1. Introduction
The COVID-19 pandemic forced the suspension of scheduled endoscopic activity in March 2020, including the one linked to the population-based organized colorectal cancer (CRC) screening programs. The resumption of endoscopic activity was one of the greatest organizational challenges for endoscopy units. Changes were implemented in the indications and prioritization of the examinations, in the appointment and reception circuits, in the restructuring of the spaces, in the measures for the protection of the personnel, in the cleaning of the spaces and in the control after the completion of the examinations.[
1] These changes initially implied a reduction in the volume of examinations carried out and an increase in delays in the completion of these examinations. In this sense, they initially reduced the diagnoses of CRC [
2] with an increase in emerging diagnoses.[
3] Modeling studies carried out in the United Kingdom suggested that the delays associated with the reduction in endoscopic capacity could represent an increase of 15.3 -16.6% mortality from CRC. [
4]
In the case of population-based CRC screening programs, the COVID-19 pandemic posed additional risk to healthy subjects invited to participate. Due to the pandemic, the activity linked to the population programs was suspended during the first wave and the resumption of the activity was heterogeneous.[
5] The main risk of the prolonged suspension of the activity of the programs was the delay in the diagnosis of CRC in patients with a positive result. Delays greater than 270 days after a positive fecal immunochemical test (FIT) increased the risk of detection of CRC and advanced CRC.[
6]
The aim of this analysis is to describe the real impact of the temporary suspension of the CRC screening program on delays, participation, and adherence to colonoscopy as process indicators. However, our main objective is to analyze the impact on the main outcome indicators of the screening program: detection of colorectal lesions, specifically CRC, and the stage at diagnosis, as the main surrogate of CRC associated mortality.
2. Materials and Methods
Study design:
We performed a retrospective observational study based on the analysis of the anonymous database of the Galician CRC screening program. As of December 31, 2019, the Galician CRC screening program had a reference population of 725,254 subjects aged 50-69 years. It is based on biennial invitation to FIT (OCsensor™, Eiken Chemical Co, Tokyo, Japan) with a 20µg/g feces threshold to refer to diagnostic colonoscopy. The information system of the screening program collects all the information related to participation, adherence to colonoscopy, delays, the colonoscopy findings as well as the CRC stage at diagnosis. No Institutional Review Board approval was required for this analysis as this analysis is part of the continuous quality evaluation of the screening program and the information was anonymized for it.
Suspension of the activity in the CRC screening program:
The Galician CRC screening program suspended its activity on March 13, 2020. At that time, the delivery of invitation letters (first screening round) and fecal collectors (successive rounds), the appointments in Primary Healthcare of the subjects with a positive FIT, and the diagnostic colonoscopies were suspended. The endoscopic activity associated with the screening program was restarted in May 2020 with restrictions and the delivery of new invitations and fecal collectors was resumed completely in January 2021.[
7]
Inclusion criteria and definition of the cohorts:
We included in this analysis all the invitations sent between January 1, 2019, and December 31, 2021. We defined as pre-pandemic invitations those sent before March 13, 2020 and as pandemic those sent after the 13th March.
Variables included in the analysis:
We calculated the delay intervals according to the data included in the information system of the screening program. The intervals were defined based on the recommendations of the Spanish network of screening programs (
https://cribadocancer.es/). In the successive rounds we calculated the delay from the previous round to the invitation, the FIT return, and the diagnostic colonoscopy. In all the rounds we calculated the delay from the invitation to the FIT return, and the diagnostic colonoscopy as well as the delay between the FIT return and the diagnostic colonoscopy.
We evaluated the participation rate in the screening program and the adherence to colonoscopy after a positive FIT. The findings in the colonoscopy were classified according to the European guidelines for quality assurance.[
8] For this analysis, we determined the rate of CRC and colorectal neoplasia (CRC and/or adenoma). Finally, we analyzed the CRC staging according to the AJCC classification.[
9]
Statistical analysis:
First, we performed a descriptive analysis. Quantitative variables were reported as the mean and standard deviation, when they presented a normal distribution or as the median and interquartile range when the distribution was not normal. To determine normality in the variables, we used the Kolmogorov-Smirnov test. The qualitative variables were represented with their frequency and percentage. Based on the proposed objectives, we used the Chi-square test to analyze the statistical relationship between the dependent variables and the pandemic, and the Student’s t-test in the quantitative variables. We calculated the Odds Ratios (OR) and the 95% Confidence Interval (CI) using the Cochran-Mantel-Haenszel statistic to determine the level of association. Finally, we performed a multivariate analysis using a multivariate logistic regression model to control the confounding variables. As a secondary analysis, we estimated the effect of the delays, at pre-stablished thresholds, on the diagnostic yield of colonoscopy, mainly CRC or colorectal neoplasia detection. The statistical analysis was performed using the IBM SPSS Statistics for Windows, Version 19.0. Armonk, NY, USA: IBM Corp.
3. Results
As we show in
Table 1, the Galician CRC screening program sent 976,187 invitations, 61% in the pandemic period. The mean age was 58 years and 52% of the invited subjects were women. The FIT was returned by 45% of the subjects, with a positive result in 5.6%. At least one colorectal adenoma was detected in 62.7% of the 23,092 subjects that performed a colonoscopy. In addition, the colonoscopy did not detect any neoplastic finding in 32.0% of the subjects. Finally, 898 (4.0%) CRCs were detected in the analyzed period, located primarily distal to the splenic flexure in 74.4% and in a TNM I tumor stage in 44.8% of the subjects.
We evaluated the impact of the pandemic in the participation and the adherence to colonoscopy after a positive FIT, as we show in
Table 1. In the multivariate logistic regression to control the potential effect of the confounding variables, we confirmed an increase in the participation (OR 1.11, 95% CI 1.09-1.116) with no changes in the adherence to colonoscopy (OR 0.93, 95% CI 0.84-1.04) during the pandemic.
- 2
Impact of the Pandemic on the Delays
In the
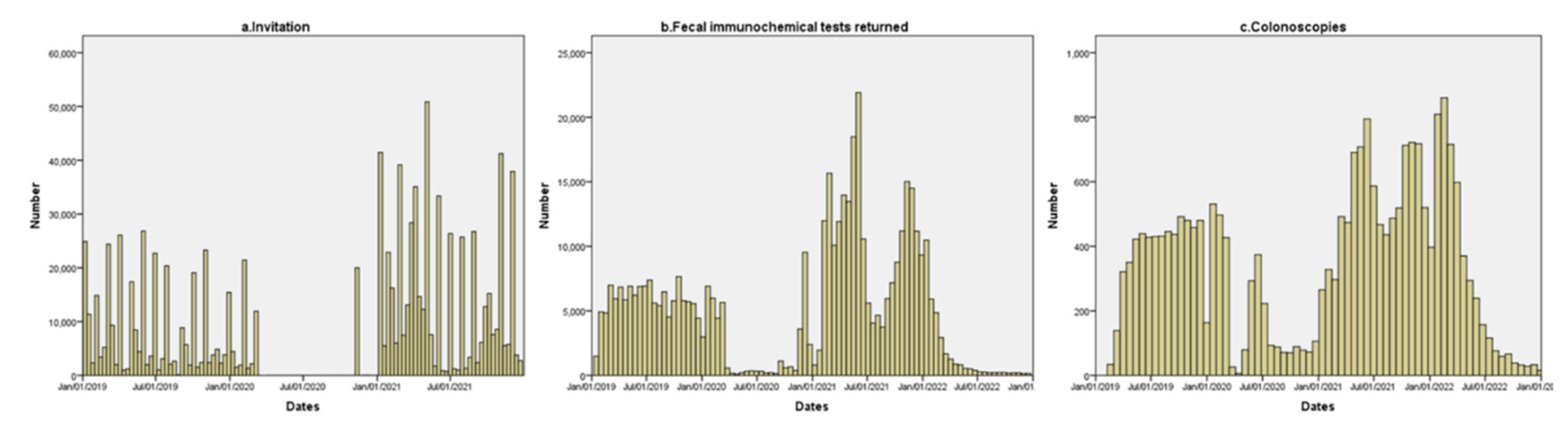
Figure 1 we show the distribution of the invitations, FIT returned, and colonoscopy performed during the evaluated period. As it is displayed, invitations were stopped for almost 9 months: from March 13, 2020, until January 11, 2021. During the initial phase of the pandemic, a small number of FITs was returned and the diagnostic colonoscopy from pending positive results was performed.
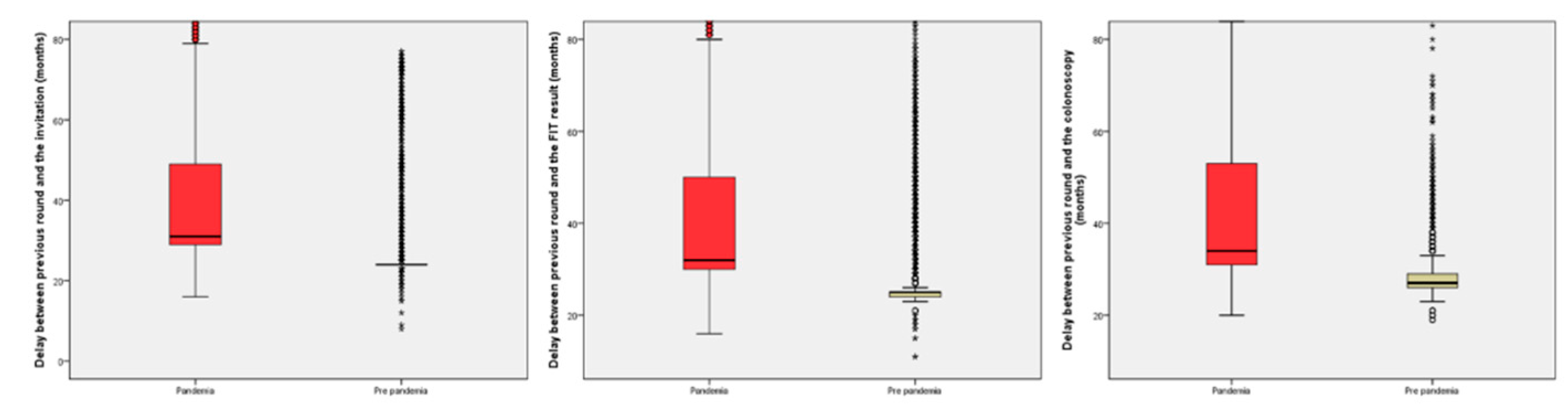
In the analysis of the delays, we found a difference of seven months in the median delay in the successive rounds in all periods evaluated with a significant increase in the subjects with delays from previous round of at least 27 or 30 months. The information regarding delays in the successive rounds is shown in
Table 2 and
Figure 2.
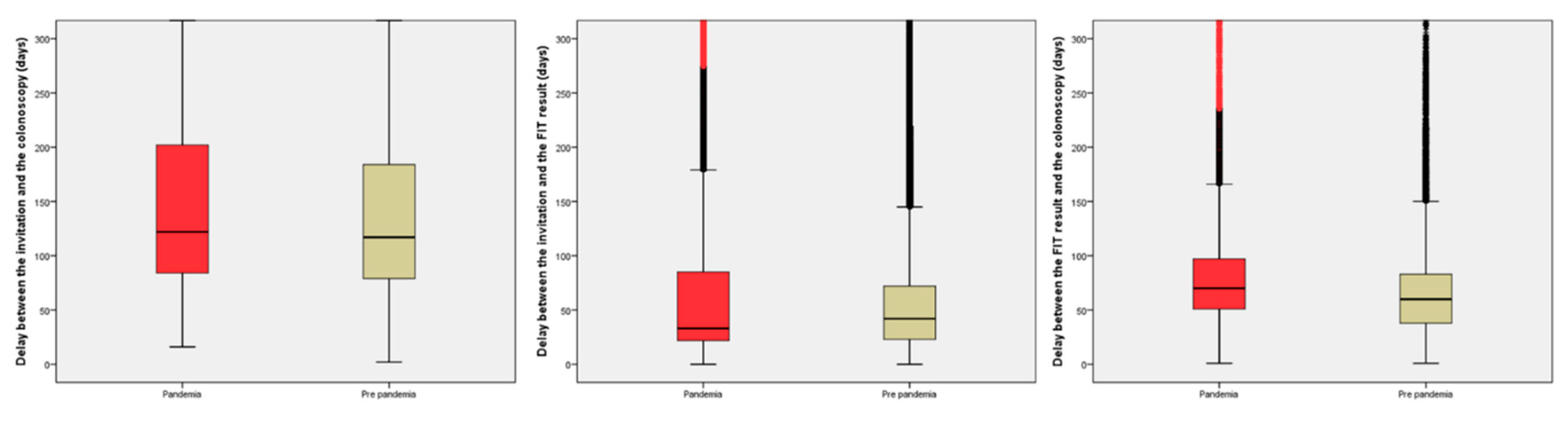
On the other hand, the delays after invitation are shown in
Table 3 and
Figure 3. To summarize the findings, the median delay from the invitation to the colonoscopy increased by 8 days, due to an increase in the delay from the positive result to the performance of the colonoscopy (10 days). In contrast, the median delay from the invitation to the FIT return was reduced by 9 days. As an example of the contradictory effect of the pandemic in the delays from the invitation, there was a significant reduction in the proportion of subjects with a delay ≥270 days from the invitation to the colonoscopy (OR 0.87, 95% CI 0.80-0.94).
3. Effect of the Pandemic on the Diagnostic Yield of Colonoscopy
As we show in
Table 1, we identified statistical differences in the proportion of lesions detected in the diagnostic colonoscopy. However, in the multivariable analysis we performed to control for the confounding variables, we found no differences in the proportion of subjects with colorectal neoplasia (OR 0.97; 95% CI 0.92-1.03) or CRC (OR 0.90; 95% CI 0.78-1.02) between both periods. Finally, the multivariable analysis confirmed there were no differences in the proportion of patients with CRC TNM IV (OR 1.87; 95% CI 0.98-3.57).
4. Effect of Delays on the Diagnostic Yield of Colonoscopy
In this secondary analysis, we evaluated the impact of the delays on the diagnostic yield of colonoscopy. As we show in
Table 4, the prolonged delays between the previous round and the colonoscopy did not modify the diagnostic yield of colonoscopy. Only, a delay between the previous round and the invitation of at least 30 months reduced the probability of colorectal neoplasia detection.
In addition, we also evaluated the impact of delays from invitation. As we present in
Table 5, the delay from the invitation to the colonoscopy produced a statistically significant increase in colorectal neoplasia detection, with no effect on the risk of CRC detection. However, we found a contradictory effect on the delay between the FIT result and the colonoscopy. In this sense, delays longer than 90 and 180 days were associated with a statistically significant reduction of the risk of CRC (OR 0.69, 95% CI 0.58-0.82; OR 0.26, 0.16-0.44) and an increase in the risk of colorectal neoplasia (OR 1.29, 95% CI 1.20-1.37; OR 3.87, 95% CI 3.25-4.59).
4. Discussion
This analysis confirms that the COVID pandemic led to a significant increase in delays in the population-based organized CRC screening programs. However, this increase in delays did not affect relevant results, such as participation, adherence after a positive test, the probability of CRC detection, and its stage at diagnosis. We have only detected a consistent association between global delays and the detection of colorectal neoplasia in screening colonoscopy. On the other hand, we have only detected a statistically significant association between CRC detection with intermediate delays and with a contradictory association.
The COVID pandemic posed the greatest challenge to healthcare systems in decades. Not only did the available resources have to be dimensioned to attend to the different waves of the pandemic, but the incident pathology was at risk of not being properly attended. In the specific case of cancer patients, the limitations for both treatment and diagnosis of incident cases were evident. In the case of the CRC, two key points occurred. On the one hand, in the first wave all screening activities were suspended to reduce exposure risks both for the healthy population and for essential health services. On the other hand, when healthcare activity was reinstated, protection measures were implemented limiting the capacity to perform colonoscopies in endoscopy units. This reduction in the diagnostic capacity of the units had a potential impact both in the evaluation of patients with symptoms and in subjects with a positive FIT in the screening programs. In this sense, the estimates posed a gloomy scenario with diagnoses at more advanced stages and an increase in mortality.[
2,
3]
In the last twelve months, several studies have been published evaluating the real impact of the pandemic on CRC screening population programs. [
10,
11] Fortunately, the most pessimistic forecasts have not been confirmed. Thus, as in our study, in the recently published Dutch study, the delays associated with the pandemic have not modified the probability of detection of CRC and, only minimally, the detection of advanced neoplasia in colonoscopy. Our results are comparable to those published by the Dutch group and contradict those of other studies with smaller sample sizes where differences were determined in participation, adherence to the screening test, and changes in the probability of detecting CRC.[
12,
13]
We have detected a result that deserves a comment. Long delays between the positive FIT and colonoscopy were significantly associated with a reduced risk of CRC detection. It has been an unexpected result and one that is difficult to justify. Our hypothesis is that these are paucisymptomatic subjects who, after obtaining a positive result, did not wait for the screening program to schedule the diagnostic colonoscopy and, therefore, were diagnosed in a shorter interval. In contrast, those subjects without any symptoms had longer delays.
It has previously been described that increases in the delay within CRC screening programs make CRC detection more likely, especially in advanced stages.[
6] Our results, as well as those obtained in the Dutch registry,[
11] clearly contradict this information. Although our data come from a retrospective and observational analysis, they are based on the systematic collection of population data. As in the Dutch study, the sample size is sufficient to rule out not only statistically significant but also clinically relevant differences. These results do not justify, in any case, an extension in the delays allowed by CRC screening programs. The satisfaction of the subjects involved in the population programs is key to increasing participation in them.
Our study has several strengths. We have been able to evaluate not only the process indicators of the screening program; participation, delays in successive rounds and from the invitation, adherence to diagnostic colonoscopy; but also, the indicators of results of the screening program: positive predictive value for CRC and lesions in colonoscopy as well as the stage of CRC at diagnosis. The available studies have only provided partial information on each of them, focusing either on the functioning or on the results. On the other hand, it is one of the few studies in which the information has been obtained from a population perspective.
On the contrary, our study has a main limitation: we were unable to evaluate the interval CRCs[
14] and, more importantly, the impact on CRC incidence in Galicia in the period of analysis. Data are only partially available in the period evaluated and will not be complete until the end of 2023.[
15] In this sense, we thought the information was relevant enough to share without the unavailable data. Besides, due to the null impact on participation, adherence to colonoscopy and CRC detection rate, we expect the impact on interval CRC will be minimal.
5. Conclusions
To conclude, the COVID pandemic had an impact on the Galician CRC screening program in terms of increases in delays. However, it did not affect the relevant results: participation in the program, adherence to diagnostic colonoscopy, detection of lesions and, above all, CRC. The pandemic has offered us a historic opportunity to understand the impact of delays in the evolution of preneoplastic lesions and CRC in asymptomatic individuals.
Author Contributions
Conceptualization: Joaquín Cubiella, Beatriz Calderón; Methodology: Joaquín Cubiella, Beatriz Calderón; Formal analysis and investigation: Joaquín Cubiella, Beatriz Calderón; Writing - original draft preparation: Joaquín Cubiella; Writing - review and editing: All authors; Funding acquisition: Joaquín Cubiella, Ángel Gómez-Amorín Resources: Raquél Almazán, Ángel Gómez-Amorín; Supervision: Raquel Almazán, Ángel Gómez-Amorín.
Funding
This research received funding from the Instituto de Salud Carlos (III) Madrid, Spain [PI21/01771, CD22/00087 and INT22/00009]. This grant is partially financed by the European Regional Development Fund of the EU (FEDER).
Institutional Review Board Statement
No Institutional Review Board approval was required for this analysis as this analysis is part of the continuous quality evaluation of the screening program and the information was anonymized for it.
Informed Consent Statement
Patient consent was waived due to reasons above explained.
Data Availability Statement
Data are available on demand.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Marín-Gabriel, J.C.; de Santiago, E.R.; en representación de la Asociación Española de Gastroenterología y la Sociedad Española de Endoscopia Digestiva AEG-SEED position paper for the resumption of endoscopic activity after the peak phase of the COVID-19 pandemic. Gastroenterologia y hepatologia 2020, 43, 389–407. [CrossRef]
- Dinmohamed, A.G.; Cellamare, M.; Visser, O.; de Munck, L.; Elferink, M.A.G.; Westenend, P.J.; Wesseling, J.; Broeders, M.J.M.; Kuipers, E.J.; Merkx, M.A.W.; et al. The impact of the temporary suspension of national cancer screening programmes due to the COVID-19 epidemic on the diagnosis of breast and colorectal cancer in the Netherlands. Journal of hematology & oncology 2020, 13, 147. [Google Scholar] [CrossRef]
- Suárez, J.; Mata, E.; Guerra, A.; Jiménez, G.; Montes, M.; Arias, F.; Ciga, M.A.; Ursúa, E.; Ederra, M.; Arín, B.; et al. Impact of the COVID-19 pandemic during Spain’s state of emergency on the diagnosis of colorectal cancer. Journal of surgical oncology 2020. [CrossRef] [PubMed]
- Maringe, C.; Spicer, J.; Morris, M.; Purushotham, A.; Nolte, E.; Sullivan, R.; Rachet, B.; Aggarwal, A. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. The Lancet Oncology 2020, 21, 1023–1034. [Google Scholar] [CrossRef]
- Dekker, E.; Chiu, H.-M.; Lansdorp-Vogelaar, I.; Expert Working Group on COVID-19 of the WEO Colorectal Cancer Screening Committee; Caro, L.E.; Dominitz, J.A.; Halloran, S.; Hassan, C.; Ismael, J.; Jover, R.; et al. Colorectal cancer screening in the COVID-19 era. Gastroenterology 2020, 19–20. [CrossRef]
- Zorzi, M.; Hassan, C.; Capodaglio, G.; Baracco, M.; Antonelli, G.; Bovo, E.; Rugge, M. Colonoscopy later than 270 days in a fecal immunochemical test-based population screening program is associated with higher prevalence of colorectal cancer. Endoscopy 2020, 52, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Peña-Rey, I.; Almazán, R.; Rodríguez-Camacho, E.; Cubiella, J. Resumption of endoscopy in the Galician colorectal cancer screening programme after the COVID-19 lock down: patient safety results. Revista espanola de enfermedades digestivas 2021, 113, 119–121. [Google Scholar] [CrossRef] [PubMed]
- European Colorectal Cancer Screening Guidelines Working, G. ; von Karsa, L.; Patnick, J.; Segnan, N.; Atkin, W.; Halloran, S.; Lansdorp-Vogelaar, I.; Malila, N.; Minozzi, S.; Moss, S.; et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis: overview and introduction to the full supplement publication. Endoscopy 2013, 45, 51–59. [Google Scholar] [CrossRef]
- AJCC Cancer Staging Manual; Edge, S.B.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.L.; Trotti, A., Ed.; 7th ed.; Springer, 2010; ISBN 978-0-387-88440-0.
- Lee, J.K.; Lam, A.Y.; Jensen, C.D.; Marks, A.R.; Badalov, J.; Layefsky, E.; Kao, K.; Ho, N.J.; Schottinger, J.E.; Ghai, N.R.; et al. Impact of the COVID-19 Pandemic on Fecal Immunochemical Testing, Colonoscopy Services, and Colorectal Neoplasia Detection in a Large United States Community-based Population. Gastroenterology 2022, 163, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Toes-Zoutendijk, E.; de Jonge, L.; van Iersel, C.A.; Spaander, M.C.W.; van Vuuren, A.J.; van Kemenade, F.; Ramakers, C.R.; Dekker, E.; Nagetaal, I.D.; van Leerdam, M.E.; et al. Impact of delayed screening invitations on screen-detected and interval cancers in the Dutch colorectal cancer screening programme: individual-level data analysis. Gut 2023, 72, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Young, S.; Williams, R.; Liang, P.S. Impact of the COVID-19 pandemic on colorectal cancer screening in New York City. Journal of medical screening 2023, 30, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, F.; Shida, D.; Suzuki, S.; Nagai, M.; Mochida, K.; Morishita, T. A delay in the diagnosis of colorectal cancer screened by fecal immunochemical tests during the COVID-19 pandemic: a longitudinal cohort study. International journal of colorectal disease 2022, 37, 2543–2546. [Google Scholar] [CrossRef] [PubMed]
- Sanduleanu, S.; le Clercq, C.M.C.; Dekker, E.; Meijer, G.A.; Rabeneck, L.; Rutter, M.D.; Valori, R.; Young, G.P.; Schoen, R.E.; Expert Working Group on ‘Right-sided lesions and interval cancers’, Colorectal Cancer Screening Committee, W. E.O. Definition and taxonomy of interval colorectal cancers: a proposal for standardising nomenclature. Gut 2015, 64, 1257–67. [Google Scholar] [CrossRef] [PubMed]
- Dirección-Xeral-de-Saude-Pública INCIDENCIA, MORTALIDADE E SUPERVIVENCIA AO CANCRO GALICIA, ANO 2021; Santiago de Compostela, Spain, 2024.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).