Submitted:
20 August 2023
Posted:
23 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Molecular Pathogenesis of Anderson-Fabry Disease
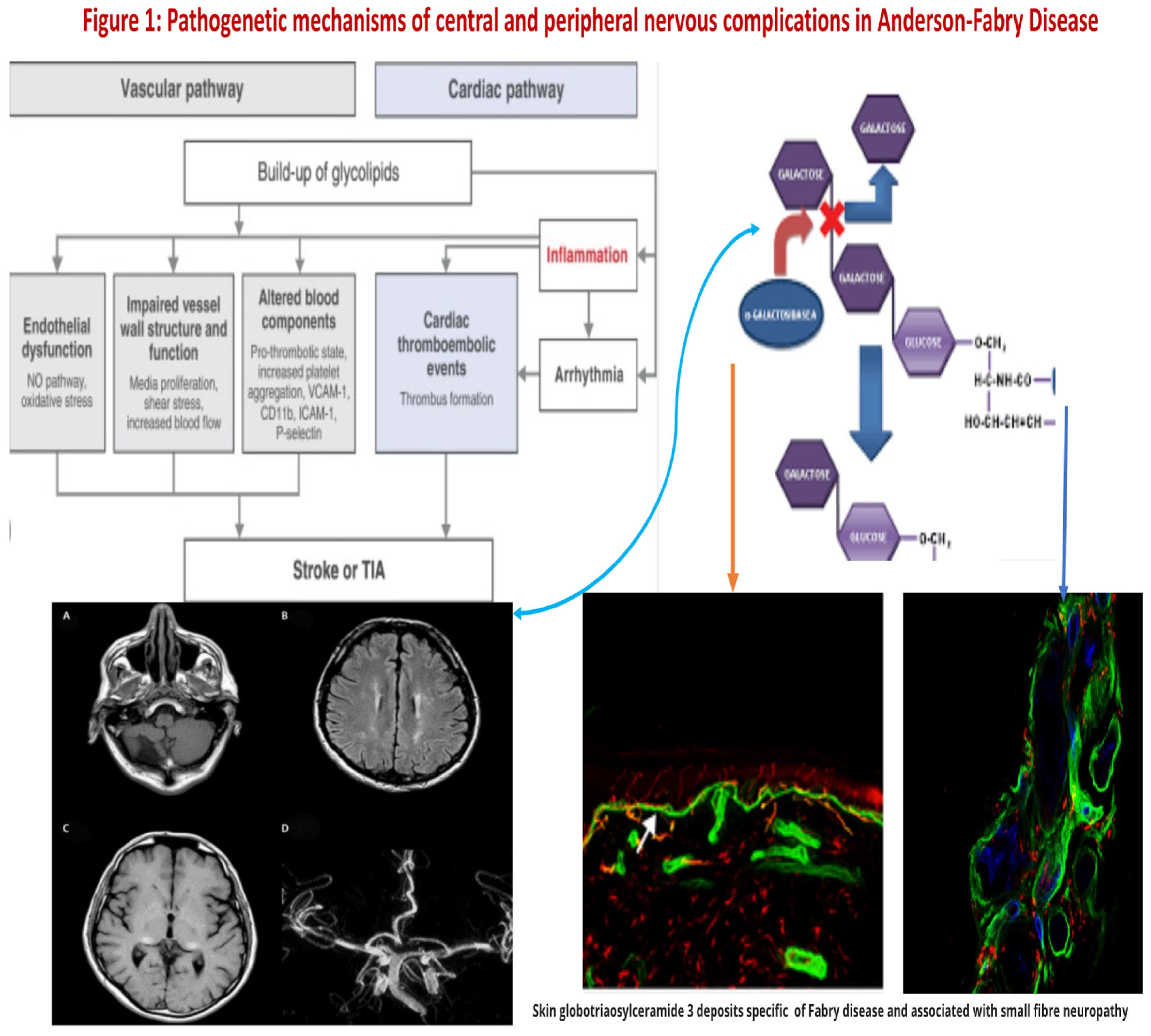
3. Molecular Pathogenesis of Central Nervous System Involvement in Anderson-Fabry Disease
4. Molecular Pathogenesis of Peripheral Nerve Involvement in Anderson-Fabry Disease
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA 1999, 281, 249–54. [Google Scholar] [CrossRef]
- Kahn, P. Anderson-Fabry disease: a histopathological study of three cases with observations on the mechanism of production of pain. J Neurol Neurosurg Psychiatry 1973, 36, 1053–62. [Google Scholar] [CrossRef]
- Gemignani F, Marbini A, Bragaglia MM, Govoni E. Pathological study of the sural nerve in Fabry’s disease. Eur Neurol 1984, 23, 173–81. [Google Scholar] [CrossRef]
- Desnick RJ, Ionnou Y, Eng CM. Fabry disease: alpha galactosidase A deficiency. In: Scriver CH, Beaudet AL, Sly WS, Valle D, eds. The metabolic and molecular bases of inherited disease. New York: McGraw Hill, 1995, 2741-84.
- Wise D, Wallace HJ, Jellinek EH. Angiokeratoma corporis diVusum. Q J Med 1962;XXXI:177‐212.
- Desnick, R.J.; Ioannou, Y.A. α-Galactosidase a Deficiency. Fabry Disease. The Metabolic and Molecular Bases of Inherited Disease, 8th ed.; Scriver, C.R., Beaudet, A.L., Sly, W.S., Valle, D., Eds.; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Sweeley, C.C.; Klionsky, B. Fabry’s Disease: Classification as a sphingolipidosis and partial char-acterization of a novel glycolipid. J. Biol. Chem. 1963, 238, 3148–3150. [Google Scholar] [CrossRef]
- Brady, R.O.; Gal, A.E.; Bradley, R.M.; Martensson, E.; Warshaw, A.L.; Laster, L. Enzymatic Defect in Fabry’s Disease. N. Engl. J. Med. 1967, 276, 1163–116. [Google Scholar] [CrossRef]
- Valbuena C, Carvalho E, Bustorff M, Ganhao M, Relvas S, Nogueira R, Carneiro F, Oliveira JP: Kidney biopsy findings in heterozygous Fabry disease females with early nephropathy. Virchows Arch 2008, 453, 329–338. [CrossRef]
- SafyanR, Whybra C, Beck M, Elstein D, Altarescu G:An association study of inflammatory cytokine gene polymorphisms in Fabry disease. Eur Cytokine Netw 2006, 17, 271–275.
- Thomaidis T, Relle M, Golbas M, Brochhausen C, Galle PR, Beck M, Schwarting A: Downregulation of alpha-galactosidase A upreg- ulates CD77: functional impact for Fabry nephropathy. Kid- ney Int 2009, 75, 399–407. [CrossRef]
- Rozenfeld P, Agriello E, De Francesco N, Martinez P, Fossati C. Leukocyte perturbation associated with Fabry disease. J Inherit Metab Dis. 2009, 32 (Suppl 1), S67–77. [Google Scholar] [CrossRef]
- Altarescu G, Chicco G, Whybra C, Delgado-Sanchez S, Sharon N, Beck M, Elstein D: Correlation between interleukin-6 pro- moter and C-reactive protein (CRP) polymorphisms and CRP levels with the Mainz Severity Score Index for Fabry disease. J Inherit Metab Dis 2008, 31, 117–123. [CrossRef]
- Moore DF, Goldin E, Gelderman MP, Robinson C, Baer J, Ries M, Elkahloun A, Brady RO, Schiffmann R: Apoptotic abnormalities in differential gene expression in peripheral blood mononuclear cells from children with Fabry disease. Acta Paediatr Suppl 2008, 97, 48–52. [CrossRef]
- Shen JS, Meng XL, Moore DF, Quirk JM, Shayman JA, Schiffmann R, Kaneski CR: Globotriaosylceramide induces oxidative stress and up-regulates cell adhesion molecule expression in Fabry disease endothelial cells. Mol Genet Metab 2008, 95, 163–16. [CrossRef]
- Rohard I, Schaefer E, Kampmann C, Beck M, Gal A. Association between polymorphisms of endothelial nitric oxide synthase gene (NOS3) and left posterior wall thickness (LPWT) of the heart in Fabry disease. J Inherit Metab Dis. 2008, 31(Suppl 2), S349-56.
- Wang RY, Abe JT, Cohen AH, Wilcox WR. Enzyme replacement therapy stabilizes obstructive pulmonary Fabry disease associated with respiratory globotriaosylceramide storage. J Inherit Metab Dis. 2008, 31 Suppl 2, S369–74. [Google Scholar]
- Aerts JM, Groener JE, Kuiper S, Donker-Koopman WE, Strijland A, Ottenhoff R, et al. Elevated globotriaosylsphingosine is a hallmark of abry disease. Proc Natl Acad Sci U S A. 2008, 105, 2812–2817. [Google Scholar] [CrossRef]
- Schiffmann, R. Fabry disease. Pharmacol Ther. 2009, 122, 65–77. [Google Scholar] [CrossRef]
- Moore DF, Kaneski CR, Askari H, Schiffmann R. The cerebral vasculopathy of Fabry disease. J Neurol Sci. 2007, 257, 258–263. [Google Scholar] [CrossRef]
- Rombach SM, Twickler TB, Aerts JM, Linthorst GE, Wijburg FA, Hollak CE. Vasculopathy in patients with Fabry disease: current controversies and research directions. Mol Genet Metab. 2010, 99, 99–108. [Google Scholar] [CrossRef]
- Namdar M, Gebhard C, Studiger R, Shi Y, Mocharla P, Schmied C, et al. Globotriaosylsphingosine accumulation and not alpha-galactosidase-A deficiency causes endothelial dysfunction in Fabry disease. PLoS One. 2012;7:e36373. doi: 10.1371/journal.pone.0036373. 8. Sestito S, Ceravolo F, Concolino D. Anderson-Fabry disease in children. Curr Pharm Des. 2013;19:6037–6045.
- Sims K, Politei J, Banikazemi M, Lee P. Stroke in Fabry disease frequently occurs before diagnosis and in the absence of other clinical events: natural history data from the Fabry Registry. Stroke. 2009, 40, 788–794. [Google Scholar] [CrossRef]
- Buechner S, Moretti M, Burlina AP, Cei G, Manara R, Ricci R, et al. Central nervous system involvement in Anderson-Fabry disease: a clinical and MRI retrospective study. J Neurol Neurosurg Psychiatry. 2008, 79, 1249–1254. [Google Scholar] [CrossRef]
- Saito S, Ohno K, Sakuraba H. Fabry-database.org: database of the clinical phenotypes, genotypes and mutant α-galactosidase A structures in Fabry disease. J Hum Genet. 2011, 56, 467–468. [Google Scholar] [CrossRef]
- Cable WJL, Kolodny EH, Adams RD. Fabry disease impaired autonomic function. Neurology. 1982, 32, 498–498. [Google Scholar] [CrossRef]
- Tabira T, Goto I, Kuroiwa Y, Kikuchi M. Neuropathological and biochemical studies in Fabry’s disease. Acta Neuropathol (Berl). 1974, 30, 345–354. [Google Scholar] [CrossRef]
- Scott LJC, Griffin JW, Luciano C, Barton NW, Banerjee T, Crawford T, McArthur JC, Tournay A, Schiffmann R. Quantitative analysis of epidermal innervation in Fabry disease. Neurology. 1999, 52, 1249–1249. [Google Scholar] [CrossRef]
- Schiffmann, R. Neuropathy and Fabry disease: pathogenesis and enzyme replacement therapy. Acta Neurol Belg. 2006, 106, 61. [Google Scholar]
- Choi L, Vernon J, Kopach O, Minett MS, Mills K, Clayton PT, Meert T, Wood JN. The Fabry disease-associated lipid Lyso-Gb3 enhances voltage-gated calcium currents in sensory neurons and causes pain. Neurosci Lett. 2015, 594, 163–168. [Google Scholar] [CrossRef]
- Prado VF, Roy A, Kolisnyk B, Gros R, Prado MAM. Regulation of cholinergic activity by the vesicular acetylcholine transporter. Biochem J. 2013, 450, 265–274. [Google Scholar] [CrossRef]
- Lücke, T. Fabry disease: reduced activities of respiratory chain enzymes with decreased levels of energy-rich phosphates in fibroblasts. Mol Genet Metab. 2004, 82, 93–97. [Google Scholar] [CrossRef]
- Yamamoto A, Abuillan W, Burk AS, Körner A, Ries A, Werz DB, Demé B, Tanaka M. Influence of length and conformation of saccharide head groups on the mechanics of glycolipid membranes: Unraveled by off-specular neutron scattering. J Chem Phys. 2015, 142, 154907. [Google Scholar] [CrossRef]
- Park S, Kim JA, Joo KY, Choi S, Choi EN, Shin JA, Han KH, Jung SC, Suh SH. Globotriaosylceramide leads to KCa3.1 channel dysfunction: a new insight into endothelial dysfunction in Fabry disease. Cardiovasc Res. 2011, 89, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Schäfer MK-H, Eiden LE, Weihe E. Cholinergic neurons and terminal fields revealed by immunohistochemistry for the vesicular acetylcholine transporter. II. The peripheral nervous system. Neuroscience. 1998, 84, 361–376. [Google Scholar]
- Moore, A.M. , Wood, M.D., Chenard, K., Hunter, D.A., Mackinnon, S.E., Sakiyama- Elbert, S.E., Borschel, G.H., 2010. Controlled delivery of glial cell line-derived neurotrophic factor enhances motor nerve regeneration. J Hand Surg Am 35(12), 2008–2017. [CrossRef] [PubMed]
- Politei JM, Bouhassira D, Germain DP, Goizet C, Guerrero-Sola A, Hilz MJ, Hutton EJ, Karaa A, Liguori R, Üçeyler N, Zeltzer LK, Burlina A. Pain in Fabry Disease: Practical Recommendations for Diagnosis and Treatment. CNS Neurosci Ther. 2016, 22(7), 568-76.
- Miller, J.J., et al., 2018. α-Galactosidase A-deficient rats accumulate glycosphingolipids and develop cardiorenal phenotypes of Fabry disease. The FASEB Journal 33, 418–429. [CrossRef] [PubMed]
- Burand AJ Jr, Stucky CL. Fabry disease pain: patient and preclinical parallels. Pain. 2021, 162(5), 1305–1321. [CrossRef]
- Kaneski CR, Brady RO, Hanover JA, Schueler UH. Development of a model system for neuronal dysfunction in Fabry disease. Mol Genet Metab. 2016, 119(1-2), 144-50.
- DeGraba T, Azhar S, Dignat-George F, Brown E, Boutière B, Altarescu G, McCarron R, Schiffmann R. Profile of endothelial and leukocyte activation in Fabry patients. Ann Neurol. 2000, 47(2), 229–33. [Google Scholar]
- Aerts JM, Groener JE, Kuiper S, Donker-Koopman WE, Strijland A, Ottenhoff R, van Roomen C, Mirzaian M, Wijburg FA, Linthorst GE, Vedder AC, Rombach SM, Cox-Brinkman J, Somerharju P, Boot RG, Hollak CE, Brady RO, Poorthuis BJ. Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc Natl Acad Sci U S A. 2008, 105(8), 2812–7. [Google Scholar]
- Rombach SM, Twickler TB, Aerts JM, Linthorst GE, Wijburg FA, Hollak CE. Vasculopathy in patients with Fabry disease: current controversies and research directions. Mol Genet Metab. 2010, 99(2), 99–108. [CrossRef]
- Rombach SM, Twickler TB, Aerts JM, Linthorst GE, Wijburg FA, Hollak CE. Vasculopathy in patients with Fabry disease: current controversies and research directions. Mol Genet Metab. 2010, 99, 99–108. [Google Scholar] [CrossRef]
- Biegstraaten M, Hollak CE, Bakkers M, Faber CG, Aerts JM, van Schaik IN. Small fiber neuropathy in Fabry disease. Mol Genet Metab. 2012, 106, 135–41. [Google Scholar] [CrossRef]
- Schuller Y, Linthorst GE, Hollak CE, Van Schaik IN, Biegstraaten M. Pain management strategies for neuropathic pain in Fabry disease--a systematic review. BMC Neurol. 2016, 16, 25. [Google Scholar]
- Geevasinga N, Tchan M, Sillence D, Vucic S. Upregulation of inward rectifying currents and Fabry disease neuropathy. J Peripher Nerv Syst. 2012, 17, 399–406. [Google Scholar] [CrossRef]
- Castellanos LCS, Rozenfeld P, Gatto RG, Reisin RC, Uchitel OD, Weissmann C. Upregulation of ASIC1a channels in an in vitro model of Fabry disease. Neurochem Int. 2020, 140, 104824. [Google Scholar] [CrossRef] [PubMed]
- Sluka KA, Winter OC, Wemmie JA. Acid-sensing ion channels: A new target for pain and CNS diseases. Curr Opin Drug Discov Devel. 2009, 12, 693–704. [Google Scholar]
- Hofmann L, Hose D, Grießhammer A, Blum R, Döring F, Dib-Hajj S, Waxman S, Sommer C, Wischmeyer E, Üçeyler N. Characterization of small fiber pathology in a mouse model of Fabry disease. Elife. 2018, 7, e39300. [Google Scholar] [CrossRef]
- Waltz TB, Burand AJ Jr, Sadler KE, Stucky CL. Sensory-specific peripheral nerve pathology in a rat model of Fabry disease. Neurobiol Pain. 2021, 10, 100074. [Google Scholar]
- Kocen, R.S. , Thomas, P.K., 1970. Peripheral Nerve Involvement in Fabry’s Disease. Archives of Neurology 22(1), 81–88. [CrossRef]
- Torvin Møller A, Winther Bach F, Feldt-Rasmussen U, Rasmussen A, Hasholt L, Lan H, Sommer C, Kølvraa S, Ballegaard M, Staehelin Jensen T. Functional and structural nerve fiber findings in heterozygote patients with Fabry disease. Pain. 2009, 145, 237–45. [Google Scholar] [CrossRef]
- Uceyler, N. , Ganendiran, S., Kramer, D., Sommer, C., 2014. Characterization of pain in fabry disease. The Clinical journal of pain 30, 915–920. [PubMed]
- Politei JM, Bouhassira D, Germain DP, Goizet C, Guerrero-Sola A, Hilz MJ, Hutton EJ, Karaa A, Liguori R, Üçeyler N, Zeltzer LK, Burlina A. Pain in Fabry Disease: Practical Recommendations for Diagnosis and Treatment. CNS Neurosci Ther. 2016, 22, 568–76. [Google Scholar] [CrossRef]
- Üçeyler N, Kahn AK, Kramer D, Zeller D, Casanova-Molla J, Wanner C, Weidemann F, Katsarava Z, Sommer C. Impaired small fiber conduction in patients with Fabry disease: a neurophysiological case-control study. BMC Neurol. 2013, 13, 47. [Google Scholar]
- Du¨tsch M, Marthol H, Stemper B, Brys M, Haendl T, Hilz MJ. Small fiber dysfunction predominates in Fabry neuropathy. J Clin Neurophysiol. 2002, 19, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Biegstraaten M, Hollak CE, Bakkers M, Faber CG, Aerts JM, van Schaik IN. Small fiber neuropathy in Fabry disease. Mol Genet Metab. 2012, 106, 135–141. [Google Scholar] [CrossRef]
- Politei JM, Pagano MA. [Peripheral neuropathy in AndersonFabry disease: its physiology, evaluation and treatment]. Rev Neurol. 2004, 38, 979–983.
- U¨ c¸eyler N, Kahn AK, Kramer D, et al. Impaired small fiber conduction in patients with Fabry disease: a neurophysiological casecontrol study. BMC Neurol. 2013, 13, 47. [Google Scholar]
- Schiffmann R, Scott LJ. Pathophysiology and assessment of neuropathic pain in Fabry disease. Acta Paediatr Suppl. 2002, 91, 48–52. [Google Scholar] [CrossRef]
- Hilz MJ, Koehn J, Kolodny EH, Brys M, Moeller S, Stemper B. Metronomic breathing shows altered parasympathetic baroreflex function in untreated Fabry patients and baroreflex improvement after enzyme replacement therapy. J Hypertens. 2011, 29, 2387–2394. [Google Scholar] [CrossRef]
- deVeber GA, Schwarting GA, Kolodny EH, Kowall NW. Fabry disease: immunocytochemical characterization of neuronal involvement. Ann Neurol. 1992, 31, 409–415. [Google Scholar] [CrossRef]
- Lao LM, Kumakiri M, Mima H, et al. The ultrastructural characteristics of eccrine sweat glands in a Fabry disease patient with hypohidrosis. J Dermatol Sci. 1998, 18, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Lim SN, Huang CC, Kuo HC, Hsieh YC, Chu CC. Subtle Changes in Cutaneous Nerves and Sural Nerve Biopsy in a Patient With Fabry’s Disease. J Clin Neuromuscul Dis. 2005, 7, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Gayathri N, Yasha TC, Kanjalkar M, et al. Fabry’s disease: An ultrastructural study of nerve biopsy. Ann Indian Acad Neurol. 2008;11(3):182-184. 29. Toyooka K, Said G. Nerve biopsy findings in hemizygous and heterozygous patients with Fabry’s disease. J Neurol. 1997, 244, 464–468. [Google Scholar]
- Kahn, P. Anderson-Fabry disease: a histopathological study of three cases with observations on the mechanism of production of pain. J Neurol Neurosurg Psychiatry. 1973, 36, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- U¨ c¸eyler N, He L, Scho¨nfeld D, et al. Small fibers in Fabry disease: baseline and follow-up data under enzyme replacement therapy. J Peripher Nerv Syst. 2011, 16, 304–314. [Google Scholar] [CrossRef]
- Brakch N, Dormond O, Bekri S, et al. Evidence for a role of sphingosine-1 phosphate in cardiovascular remodelling in Fabry disease. Eur Heart J. 2010, 31, 67–76. [Google Scholar] [CrossRef]
- Tom´e FM, Fardeau M, Lenoir G. Ultrastructure of muscle and sensory nerve in Fabry’s disease. Acta Neuropathol. 1977, 38, 187–194. [Google Scholar] [CrossRef]
- ilz MJ, Kolodny EH, Brys M, Stemper B, Haendl T, Marthol H. Reduced cerebral blood flow velocity and impaired cerebral autoregulation in patients with Fabry disease. Journal of Neurology. 2004, 251, 564–570.
- Moore DF, Ye F, Brennan ML, et al. Ascorbate decreases fabry cerebral hyperperfusion suggesting a reactive oxygen species abnormality: an arterial spin tagging study. Journal of Magnetic Resonance Imaging. 2004, 20, 674–683. [Google Scholar] [CrossRef]
- Azevedo E, Mendes A, Seixas D, et al. Functional transcranial Doppler: presymptomatic changes in Fabry disease. Eur Neurol 2012, 67, 331–7. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




