1. Introduction
Cashew (
Anacardium occidentale) is a non-climacteric fruit that is traditionally endemic to northeastern Brazil. The fruit is composed of both a true fruit, which is the chestnut, and a pseudo fruit, which is the pulpy part or apple. It is commonly used by the Brazilian population culinary in the production of sweets, juices, and primarily, dried and/or roasted chestnuts. However, due to the high economic value of the chestnut, a significant amount of pseudo fruit is wasted during processing. This waste occurs either because of the fruit’s high perishability or after juice extraction, which generates a fibrous matrix with no specific use [
1,
2]. Therefore, preliminary studies have been conducted to explore the potential uses of the pseudo fruit in human food [
1].
Therefore, the by-products of tropical fruits, such as cashew, can be regarded as potential sources of dietary fibers and natural pigments, supporting international recommendations to increase fiber consumption [
3,
4,
5]. Compounds found in the peel, seeds, and residual pulp of fruits can provide energy for the intestinal microbiota, leading to the generation of several bioactive metabolites with increased bioavailability and/or bioactivity [
6]. It’s important to highlight that e gut microbiota plays a crucial role in nutrient synthesis, digestion and absorption, immune modulation, pathogen control, modulation of energy extraction from food, and regulation of appetite [
7].
In addition to improve the gut microbiota, the use of fruit by-products as potential prebiotic ingredients that are selectively utilized by allochthonous beneficial microorganisms has been studied [
8,
9]. Prebiotics are substrates that can
‘stimulate the growth of beneficial microorganisms, which confer health benefits to the host
’ [
10]. In contrast, probiotics are
‘live microorganisms that confer a health benefit on the host when administered in adequate amounts
’ [
11], with many of them belonging to the lactic acid bacteria group [
12]. For probiotics, their survival in the food matrix is essential.
Although there is no consensus on the physiological dose for a probiotic benefit, institutions and researchers have suggested that populations ranging from 6.0 - 9.0 log of Colony-Forming Units (CFU) per gram or serving portion could assist in maintaining a healthier gut microbiota [
11,
13].
Furthermore, fruit by-products may contain various phenolic compounds, including those of the flavonoid type (catechin, quercetin, procyanidin dimers, and proanthocyanidins) and those of the non-flavonoid type (phenolic acids, stilbenes, tannins, coumarins, etc.) [
14,
15,
16].
The diverse chemical composition of fruit by-products, including cashew pseudofruit, could assure significant prebiotic potential, and be a food matrix for probiotics. Therefore, this study aims to evaluate the functional aspects of cashew apple by-product (CB), involving its application in fermented milk with a probiotic strain and a starter culture.
2. Materials and Methods
2.1. Cashew by-product (CB)
Approximately 15 kg of CB were obtained from a local juice processing industry in Natal, Rio Grande do Norte, Brazil. The collection, storage, and transportation to São Paulo, Brazil, where it was dehydrated, were performed with this matrix frozen at -18 °C. First, portions of 500 g were hermetically packed and heated in boiling water for 2 minutes, followed by an ice bath cooling. Next, they were dehydrated at 60 °C in an oven with air circulation (Solab, São Paulo, Brazil) until constant weight, milled and sieved to obtain flour with an approximate particle size of ≤ 0.42 mm as described by Praia et al. (2022), and stored in hermetically closed glass bottles, under light protection at -20 °C. A 10 g portion of the dehydrated CB, kept for the fermentability study, was sterilized with irradiation in Gammacell 220 (Atomic Energy Canada Ltd Ottawa, Canada) with activity of 1287.6 Ci and dose of 25 kGy, at a rate of 1.089 kGy/h, according to Albuquerque et al. [
8].
The non-irradiated CB were evaluated by antioxidant activity, total phenolic compounds using Folin-Ciocalteu reagent, DPPH, and gallic acid (Sigma-Aldrich, St. Louis, MA, USA). The characterization of the phenolic compound profile of CB was also conducted.
2.2. Fermentability assay in CB by probiotic and starter bacteria
CB was evaluated for its ability to interfere with the growth of a range of bacteria using in vitro fermentability as described by Vieira et al. [
9]. A total of 10 probiotic strains and 3 starters were selected (
Table 1) to achieve an inoculum of 4-5 log CFU/mL, determined by traditional plating methods as described below.
The frozen strains were thawed and subsequently reactivated in 5 mL of specific broth under specific cultivation conditions (
Table 1). Aliquots of 100 µL were added to 5 mL of modified MRS broth - MRSm supplemented with 1% of CB previously sterilized by irradiation, as described above, following incubation at 37 °C for up to 48 hours.
The microorganisms in MRSm + 1% CB were enumerated by colony counting on selective agar at the initial inoculum (0 h), and after 24 h, and 48 h of incubation (
Table 2). Control tests were also conducted to verify the effectiveness of CB sterilization and fermentative capacity of all strains in pure MRSm broth (without CB). The results were expressed as the variation in microorganisms’ populations (delta - Δ) between 0 and 24 h of fermentation (Δ24) and between 0 to 48 h of fermentation (Δ48) (
Table 2). Based on these population variation results, combined with scientific research on the tested strains, the probiotic strain
Lacticaseibacillus paracasei subsp.
paracasei F19
® and the starter
Streptococcus thermophilus ST-M6
® were selected for use in fermented milk formulations.
2.3. Cultures employed, and the fermented milk production
The probiotic strains
Lacticaseibacillus paracasei subsp.
paracasei F19® (F19) and the starter culture
Streptococcus thermophilus ST-M6® (ST-M6), both from Chr. Hansen (Hørsholm, Denmark), were chosen for use due to their known abilities and survival rates in various food matrices. The probiotic and the starter strains, frozen probiotics, and starter cultures (preserved at −80 °C with 20% glycerol) were activated at 37 °C for 24 hours in the specific broth for each strain. F19 strains were activated in MRS (De Man-Rogosa and Sharp, Oxoid) broth under anaerobic conditions with the AnaeroGen
TM Anaerobic System (Oxoid), and ST-M6 strains were activated in Hogg-Jago Glucose (HJ) broth under aerobic conditions, following the methodology described by Battistini et al. [
17].
For the formulation of fermented milk, partially skimmed (2.4% of milk fat) powdered milk, lactose-free sterilized milk (Ninho®, Nestlé, Vevey, Vaud, Switzerland) (
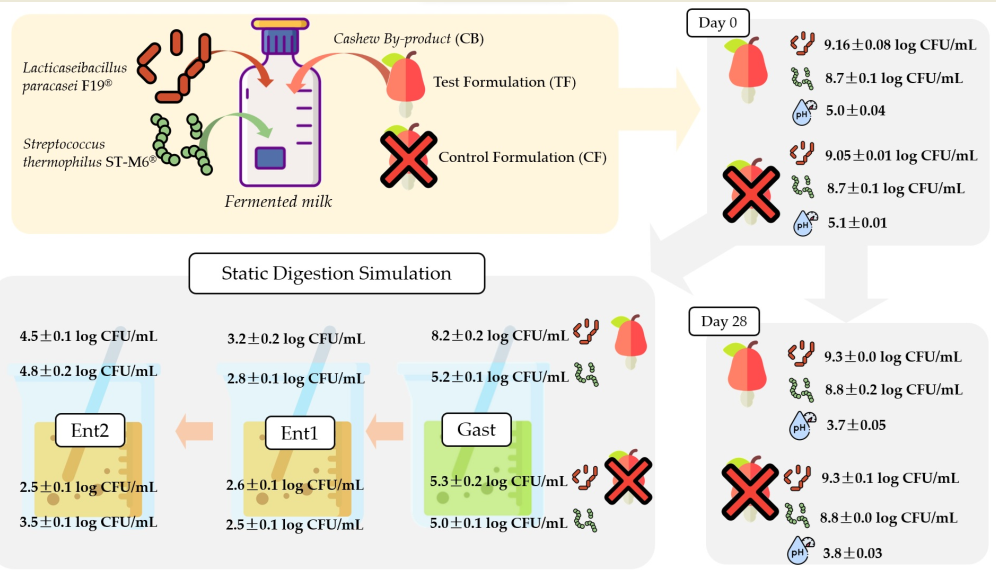
Table 3). The reconstituted milk was heated with agitation in a food processor (Thermomix, Vorweck®, Germany) with 5% of sugar (União®, Brazil), 2,5% of powdered milk (Ninho®, Nestlé, lactose-free), and 0,1% of unflavored gelatin (Royal®, Mondelez). A 2,5% of non-sterilized CB was added only to the FT, and along with the CF, they were both pasteurized for 5 minutes at 90 °C and cooled in an ice bath to 37 °C. At last, the probiotic and starter cultures were added resulting in a pre-fermentation inoculum of at least 8.0 log CFU/mL of each strain.
The formulations were incubated at 37 °C until reaching pH 5.5 and refrigerated at 4 °C overnight. After overnight, the fermented milk formulations were homogenized with the aid of sanitized spatulas. Portions of 25 g were subdivided into polypropylene cups (Tries Aditivos Plásticos, São Paulo, Brazil), sealed and stored under refrigeration (4 °C) for up to 28 days.
2.4. Evaluation of microorganism survival during storage
Three batches of each formulation were produced and the evaluation during shelf life was conducted using the parameters described next from the first day after obtaining the product and weekly on days 7, 14, 21, and 28 of storage. The viability of probiotic and starter microorganisms in the 3 batches of fermented milk was determined during refrigerated storage in the intervals cited above for both formulations. Three portions of 10 g (taken from independent plastic pots) were mixed with 90 mL of sterile saline solution (0.85%) following subsequent serial dilutions. The microbial counts were expressed in log CFU/g using the pour plate technique where 1 mL of the dilutions was thoroughly mixed with the appropriate agar culture medium for each species (
Table 1).
2.5. Physicochemical characterization
The chemical composition of the CB and the fermented milk formulations (CF and TF) was determined, following recommendations from the Official Methods of Analysis of the Association of Official Agricultural Chemists (2012). The following parameters were determined: moisture (925.09 - AOAC 2012), fixed mineral residue (930.30 - AOAC 2012), total fats (Bligh and Dyer, 1959), proteins (990.03 - AOAC 2012), total and insoluble dietary fibers (respectively, 985.29 and 991.42 - AOAC 2012).
The available carbohydrate content was calculated by difference. The fiber content for TF was estimated based on the amount of CB added in the test formulation (2.5%). Direct pH measurement was performed on CB solution, CF, and TF with Orion Three Stars equipment (Thermo Fisher Scientific, Waltham, MA, USA) using a penetration electrode, model 2A04-GF (Analyzer), in triplicate. The CB acidity was quantified using the titration method for expression in equivalents of malic acid (IAL, 2008). For this, the measurement was carried out in a solution of 10 g in 100 mL of distilled water homogenized in BagMixer 400 (Inter Science, St. Nom, France) for 2 minutes (IAL, 2008).
The CB were subjected to 2 consecutive extractions under stirring at room temperature in a ratio of 1:20 with 70% methanol. The extract was subjected to analysis of total phenolic compounds using Folin-Ciocalteu reagent (Sigma-Aldrich, Brazil) and to the DPPH free radical antioxidant potential (Magalhães et al., 2010, Medeiros et al., 2019). The methanolic extraction of the CB was subjected to a solid phase extraction, with CC 6 polyamide columns (1g / 6mL) (Macherey-Nagel Gmbh and Co., Duren, Germany) conditioned with methanol and distilled water. Methanol and methanol-ammonia were extracted and completely evaporated under reduced pressure at 40 °C, dissolved in methanol and filtered through a 0.22 µm filter membrane (polytetrafluoroethylene – PTFE, Millipore).
Phenolic compounds of CB were profiled in reversed phase HPLC (Hewlett-Packard 1100 system) with an autosampler, and a quaternary pump coupled to a diode array detector. The eluent solvents were A (water/tetrahydrofuran/trifluoroacetic acid 98:2:01) and B (acetonitrile). Extracts were monitored at 270 nm and injected in triplicate for each eluate (methanol and methanol/ammonia). To identify the phenolic compounds of interest, the peak identification, retention times and spectral characteristics of the diode matrix were compared to standards and spectra from the database.
2.6. Evaluation of fatty acid profile
Lipids extracted from CB, TF, CF samples were submitted to a cold extraction of total lipids [
18] and detection of fatty acid profile, followed by the esterification of fatty acids with methanolic boron trifluoride and quantification with Gas Chromatography coupled to Mass Spectrometry (GC-MS, Agilent 7890a GC, Santa Clara, CA, USA) (Iverson et al., 2001) with C23:0 methyl trichosonoate as internal standard and values were expressed as percentage.
2.7. Viability of microorganisms during in vitro-simulated gastrointestinal conditions
This parameter was investigated in fermented milk samples, at four storage times (days 1, 7, 14, and 28). For this purpose, in vitro-simulated gastrointestinal conditions adapted by Bedani et al. [
19] was used to assess the survival of the probiotic and starter strainss. The samples were diluted 1:10 in sterilized peptone water (0.1%), and aliquots of 10 mL were passed through three sequential phases: Gastric Phase (Gast), and Enteric Phase 1 and 2 (Ent1 and Ent2). All phases were performed during 2 hours in a metabolic bath (MA-095, Marconi, Brazil) at 37 °C and 150 RPM of agitation with the following parameters: Gast with pH 2.0-2.5 with HCl, pepsin and lipase at concentrations of 3 g/L and 0.9 mg/L; Ent1 with pH 4.5-5.5 with buffer solution, bile salts and pancreatin at concentrations of 10 g/L and 1 g/L; and last Ent2 with pH to 6.5-7.5 and the same concentrations of bile salts and pancreatin as in the previous phase.
The counts of probiotic and starter cultures was obtained with aliquots collected from triplicates at time 0 and after each phase. Aliquots of 1 mL of serial dilutions in sterile buffered peptone solution (0.1%) were used in the phases that simulated gastrointestinal conditions (and sterile 0.85% saline solution for time 0). Aliquots were submitted to counting using the pour plate technique in specific agar and incubation conditions (
Table 1). Results were expressed in log CFU/g of each fermented milk formulation.
2.8. Statistical analysis
All statistical analyzes were performed using the Minitab statistical package, version 17.3.1 for Windows (Minitab Inc. 2013, USA). The results were submitted to descriptive analysis as mean, standard deviation, or standard error of the mean. For non-parametric data, the median was presented followed by the first and third quartiles.
Levene’s tests were performed for equality of variances and Shapiro-Wilk’s tests to evaluate normal distribution. Depending on these results, parametric or nonparametric tests with a significance level of 95 or 99% were used, followed by the appropriate mean or median comparison. If necessary, data that did not assume a normal distribution were subjected to Johnson’s transformation and, in this case, if there was no equality of variances, the means were paired and compared using the Games-Howell test with a significance level of 95 or 99%.
3. Results and Discussion
3.1. Physical-chemical and functional characteristics of the cashew by-product (CB)
A high protein content profile was observed in the cashew by-product, with 10.67 (± 0.02) g of protein per 100 g of by-product (
Table 5), which is higher than certain cereals [
20]. Since the evaluated material represents the fibrous portion retained after pressing the pseudofruit, emphasis should be given to the dietary fiber values, with higher values of insoluble fiber expected.
Applying this fibrous matrix as an ingredient can be an important factor in increasing beneficial health effects, such as water retention and satiety (soluble fiber) or regulation of intestinal effects and increased stool volume (insoluble fraction) [
21,
22]. In fact, the total dietary fiber values were higher than those of other by-products, such as apple (55.48 g/100 g), guava (44.30 g/100 g), and acerola (48.60 g/100 g) [
5,
23].
Phenolic compounds in cashew apples tend to be retained in husks, seeds, or residual fibrous pulp [
24,
25]. Phenolic compounds and dietary fiber from fruits, whether by-products or not, have a positive relationship with the modulation ability of the intestinal microbiota, integrity of the intestinal mucosa, serum levels, and insulin resistance [
26,
27,
28]. In this study, cashew by-products presented 486.6 mg EAG/100g of total phenolic compounds, which directly impacted increased antioxidant activity measured by the DPPH free radical assay (88.8% inhibition and IC50 of 1.16 mg/ml). These values are higher than those found in apple, banana, and orange by-products [
29].
Syringic and ellagic acid values exceeded those found in a study with lyophilized by-products of cashew, acerola, and guava, which ranged from 1.0 to 49.0 mg/100g for ellagic acid and 5.0 to 50.6 mg/100g for syringic acid [
30]. Lower values of ellagic acid were identified in cashew nut extracts [
31], as well as salicylic and vanillic acids, and flavonoids (quercetin, myricetin, narigenin, etc.) in lyophilized cashew by-products [
5].
The most abundant fatty acids were oleic, palmitic, stearic, linolenic, and linoleic acids (
Table 5). Cashew by-products showed a higher amount of oleic acid than soy, hemp, and lupine, as well as a higher linolenic acid content than that reported for oats [
32].
3.2. Probiotic selection
Due to the large number of probiotic strains, the choice of the most adapted one to CB was decided in two stages, as described in
Table 2: Δ 48 and Δ 24. In Δ48 of fermentation in MRSm + CB,
Limosilactobacillus reuteri RC-14® (RC-14) strain showed the highest value (3.32 log UCF/mL), but withoutstatistical significance (p < 0.05) when compared to
Lacticaseibacillus paracasei subsp.
paracasei 431® (431),
Lactobacillus acidophilus LA-5® (LA-5),
Lacticaseibacillus paracasei subsp. paracasei F19® (F19) and
Bifidobacterium animalis subsp.
lactis BB-12. These five strains with the best Δ48 value were selected for comparison with their respective MRSm Control groups. At this point, it was observed that only strains L. reuteri RC-14 and
L. paracasei subsp.
paracasei (F19) exhibited significantly higher values (p < 0.05) compared to their respective controls. Therefore, further comparisons were conducted exclusively between these strains in terms of Δ24 multiplication.
The Δ24 of these two strains were statistically similar (p > 0.05) in MRSm + CB compared to each other, and higher (p < 0.05) when compared with their respective MRSm Control. However, as the objective of the work was to choose only one probiotic strain.
L. paracasei subsp.
paracasei (F19) strain has interesting characteristics of genetic stability, survival to digestive conditions and beneficial interaction with the intestinal epithelium related to regulation of genes associated with gastrointestinal pathologies [
33].
Other evidence from clinical studies summarized by Jones [
34] corroborates more positive claims of
L. paracasei F19 associated with a high-fiber diet in improving swelling and abdominal pain, as well as the influence of this probiotic in reducing the accumulation of fat by modulating transcription factors in energy metabolism.
In another study with probiotic strains, both RC-14 and F19 showed good adaptability in fruit by-products, okara or amaranth flour after 24 and 48 hours of fermentation [
9]. On the other hand, another report demonstrated a low viability of the RC-14 strain in fermented milk throughout its shelf life [
35]. Thus, summing up all this evidence, it was decided to choose the F19 strain for application in fermented milk formulations.
Starter strains were also chosen in stages (
Table 2). However, unlike probiotics, the number of starters was considerably smaller. A promising behavior of
S. thermophilus ST-M6 was observed. This strain had a higher Δ48 and Δ 24 multiplication values in the MRSm + CB compared with the other strains (p < 0,05). When compared to their respective MRSm Control groups, S. thermophilus ST-M6 also showed significantly higher values (p < 0.05) both in Δ48 and Δ 24. Therefore, this strain was chosen to be the starter in the fermented milk formulation.
3.3. Viability of microorganisms used in fermented milk during storage, pH and titratable acidity
The presence of CB had little effect on the microorganism’s viability (
Table 4). In both formulations, values above 9.0 log CFU/mL for F19® and above 8.6 log CFU/mL for ST-M6 were found during 28 days in refrigeration. During storage, higher values (p < 0.01) were found in the TF for the probiotic strain (day 14) and starter (day 7), although both increases were lower than 0.5 log CFU/mL. Vieira et al. [
36], using probiotic Lactobacillus genus in a plant-based beverage with acerola fibers, obtained lower values than those in the present study over a 28-day refrigeration period (minimum of 7.35 ± 0.56 log.CFU/mL).
The ST-M6® strain had a count of 8.8 CFU/mL, after 28 days in both CF and TF formulations. ST-M6 showed an increasing trend until day 21, followed by a decline on day 28 in both formulations.
The viability of probiotic microorganisms in the refrigerated storage of both CF and TF was acceptable. International institutions such as the Canadian [
37] consider adequate amounts of more than 9 log CFU/portion of the more traditional probiotics (
Lactobacillus spp. or
Bifidobacterium spp.) to claim a general benefit in improving the health of the intestinal microbiota [
11].
The active metabolism of microorganisms presents in fermented milk also resulted in acidification during their storage (
Table 4) (p < 0.05). At the end of 28 days, free acidity (grams of lactic acid/100 mL) and pH were, respectively, 1.4 and 3.7 for TF and 1.5 and 3.8 for CF. It should be noted that both the pH and the titratable acidity of the two formulations is according to national and international quality standards for fermented milks throughout their shelf life (FAO, 2023; Brazil, 2007).
Vieira et al. [
38] observed the same acidification trend during refrigeration in probiotic plant-based beverages with acerola fiber (reaching pH 4.2 in some formulations on day 28). Karaka et al. [
39] developed a probiotic yogurt supplemented with 1 or 2% apricot fiber with probiotic strains from the
Lactobacillus genus and observed similar titratable acidity results to our study after 20 days of storage (1.153 – 1.265). Even though the viability of the strains was at most 7.42 ± 0.14 log CFU/mL at the same time. It is presumed that the dairy matrix and higher initial inoculum of probiotics and starter strains in our study were factors that directly influenced the higher production of lactic acid during refrigeration, consequently leading to highlighted values of titratable acidity and pH.
3.4. Physical-chemical composition of fermented milk
As expected, there was an increase in total fiber content and, mainly, insoluble fiber content in Test Formulation (TF) and compared to Control Formulation (CF) (p < 0.05) (
Table 5). It can be expected that the microbial enzymatic metabolism acts on this added matrix, favoring the production of new derived compounds with an impact on aroma and functionality during fermentation and storage [
40,
41].
Another point observed was the fatty acid composition (
Table 5). CB had little effect on the diversity of these compounds observed, which was like what was observed for dairy bases [
42,
43]. CF and TF showed a predominance of palmitic, myristic and stearic saturated fatty acids. Yoghurt formulations showed equivalence in the values of fatty acids [
44,
45].
Evidence has shown that species from genera such as Lactobacillus spp., Bifidobacterium spp. and Streptococcus thermophilus can interfere with the production of conjugated linolenic acid [
46], like rumenic acid, which may have interfered with TF. Together, rumenic and linolenic acid are compounds with a proven positive influence on reducing the risk of cardiovascular diseases [
47].
In CB, the Total Phenolic Compounds was 486.57 ± 10.06 (mg AGE/100g), which were composed of syringic acid 146.8 ± 7.1, Quercetin glycoside 16.8 ± 1.2, Catechin 40 .4 ± 5.4 and Ellagic Acid 122.0 ± 1.4. Antioxidant activity was 1.16 ± 0.03 (DPPH· IC50) and 88.78 ± 0.13 (DPPH). Total phenolic compounds (TPC) identified by the Folin-Ciocalteu assay were significantly higher (p < 0.05) in TF during refrigerated storage. On the other hand, a significant increase was observed on days 14 and 28 in CF (
Table 4). The acidification process may have resulted in higher TPC and DPPH values for both formulations, due to the influence of the pH in these parameters [
48].
As for the antioxidant potential measured by DPPH inhibition, a direct proportionality was observed with higher TPC values. Despite a slight oscillation in the percentage of inhibition for TF on day 14, all values observed during storage were greater than 66% and significantly higher (p < 0.05) than those for CF.
In the present study, the addition of CB resulted in better values of antioxidant activity with a tendency to improve during shelf life. This possible oxidative stability is most likely due to the phenolic compounds [
49] naturally present in cashew fiber [
50] associated with the active metabolism of the microorganisms used.
It is hypothesized that the fermentation and consequent acidification of the dairy base, as well as the addition of fibrous matrix from the CB, may have acted synergistically, resulting in the formation and release of metabolites with greater antioxidant capacity. When F19, is present in fermented milk formulations, exhibited the capacity to degrade fiber and generate antioxidant compounds [
51]. Consequently, the inclusion of F19 may have had a favorable impact on enhancing the antioxidant profile throughout the shelf life of TF.
3.5. Survival of microorganisms in fermented milk under in vitro-simulated gastrointestinal conditions
Probiotic and starter strains was evaluated under in vitro-simulated gastrointestinal conditions (
Table 6), in the F19 population always above 9.00 log CFU/mL (F0), variable survival responses were observed in the formulations stored up to 7, 14, and 28 days under refrigeration. It is interesting to note that the three highest values (p < 0.05) of survival to gastric conditions (FG) were respectively for TF on days 14, 7 and 28. Resistance of F19 has already been observed in static digestion simulation model.
Considering these periods, TF was superior to CF by an average of 2,91 log CFU/mL. Therefore, cashew fiber may be acting as a stress shield against tension caused by stomach acidity. Also, higher populations of F19 surviving microorganisms (p < 0.05) were also detected for days 7 and 28 after enteric phase II (Ent2). Thus, dietary fiber may have conferred a potential for bacterial survival during gastrointestinal conditions and improved adhesion to the intestinal epithelium [
52,
53].
In addition to the investigation of
L. paracasei subsp.
paracasei F19, the survival of the
S. thermophilus ST-M6® strain was also investigated (
Table 6), where a trend towards a progressive decrease in the microorganism population was observed after the gastric and enteric phases I and a positive interference of the presence of CB in recovery at the end of enteral phase II (p < 0.05).
Vieira et al. [
36] developed a fermented soy beverage with the co-culture of
Bifidobacterium longum BB-46®,
Lactobacillus acidophilus LA-5® and
Streptocuccus thermophilus TH-4® enriched with acerola by-product. The authors evaluated the survival of strains under simulated gastrointestinal conditions in the same static model used in this study. A better survival of BB-46® was observed after gastric, enteric phase I and II (greater than 5.5 log CFU/mL) during the entire storage period. For the LA-5® strain, on the other hand, survival after gastric, enteric phase I and II was only higher until day 14 of storage.
4. Conclusions
The dehydrated cashew by-product (CB) revealed to be a promissing functional substrate for Lacticaseibacillus paracasei subsp. paracasei F19® (F19) and Streptococcus thermophilus ST-M6® (ST-M6). ST-M6 showed good viability in fermented milk when CB was added or not, but the presence of the by-product significantly increased the composition of phenolic compounds and antioxidant activity during storage and also confered increased gastric resistance to the probiotic strain.
Finally, in line with the trend towards sustainable development and use of by-products, the development of a probiotic fermented milk with dehydrated cashew by-product has proven to be a promising alternative as a healthy food product.
Author Contributions
Conceptualization, M.E.H. and I.U.D.M.; methodology, M.E.H., I.U.D.M. and L.H.G.G.; validation, M.E.H., I.U.D.M. and L.H.G.G.; formal analysis, M.E.H., I.U.D.M., L.H.G.G. and M.K.S.; investigation, M.E.H., I.U.D.M., L.H.G.G. and M.K.S.; data curation, M.E.H. and I.U.D.M.; writing—original draft preparation, M.E.H.; writing—review and editing, M.E.H., I.U.D.M., K.S. and S.M.I.S.; visualization, M.E.H., I.U.D.M., L.H.G.G., M.K.S., K.S. and S.M.I.S.; supervision, S.M.I.S.; project administration, S.M.I.S.; funding acquisition, S.M.I.S. All authors have read and agreed to the published version of the manuscript.”
Data Availability Statement
All related data and methods are presented in this paper. Additional inquiries should be addressed to the corresponding author.
Acknowledgments
The authors wish to thank Chr. Hansen for providing the probiotic cultures.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Siqueira, A.M.de A.; Brito, E.S.. Aproveitamento do bagaço do caju para alimentação humana e utilização em outras indústrias de alimentos. In: Araújo, J.P.P. (Ed.). Agronegócio caju: práticas e inovações. [s.l.] Embrapa 2003. p. 351–376. de A. Araújo.
- Berry, A.D.; Sargent, S.A. Cashew apple and nut (Anacardium occidentale L.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits. Woodhead Publishing Limited 2011, p 414-422. [CrossRef]
- Kowalska, H.; Czajkowska, K.; Cichowska, J.; Lenart, A. (2017). What’s new in biopotential of fruit and vegetable by-products applied in the food processing industry. Trends in Food Science and Technology 2017 67, 150–159. [CrossRef]
- Bussolo de Souza, C.; Jonathan, M.; Saad, S.M.I.; Schols, H.A.; Venema, K. Degradation of fibres from fruit by-products allows selective modulation of the gut bacteria in an in vitro model of the proximal colon. Journal of Functional Foods 2019, 57, 275–285. [CrossRef]
- Medeiros, I.U.D.; Aquino, J.S.; Cavalcanti, N.S.H.; Campos, A.R.N.; Cordeiro, A.M.T.M.; Damasceno, K.S.F.S.C., ..., Hoskin, R.T. (2019). Characterization and functionality of fibre-rich pomaces from the tropical fruit pulp industry. British Food Journal 2019. 122(3), 813–826. [CrossRef]
- Zhang, L. , Carmody, R.N., Kalariya, H.M., Duran, R.M., Moskal, K., Poulev, A., …; Roopchand, D.E. Grape proanthocyanidin-induced intestinal bloom of Akkermansia muciniphila is dependent on its baseline abundance and precedes activation of host genes related to metabolic health. The Journal of Nutritional Biochemistry 2018. 56, 142–151. [CrossRef]
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The Human Microbiota in Health and Disease. Engineering 2017, 3, 71–82. [CrossRef]
- Albuquerque, M.A.; Bedani, R.; Vieira, A.D.; LeBlanc, J.G.; Saad, S.M.I. Supplementation with fruit and okara soybean by-products and amaranth flour increases the folate production by starter and probiotic cultures. Int J Food Microbiol 2016. 236, 26-32. [CrossRef]
- Vieira, A.D.S.; Bedani, R.; Albuquerque, M.A.C., Biscola, V., & Saad, S.M.I. The impact of fruit and soybean by-products and amaranth on the growth of probiotic and starter microorganisms. Food Research International 2017, 97, 356–363. [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A., Salminen, S.J.; …, Reid, G. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nature Reviews Gastroenterology and Hepatology 2017, 14(8), 491–502. [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; …, Sanders, M.E. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology and Hepatology 2014, 11(8), 506–514. [CrossRef]
- Ballan, R.; Battistini, C.; Xavier-Santos, D.; Saad S.M.I. Interactions of probiotics and prebiotics with the gut microbiota. Prog Mol Biol Transl Sci 2020, 171:265-300. [CrossRef]
- Gallina, D. A.; Alves, A. T. S.; Trento, F. K. H. de S.; Carusi, J. Caracterização de Leites Fermentados Com e Sem Adição de Probióticos e Prebióticos e Avaliação da Viabilidade de Bactérias Láticas e Probióticas Durante a Vida-de-Prateleira. Unopar Cientifica. Ciencias Biologicas e Da Saude 2011, 13(4), 239–244. [CrossRef]
- Gómez-García, R.; Campos, D.A.; Aguilar, C.N.; Madureira, A.R.; Pintado, M. Valorization of melon fruit (Cucumis melo L.) by-products: Phytochemical and Biofunctional properties with Emphasis on Recent Trends and Advances. Trends in Food Science and Technology 2020, 99, 507–519. [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects – A review. Journal of Functional Foods 2015, 18, 820–897. [CrossRef]
- Gil-Sánchez, I.; Ayuda-Durán, B.; González-Manzano, S.; Santos-Buelga, C.; Cueva, C.; Martín-Cabrejas, M.A.; ..., Bartolomé, B. Chemical characterization and in vitro colonic fermentation of grape pomace extracts. Journal of the Science of Food and Agriculture 2017, 97(10), 3433–3444. 10. [CrossRef]
- Battistini, C.; Herkenhoff, M. E.; de Souza Leite, M.; Vieira, A. D. S.; Bedani, R.; Saad, S. M. I. Brewer’s Spent Grain Enhanced the Recovery of Potential Probiotic Strains in Fermented Milk After Exposure to In Vitro-Simulated Gastrointestinal Conditions. Probiotics Antimicrob Proteins 2023, 15, 326–337. [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology 1959, 37, 911-917. [CrossRef]
- Bedani, R.; Campos, M.M.; Castro, I.A.; Rossi, E.A.; Saad, S.M.I. Incorporation of soybean by-product okara and inulin in a probiotic soy yoghurt: texture profile and sensory acceptance. J Sci Food Agric 2014, 94(1):119-25. [CrossRef]
- Saldivar, S.O.S. Cereals: Dietary Importance. In B. Caballero, P. M. Finglas, & F. Toldrá (Eds.), Encyclopedia of Food and Health. Elsevier 2016. pp. 703–711. [CrossRef]
- O’Shea, N.; Ktenioudaki, A.; Smyth, T. P.; McLoughlin, P.; Doran, L.; Auty, M. a E.; …, Gallagher, E. Physicochemical assessment of two fruit by-products as functional ingredients: Apple and orange pomace. Journal of Food Engineering 2015, 153, 89–95. [CrossRef]
- Kamal-Eldin, A. (2016). Dietary Fiber: Bran. In B. Caballero; P. M. Finglas; F. Toldrá (Eds.), Encyclopedia of Food and Health. Elsevier 2016. pp. 378–382. [CrossRef]
- Mateos-Aparicio, I.; Armada, R.D.P.; Pérez-Cózar, M.L.; Rupérez, P.; Redondo-Cuenca, A.; Villanueva-Suárez, M.J. Apple by-product dietary fibre exhibits potential prebiotic and hypolipidemic effectsin high-fat fed Wistar rats. Bioactive Carbohydrates and Dietary Fibre 2019, 23, 100219. [CrossRef]
- González-Aguilar, G.A.; Blancas-Benítez, F.J.; Sáyago-Ayerdi, S.G. Polyphenols associated with dietary fibers in plant foods: molecular interactions and bioaccessibility. Current Opinion in Food Science 2017, 13, 84–88. [CrossRef]
- akobek, L.; Matić, P. Non-covalent dietary fiber - Polyphenol interactions and their influence on polyphenol bioaccessibility. Trends in Food Science & Technology 2019, 83, 235–247.
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.T.; Portillo, M.P.; Martínez, J.A.; ..., Milagro, F.I. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. Journal of Nutritional Biochemistry 2015, 26(6), 651–660. [CrossRef]
- Koutsos, A.; Lima, M.; Conterno, L.; Gasperotti, M.; Bianchi, M.; Fava, F.; …, Tuohy, K.M. Effects of commercial apple varieties on human gut microbiota composition and metabolic output using an in vitro colonic model. Nutrients 2017, 9(6), 1–23. 6. [CrossRef]
- Chacar, S.; Itani, T.; Hajal, J.; Saliba, Y.; Louka, N.; Faivre, J.F.; …, Fares, N. The Impact of Long-Term Intake of Phenolic Compounds-Rich Grape Pomace on Rat Gut Microbiota. Journal of Food Science 2018, 83, 246–251. [CrossRef]
- Hernández-Carranza, P.; Ávila-Sosa, R.; Guerrero-Beltrán, J. A.; Navarro-Cruz, A. R.; Corona-Jiménez, E.; Ochoa-Velasco, C. E. Optimization of Antioxidant Compounds Extraction from Fruit By-Products: Apple Pomace, Orange and Banana Peel. Journal of Food Processing and Preservation 2016, 40(1), 103–115. [CrossRef]
- Sabino Batista, K.; Alves, A.F.; Lima, M.D.S.; da Silva, L.A.; Lins, P.P.; de Sousa Gomes, J.A.; ..., Aquino, J.S. Beneficial effects of consumption of acerola, cashew or guava processing by-products on intestinal health and lipid metabolism in dyslipidaemic female Wistar rats. British Journal of Nutrition 2018, 119, 30–41. [CrossRef]
- Moo-Huchin, V. M.; Estrada-Mota, I.; Estrada-León, R.; Cuevas-Glory, L.; Ortiz-Vázquez, E.; de Lourdes Vargas, Y.; ..., Sauri-Duch, E. Determination of some physicochemical characteristics, bioactive compounds and antioxidant activity of tropical fruits from Yucatan, Mexico. Food Chemistry 2014, 152, 508–515. [CrossRef]
- Multari, S.; Marsol-Vall, A.; Heponiemi, P.; Suomela, J.P.; Yang, B. Changes in the volatile profile, fatty acid composition and other markers of lipid oxidation of six different vegetable oils during short-term deep-frying. Food Research International 2019, 122, 318-329. [CrossRef]
- Di Cerbo, A.; Palmieri, B. Lactobacillus paracasei subsp. paracasei f19; A farmacogenomic and clinical update. Nutricion Hospitalaria 2013, 28(6), 1842–1850. [CrossRef]
- Jones, R. M. The Use of Lactobacillus casei and Lactobacillus paracasei in Clinical Trials for the Improvement of Human Health. In: The Microbiota in Gastrointestinal Pathophysiology: Implications for Human Health, Prebiotics, Probiotics, and Dysbiosis. [s.l.] Elsevier 2017. p. 99–108.
- Hekmat, S., Soltani, H.; Reid, G. Growth and survival of Lactobacillus reuteri RC-14 and Lactobacillus rhamnosus GR-1 in yogurt for use as functional food. Inn. Food Sci. Emerg. Technol. 2009, 10, p. 293-296.
- Vieira, A.D.S; Battistini, C.; Bedani, R.; Saad, S.M.I. Acerola by-product may improve the in vitro gastrointestinal resistance of probiotic strains in a plant-based fermented beverage. LWT - Food Science and Technology 2021, 141, p. 110858. [CrossRef]
- Health Canada (2019) Probiotic Claims. https://inspection.canada.ca/eng/1297964599443/1297965645317. Accessed 04 Aug 2023.
- Vieira, A.D.S.; de Souza, C.B.; Padilha, M.; Zoetendal, E.G.; Smidt, H.; Saad, S.M.I.; ..., Venema, K. (2021). Impact of a fermented soy beverage supplemented with acerola by-product on the gut microbiota from lean and obese subjects using an in vitro model of the human colon. Applied Microbiology and Biotechnology 2021, 105(9), p. 3771–3785. [CrossRef]
- Karaka, O.B.; Güzeler, N.; Tangüler H.; Yasar, K.; Akın, M.B. Effects of Apricot Fibre on the Physicochemical Characteristics, the Sensory Properties and Bacterial Viability of Nonfat Probiotic Yoghurts. Foods 2019, 8(33), 1-15. 33. [CrossRef]
- Herkenhoff, M.E.; Battistini, C.; Praia, A.B.; Rossini, B.C.; Dos Santos, L.D.; Brödel, O.; Frohme, M.; Saad, S.M.I. The combination of omics strategies to evaluate starter and probiotic strains in the Catharina sour Brazilian-style beer. Food Res Int 2023, 167, 112704. [CrossRef]
- Praia, A.B.; Herkenhoff, M.E.; Broedel, O.; Frohme, M.; Saad, S.M.I. Sour Beer with Lacticaseibacillus paracasei subsp. paracasei F19: Feasibility and Influence of Supplementation with Spondias mombin L. Juice and/or By-Product. Foods 2022, 11(24), 4068. [CrossRef]
- Churakov, M.; Karlsson, J.; Edvardsson Rasmussen, A.; Holtenius, K. Milk fatty acids as indicators of negative energy balance of dairy cows in early lactation. Animal 2021, 15(7), 100253. 7. [CrossRef]
- Unger, A.L.; Torres-Gonzalez, M.; Kraft, J. Dairy Fat Consumption and the Risk of Metabolic Syndrome: An Examination of the Saturated Fatty Acids in Dairy. Nutrients 2019, 11(9), 2200. Nutrients, 2019; 11, 9, 2200. [CrossRef]
- Serafeimidou, A.; Zlatanos, S.; Laskaridis, K.; Sagredos, A. Chemical characteristics, fatty acid composition and conjugated linoleic acid (CLA) content of traditional Greek yogurts. Food Chemistry 2012, 134(4), 1839–1846. 4. [CrossRef]
- Gu, Y.; Li, X.; Chen, H.; Guan, K.; Qi, X.; Yang, L.; Ma, Y. Evaluation of FAAs and FFAs in yogurts fermented with different starter cultures during storage. Journal of Food Composition and Analysis 2021, 96, 103666. [CrossRef]
- Gholami, Z.; Khosravi-Darani, K. An Overview of Conjugated Linoleic Acid: Microbial Production and Application. Mini-Reviews in Medicinal Chemistry 2014, 14(9), 734–746. [CrossRef]
- Marand, M.A.; Amjabi, S.; Roufegarinejad, L.; Jafari, S.M. Fortification of yogurt with flaxseed powder and evaluation of its fatty acid profile, physicochemical, antioxidant, and sensory properties. Powder Technology 2020, 359, 76–84. [CrossRef]
- Pérez-Burillo, S.; Mehta, T.; Pastoriza, S.; Kramer, D.L.; Paliy, O.; Rufián-Henares, J.A. Potential probiotic salami with dietary fiber modulates antioxidant capacity, short chain fatty acid production and gut microbiota community structure. LWT - Food Science and Technology 2019, 105, 355–362. [CrossRef]
- Zhang, S.; Willett, S.A.; Hyatt, J.R.; Martini, S.; Akoh, C.C. Phenolic compounds as antioxidants to improve oxidative stability of menhaden oil-based structured lipid as butterfat analog. Food Chemistry 2021, 334, 127584. [CrossRef]
- Menezes, F.N.D.D.; da Cruz Almeida, E.T.; da Silva Vieira, A.R.; de Souza Aquino, J.; Dos Santos Lima, M.; Magnani, M.; de Souza, E.L. (2021). Impact of Cashew (Anacardium occidentale L.) by-Product on Composition and Metabolic Activity of Human Colonic Microbiota In Vitro Indicates Prebiotic Properties. Curr Microbiol 2021, 78(6), 2264-2274. [CrossRef]
- Ljungh, Å.; Lan, J.; Yanagisawa, N. Isolation, Selection and Characteristics of Lactobacillus paracasei subsp. paracasei F19. Microbial Ecology in Health and Disease 2002, 14(1), 4–6. [CrossRef]
- Beukema, M.; Faas, M. M.; De Vos, P. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: impact via gut microbiota and direct effects on immune cells. Experimental and Molecular Medicine 2020, 52(9), 1364–1376. 9. [CrossRef]
- Larsen, N.; Cahú, T.B.; Saad, S.M.I.; Blennow, A.; Jespersen, L. The effect of pectins on survival of probiotic Lactobacillus spp. in gastrointestinal juices is related to their structure and physical properties. Food Microbiology 2018, 74, 11–20. [CrossRef]
Table 1.
Microorganisms evaluated in the present study and their cultivation conditions for each strain.
Table 1.
Microorganisms evaluated in the present study and their cultivation conditions for each strain.
| Strain |
Broth |
Agar |
Incubation (at 37 oC) |
|
Streptococcus thermophilus TH-4® a
|
HJ1
|
M172
|
Aerobiosis |
|
Streptococcus. thermophilus ST-M6® a
|
|
Streptococcus thermophilus TA-40® b
|
|
Lactobacillus acidophilus LA-5® c
|
MRS3
|
MRS maltose4
|
Aerobiosis |
|
Limosilactobacillus fermentum PCC® c
|
MRS3
|
MRS5
|
Anaerobiosis7
|
|
Limosilactobacillus reuteri RC-14® c
|
MRS3
|
Acidified MRS 6
|
Anaerobiosis7
|
|
Lacticaseibacillus. paracasei subsp. paracasei 431® c
|
|
Lacticaseibacillus. paracasei subsp. paracasei F-19® c
|
|
Lacticaseibacillus. rhamnosus GR-1® c
|
MRS3
|
Acidified MRS 6
|
Aerobiosis |
|
Lacticaseibacillus. rhamnosus LGG® c
|
|
Bifidobacterium animalis subsp. lactis BB-12® c
|
MRS cistein8
|
LP-MRS9
|
Anaerobiosis7
|
|
Bifidobacterium. longum BB-46® c
|
|
Bifidobacterium longum subsp. infantis BB-02® c
|
Table 2.
Population (log CFU/mL) variation of probiotic and starter microorganisms after 24 and 48 hours of fermentation in MRSm broth with (+CB) and without (Control) cashew by-product.
Table 2.
Population (log CFU/mL) variation of probiotic and starter microorganisms after 24 and 48 hours of fermentation in MRSm broth with (+CB) and without (Control) cashew by-product.
| Strains |
Δ48 |
Δ24 |
| MRSm + CB |
MRSm Control |
MRSm + CB |
MRSm Control |
|
L. reuteri RC-14 |
3.32 ± 0.13ªA
|
2.57 ± 0.13B
|
3.64 ± 0.07ªA
|
3.27 ± 0.02B
|
|
L.paracasei subsp. paracasei 431 |
2.92 ± 0.17abA
|
2.79 ± 0.06A
|
- |
- |
|
L. acidophilus LA-5 |
2.87 ± 0.09abB
|
3.14 ± 0.02A
|
- |
- |
|
L. paracasei subsp. paracasei F19 |
2.80 ± 0.10abA
|
2.35 ± 0.03B
|
3.36 ± 0.22abA
|
2.55 ± 0.08B
|
|
B. animalis subsp lactis BB-12 |
2.79 ± 0.33abA
|
2.62 ±0.06A
|
- |
- |
|
L. rhamnosus GR-1 |
2.56 ± 0.08bc
|
- |
- |
- |
|
L. rhamnosus LGG |
2.56 ± 0.13bc
|
- |
- |
- |
|
B. longum subsp. infantis BB-02 |
2.28 ± 0.19bc
|
- |
- |
- |
|
L. fermentum PCC |
2.23 ± 0.10bc
|
- |
- |
- |
|
B. longum BB-46 |
2.02 ± 0.16c
|
- |
- |
- |
|
S. thermophilus ST-M6 |
3.74 ± 0.12A
|
-0.21 ± 0.15B
|
0.85 ± 0.13A
|
0.11 ± 0.04B
|
|
S. thermophilus TA-40 |
-1.56 ± 0.11A
|
-2.19 ± 0.10B
|
-0.32 ± 0.04A
|
-0.76 ± 0.08B |
|
S. thermophilus TH-04 |
-0.32 (-0.52 – -0.02)A
|
-2.20 (-2.43 – -2.11)B
|
-0.52 ± 0.03A
|
-0.71 ± 0.17A
|
Table 3.
Ingredients and proportions used in the formulation of fermented milk.
Table 3.
Ingredients and proportions used in the formulation of fermented milk.
| Ingredients |
Formulations (g/ 100 mL of milk) |
| Test (TF) |
Control (CT) |
| Dehydrated Cashew By-product (CB) |
2.5 |
- |
| Skimmed Milk Powder without Lactose (Ninho®, Nestlé, Brazil) |
2.5 |
2.5 |
| Sucrose (União®, Brazil) |
5.0 |
5.0 |
| Unflavored gelatin (Modelez®, Brazil) |
0.1 |
0.1 |
Table 4.
Population (log CFU/mL) variation of probiotic and starter strains, pH values, titratable acidity, total phenolic compounds, and antioxidant activity in fermented milk with and without addition of cashew by-product during 28 days in refrigeration.
Table 4.
Population (log CFU/mL) variation of probiotic and starter strains, pH values, titratable acidity, total phenolic compounds, and antioxidant activity in fermented milk with and without addition of cashew by-product during 28 days in refrigeration.
| Parameter |
Formulation |
Inoculation |
Day 1 |
Day 7 |
Day 14 |
Day 21 |
Day 28 |
| F-19® count |
CF |
8.7 ± 0.2abA
|
9.1 ± 0.2abA |
9.1 ± 0.1bA
|
9.2 ± 0.1bB
|
9.4 ± 0.1aA
|
9.3 ± 0.1abA
|
| TF |
8.6 ± 0.1cA
|
9.1 ± 0.2abcA
|
9.2 ± 0.1bA
|
9.4 ± 0.1abA
|
9.4 ± 0.0aA
|
9.3 ± 0.0abA
|
| ST-M6® count |
CF |
8.0 ± 0.2bA
|
8.7 ± 0.1abA
|
8.6 ± 0.1abB
|
9.0 ± 0.1aA
|
9.0 ± 0.2aA
|
8.8 ± 0.0aA
|
| TF |
8.0 ± 0.1bA
|
8.7 ± 0.1aA
|
8.8 ± 0.1aA
|
8.8 ± 0.2aA
|
9.0 ± 0.2aA
|
8.8 ± 0.2aA
|
| pH |
CF |
- |
5.1 ± 0.01aA
|
4.3 ± 0.04bA
|
4.0 ± 0.02cA
|
3.9 ± 0.01dA
|
3.8 ± 0.03dA
|
| TF |
- |
5.0 ± 0.04aA
|
4.1 ± 0.03bB
|
3.9 ± 0.01cA |
3.8 ± 0.01dA
|
3.7 ± 0.05dA
|
| - |
| Acidity |
CF |
- |
0.6 ± 0.01dA
|
1.0 ± 0.02cA
|
1.2 ± 0.01bB
|
1.3 ± 0.02bA
|
1.5 ± 0.02aA
|
| TF |
- |
0.7 ± 0.03dA
|
1.1 ± 0.01cA
|
1.3 ± 0.02bA
|
1.3 ± 0.03bA
|
1.4 ± 0.02aA
|
| TPC |
CF |
- |
1145.4 ± 26.1cB
|
- |
1669.8 ± 14.4bB
|
- |
1722.1 ± 12.1aB
|
| TF |
- |
2250.5 ± 16,4aA
|
- |
2459.5 ± 7.1aA
|
- |
2441.2 ± 25.3aA
|
| DPPH IC50
|
CF |
- |
102.07 ± 3.2aA
|
- |
83.77 ± 2.8bA
|
- |
112.23 ± 4.0aA
|
| TF |
- |
49.83 ± 2.0aB
|
- |
29.41± 1.0bB
|
- |
17.28 ± 0.6cB
|
| I. DPPH |
CF |
- |
50.57 ± 0.4abB
|
- |
52.02 ± 0.5aB
|
- |
48.3 6± 0.2bB
|
| TF |
- |
76.27 ± 1.4aA
|
- |
66.47 ± 0.9bA
|
- |
71.01 ± 0.8abA
|
Table 5.
Physical-chemical composition of cashew by-product (CB), test formulation (TF) with 2.5% cashew by-product and control formulation (CF) without cashew by-product.
Table 5.
Physical-chemical composition of cashew by-product (CB), test formulation (TF) with 2.5% cashew by-product and control formulation (CF) without cashew by-product.
| Parameter or composite |
CB |
CF |
TF |
| Humidity g/100g |
2.85 ± 0.11 |
80.57 ± 0.15* |
78.36 ± 0.27 |
| Ashes g/100g |
1.25 ± 0.02 |
0.79 ± 0.02 |
0.83 ± 0.01* |
| Proteins g/100g |
10.67 ± 0.02 |
3.69 ± 0.03 |
3.74 ± 0.30 |
| Carbohydrates g/100g |
9.52 ± 0.13 |
10.89 ± 0.15 |
11.18 ± 0.28 |
| Total fat g/100g |
4.47 ± 0.11 |
4.06 ± 0.03 |
4.11 ± 0.23 |
| Palmitic acid (C16:0) µg/ g fat |
867.6 |
1449.0 ± 177 |
1341.0 ± 85.0 |
| Palmitoleic acid (C16:1) µg/ g fat |
43.8 |
45.8 ± 8.1 |
43.8 ± 3.3 |
| Stearic acid (C18:0) µg/ g fat |
350.2 |
691.1 ± 75.3 |
620.0 ± 38.0 |
| Oleic acid (C18:1 ɷ-9) µg/ g fat |
1336.6 |
643.0 ± 109 |
708.3 ± 61.0 |
| Linoleic acid (C18:2 ɷ-6) µg/ g fat |
90.2 |
77.0 ± 13.9 |
77.0 ± 6.3 |
| Linolenic acid (C18:3 ɷ-3) µg/ g fat |
54.5 |
- |
8.6 ± 0.9 |
| cis-13-eicosanoic acid µg/ g fat |
33.4 |
- |
- |
| Rumenic Acid (C18:2) µg/ g fat |
- |
8.4 ± 1.5a
|
7.8 ± 1.0a
|
Table 6.
Population survival (log CFU/mL) of Lacticaseibacillus paracasei subsp. paracasei F-19® and Streptococcus thermophilus ST-M6® during simulated gastrointestinal conditions in the in vitro static system in fermented milk with and without cashew by-product.
Table 6.
Population survival (log CFU/mL) of Lacticaseibacillus paracasei subsp. paracasei F-19® and Streptococcus thermophilus ST-M6® during simulated gastrointestinal conditions in the in vitro static system in fermented milk with and without cashew by-product.
| Strain |
Period |
Formulation |
F0 |
Gast |
Ent1 |
Ent2 |
| F19 |
D7 |
CF |
9.05 ± 0.01aB
|
5.28 ± 0.23bD
|
2.55 ± 0.06cB
|
2.53± 0.08cC
|
| |
TF |
9.16 ± 0.08aAB
|
8.21 ± 0.17bB
|
3.15 ± 0.24dAB
|
4.52 ± 0.05cB
|
| |
D14 |
CF |
9.18 ± 0.01aAB
|
5.20 ± 0.16bD
|
3.67 ± 0.11cA
|
3.93 ± 0.16cB
|
| |
TF |
9.42 ± 0.09aA
|
9.26 ± 0.01aA
|
3.66 ± 0.08dA
|
4.43 ± 0.11cB
|
| |
D28 |
CF |
9.26 ± 0.01aAB
|
4.81 ± 0.09bD
|
3.26 ± 0.11dA
|
4.19 ± 0.13cB
|
| |
TF |
9.23 ± 0.07aAB
|
6.55 ± 0.05bC
|
3.59 ± 0.06dA
|
4.65 ± 0.17cA
|
| ST-M6 |
D7 |
CF |
8.70 ± 0.09aA
|
5.01 ± 0.08bA
|
2.54 ± 0.11dB
|
3.55 ± 0.04cB
|
| |
TF |
8.81 ± 0.08aA
|
5.14 ± 0.07bA
|
2.75 ± 0.34cAB
|
4.73 ± 0.25bA
|
| |
D14 |
CF |
9.06 ± 0.11aA
|
5.07 ± 0.15bA
|
3.73 ± 0.08cA
|
3.78 ± 0.06cAB
|
| |
TF |
8.85 ± 0.22aA
|
2.97 ± 0.20cB
|
2.52 ± 0.18cB
|
4.29 ± 0.10bA
|
| |
D28 |
CF |
8.58 ± 0.31aA
|
5.11 ± 0.21bA
|
3.58 ± 0.17cA
|
4.19 ± 0.16cAB
|
| |
TF |
8.52 ± 0.28aA
|
5.20 ± 0.16bA
|
3.50 ± 0.06dA
|
4.49 ± 0.13cA
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).