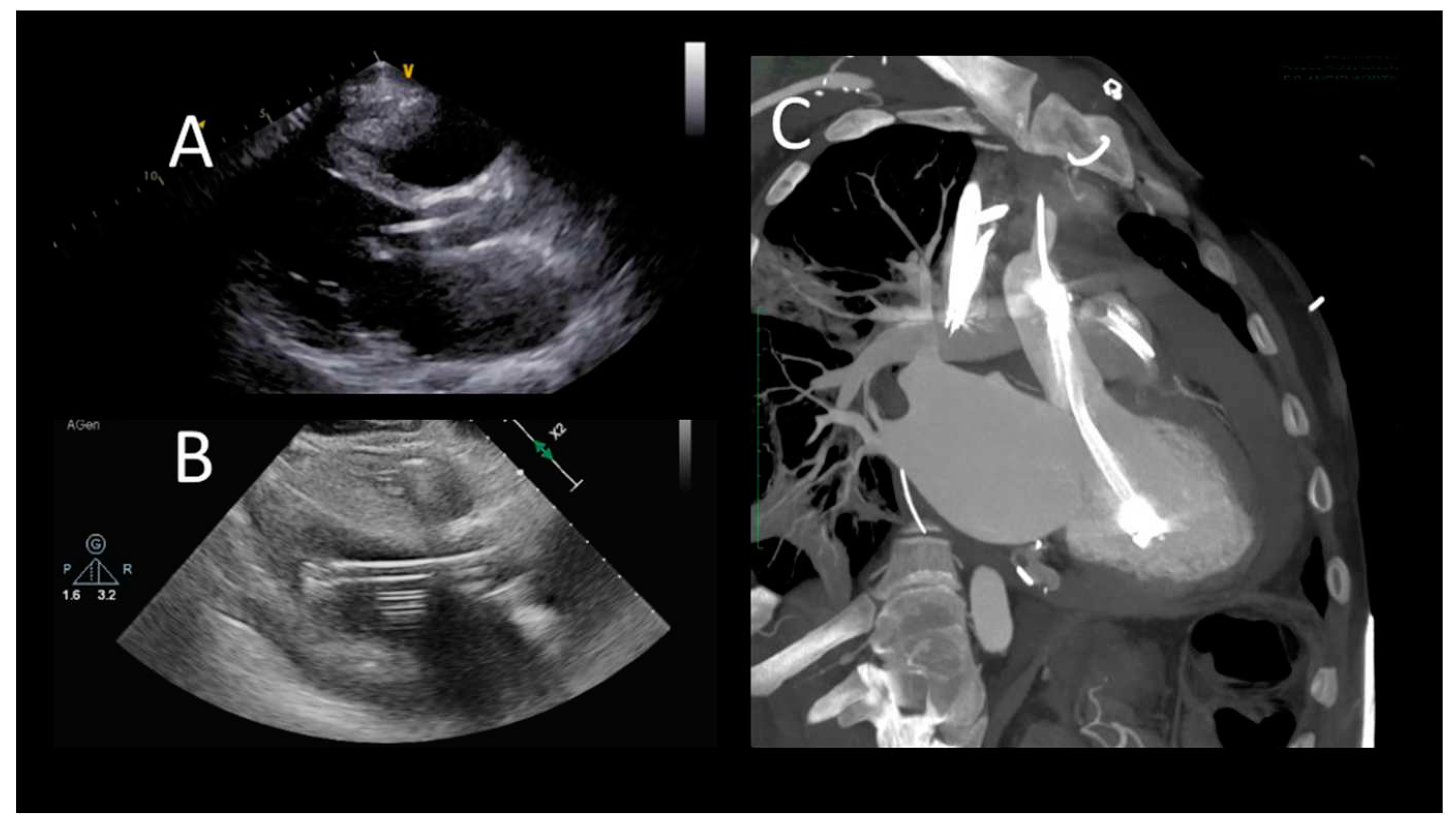
Figure 2.
Transthoracic echocardiographic evaluation after Impella implantation A) correct device's position; B) uncorrect position (towards the LV apex), C) CCT showing incorrect, apical position.
Figure 2.
Transthoracic echocardiographic evaluation after Impella implantation A) correct device's position; B) uncorrect position (towards the LV apex), C) CCT showing incorrect, apical position.
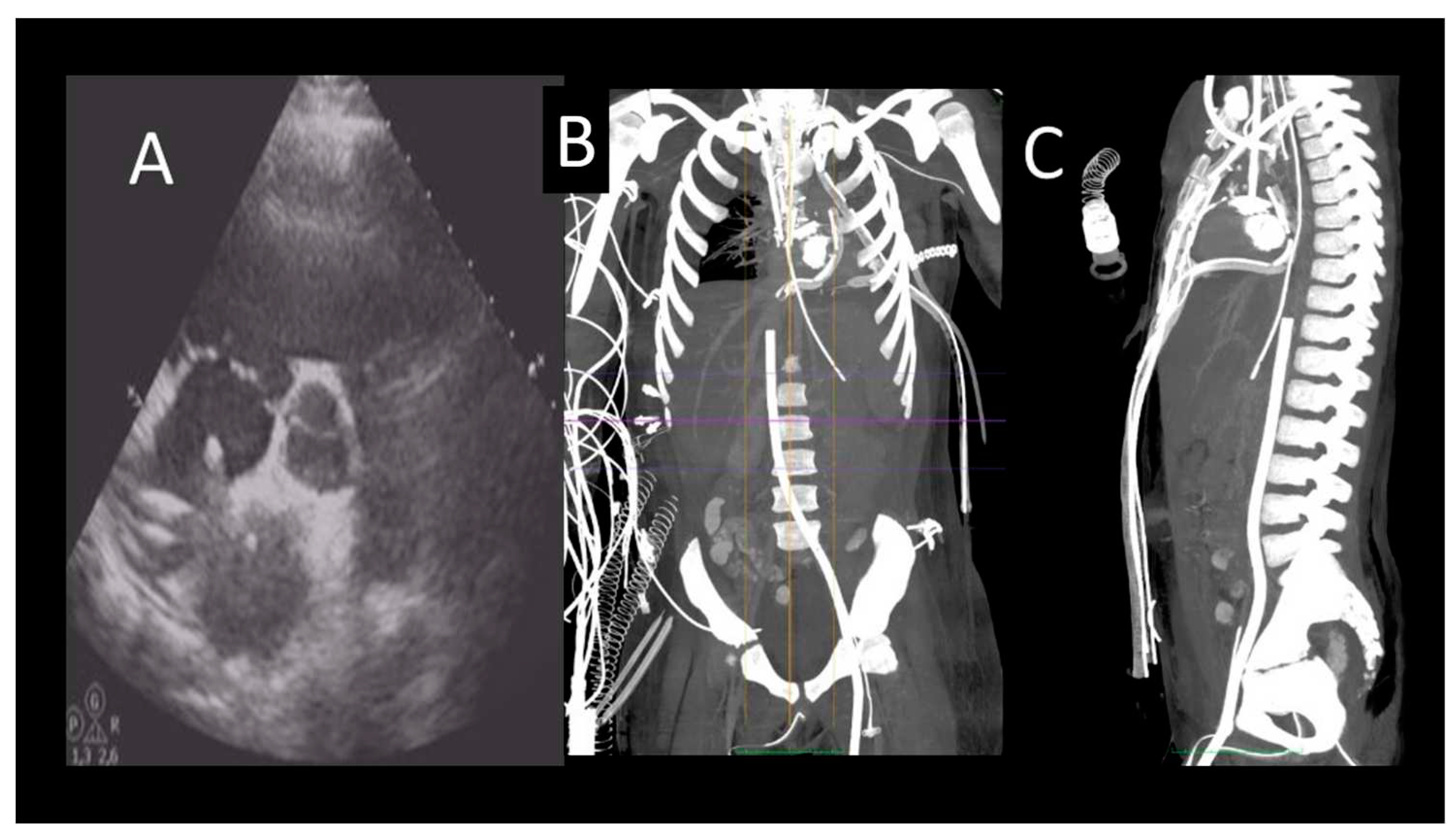
Figure 3.
Evaluation after VA ECMO implantation. A) TTE (PSAX) showing correct position of RA cannula; B) CCT showing correct position of RA and femoral vein ECMO cannulas.
Figure 3.
Evaluation after VA ECMO implantation. A) TTE (PSAX) showing correct position of RA cannula; B) CCT showing correct position of RA and femoral vein ECMO cannulas.
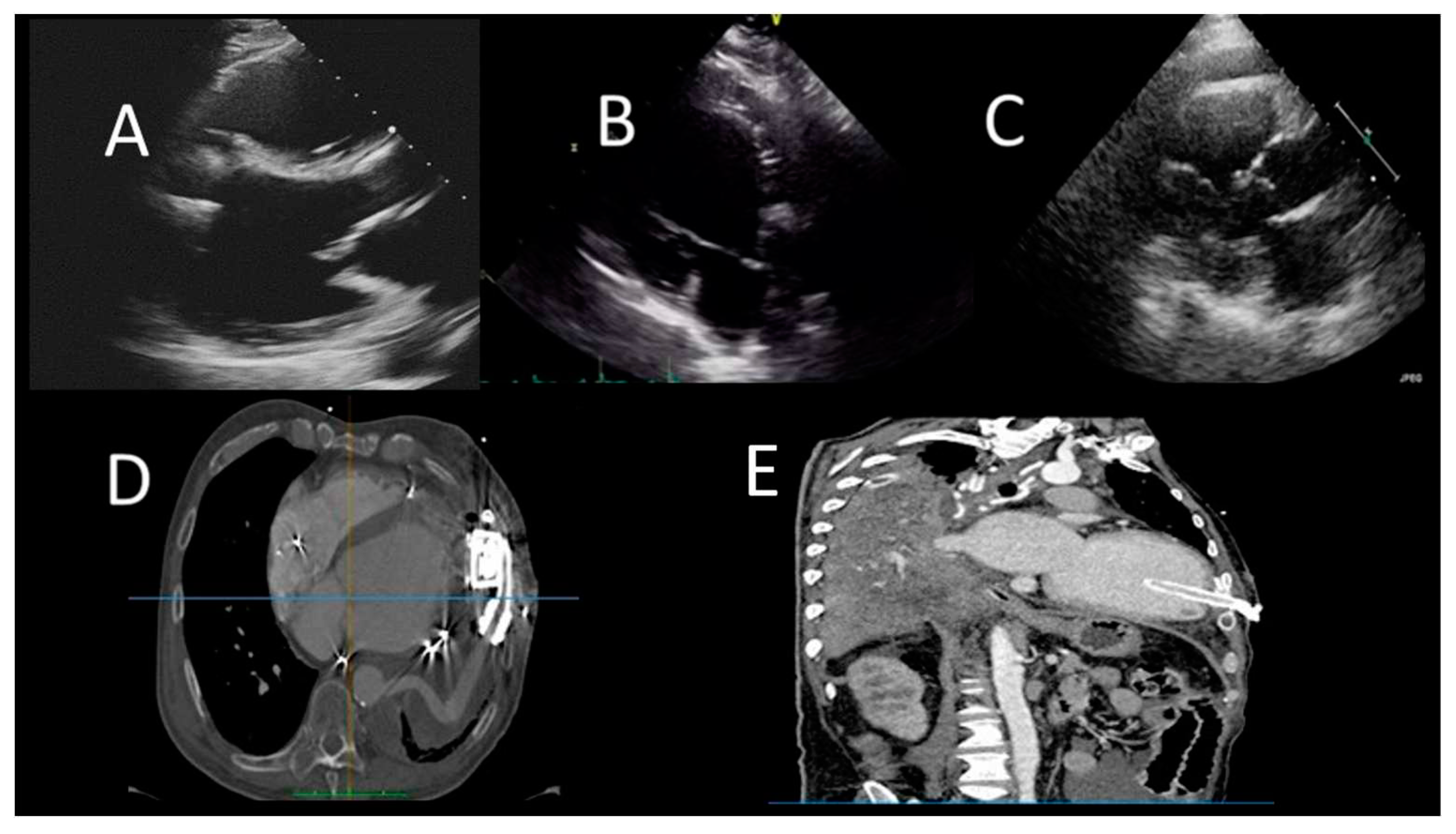
Figure 4.
Evaluation after LVAD assist device. A) TTE evaluation (PLAX) shows normal position of IVS and the inflow cannula; B) TTE (PLAX) of LVAD patient, showing incorrect right-convex position of the IVS; C) TTE evaluation (PLAX) of LVAD patient, showing incorrect left-convex position of the IVS; D) CCT showing hematoma around LV cannula; E) CCT showing small LV apical thrombus.
Figure 4.
Evaluation after LVAD assist device. A) TTE evaluation (PLAX) shows normal position of IVS and the inflow cannula; B) TTE (PLAX) of LVAD patient, showing incorrect right-convex position of the IVS; C) TTE evaluation (PLAX) of LVAD patient, showing incorrect left-convex position of the IVS; D) CCT showing hematoma around LV cannula; E) CCT showing small LV apical thrombus.
Figure 5.
Representative case of use of CMR in OTC patients. A, B) immuno-mediated rejection with pericardial and pleural effusion (diastolic frame on A and B, respectively four- and three long-axis views) ; C) Post-contrast sequences (short axis view) demonstrated the absence of late gadolinium enhancement (LGE) and the T2-mapping was negative for inflammation. The absence of LGE and normal mapping confirmed their prognostic role since the patient demonstrated a fully recovery after modification of immunosoppressive therapy.
Figure 5.
Representative case of use of CMR in OTC patients. A, B) immuno-mediated rejection with pericardial and pleural effusion (diastolic frame on A and B, respectively four- and three long-axis views) ; C) Post-contrast sequences (short axis view) demonstrated the absence of late gadolinium enhancement (LGE) and the T2-mapping was negative for inflammation. The absence of LGE and normal mapping confirmed their prognostic role since the patient demonstrated a fully recovery after modification of immunosoppressive therapy.
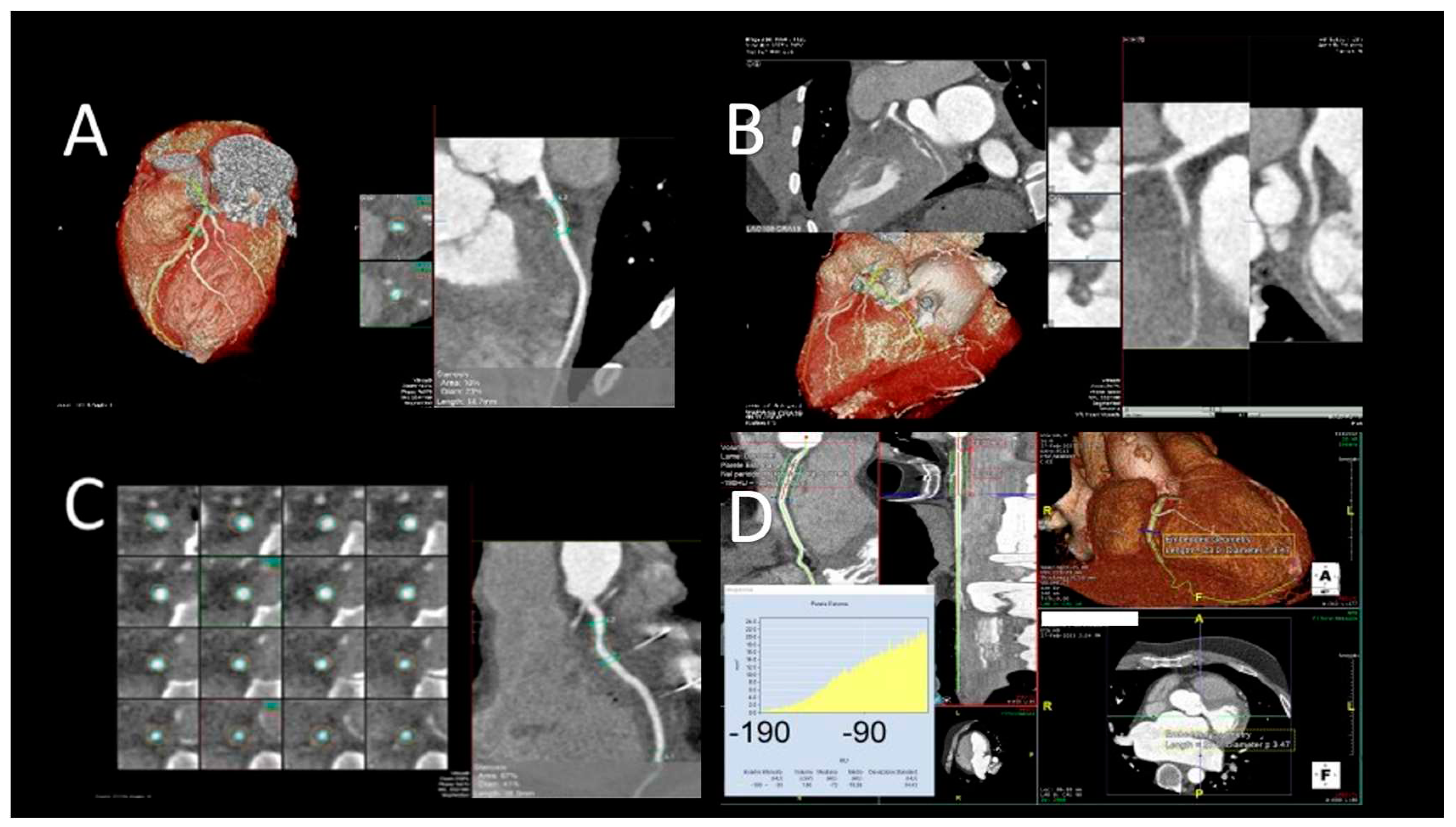
Figure 6.
Evaluation of cardiac plaques in OTC patients by CCT. A) mild LAD circumferential soft plaque; B) occlusion of LCx artery; C) mild RCA soft plaque; D) Increased pFAI values suggestive of coronary artery inflammation.
Figure 6.
Evaluation of cardiac plaques in OTC patients by CCT. A) mild LAD circumferential soft plaque; B) occlusion of LCx artery; C) mild RCA soft plaque; D) Increased pFAI values suggestive of coronary artery inflammation.
Table 1.
Cut off reference for Left Ventricular Ejection Fraction (LV EF) and their clinical implications (1).
Table 1.
Cut off reference for Left Ventricular Ejection Fraction (LV EF) and their clinical implications (1).
| LVEF |
Clinical indication |
| ≤35% |
ICD implantation in primary prevention |
| Role |
Severe cardiac dysfunction |
| Role |
Role |
| Role |
Role |
Table 2.
Timing and role of TTE (transthoracic echocardiography), TEE (transesophageal echocardiography) and CCT (cardiac computed tomography) in IAPB (intra-aortic balloon pump).
Table 2.
Timing and role of TTE (transthoracic echocardiography), TEE (transesophageal echocardiography) and CCT (cardiac computed tomography) in IAPB (intra-aortic balloon pump).
| |
TTE |
TEE |
CT |
| Role |
Monitor LV function.
Guide the weaning of IABP support. |
Guide positioning. |
Indicated in the suspicion of complications. |
| Timing |
Post operative. |
Intra operative. |
Post operative. |
| Identification of complications |
New or worsening aortic regurgitations. |
|
Aortic dissection.
Displacement of aortic balloon.
Arterial embolizations and in organ parenchyma. |
Table 3.
Timing and role of TTE (transthoracic echocardiography), TEE (transesophageal echocardiography), and CCT (cardiac computed tomography) in Impella patients.
Table 3.
Timing and role of TTE (transthoracic echocardiography), TEE (transesophageal echocardiography), and CCT (cardiac computed tomography) in Impella patients.
| |
TTE |
TEE |
CT |
| Role |
Selection of candidates.
Guide the placement |
Selection of candidates.
Guide the placement |
To exclude complications. |
| Timing |
Pre operative.
Post operative. |
Pre operative.
Intra operative.
Post operative. |
Post operative. |
| Identification of complications |
Mitral and aortic regurgitations.
Pericardial effusion.
Rupture of LV free wall. |
Exclude iatrogenic aortic dissection.
Damage of mitralic and aortic valve. |
Aortic dissection.
Damage of mitral and aortic valve system. |
| |
|
|
|
Table 4.
Timing and role of TTE (transthoracic echocardiography), TEE (transesophageal echocardiography), and CCT (cardiac computed tomography) in veno-arterial extracorporeal membrane oxygenation.
Table 4.
Timing and role of TTE (transthoracic echocardiography), TEE (transesophageal echocardiography), and CCT (cardiac computed tomography) in veno-arterial extracorporeal membrane oxygenation.
| |
TTE |
TEE |
CT |
| Role |
Selection of candidates.
Identification of complications.
Weaning. |
Guide the placement.
Identification of complications. |
Identification of complications. |
| Timing |
Pre operative.
Post operative. |
Intra operative.
Post operative. |
Post operative. |
| Identification of complications |
Aortic dissections.
Mitral and aortic regurgitations.
|
Cannula malposition.
Plaque embolizations.
Aortic dissections.
Mitral and aortic regurgitations.
|
Defect of opacification of arterial system.
Cannula malposition.
Hematoma.
Haemothorax;
Thrombosis of arterial system. |
Table 5.
Parameters to be evaluated in LVAD candidates and their influence on LVAD placement.
Table 5.
Parameters to be evaluated in LVAD candidates and their influence on LVAD placement.
| Parameter |
Influence on LVAD placement |
| FE |
< 25% indicates LVAD placement. |
| LV Size |
An adeguate volume is essential to LVAD placement. |
| Intra-cardiac thrombi |
Exclude LVAD placement. |
| RV function |
The presence of severe RV dysfunction may suggest a biventricular support. |
| Valve abnormalities |
Signicant aortic regurgitation, moderate to severe mitral stenosis and moderate to severe tricuspid regurgitation exclude LVAD placement. |
| Congenital heart disease |
Shunt lesions exclude LVAD placement. |
Table 6.
Timing and role of TTE (transthoracic echocardiography), advanced echocardiography, and CCT (cardiac computed tomography) in LVAD patients.
Table 6.
Timing and role of TTE (transthoracic echocardiography), advanced echocardiography, and CCT (cardiac computed tomography) in LVAD patients.
| |
TTE |
TTE with Echocontrast |
CT |
| Role |
LV volume and function.
Position of interventricular septum and cannula.
Right ventricular size and function. |
Better definition of endocardial border for quantification of LV volume and residual function.
Identification of patients at higher risk of RV dysfunction. |
Identification of specific complications. |
| Identification of complications |
Evidence of the intracardiac thrombi. |
Increases the possibility to detect intracavitary thrombi. |
Compression of right ventricle.
Thrombosis.
Malposition and kinking of outflow cannula. |
Table 6.
Timing and role of TTE (transthoracic echocardiography), advanced echocardiography,and CCT (cardiac computed tomography) in HT (heart transplant) patients.
Table 6.
Timing and role of TTE (transthoracic echocardiography), advanced echocardiography,and CCT (cardiac computed tomography) in HT (heart transplant) patients.
| |
TTE |
Advanced echo |
CMR |
CT |
| Role |
LV wall thickness and mass.
LV volume and function.
Diastolic function.
Valve morphology and function.
Pericardium. |
LV torsion (speckle tracking).
Cardiac ischaemia (stress echocardiography).
|
LV wall thickness and mass.
LV volume and function.
Myocardial perfusion reserve (stress). |
Coronary stenosis.
Coronary plaque. |
| Timing |
Immediate post operative.
Short-term period.
Long-term period. |
Short-term period.
Long-term period. |
Short-term period.
Long-term period |
Short-term period.
Long-term period. |
| Identification of complications |
Allograft rejection.
Primary or secondary valvopathies.
Pericardial effusion. |
Acute cell rejection.
Cardiac allograft vasculopathy. |
Acute cell rejection.
Cardiac allograft vasculopathy. |
Cardiac allograft vasculopathy. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).