Submitted:
22 August 2023
Posted:
23 August 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Nitrogen uptake
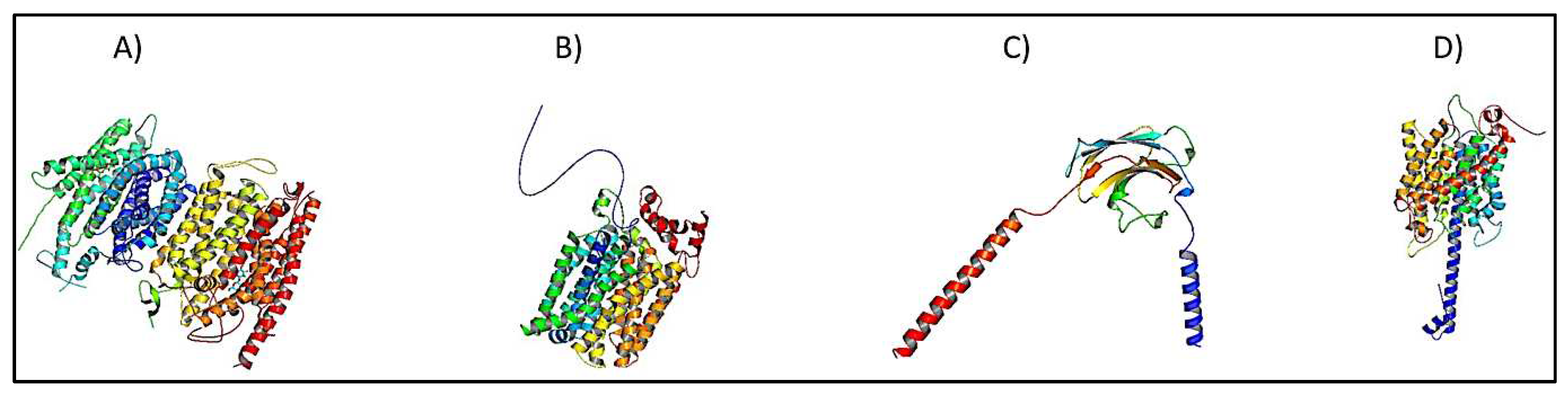
Plant nitrate transporters (NRTs)
NRT Structural analysis
Plant ammonium transporters (AMTs)
AMTs Structural analysis
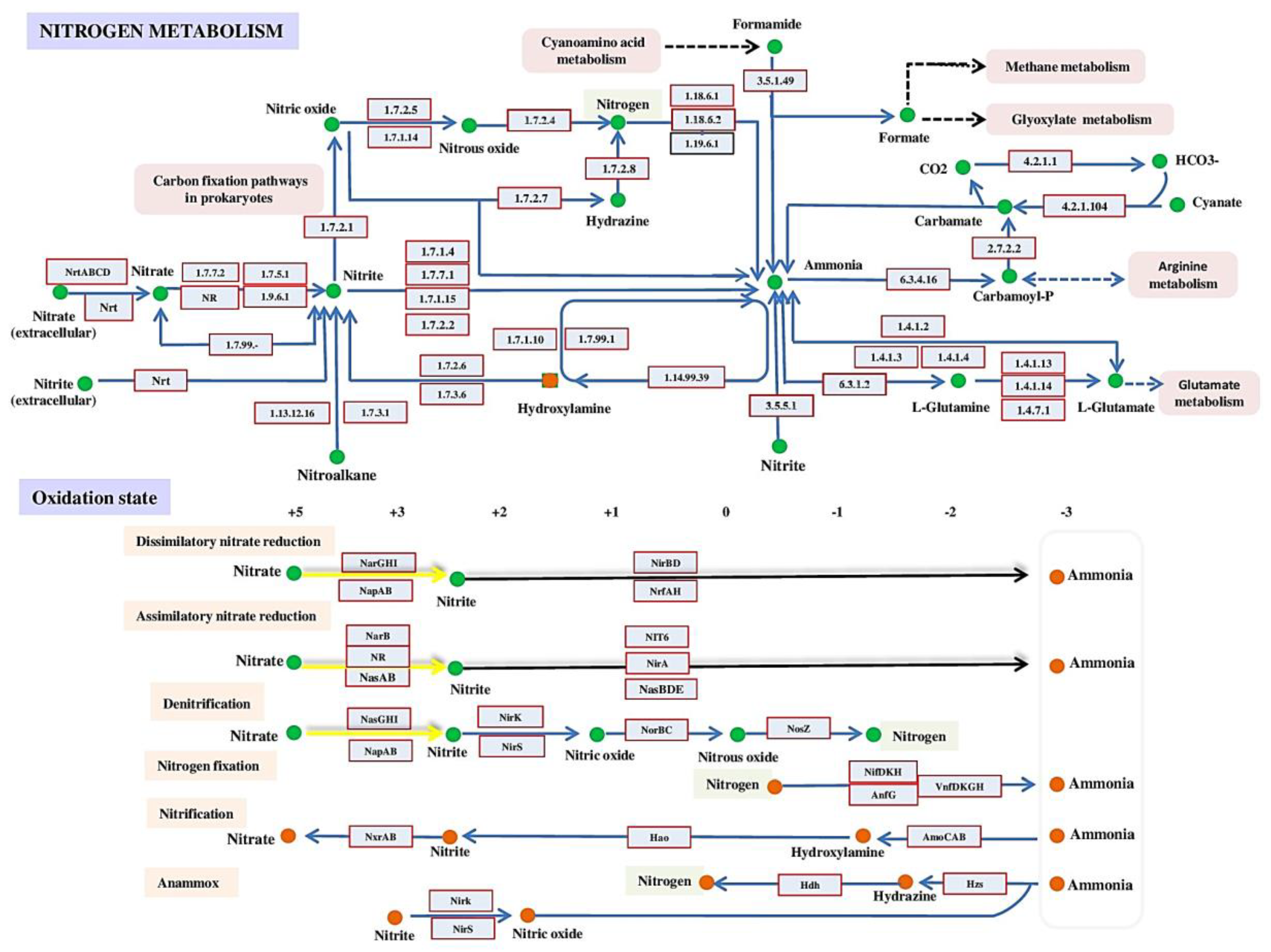
Nitrogen metabolism pathway
| No. | KEGG ID | Gene Symbol | Gene Name | EC number | Reference | |
| 1 | K04561 | NORB | Nitric oxide reductase subunit B | 1.7.2.5 | Heiss et al., 1989; Suzuki et al., 2006 | |
| 2 | K15877 | CYP55 | Fungal nitric oxide reductase | 1.7.1.14 | Kizawa et al., 1991; Zhang and Shoun, 2008 | |
| 3 | K00376 | NOSZ | Nitrous-oxide reductase | 1.7.2.4 | Coyleet al., 1985; Arai et al., 2003; Zumft and Kroneck, 2007 | |
| 4 | K02586 | NIFD | Nitrogenase molybdenum-iron protein alpha chain | 1.18.6.1 | Joerger et al., 1989; Fani et al., 2000 | |
| 5 | K22896 | VNFD | Vanadium-dependent nitrogenase alpha chain | 1.18.6.2 | Joerger et al., 1990 | |
| 6 | R05186 | NIFF | Nitrogenase | 1.19.6.1 | Deistung et al., 1985 | |
| 7 | K01455 | FORMAMIDASE | Formamidase | 3.5.1.49 | ||
| 8 | K20935 | HDH | Hydrazine dehydrogenase | 1.7.2.8 | Kartal et al., 2011 | |
| 9 | K20932 | K20932 | Hydrazine synthase subunit | 1.7.2.7 | 1.7.2.7 | |
| 10 | K01672 | CA | Carbonic anhydrase | 4.2.1.1 | Breton, 2001; Pastorekova et al., 2004 | |
| 11 | K01725 | CYNS | Cyanate lyase | 4.2.1.104 | Harano et al., 1997 | |
| 12 | K00368 | NIRK | Nitrite reductase (NO-forming) | 1.7.2.1 | Cantera and Stein, 2007 | |
| 13 | K02575 | NRT2, NARK, NRTP, NASA | MFS transporter, NNP family, nitrate/nitrite transporter | ------ | Noji et al., 1989 | |
| 14 | K15576 | NRTA, NRTB, NRTC, NASD | Nitrate/nitrite transport system substrate-binding protein | 7.3.2.4 | Maeda and Omata, 2009 | |
| 15 | K00370 | NARG, NARZ, NXRA | Nitrate reductase/nitrite oxidoreductase, alpha subunit | 1.7.5.11.7.99.- | Blasco et al., 1990; Sohaskey and Wayne, 2003 | |
| 16 | K10534 | NR | Nitrate reductase (NAD(P)H) | 1.7.1.1 1.7.1.2 1.7.1.3 | Okamoto and Marzluf, 1993 | |
| 17 | K00367 | NARB | Ferredoxin-nitrate reductase | 1.7.7.2 | Rubio et al., 1996 | |
| 18 | K02567 | NAPA | Nitrate reductase (cytochrome) | 1.9.6.1 | Stolz and Basu, 2002; Jepson et al., 2007 | |
| 19 | K17877 | NIT-6 | Nitrite reductase (NAD(P)H) | 1.7.1.4 | Exley et al., 1993 | |
| 20 | K00366 | NIRA | Ferredoxin-nitrite reductase | 1.7.7.1 | Takahashi et al., 2001 | |
| 21 | K00362 | NIRB | Nitrite reductase (NADH) large subunit | 1.7.1.15 | Harborne et al., 1992 | |
| 22 | K03385 | NRFA | Nitrite reductase (cytochrome c-552) | 1.7.2.2 | Einsle et al., 1999 | |
| 23 | K00459 | NCD2, NPD | Nitronate monooxygenase | 1.13.12.16 | Gadda and Francis, 2010 | |
| 24 | K19823 | NAO | Nitroalkane oxidase | 1.7.3.1 | Daubner et al., 2002 | |
| No. | KEGG ID | Gene Symbol | Gene Name | EC number | Reference |
| 25 | R00143 | Hydroxylamine reductase (NADH) | 1.7.1.10 | ||
| 26 | K05601 | HCP | Hydroxylamine reductase | 1.7.99.1 | Wolfe et al., 2002 |
| 27 | K10535 | HAO | Hydroxylamine dehydrogenase | 1.7.2.6 | Hommes et al., 2001 |
| 28 | R10230 | Hydroxylamine oxidase (cytochrome) | 1.7.3.6 | Wehrfritz et al., 1996 | |
| 29 | K10944 | PMOA-AMOA | Methane/ammonia monooxygenase subunit A | 1.14.18.3 1.14.99.39 | Norton et al., 2002 |
| 30 | K00926 | ARCC | Carbamate kinase | 2.7.2.2 | Durbecq et al., 1997 |
| 31 | K01948 | CPS1 | Carbamoyl-phosphate synthase (ammonia) | 6.3.4.16 | Haraguchi et al., 1991 |
| 32 | K00260 | GUDB, ROCG | Glutamate dehydrogenase | 1.4.1.2 | Belitsky and Sonenshein, 1998 |
| 33 | K00261 | GLUD1_2, GDHA | Glutamate dehydrogenase (NAD(P)+) | 1.4.1.3 | Frigerio et al., 2008 |
| 34 | K00262 | E1.4.1.4, GDHA | Glutamate dehydrogenase (NADP+) | 1.4.1.4 | Riba et al., 1988 |
| 35 | K01915 | GLNA, GLUL | Glutamine synthetase | 6.3.1.2 | Newsholme et al., 2003 |
| 36 | K01501 | E3.5.5.1 | Nitrilase | 3.5.5.1 | Zhu et al., 2008 |
| 37 | K00265 | GLTB | Glutamate synthase (NADPH) large chain | 1.4.1.13 | Sonawane and Röhm, 2004 |
| 38 | K00264 | GLT1 | Glutamate synthase (NADH) | 1.4.1.14 | Lancien et al., 2002 |
| 39 | K00284 | GLU, GLTS | Glutamate synthase (ferredoxin) | 1.4.7.1 | Coschigano et al., 1998 |
| 40 | K00372 | NASC, NASA | Assimilatory nitrate reductase catalytic subunit | 1.7.99.- | Ogawa et al., 1995; Lin et al., 1993 |
| 41 | K26139 | NASD, NASB | Nitrite reductase [NAD(P)H] large subunit | 1.7.1.4 | Ogawa et al., 1995 |
| 42 | K15864 | NIRS | Nitrite reductase (NO-forming) / hydroxylamine reductase | 1.7.2.1 1.7.99.1 | Rees et al., 1997; Hole et al., 1996 |
| 43 | K00531 | ANFG | Nitrogenase delta subunit | 1.18.6.1 | Joerger et al., 1989 |
| 44 | K04561 | NORB | Nitric oxide reductase subunit B | 1.7.2.5 | Heiss et al., 1989; Suzuki et al., 2006 |
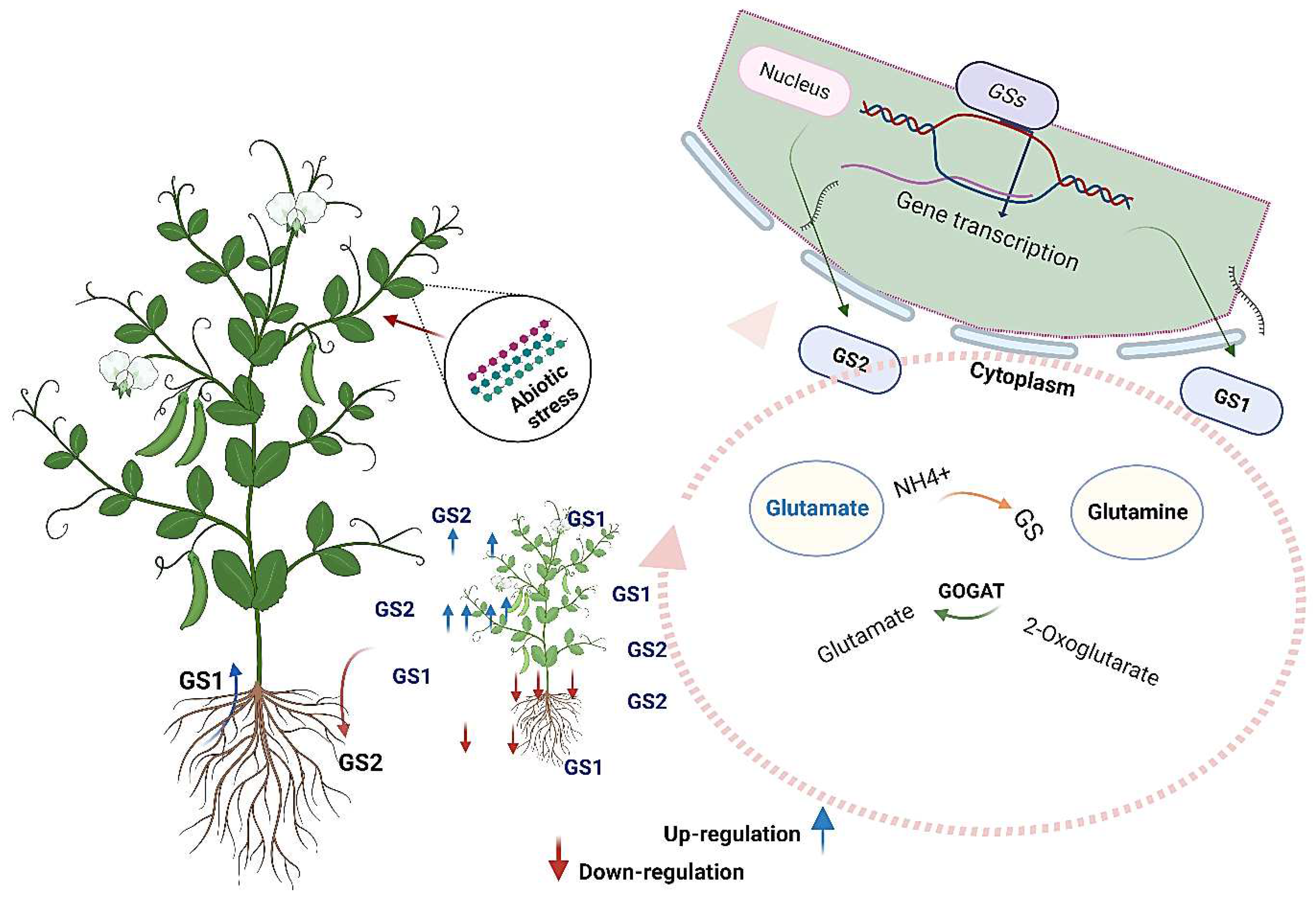
Function and regulation of glutamine synthetase and glutamate synthase in nitrogen metabolism
Isoforms of GS and GoGAT
Biological function of GS in response to abiotic stress
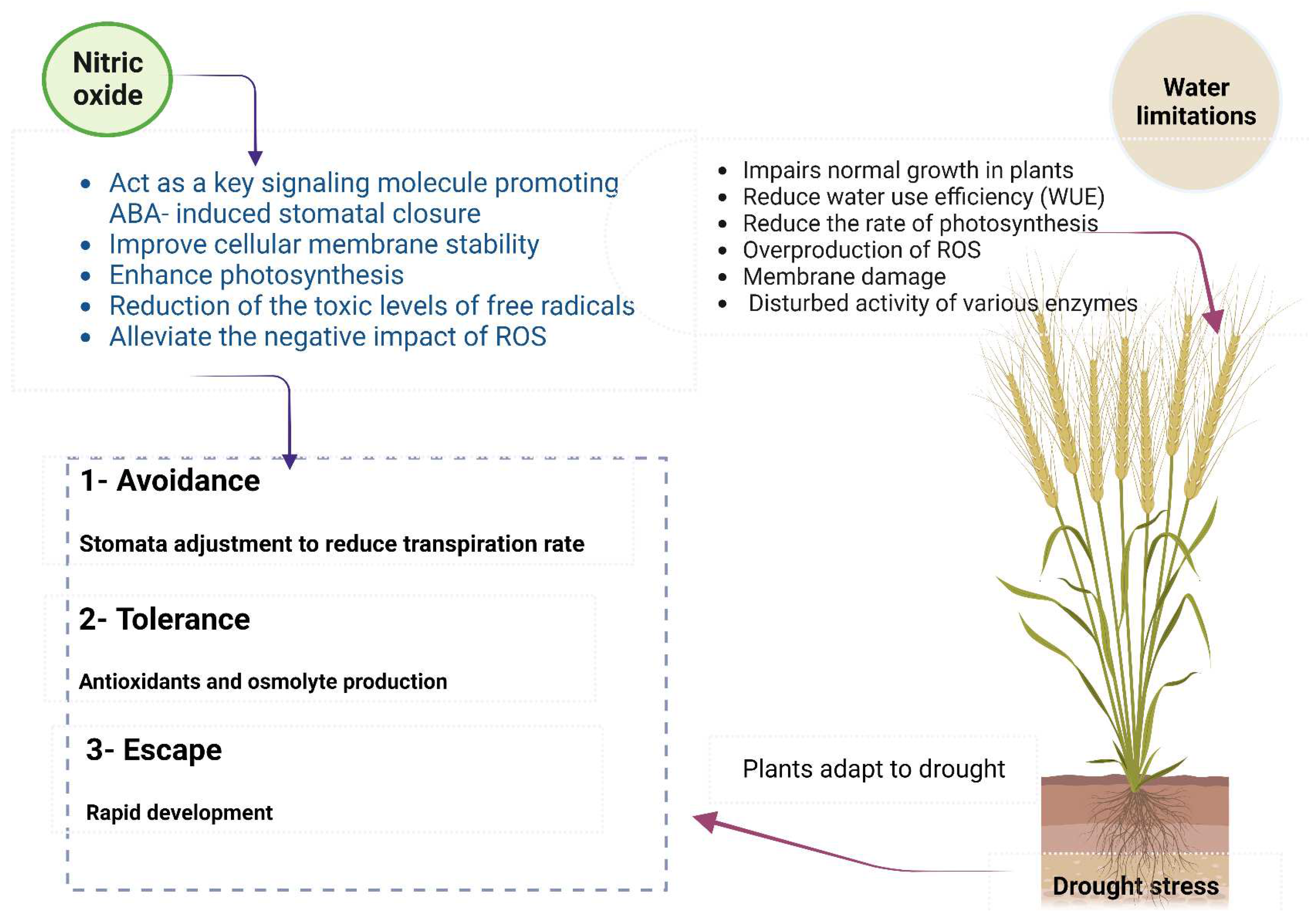
Nitrogen-induced resilience in plants against drought stress
Nitric oxide enhances plant survival under drought stress
Salinity stress and nitrogen in plant
Nitric oxide and salt stress
Nitrate fertilizers have hazardous health issues
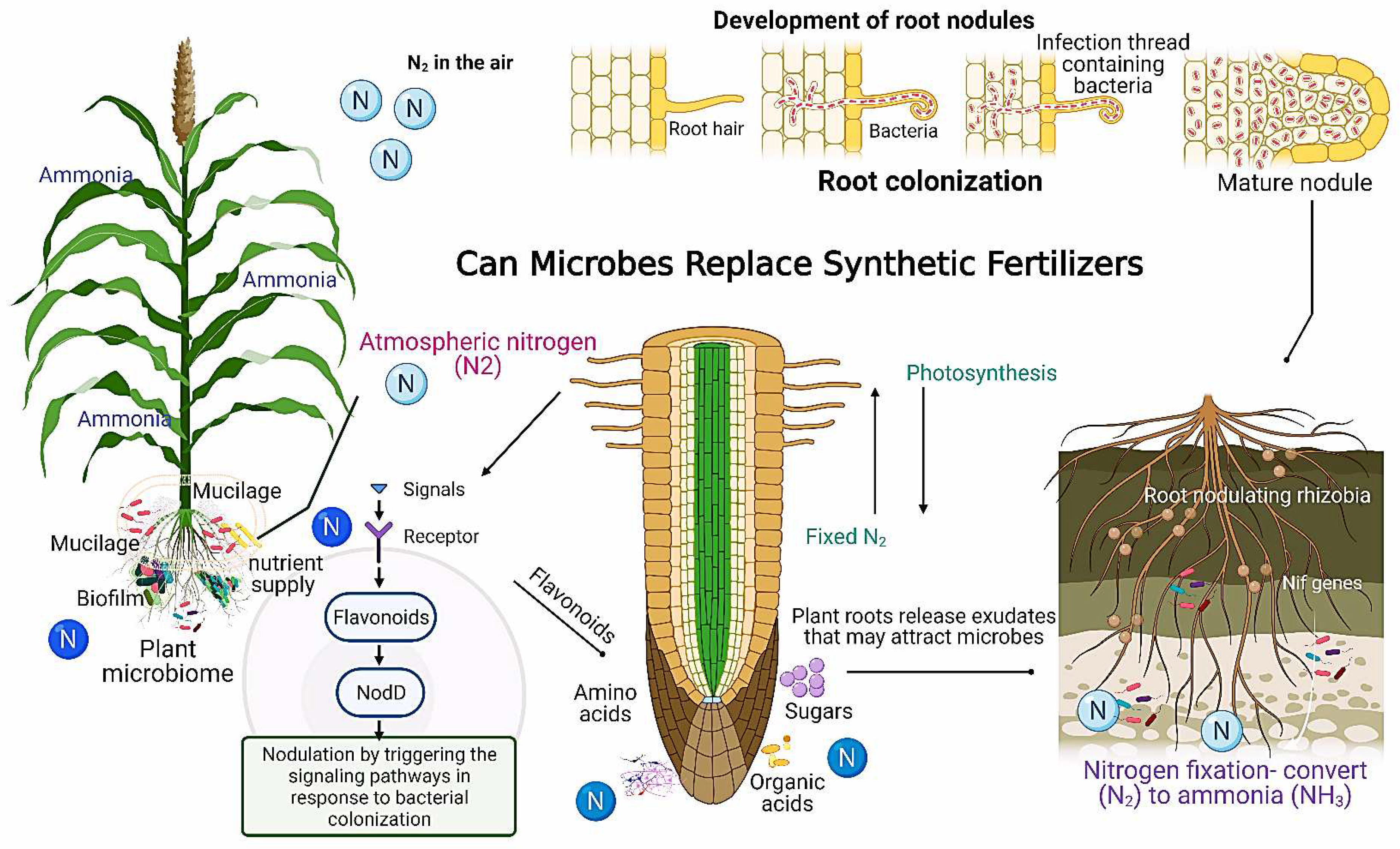
Establishing nitrogen fixation in plants by diazotrophic microbiota
Can microbes replace synthetic fertilizers?
Arctic N cycling microbiota
Genomic approaches have a role in identifying new genes to improve nitrogen fixation
Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Lal, R. Restoring Soil Quality to Mitigate Soil Degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Menzies, N.W.; Wang, P.; McKenna, B.A.; Lombi, E. Soil and the intensification of agriculture for global food security. Environment International 2019, 132, 105078. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.; Bogunovic, I.; Muñoz-Rojas, M.; Brevik, E.C. Soil ecosystem services, sustainability, valuation and management. Current Opinion in Environmental Science & Health 2018, 5, 7–13. [Google Scholar] [CrossRef]
- Pimentel, D.; Burgess, M. Soil Erosion Threatens Food Production. Agriculture 2013, 3, 443–463. [Google Scholar] [CrossRef]
- Díaz-Rodríguez, A.M.; Salcedo Gastelum, L.A.; Félix Pablos, C.M.; Parra-Cota, F.I.; Santoyo, G.; Puente, M.L.; Bhattacharya, D.; Mukherjee, J.; de los Santos-Villalobos, S. The Current and Future Role of Microbial Culture Collections in Food Security Worldwide. Frontiers in Sustainable Food Systems 2021, 4. [Google Scholar] [CrossRef]
- Arora, N.K.; Khare, E.; Maheshwari, D.K. Plant Growth Promoting Rhizobacteria: Constraints in Bioformulation, Commercialization, and Future Strategies. In Plant Growth and Health Promoting Bacteria; Maheshwari, D.K., Ed.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2011; pp. 97–116. [Google Scholar] [CrossRef]
- Hirel, B.; Tétu, T.; Lea, P.J.; Dubois, F. Improving Nitrogen Use Efficiency in Crops for Sustainable Agriculture. Sustainability 2011, 3, 1452–1485. [Google Scholar] [CrossRef]
- Akinnifesi, F.K.; Ajayi, O.C.; Sileshi, G.; Chirwa, P.W.; Chianu, J. Fertiliser trees for sustainable food security in the maize-based production systems of East and Southern Africa. A review. Agronomy for Sustainable Development 2010, 30, 615–629. [Google Scholar] [CrossRef]
- Spiertz, H. Avenues to meet food security. The role of agronomy on solving complexity in food production and resource use. European Journal of Agronomy 2012, 43, 1–8. [Google Scholar] [CrossRef]
- Azadi, H.; Ho, P. Genetically modified and organic crops in developing countries: A review of options for food security. Biotechnology Advances 2010, 28, 160–168. [Google Scholar] [CrossRef]
- Singh, J.S.; Gupta, V.K. Soil microbial biomass: A key soil driver in management of ecosystem functioning. Science of the Total Environment 2018, 634, 497–500. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Annals of botany 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.Y.; Tian, W.H.; Jin, C.W. Nitrogen in plants: from nutrition to the modulation of abiotic stress adaptation. Stress Biology 2022, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, N.; Sethi, M.; Kumar, A.; Dang, D.; Singh, J.; Chhuneja, P. Biochemical and genetic approaches improving nitrogen use efficiency in cereal crops: A review. Frontiers in plant science 2021, 12, 657629. [Google Scholar] [CrossRef]
- Delin, S.; Stenberg, B.; Nyberg, A.; Brohede, L. Potential methods for estimating nitrogen fertilizer value of organic residues. Soil Use and Management 2012, 28, 283–291. [Google Scholar] [CrossRef]
- Yao, X.; Nie, J.; Bai, R.; Sui, X. Amino acid transporters in plants: Identification and function. Plants 2020, 9, 972. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Dong, C.; Yang, T.; Bao, S.; Fang, W.; Lucas, W.J.; Zhang, Z. The tea plant CsLHT1 and CsLHT6 transporters take up amino acids, as a nitrogen source, from the soil of organic tea plantations. Horticulture Research 2021, 8. [Google Scholar] [CrossRef]
- Feng, H.; Fan, X.; Miller, A.J.; Xu, G. Plant nitrogen uptake and assimilation: regulation of cellular pH homeostasis. Journal of Experimental Botany 2020, 71, 4380–4392. [Google Scholar] [CrossRef]
- Forde, B.G. Nitrate transporters in plants: structure, function and regulation. Biochimica et Biophysica Acta (BBA)-Biomembranes 2000, 1465, 219–235. [Google Scholar] [CrossRef]
- Iqbal, A.; Qiang, D.; Zhun, W.; Xiangru, W.; Huiping, G.; Hengheng, Z.; Nianchang, P.; Xiling, Z.; Meizhen, S. Growth and nitrogen metabolism are associated with nitrogen-use efficiency in cotton genotypes. Plant Physiology and Biochemistry 2020, 149, 61–74. [Google Scholar] [CrossRef]
- Léran, S.; Varala, K.; Boyer, J.-C.; Chiurazzi, M.; Crawford, N.; Daniel-Vedele, F.; David, L.; Dickstein, R.; Fernandez, E.; Forde, B. A unified nomenclature of NITRATE TRANSPORTER 1/PEPTIDE TRANSPORTER family members in plants. Trends in plant science 2014, 19, 5–9. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Hsu, P.-K.; Tsay, Y.-F. Uptake, allocation and signaling of nitrate. Trends in plant science 2012, 17, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Cheng, Y.-H.; Chen, K.-E.; Tsay, Y.-F. Nitrate transport, signaling, and use efficiency. Annual review of plant biology 2018, 69, 85–122. [Google Scholar] [CrossRef]
- Kiba, T.; Feria-Bourrellier, A.-B.; Lafouge, F.; Lezhneva, L.; Boutet-Mercey, S.; Orsel, M.; Bréhaut, V.; Miller, A.; Daniel-Vedele, F.; Sakakibara, H. The Arabidopsis nitrate transporter NRT2. 4 plays a double role in roots and shoots of nitrogen-starved plants. The Plant Cell 2012, 24, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Chattha, M.S.; Ali, Q.; Haroon, M.; Afzal, M.J.; Javed, T.; Hussain, S.; Mahmood, T.; Solanki, M.K.; Umar, A.; Abbas, W. Enhancement of nitrogen use efficiency through agronomic and molecular based approaches in cotton. Frontiers in Plant Science 2022, 13, 994306. [Google Scholar] [CrossRef] [PubMed]
- Maghiaoui, A.; Gojon, A.; Bach, L. NRT1. 1-centered nitrate signaling in plants. Journal of Experimental Botany 2020, 71, 6226–6237. [Google Scholar] [CrossRef] [PubMed]
- Izmailov, S.; Nikitin, A. Nitrate signaling in plants: mechanisms of implementation. Russian journal of plant physiology 2020, 67, 31–44. [Google Scholar] [CrossRef]
- O'Brien, J.A.; Vega, A.; Bouguyon, E.; Krouk, G.; Gojon, A.; Coruzzi, G.; Gutiérrez, R.A. Nitrate transport, sensing, and responses in plants. Molecular plant 2016, 9, 837–856. [Google Scholar] [CrossRef]
- Fang, X.Z.; Fang, S.Q.; Ye, Z.Q.; Liu, D.; Zhao, K.L.; Jin, C.W. NRT1. 1 dual-affinity nitrate transport/signalling and its roles in plant abiotic stress resistance. Frontiers in plant science 2021, 12, 715694. [Google Scholar] [CrossRef]
- Chen, C.-Z.; Lv, X.-F.; Li, J.-Y.; Yi, H.-Y.; Gong, J.-M. Arabidopsis NRT1. 5 is another essential component in the regulation of nitrate reallocation and stress tolerance. Plant Physiology 2012, 159, 1582–1590. [Google Scholar] [CrossRef]
- Nazish, T.; Arshad, M.; Jan, S.U.; Javaid, A.; Khan, M.H.; Naeem, M.A.; Baber, M.; Ali, M. Transporters and transcription factors gene families involved in improving nitrogen use efficiency (NUE) and assimilation in rice (Oryza sativa L.). Transgenic Research 2021, 1–20. [Google Scholar] [CrossRef]
- Guo, B.; Li, Y.; Wang, S.; Li, D.; Lv, C.; Xu, R. Characterization of the Nitrate Transporter gene family and functional identification of HvNRT2. 1 in barley (Hordeum vulgare L.). PLoS One 2020, 15, e0232056. [Google Scholar]
- Aluko, O.O.; Kant, S.; Adedire, O.M.; Li, C.; Yuan, G.; Liu, H.; Wang, Q. Unlocking the potentials of nitrate transporters at improving plant nitrogen use efficiency. Frontiers in Plant Science 2023, 14, 1074839. [Google Scholar] [CrossRef]
- Ji, L.; Song, L.; Zou, L.; Li, S.; Zhang, R.; Yang, J.; Wang, C.; Zhang, Y.; Wang, X.; Yun, L. Cassava Nitrate Transporter NPF5. 4 promotes both yield potential and salt tolerance in rice. 2022. [Google Scholar]
- Zhao, L.; Chen, P.; Liu, P.; Song, Y.; Zhang, D. Genetic effects and expression patterns of the nitrate transporter (NRT) gene family in Populus tomentosa. Frontiers in Plant Science 2021, 12, 661635. [Google Scholar] [CrossRef] [PubMed]
- Qi(杞金芳), J.; Yu(郁露), L.; Ding(丁静丽), J.; Ji(姬晨晨), C.; Wang(汪社亮), S.; Wang(王创), C.; Ding(丁广大), G.; Shi(石磊), L.; Xu(徐芳森), F.; Cai(蔡红梅), H. Transcription factor OsSNAC1 positively regulates nitrate transporter gene expression in rice. Plant Physiology 2023, 192, 2923–2942. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, K.; Xie, J.; Liu, J.; Tan, P.; Peng, F. Genome-Wide Identification and Expression Analysis of AMT and NRT Gene Family in Pecan (Carya illinoinensis) Seedlings Revealed a Preference for NH4+-N. International Journal of Molecular Sciences 2022, 23, 13314. [Google Scholar] [CrossRef]
- Schuldiner, S.; Shirvan, A.; Linial, M. Vesicular neurotransmitter transporters: from bacteria to humans. Physiological reviews 1995, 75, 369–392. [Google Scholar] [CrossRef]
- Tsay, Y.-F.; Chiu, C.-C.; Tsai, C.-B.; Ho, C.-H.; Hsu, P.-K. Nitrate transporters and peptide transporters. FEBS letters 2007, 581, 2290–2300. [Google Scholar] [CrossRef]
- Sun, J.; Bankston, J.R.; Payandeh, J.; Hinds, T.R.; Zagotta, W.N.; Zheng, N. Crystal structure of the plant dual-affinity nitrate transporter NRT1. 1. Nature 2014, 507, 73–77. [Google Scholar] [CrossRef]
- Madej, M.G.; Dang, S.; Yan, N.; Kaback, H.R. Evolutionary mix-and-match with MFS transporters. Proceedings of the National Academy of Sciences 2013, 110, 5870–5874. [Google Scholar] [CrossRef] [PubMed]
- Orsel, M.; Chopin, F.; Leleu, O.; Smith, S.J.; Krapp, A.; Daniel-Vedele, F.o.; Miller, A.J. Characterization of a Two-Component High-Affinity Nitrate Uptake System in Arabidopsis. Physiology and Protein-Protein Interaction. Plant Physiology 2006, 142, 1304–1317. [Google Scholar] [CrossRef]
- Okamoto, M.; Kumar, A.; Li, W.; Wang, Y.; Siddiqi, M.Y.; Crawford, N.M.; Glass, A.D.M. High-Affinity Nitrate Transport in Roots of Arabidopsis Depends on Expression of the NAR2-Like Gene AtNRT3.1. Plant Physiology 2006, 140, 1036–1046. [Google Scholar] [CrossRef]
- Benková, E.; Bielach, A. Lateral root organogenesis — from cell to organ. Current Opinion in Plant Biology 2010, 13, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Yong, Z.; Kotur, Z.; Glass, A.D. Characterization of an intact two-component high-affinity nitrate transporter from Arabidopsis roots. The Plant Journal 2010, 63, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Bowen, G.D.; Smith, S.E. THE EFFECTS OF MYCORRHIZAS ON NITROGEN UPTAKE BY PLANTS. Ecological Bulletins 1981, 237–247. [Google Scholar]
- Öhlund, J.; Näsholm, T. Growth of conifer seedlings on organic and inorganic nitrogen sources. Tree Physiology 2001, 21, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Gazzarrini, S.; Lejay, L.; Gojon, A.; Ninnemann, O.; Frommer, W.B.; von Wirén, N. Three Functional Transporters for Constitutive, Diurnally Regulated, and Starvation-Induced Uptake of Ammonium into Arabidopsis Roots. The Plant Cell 1999, 11, 937–947. [Google Scholar] [CrossRef]
- Bloom, A.J.; Meyerhoff, P.A.; Taylor, A.R.; Rost, T.L. Root Development and Absorption of Ammonium and Nitrate from the Rhizosphere. Journal of Plant Growth Regulation 2002, 21, 416–431. [Google Scholar] [CrossRef]
- Bittsánszky, A.; Pilinszky, K.; Gyulai, G.; Komives, T. Overcoming ammonium toxicity. Plant Science 2015, 231, 184–190. [Google Scholar] [CrossRef]
- Renard, J.J.; Calidonna, S.E.; Henley, M.V. Fate of ammonia in the atmosphere—a review for applicability to hazardous releases. Journal of Hazardous Materials 2004, 108, 29–60. [Google Scholar] [CrossRef]
- Hachiya, T.; Watanabe, C.K.; Fujimoto, M.; Ishikawa, T.; Takahara, K.; Kawai-Yamada, M.; Uchimiya, H.; Uesono, Y.; Terashima, I.; Noguchi, K. Nitrate Addition Alleviates Ammonium Toxicity Without Lessening Ammonium Accumulation, Organic Acid Depletion and Inorganic Cation Depletion in Arabidopsis thaliana Shoots. Plant and Cell Physiology 2012, 53, 577–591. [Google Scholar] [CrossRef]
- von Wirén, N.; Gazzarrini, S.; Gojon, A.; Frommer, W.B. The molecular physiology of ammonium uptake and retrieval. Current Opinion in Plant Biology 2000, 3, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Loqué, D.; von Wirén, N. Regulatory levels for the transport of ammonium in plant roots. Journal of Experimental Botany 2004, 55, 1293–1305. [Google Scholar] [CrossRef]
- Glass, A.D.M.; Britto, D.T.; Kaiser, B.N.; Kinghorn, J.R.; Kronzucker, H.J.; Kumar, A.; Okamoto, M.; Rawat, S.; Siddiqi, M.Y.; Unkles, S.E.; et al. The regulation of nitrate and ammonium transport systems in plants. Journal of Experimental Botany 2002, 53, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.-L.; Zhou, J.-Y.; Yang, S.-Y.; Qi, W.; Yang, K.-J.; Su, Y.-H. Function and Regulation of Ammonium Transporters in Plants. International Journal of Molecular Sciences 2020, 21, 3557. [Google Scholar] [CrossRef]
- Behie, S.W.; Bidochka, M.J. Ubiquity of Insect-Derived Nitrogen Transfer to Plants by Endophytic Insect-Pathogenic Fungi: an Additional Branch of the Soil Nitrogen Cycle. Applied and Environmental Microbiology 2014, 80, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Giehl, R.F.H.; Laginha, A.M.; Duan, F.; Rentsch, D.; Yuan, L.; von Wirén, N. A Critical Role of AMT2;1 in Root-To-Shoot Translocation of Ammonium in Arabidopsis. Molecular Plant 2017, 10, 1449–1460. [Google Scholar] [CrossRef]
- Couturier, J.; Montanini, B.; Martin, F.; Brun, A.; Blaudez, D.; Chalot, M. The expanded family of ammonium transporters in the perennial poplar plant. New Phytologist 2007, 174, 137–150. [Google Scholar] [CrossRef]
- Esmaeilzadeh-Salestani, K.; Samandari_Bahraseman, M.R.; Tohidfar, M.; Khaleghdoust, B.; Keres, I.; Mõttus, A.; Loit, E. Expression of AMT1;1 and AMT2;1 is stimulated by mineral nitrogen and reproductive growth stage in barley under field conditions. Journal of Plant Nutrition 2023, 46, 1246–1258. [Google Scholar] [CrossRef]
- Wu, X.; Yang, H.; Qu, C.; Xu, Z.; Li, W.; Hao, B.; Yang, C.; Sun, G.; Liu, G. Sequence and expression analysis of the AMT gene family in poplar. Frontiers in Plant Science 2015, 6. [Google Scholar] [CrossRef]
- Wu, X.X.; Yuan, D.P.; Chen, H.; Kumar, V.; Kang, S.M.; Jia, B.; Xuan, Y.H. Ammonium transporter 1 increases rice resistance to sheath blight by promoting nitrogen assimilation and ethylene signalling. Plant Biotechnology Journal 2022, 20, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Pastor, V.; Gamir, J.; Camañes, G.; Cerezo, M.; Sánchez-Bel, P.; Flors, V. Disruption of the ammonium transporter AMT1.1 alters basal defenses generating resistance against Pseudomonas syringae and Plectosphaerella cucumerina. Frontiers in Plant Science 2014, 5. [Google Scholar] [CrossRef]
- Lima, J.E.; Kojima, S.; Takahashi, H.; von Wirén, N. Ammonium Triggers Lateral Root Branching in Arabidopsis in an AMMONIUM TRANSPORTER1;3-Dependent Manner. The Plant Cell 2010, 22, 3621–3633. [Google Scholar] [CrossRef] [PubMed]
- Rogato, A.; D’Apuzzo, E.; Chiurazzi, M. The multiple plant response to high ammonium conditions. Plant Signaling & Behavior 2010, 5, 1594–1596. [Google Scholar] [CrossRef]
- Rogato, A.; D’Apuzzo, E.; Barbulova, A.; Omrane, S.; Parlati, A.; Carfagna, S.; Costa, A.; Schiavo, F.L.; Esposito, S.; Chiurazzi, M. Characterization of a Developmental Root Response Caused by External Ammonium Supply in Lotus japonicus. Plant Physiology 2010, 154, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Liu, Q.; Liu, X.; Ma, Z.; Zhang, J.; Li, X.; Shen, L.; Yuan, J.; Zhang, Q. Phosphatidic acid regulates ammonium uptake by interacting with AMMONIUM TRANSPORTER 1;1 in Arabidopsis. Plant Physiology 2023, 10.1093/plphys/kiad421. [Google Scholar] [CrossRef]
- Giovannetti, M.; Volpe, V.; Salvioli, A.; Bonfante, P. Chapter 7 - Fungal and Plant Tools for the Uptake of Nutrients in Arbuscular Mycorrhizas: A Molecular View. In Mycorrhizal Mediation of Soil; Johnson, N.C., Gehring, C., Jansa, J., Eds.; Elsevier, 2017; pp. 107–128. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, W.; Wei, J.; Yoon, H.; An, G. Transcription Factor OsDOF18 Controls Ammonium Uptake by Inducing Ammonium Transporters in Rice Roots. Mol Cells 2017, 40, 178–185. [Google Scholar] [CrossRef]
- Yuan, L.; Gu, R.; Xuan, Y.; Smith-Valle, E.; Loqué, D.; Frommer, W.B.; von Wirén, N. Allosteric Regulation of Transport Activity by Heterotrimerization of Arabidopsis Ammonium Transporter Complexes in Vivo. The Plant Cell 2013, 25, 974–984. [Google Scholar] [CrossRef]
- Lanquar, V.; Loqué, D.; Hörmann, F.; Yuan, L.; Bohner, A.; Engelsberger, W.R.; Lalonde, S.; Schulze, W.X.; von Wirén, N.; Frommer, W.B. Feedback Inhibition of Ammonium Uptake by a Phospho-Dependent Allosteric Mechanism in Arabidopsis. The Plant Cell 2009, 21, 3610–3622. [Google Scholar] [CrossRef]
- Loqué, D.; Lalonde, S.; Looger, L.L.; von Wirén, N.; Frommer, W.B. A cytosolic trans-activation domain essential for ammonium uptake. Nature 2007, 446, 195–198. [Google Scholar] [CrossRef]
- Søgaard, R.; Alsterfjord, M.; MacAulay, N.; Zeuthen, T. Ammonium ion transport by the AMT/Rh homolog TaAMT1;1 is stimulated by acidic pH. Pflügers Archiv - European Journal of Physiology 2009, 458, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhao, Y.; Luo, W.; Li, R.; He, Q.; Fang, X.; Michele, R.D.; Ast, C.; von Wirén, N.; Lin, J. Single-particle analysis reveals shutoff control of the <i>Arabidopsis</i> ammonium transporter AMT1;3 by clustering and internalization. Proceedings of the National Academy of Sciences 2013, 110, 13204–13209. [Google Scholar] [CrossRef]
- Koltun, A.; Maniero, R.A.; Vitti, M.; de Setta, N.; Giehl, R.F.H.; Lima, J.E.; Figueira, A. Functional characterization of the sugarcane (Saccharum spp.) ammonium transporter AMT2;1 suggests a role in ammonium root-to-shoot translocation. Frontiers in Plant Science 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Mengel, K.; Kirkby, E.A.; Kosegarten, H.; Appel, T. Nitrogen. Principles of plant nutrition 2001, 397–434. [Google Scholar]
- Sparacino-Watkins, C.; Stolz, J.F.; Basu, P. Nitrate and periplasmic nitrate reductases. Chemical Society Reviews 2014, 43, 676–706. [Google Scholar] [CrossRef] [PubMed]
- Rosswall, T. Some perspectives of the major biogeochemical cycles. Likens, GE., editor. Vol. 2. New York, USA: John Wiley & Sons Ithaca: 1981.
- Ward, M.H.; DeKok, T.M.; Levallois, P.; Brender, J.; Gulis, G.; Nolan, B.T.; VanDerslice, J. Workgroup report: drinking-water nitrate and health—recent findings and research needs. Environmental health perspectives 2005, 113, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Powlson, D.S.; Addiscott, T.M.; Benjamin, N.; Cassman, K.G.; de Kok, T.M.; van Grinsven, H.; L'hirondel, J.L.; Avery, A.A.; Van Kessel, C. When does nitrate become a risk for humans? Journal of environmental quality 2008, 37, 291–295. [Google Scholar] [CrossRef]
- Bonner, F.T.; Hughes, M.N. The aqueous solution chemistry of nitrogen in low positive oxidation states. Comments on Inorganic Chemistry 1988, 7, 215–234. [Google Scholar] [CrossRef]
- Jaffe, D.A. 12 The Nitrogen Cycle. In International Geophysics, Butcher, S.S., Charlson, R.J., Orians, G.H., Wolfe, G.V., Eds. Academic Press: 1992; Vol. 50, pp. 263–284.
- Canfield, D.E.; Glazer, A.N.; Falkowski, P.G. The evolution and future of Earth’s nitrogen cycle. science 2010, 330, 192–196. [Google Scholar] [CrossRef]
- Ishii, S.; Ikeda, S.; Minamisawa, K.; Senoo, K. Nitrogen cycling in rice paddy environments: past achievements and future challenges. Microbes and environments 2011, 26, 282–292. [Google Scholar] [CrossRef]
- Elrys, A.S.; Zhu, Q.; Jiang, C.; Liu, J.; Sobhy, H.H.; Shen, Q.; Uwiragiye, Y.; Wu, Y.; El-Tarabily, K.A.; Meng, L. Global soil nitrogen cycle pattern and nitrogen enrichment effects: Tropical versus subtropical forests. Global Change Biology 2023, 29, 1905–1921. [Google Scholar] [CrossRef] [PubMed]
- Joerger, R.; Jacobson, M.; Premakumar, R.; Wolfinger, E.; Bishop, P. Nucleotide sequence and mutational analysis of the structural genes (anfHDGK) for the second alternative nitrogenase from Azotobacter vinelandii. Journal of bacteriology 1989, 171, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Joerger, R.; Loveless, T.; Pau, R.; Mitchenall, L.; Simon, B.; Bishop, P. Nucleotide sequences and mutational analysis of the structural genes for nitrogenase 2 of Azotobacter vinelandii. Journal of bacteriology 1990, 172, 3400–3408. [Google Scholar] [CrossRef]
- Fani, R.; Gallo, R.; Liò, P. Molecular evolution of nitrogen fixation: the evolutionary history of the nifD, nifK, nifE, and nifN genes. Journal of molecular evolution 2000, 51, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cantera, J.J.L.; Stein, L.Y. Molecular diversity of nitrite reductase genes (nirK) in nitrifying bacteria. Environmental microbiology 2007, 9, 765–776. [Google Scholar] [CrossRef]
- Kartal, B.; Maalcke, W.J.; De Almeida, N.M.; Cirpus, I.; Gloerich, J.; Geerts, W.; Op den Camp, H.J.; Harhangi, H.R.; Janssen-Megens, E.M.; Francoijs, K.-J. Molecular mechanism of anaerobic ammonium oxidation. Nature 2011, 479, 127–130. [Google Scholar] [CrossRef]
- Jepson, B.J.; Mohan, S.; Clarke, T.A.; Gates, A.J.; Cole, J.A.; Butler, C.S.; Butt, J.N.; Hemmings, A.M.; Richardson, D.J. Spectropotentiometric and structural analysis of the periplasmic nitrate reductase from Escherichia coli. Journal of Biological Chemistry 2007, 282, 6425–6437. [Google Scholar] [CrossRef]
- Heiss, B.; Frunzke, K.; Zumft, W. Formation of the NN bond from nitric oxide by a membrane-bound cytochrome bc complex of nitrate-respiring (denitrifying) Pseudomonas stutzeri. Journal of bacteriology 1989, 171, 3288–3297. [Google Scholar] [CrossRef]
- Suzuki, M.; Arai, H.; Ishii, M.; Igarashi, Y. Gene structure and expression profile of cytochrome bc nitric oxide reductase from Hydrogenobacter thermophilus TK-6. Bioscience, biotechnology, and biochemistry 2006, 70, 1666–1671. [Google Scholar] [CrossRef]
- Ogawa, K.-i.; Akagawa, E.; Yamane, K.; Sun, Z.-W.; LaCelle, M.; Zuber, P.; Nakano, M.M. The nasB operon and nasA gene are required for nitrate/nitrite assimilation in Bacillus subtilis. Journal of bacteriology 1995, 177, 1409–1413. [Google Scholar] [CrossRef]
- Exley, G.E.; Colandene, J.D.; Garrett, R.H. Molecular cloning, characterization, and nucleotide sequence of nit-6, the structural gene for nitrite reductase in Neurospora crassa. Journal of bacteriology 1993, 175, 2379–2392. [Google Scholar] [CrossRef] [PubMed]
- Sohaskey, C.D.; Wayne, L.G. Role of narK2X and narGHJI inHypoxic Upregulation of Nitrate Reduction by Mycobacteriumtuberculosis. Journal of bacteriology 2003, 185, 7247–7256. [Google Scholar] [CrossRef] [PubMed]
- Stolz, J.F.; Basu, P. Evolution of nitrate reductase: molecular and structural variations on a common function. Chembiochem 2002, 3, 198–206. [Google Scholar] [CrossRef]
- Engels, C.; Marschner, H. Plant uptake and utilization of nitrogen. Nitrogen fertilization in the environment 1995, 41–81. [Google Scholar]
- Vega-Mas, I.; Cukier, C.; Coleto, I.; González-Murua, C.; Limami, A.M.; González-Moro, M.B.; Marino, D. Isotopic labelling reveals the efficient adaptation of wheat root TCA cycle flux modes to match carbon demand under ammonium nutrition. Scientific Reports 2019, 9, 8925. [Google Scholar] [CrossRef]
- Fujita, T.; Beier, M.P.; Tabuchi-Kobayashi, M.; Hayatsu, Y.; Nakamura, H.; Umetsu-Ohashi, T.; Sasaki, K.; Ishiyama, K.; Murozuka, E.; Kojima, M. Cytosolic glutamine synthetase GS1; 3 is involved in rice grain ripening and germination. Frontiers in Plant Science 2022, 13, 835835. [Google Scholar] [CrossRef]
- Lea, P.J.; Miflin, B.J. Nitrogen assimilation and its relevance to crop improvement. Annu. Plant Rev. Volume 2011, 42, 1–40. [Google Scholar]
- Miflin, B.J.; Lea, P.J. The pathway of nitrogen assimilation in plants. Phytochemistry 1976, 15, 873–885. [Google Scholar] [CrossRef]
- Oaks, A.; Stulen, I.; Jones, K.; Winspear, M.J.; Misra, S.; Boesel, I.L. Enzymes of nitrogen assimilation in maize roots. Planta 1980, 148, 477–484. [Google Scholar] [CrossRef]
- Tamura, W.; Hidaka, Y.; Tabuchi, M.; Kojima, S.; Hayakawa, T.; Sato, T.; Obara, M.; Kojima, M.; Sakakibara, H.; Yamaya, T. Reverse genetics approach to characterize a function of NADH-glutamate synthase1 in rice plants. Amino Acids 2010, 39, 1003–1012. [Google Scholar] [CrossRef]
- Kojima, S.; Minagawa, H.; Yoshida, C.; Inoue, E.; Takahashi, H.; Ishiyama, K. Coregulation of glutamine synthetase1; 2 (GLN1; 2) and NADH-dependent glutamate synthase (GLT1) gene expression in Arabidopsis roots in response to ammonium supply. Frontiers in Plant Science 2023, 14, 1127006. [Google Scholar] [CrossRef]
- Yasuda, T.; Konishi, N.; Kojima, S. Ammonium uptake capacity and response of cytosolic glutamine synthetase 1; 2 to ammonium supply are key factors for the adaptation of ammonium nutrition in Arabidopsis thaliana. Soil Science and Plant Nutrition 2017, 63, 553–560. [Google Scholar] [CrossRef]
- Konishi, N.; Saito, M.; Imagawa, F.; Kanno, K.; Yamaya, T.; Kojima, S. Cytosolic glutamine synthetase isozymes play redundant roles in ammonium assimilation under low-ammonium conditions in roots of Arabidopsis thaliana. Plant and Cell Physiology 2018, 59, 601–613. [Google Scholar] [CrossRef]
- Yamaya, T.; Kusano, M. Evidence supporting distinct functions of three cytosolic glutamine synthetases and two NADH-glutamate synthases in rice. Journal of experimental botany 2014, 65, 5519–5525. [Google Scholar] [CrossRef]
- Kusano, M.; Fukushima, A.; Redestig, H.; Saito, K. Metabolomic approaches toward understanding nitrogen metabolism in plants. Journal of Experimental Botany 2011, 62, 1439–1453. [Google Scholar] [CrossRef]
- Forde, B.G.; Lea, P.J. Glutamate in plants: metabolism, regulation, and signalling. Journal of experimental botany 2007, 58, 2339–2358. [Google Scholar] [CrossRef] [PubMed]
- Bowsher, C.G.; Lacey, A.E.; Hanke, G.T.; Clarkson, D.T.; Saker, L.R.; Stulen, I.; Emes, M.J. The effect of Glc6P uptake and its subsequent oxidation within pea root plastids on nitrite reduction and glutamate synthesis. Journal of experimental botany 2007, 58, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Habash, D.Z.; Bernard, S.; Schondelmaier, J.; Weyen, J.; Quarrie, S.A. The genetics of nitrogen use in hexaploid wheat: N utilisation, development and yield. Theoretical and Applied Genetics 2007, 114, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Yang, F.; He, X.; Du, X.; Mu, P.; Ma, W. Advances in the functional study of glutamine synthetase in plant abiotic stress tolerance response. The Crop Journal 2022, 10, 917–923. [Google Scholar] [CrossRef]
- Prinsi, B.; Espen, L. Mineral nitrogen sources differently affect root glutamine synthetase isoforms and amino acid balance among organs in maize. BMC plant biology 2015, 15, 1–13. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiong, S.; Wei, Y.; Meng, X.; Wang, X.; Ma, X. The role of glutamine synthetase isozymes in enhancing nitrogen use efficiency of N-efficient winter wheat. Scientific Reports 2017, 7, 1000. [Google Scholar] [CrossRef] [PubMed]
- Habash, D.; Massiah, A.; Rong, H.; Wallsgrove, R.; Leigh, R. The role of cytosolic glutamine synthetase in wheat. Annals of Applied Biology 2001, 138, 83–89. [Google Scholar] [CrossRef]
- Gadaleta, A.; Nigro, D.; Marcotuli, I.; Giancaspro, A.; Giove, S.L.; Blanco, A. Isolation and characterisation of cytosolic glutamine synthetase (<i>GSe</i>) genes and association with grain protein content in durum wheat. Crop and Pasture Science 2014, 65, 38–45. [Google Scholar]
- Tabuchi, M.; Abiko, T.; Yamaya, T. Assimilation of ammonium ions and reutilization of nitrogen in rice (Oryza sativa L.)*. Journal of Experimental Botany 2007, 58, 2319–2327. [Google Scholar] [CrossRef]
- Ishiyama, K.; Inoue, E.; Watanabe-Takahashi, A.; Obara, M.; Yamaya, T.; Takahashi, H. Kinetic Properties and Ammonium-dependent Regulation of Cytosolic Isoenzymes of Glutamine Synthetase in <em>Arabidopsis</em>*. Journal of Biological Chemistry 2004, 279, 16598–16605. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Minagawa, H.; Yoshida, C.; Inoue, E.; Takahashi, H.; Ishiyama, K. Coregulation of glutamine synthetase1;2 (GLN1;2) and NADH-dependent glutamate synthase (GLT1) gene expression in Arabidopsis roots in response to ammonium supply. Frontiers in Plant Science 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Li, Q.; Liu, G.; Selvaraj, G.; Zheng, Z.; Zou, J.; Wei, Y. Roles of Cytosolic Glutamine Synthetases in Arabidopsis Development and Stress Responses. Plant and Cell Physiology 2019, 60, 657–671. [Google Scholar] [CrossRef]
- Yan, S.; Tang, Z.; Su, W.; Sun, W. Proteomic analysis of salt stress-responsive proteins in rice root. PROTEOMICS 2005, 5, 235–244. [Google Scholar] [CrossRef]
- Yousfi, S.; Márquez, A.J.; Betti, M.; Araus, J.L.; Serret, M.D. Gene expression and physiological responses to salinity and water stress of contrasting durum wheat genotypes. Journal of Integrative Plant Biology 2016, 58, 48–66. [Google Scholar] [CrossRef]
- Teixeira, J.; Pereira, S. High salinity and drought act on an organ-dependent manner on potato glutamine synthetase expression and accumulation. Environmental and Experimental Botany 2007, 60, 121–126. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, Y.; He, Q.; Li, H.; Zhang, X.; Zhang, F. Comparative proteomics illustrates the complexity of drought resistance mechanisms in two wheat (Triticum aestivum L.) cultivars under dehydration and rehydration. BMC Plant Biology 2016, 16, 188. [Google Scholar] [CrossRef]
- Nagy, Z.; Németh, E.; Guóth, A.; Bona, L.; Wodala, B.; Pécsváradi, A. Metabolic indicators of drought stress tolerance in wheat: Glutamine synthetase isoenzymes and Rubisco. Plant Physiology and Biochemistry 2013, 67, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.-F.; Lin, C.C.; Wang, J.-W.; Chen, C.T.; Kao, C.H. Changes in ammonium ion content and glutamine synthetase activity in rice leaves caused by excess cadmium are a consequence of oxidative damage. Plant Growth Regulation 2002, 36, 41–47. [Google Scholar] [CrossRef]
- Balestrasse, K.B.; Benavides, M.P.; Gallego, S.M.; Tomaro, M.L. Effect of cadmium stress on nitrogen metabolism in nodules and roots of soybean plants. Functional Plant Biology 2003, 30, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Chaffei, C.; Pageau, K.; Suzuki, A.; Gouia, H.; Ghorbel, M.H.; Masclaux-Daubresse, C. Cadmium Toxicity Induced Changes in Nitrogen Management in Lycopersicon esculentum Leading to a Metabolic Safeguard Through an Amino Acid Storage Strategy. Plant and Cell Physiology 2004, 45, 1681–1693. [Google Scholar] [CrossRef] [PubMed]
- Rana, N.K.; Mohanpuria, P.; Yadav, S.K. Expression of tea cytosolic glutamine synthetase is tissue specific and induced by cadmium and salt stress. Biologia Plantarum 2008, 52, 361–364. [Google Scholar] [CrossRef]
- Sedri, M.H.; Amini, A.; Golchin, A. Evaluation of Nitrogen Effects on Yield and Drought Tolerance of Rainfed Wheat using Drought Stress Indices. Journal of Crop Science and Biotechnology 2019, 22, 235–242. [Google Scholar] [CrossRef]
- Song, J.; Wang, Y.; Pan, Y.; Pang, J.; Zhang, X.; Fan, J.; Zhang, Y. The influence of nitrogen availability on anatomical and physiological responses of Populus alba × P. glandulosa to drought stress. BMC Plant Biology 2019, 19, 63. [Google Scholar] [CrossRef]
- Xu, N.; Guo, W.; Liu, J.; Du, N.; Wang, R. Increased nitrogen deposition alleviated the adverse effects of drought stress on Quercus variabilis and Quercus mongolica seedlings. Acta Physiologiae Plantarum 2015, 37, 107. [Google Scholar] [CrossRef]
- Gessler, A.; Schaub, M.; McDowell, N.G. The role of nutrients in drought-induced tree mortality and recovery. New Phytologist 2017, 214, 513–520. [Google Scholar] [CrossRef]
- Li, S.; Zhou, L.; Addo-Danso, S.D.; Ding, G.; Sun, M.; Wu, S.; Lin, S. Nitrogen supply enhances the physiological resistance of Chinese fir plantlets under polyethylene glycol (PEG)-induced drought stress. Scientific Reports 2020, 10, 7509. [Google Scholar] [CrossRef]
- Safavi-Rizi, V.; Uellendahl, K.; Öhrlein, B.; Safavi-Rizi, H.; Stöhr, C. Cross-stress tolerance: Mild nitrogen (N) deficiency effects on drought stress response of tomato (Solanum lycopersicum L.). Plant-Environment Interactions 2021, 2, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Cao, X.; Hu, J.; Zhu, L.; Zhang, J.; Huang, J.; Jin, Q. Nitrogen Metabolism in Adaptation of Photosynthesis to Water Stress in Rice Grown under Different Nitrogen Levels. Frontiers in Plant Science 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Singh, V.P.; Prasad, S.M. Responses of photosynthesis, nitrogen and proline metabolism to salinity stress in Solanum lycopersicum under different levels of nitrogen supplementation. Plant Physiology and Biochemistry 2016, 109, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Tan, D.K.Y.; Afridi, M.Z.; Luo, H.; Tung, S.A.; Ajab, M.; Fahad, S. Nitrogen fertility and abiotic stresses management in cotton crop: a review. Environmental Science and Pollution Research 2017, 24, 14551–14566. [Google Scholar] [CrossRef]
- Diao, M.; Ma, L.; Wang, J.; Cui, J.; Fu, A.; Liu, H.-y. Selenium Promotes the Growth and Photosynthesis of Tomato Seedlings Under Salt Stress by Enhancing Chloroplast Antioxidant Defense System. Journal of Plant Growth Regulation 2014, 33, 671–682. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, B.; Zhang, H.-J.; Weeda, S.; Yang, C.; Yang, Z.-C.; Ren, S.; Guo, Y.-D. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). Journal of Pineal Research 2013, 54, 15–23. [Google Scholar] [CrossRef]
- Nabi, R.B.S.; Tayade, R.; Hussain, A.; Kulkarni, K.P.; Imran, Q.M.; Mun, B.-G.; Yun, B.-W. Nitric oxide regulates plant responses to drought, salinity, and heavy metal stress. Environmental and Experimental Botany 2019, 161, 120–133. [Google Scholar] [CrossRef]
- Pissolato, M.D.; Silveira, N.M.; Prataviera, P.J.C.; Machado, E.C.; Seabra, A.B.; Pelegrino, M.T.; Sodek, L.; Ribeiro, R.V. Enhanced Nitric Oxide Synthesis Through Nitrate Supply Improves Drought Tolerance of Sugarcane Plants. Frontiers in Plant Science 2020, 11. [Google Scholar] [CrossRef]
- Procházková, D.; Sumaira, J.; Wilhelmová, N.a.; Pavlíková, D.; Száková, J. Chapter 11 - Reactive Nitrogen Species and the Role of NO in Abiotic Stress. In Emerging Technologies and Management of Crop Stress Tolerance; Ahmad, P., Rasool, S., Eds.; Academic Press: San Diego, 2014; pp. 249–266. [Google Scholar] [CrossRef]
- Simontacchi, M.; Galatro, A.; Ramos-Artuso, F.; Santa-María, G.E. Plant Survival in a Changing Environment: The Role of Nitric Oxide in Plant Responses to Abiotic Stress. Frontiers in Plant Science 2015, 6. [Google Scholar] [CrossRef]
- Lau, S.-E.; Hamdan, M.F.; Pua, T.-L.; Saidi, N.B.; Tan, B.C. Plant Nitric Oxide Signaling under Drought Stress. Plants 2021, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Jiang, M.; Zhang, J.; Ding, H.; Xu, S.; Hu, X.; Tan, M. Nitric oxide induced by hydrogen peroxide mediates abscisic acid-induced activation of the mitogen-activated protein kinase cascade involved in antioxidant defense in maize leaves. New Phytologist 2007, 175, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Wu, X.; Zhong, Y. Exogenously Applied Nitric Oxide Enhances the Drought Tolerance in Hulless Barley. Plant Production Science 2015, 18, 52–56. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Qin, C.; Begum, N.; Maodong, Q.; Dong, X.X.; El-Esawi, M.; El-Sheikh, M.A.; Alatar, A.A.; Zhang, L. Nitrogen availability prevents oxidative effects of salinity on wheat growth and photosynthesis by up-regulating the antioxidants and osmolytes metabolism, and secondary metabolite accumulation. BMC Plant Biology 2019, 19, 479. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, J.; Zhou, G.; Li, J.; Qian, C.; Lin, G.; Li, Y.; Zuo, Q. Moderate nitrogen application improved salt tolerance by enhancing photosynthesis, antioxidants, and osmotic adjustment in rapeseed (Brassica napus L.). Frontiers in Plant Science 2023, 14. [Google Scholar] [CrossRef]
- Nazir, F.; Mahajan, M.; Khatoon, S.; Albaqami, M.; Ashfaque, F.; Chhillar, H.; Chopra, P.; Khan, M.I.R. Sustaining nitrogen dynamics: A critical aspect for improving salt tolerance in plants. Frontiers in Plant Science 2023, 14. [Google Scholar] [CrossRef]
- Han, M.-L.; Lv, Q.-Y.; Zhang, J.; Wang, T.; Zhang, C.-X.; Tan, R.-J.; Wang, Y.-L.; Zhong, L.-Y.; Gao, Y.-Q.; Chao, Z.-F.; et al. Decreasing nitrogen assimilation under drought stress by suppressing DST-mediated activation of Nitrate Reductase 1.2 in rice. Molecular Plant 2022, 15, 167–178. [Google Scholar] [CrossRef]
- Hessini, K.; Issaoui, K.; Ferchichi, S.; Saif, T.; Abdelly, C.; Siddique, K.H.M.; Cruz, C. Interactive effects of salinity and nitrogen forms on plant growth, photosynthesis and osmotic adjustment in maize. Plant Physiology and Biochemistry 2019, 139, 171–178. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, Y.; Liu, X.; Korpelainen, H.; Li, C. Ammonium and nitrate affect sexually different responses to salt stress in Populus cathayana. Physiologia Plantarum 2022, 174, e13626. [Google Scholar] [CrossRef]
- Qu, C.; Liu, C.; Ze, Y.; Gong, X.; Hong, M.; Wang, L.; Hong, F. Inhibition of Nitrogen and Photosynthetic Carbon Assimilation of Maize Seedlings by Exposure to a Combination of Salt Stress and Potassium-Deficient Stress. Biological Trace Element Research 2011, 144, 1159–1174. [Google Scholar] [CrossRef]
- Akram, M. Effect of Nitrogen Nutrition on Solute Accumulation and Ion Contents of Maize under Sodium Chloride Stress. Communications in Soil Science and Plant Analysis 2014, 45, 86–100. [Google Scholar] [CrossRef]
- Zhao, F.G.; Qin, P. Protective effect of exogenous polyamines on root tonoplast function against salt stress in barley seedlings. Plant Growth Regulation 2004, 42, 97–103. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Oku, H.; Nahar, K.; Bhuyan, M.H.M.B.; Mahmud, J.A.; Baluska, F.; Fujita, M. Nitric oxide-induced salt stress tolerance in plants: ROS metabolism, signaling, and molecular interactions. Plant Biotechnology Reports 2018, 12, 77–92. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Kapoor, D.; Singh, S.; Gautam, V.; Dhanjal, D.S.; Jan, S.; Ramamurthy, P.C.; Prasad, R.; Singh, J. Nitric Oxide: A Ubiquitous Signal Molecule for Enhancing Plant Tolerance to Salinity Stress and Their Molecular Mechanisms. Journal of Plant Growth Regulation 2021, 40, 2329–2341. [Google Scholar] [CrossRef]
- Ahmad, P.; Abdel Latef, A.A.; Hashem, A.; Abd_Allah, E.F.; Gucel, S.; Tran, L.-S.P. Nitric Oxide Mitigates Salt Stress by Regulating Levels of Osmolytes and Antioxidant Enzymes in Chickpea. Frontiers in Plant Science 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Jiang, D.; Liu, F.; Dai, T.; Liu, W.; Jing, Q.; Cao, W. Exogenous nitric oxide improves seed germination in wheat against mitochondrial oxidative damage induced by high salinity. Environmental and Experimental Botany 2009, 67, 222–227. [Google Scholar] [CrossRef]
- Lopez, G.; Ahmadi, S.H.; Amelung, W.; Athmann, M.; Ewert, F.; Gaiser, T.; Gocke, M.I.; Kautz, T.; Postma, J.; Rachmilevitch, S.; et al. Nutrient deficiency effects on root architecture and root-to-shoot ratio in arable crops. Frontiers in Plant Science 2023, 13. [Google Scholar] [CrossRef]
- Falcioni, R.; Moriwaki, T.; Rodrigues, M.; de Oliveira, K.M.; Furlanetto, R.H.; dos Reis, A.S.; dos Santos, G.L.A.A.; Mendonça, W.A.; Crusiol, L.G.T.; Gonçalves, J.V.F.; et al. Nutrient deficiency lowers photochemical and carboxylation efficiency in tobacco. Theoretical and Experimental Plant Physiology 2023, 35, 81–97. [Google Scholar] [CrossRef]
- Sattelmacher, B. Addiscott, TM, AP whitmore and DS powlson: Farming, fertilizers and the nitrate problem. CAB international, 1991; 176 seiten. Paperback,£ 12.95. ISBN 0 85198 658 7. Wiley Online Library: 1993.
- Aparicio, V.; Costa, J.L.; Zamora, M. Nitrate leaching assessment in a long-term experiment under supplementary irrigation in humid Argentina. Agricultural water management 2008, 95, 1361–1372. [Google Scholar] [CrossRef]
- Mary, H. Ward. Too Much of a Good Thing? Nitrate from Nitrogen Fertilizers and Cancer. Reviews on Environmental Health 2009, 24, 357–363. [Google Scholar] [CrossRef]
- Ju, X.T.; Kou, C.L.; Zhang, F.S.; Christie, P. Nitrogen balance and groundwater nitrate contamination: Comparison among three intensive cropping systems on the North China Plain. Environmental Pollution 2006, 143, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Maghanga, J.K.; Kituyi, J.L.; Kisinyo, P.O.; Ng’etich, W.K. Impact of Nitrogen Fertilizer Applications on Surface Water Nitrate Levels within a Kenyan Tea Plantation. Journal of Chemistry 2013, 2013, 196516. [Google Scholar] [CrossRef]
- Bryan, N.S.; van Grinsven, H. Chapter Three - The Role of Nitrate in Human Health. In Advances in Agronomy, Sparks, D.L., Ed. Academic Press: 2013; Vol. 119, pp. 153–182.
- FRASER, P.; CHILVERS, C.; BERAL, V.; HILL, M.J. Nitrata and Human Cancer: A Review of the Evidence. International Journal of Epidemiology 1980, 9, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Knobeloch, L.; Salna, B.; Hogan, A.; Postle, J.; Anderson, H. Blue babies and nitrate-contaminated well water. Environmental Health Perspectives 2000, 108, 675–678. [Google Scholar] [CrossRef]
- Maghanga, J.; Kituyi, J.; Kisinyo, P.; Ng’Etich, W. Impact of nitrogen fertilizer applications on surface water nitrate levels within a Kenyan tea plantation. Journal of chemistry 2013, 2013. [Google Scholar] [CrossRef]
- Díaz-Rodríguez, A.M.; Salcedo Gastelum, L.A.; Félix Pablos, C.M.; Parra-Cota, F.I.; Santoyo, G.; Puente, M.L.; Bhattacharya, D.; Mukherjee, J.; de los Santos-Villalobos, S. The current and future role of microbial culture collections in food security worldwide. Frontiers in Sustainable Food Systems 2021, 4, 614739. [Google Scholar] [CrossRef]
- Fischer, R.A.; Connor, D.J. Issues for cropping and agricultural science in the next 20 years. Field Crops Research 2018, 222, 121–142. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, X.; Wu, K.; Fu, X. Nitrogen signaling and use efficiency in plants: what's new? Current Opinion in Plant Biology 2015, 27, 192–198. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant Nitrogen Assimilation and Use Efficiency. Annual Review of Plant Biology 2012, 63, 153–182. [Google Scholar] [CrossRef]
- Motavalli, P.P.; Goyne, K.W.; Udawatta, R.P. Environmental Impacts of Enhanced-Efficiency Nitrogen Fertilizers. Crop Management 2008, 7, 1–15. [Google Scholar] [CrossRef]
- Woods, J.; Williams, A.; Hughes, J.K.; Black, M.; Murphy, R. Energy and the food system. Philosophical Transactions of the Royal Society B: Biological Sciences 2010, 365, 2991–3006. [Google Scholar] [CrossRef] [PubMed]
- Ntatsi, G.; Karkanis, A.; Yfantopoulos, D.; Pappa, V.; Konosonoka, I.H.; Travlos, I.; Bilalis, D.; Bebeli, P.; Savvas, D. Evaluation of the field performance, nitrogen fixation efficiency and competitive ability of pea landraces grown under organic and conventional farming systems. Archives of Agronomy and Soil Science 2019, 65, 294–307. [Google Scholar] [CrossRef]
- Van Deynze, A.; Zamora, P.; Delaux, P.-M.; Heitmann, C.; Jayaraman, D.; Rajasekar, S.; Graham, D.; Maeda, J.; Gibson, D.; Schwartz, K.D.; et al. Nitrogen fixation in a landrace of maize is supported by a mucilage-associated diazotrophic microbiota. PLOS Biology 2018, 16, e2006352. [Google Scholar] [CrossRef]
- Santi, C.; Bogusz, D.; Franche, C. Biological nitrogen fixation in non-legume plants. Annals of Botany 2013, 111, 743–767. [Google Scholar] [CrossRef]
- Mus, F.; Crook, M.B.; Garcia, K.; Costas, A.G.; Geddes, B.A.; Kouri, E.D.; Paramasivan, P.; Ryu, M.-H.; Oldroyd, G.E.D.; Poole, P.S.; et al. Symbiotic Nitrogen Fixation and the Challenges to Its Extension to Nonlegumes. Applied and Environmental Microbiology 2016, 82, 3698–3710. [Google Scholar] [CrossRef] [PubMed]
- Barnawal, D.; Maji, D.; Bharti, N.; Chanotiya, C.S.; Kalra, A. ACC Deaminase-Containing Bacillus subtilis Reduces Stress Ethylene-Induced Damage and Improves Mycorrhizal Colonization and Rhizobial Nodulation in Trigonella foenum-graecum Under Drought Stress. Journal of Plant Growth Regulation 2013, 32, 809–822. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Yadav, A.N.; Yadav, N.; Kumar, M.; Kumar, V.; Vyas, P.; Dhaliwal, H.S.; Saxena, A.K. Microbial biofertilizers: Bioresources and eco-friendly technologies for agricultural and environmental sustainability. Biocatalysis and Agricultural Biotechnology 2020, 23, 101487. [Google Scholar] [CrossRef]
- Wen, A.; Havens, K.L.; Bloch, S.E.; Shah, N.; Higgins, D.A.; Davis-Richardson, A.G.; Sharon, J.; Rezaei, F.; Mohiti-Asli, M.; Johnson, A.; et al. Enabling Biological Nitrogen Fixation for Cereal Crops in Fertilized Fields. ACS Synthetic Biology 2021, 10, 3264–3277. [Google Scholar] [CrossRef]
- Compant, S.; Cambon, M.C.; Vacher, C.; Mitter, B.; Samad, A.; Sessitsch, A. The plant endosphere world – bacterial life within plants. Environmental Microbiology 2021, 23, 1812–1829. [Google Scholar] [CrossRef]
- Bacic, A.; Moody, S.F.; Clarke, A.E. Structural Analysis of Secreted Root Slime from Maize (Zea mays L.). Plant Physiology 1986, 80, 771–777. [Google Scholar] [CrossRef]
- Galloway, A.F.; Akhtar, J.; Marcus, S.E.; Fletcher, N.; Field, K.; Knox, P. Cereal root exudates contain highly structurally complex polysaccharides with soil-binding properties. The Plant Journal 2020, 103, 1666–1678. [Google Scholar] [CrossRef] [PubMed]
- Okon, Y.; Heytler, P.G.; Hardy, R.W.F. N<sub>2</sub> Fixation by <i>Azospirillum brasilense</i> and Its Incorporation into Host <i>Setaria italica</i>. Applied and Environmental Microbiology 1983, 46, 694–697. [Google Scholar] [CrossRef]
- Pankievicz, V.C.S.; do Amaral, F.P.; Santos, K.F.D.N.; Agtuca, B.; Xu, Y.; Schueller, M.J.; Arisi, A.C.M.; Steffens, M.B.R.; de Souza, E.M.; Pedrosa, F.O.; et al. Robust biological nitrogen fixation in a model grass–bacterial association. The Plant Journal 2015, 81, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.A.; Holz, M.; Woche, S.K.; Bachmann, J.; Carminati, A. Effect of soil drying on mucilage exudation and its water repellency: a new method to collect mucilage. Journal of Plant Nutrition and Soil Science 2015, 178, 821–824. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Badri, D.V.; Bakker, M.G.; Sugiyama, A.; Manter, D.K.; Vivanco, J.M. Root exudation of phytochemicals in Arabidopsis follows specific patterns that are developmentally programmed and correlate with soil microbial functions. PloS one 2013, 8, e55731. [Google Scholar] [CrossRef]
- Rane, N.R.; Tapase, S.; Kanojia, A.; Watharkar, A.; Salama, E.-S.; Jang, M.; Kumar Yadav, K.; Amin, M.A.; Cabral-Pinto, M.M.S.; Jadhav, J.P.; et al. Molecular insights into plant–microbe interactions for sustainable remediation of contaminated environment. Bioresource Technology 2022, 344, 126246. [Google Scholar] [CrossRef]
- Dubois, O. The state of the world's land and water resources for food and agriculture: managing systems at risk; Earthscan: 2011.
- Francis, B.; Aravindakumar, C.T.; Brewer, P.B.; Simon, S. Plant nutrient stress adaptation: A prospect for fertilizer limited agriculture. Environmental and Experimental Botany 2023, 213, 105431. [Google Scholar] [CrossRef]
- Igiehon, N.O.; Babalola, O.O. Biofertilizers and sustainable agriculture: exploring arbuscular mycorrhizal fungi. Applied Microbiology and Biotechnology 2017, 101, 4871–4881. [Google Scholar] [CrossRef]
- Meena, V.S.; Meena, S.K.; Verma, J.P.; Kumar, A.; Aeron, A.; Mishra, P.K.; Bisht, J.K.; Pattanayak, A.; Naveed, M.; Dotaniya, M.L. Plant beneficial rhizospheric microorganism (PBRM) strategies to improve nutrients use efficiency: A review. Ecological Engineering 2017, 107, 8–32. [Google Scholar] [CrossRef]
- Hartemink, A.E. The definition of soil since the early 1800s. Advances in Agronomy 2016, 137, 73–126. [Google Scholar]
- Campanhola, C.; Pandey, S. Sustainable food and agriculture: An integrated approach; Academic Press: 2018.
- Igiehon, N.O.; Babalola, O.O. Biofertilizers and sustainable agriculture: exploring arbuscular mycorrhizal fungi. Applied microbiology and biotechnology 2017, 101, 4871–4881. [Google Scholar] [CrossRef] [PubMed]
- Bodelier, P.L. Toward understanding, managing, and protecting microbial ecosystems. Frontiers in microbiology 2011, 2, 80. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, A.; Bhattarai, B.; Pandey, S. Variation of soil microbial population in different soil horizons. J Microbiol Exp 2015, 2, 00044. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Zhang, P.; Trivedi, P.; Riera, N.; Wang, Y.; Liu, X.; Fan, G.; Tang, J.; Coletta-Filho, H.D. The structure and function of the global citrus rhizosphere microbiome. Nature communications 2018, 9, 4894. [Google Scholar] [CrossRef]
- Hu, J.; Wei, Z.; Friman, V.-P.; Gu, S.-h.; Wang, X.-f.; Eisenhauer, N.; Yang, T.-j.; Ma, J.; Shen, Q.-r.; Xu, Y.-c. Probiotic diversity enhances rhizosphere microbiome function and plant disease suppression. MBio 2016, 7, 10–1128. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS microbiology reviews 2013, 37, 634–663. [Google Scholar] [CrossRef]
- Mitter, E.K.; Tosi, M.; Obregón, D.; Dunfield, K.E.; Germida, J.J. Rethinking crop nutrition in times of modern microbiology: innovative biofertilizer technologies. Frontiers in Sustainable Food Systems 2021, 5, 606815. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher-Jenull, J.; Ceccherini, M.T.; Pietramellara, G.; Renella, G.; Schloter, M. Beyond microbial diversity for predicting soil functions: A mini review. Pedosphere 2020, 30, 5–17. [Google Scholar] [CrossRef]
- Johansson, J.F.; Paul, L.R.; Finlay, R.D. Microbial interactions in the mycorrhizosphere and their significance for sustainable agriculture. FEMS microbiology ecology 2004, 48, 1–13. [Google Scholar] [CrossRef]
- Saccá, M.L.; Barra Caracciolo, A.; Di Lenola, M.; Grenni, P. Ecosystem services provided by soil microorganisms. Proceedings of Soil biological communities and ecosystem resilience; pp. 9–24.
- Nannipieri, P.; Ascher, J.; Ceccherini, M.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. European journal of soil science 2003, 54, 655–670. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Cross, H.R. Land-based production of animal protein: impacts, efficiency, and sustainability. Annals of the New York Academy of Sciences 2014, 1328, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G.; Orozco-Mosqueda, M.d.C.; Govindappa, M. Mechanisms of biocontrol and plant growth-promoting activity in soil bacterial species of Bacillus and Pseudomonas: a review. Biocontrol Science and Technology 2012, 22, 855–872. [Google Scholar] [CrossRef]
- Bais, H.P.; Park, S.-W.; Weir, T.L.; Callaway, R.M.; Vivanco, J.M. How plants communicate using the underground information superhighway. Trends in plant science 2004, 9, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Soil health and human nutrition. In The Soil-Human Health-Nexus, CRC Press: 2020; pp. 315–325.
- Brunelle, T.; Dumas, P.; Souty, F.; Dorin, B.; Nadaud, F. Evaluating the impact of rising fertilizer prices on crop yields. Agricultural economics 2015, 46, 653–666. [Google Scholar] [CrossRef]
- Samberg, L.H.; Gerber, J.S.; Ramankutty, N.; Herrero, M.; West, P.C. Subnational distribution of average farm size and smallholder contributions to global food production. Environmental Research Letters 2016, 11, 124010. [Google Scholar] [CrossRef]
- Igiehon, N.O.; Babalola, O.O. Rhizosphere microbiome modulators: contributions of nitrogen fixing bacteria towards sustainable agriculture. International journal of environmental research and public health 2018, 15, 574. [Google Scholar] [CrossRef]
- Masson-Boivin, C.; Giraud, E.; Perret, X.; Batut, J. Establishing nitrogen-fixing symbiosis with legumes: how many rhizobium recipes? Trends in microbiology 2009, 17, 458–466. [Google Scholar] [CrossRef]
- Mendoza-Suárez, M.; Andersen, S.U.; Poole, P.S.; Sánchez-Cañizares, C. Competition, nodule occupancy, and persistence of inoculant strains: key factors in the rhizobium-legume symbioses. Frontiers in plant science 2021, 12, 690567. [Google Scholar] [CrossRef]
- Holland, P.L. Introduction: Reactivity of Nitrogen from the Ground to the Atmosphere. Chemical reviews 2020, 120, 4919–4920. [Google Scholar] [CrossRef]
- Nyaga, J.W.; Njeru, E.M. Potential of native rhizobia to improve cowpea growth and production in semiarid regions of Kenya. Frontiers in Agronomy 2020, 2, 606293. [Google Scholar] [CrossRef]
- Bogino, P.C.; Oliva, M.D.l.M.; Sorroche, F.G.; Giordano, W. The Role of Bacterial Biofilms and Surface Components in Plant-Bacterial Associations. International Journal of Molecular Sciences 2013, 14, 15838–15859. [Google Scholar] [CrossRef]
- Cesco, S.; Mimmo, T.; Tonon, G.; Tomasi, N.; Pinton, R.; Terzano, R.; Neumann, G.; Weisskopf, L.; Renella, G.; Landi, L.; et al. Plant-borne flavonoids released into the rhizosphere: impact on soil bio-activities related to plant nutrition. A review. Biology and Fertility of Soils 2012, 48, 123–149. [Google Scholar] [CrossRef]
- Yamada, Y.; Hoshino, K.-i.; Ishikawa, T. The phylogeny of acetic acid bacteria based on the partial sequences of 16S ribosomal RNA: the elevation of the subgenus Gluconoacetobacter to the generic level. Bioscience, biotechnology, and biochemistry 1997, 61, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Tajima, H.; Cline, L.C.; Fong, R.Y.; Ottaviani, J.I.; Shapiro, H.Y.; Blumwald, E. Genetic modification of flavone biosynthesis in rice enhances biofilm formation of soil diazotrophic bacteria and biological nitrogen fixation. Plant Biotechnology Journal 2022, 20, 2135–2148. [Google Scholar] [CrossRef] [PubMed]
- Cesco, S.; Mimmo, T.; Tonon, G.; Tomasi, N.; Pinton, R.; Terzano, R.; Neumann, G.; Weisskopf, L.; Renella, G.; Landi, L. Plant-borne flavonoids released into the rhizosphere: impact on soil bio-activities related to plant nutrition. A review. Biology and Fertility of Soils 2012, 48, 123–149. [Google Scholar] [CrossRef]
- Zhang, J.; Subramanian, S.; Stacey, G.; Yu, O. Flavones and flavonols play distinct critical roles during nodulation of Medicago truncatula by Sinorhizobium meliloti. The Plant Journal 2009, 57, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xu, A.; Elmerich, C.; Ma, L.Z. Biofilm formation enables free-living nitrogen-fixing rhizobacteria to fix nitrogen under aerobic conditions. The ISME journal 2017, 11, 1602–1613. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: an emergent form of bacterial life. Nature Reviews Microbiology 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Young, J.M.; Leschine, S.B.; Reguera, G. Reversible control of biofilm formation by Cellulomonas spp. in response to nitrogen availability. Environmental microbiology 2012, 14, 594–604. [Google Scholar] [CrossRef]
- Pankievicz, V.; Irving, T.B.; Maia, L.G.; Ané, J.-M. Are we there yet? The long walk towards the development of efficient symbiotic associations between nitrogen-fixing bacteria and non-leguminous crops. BMC biology 2019, 17, 1–17. [Google Scholar] [CrossRef]
- Ike, A.; Sriprang, R.; Ono, H.; Murooka, Y.; Yamashita, M. Bioremediation of cadmium contaminated soil using symbiosis between leguminous plant and recombinant rhizobia with the MTL4 and the PCS genes. Chemosphere 2007, 66, 1670–1676. [Google Scholar] [CrossRef] [PubMed]
- Rosenblueth, M.; Ormeño-Orrillo, E.; López-López, A.; Rogel, M.A.; Reyes-Hernández, B.J.; Martínez-Romero, J.C.; Reddy, P.M.; Martínez-Romero, E. Nitrogen fixation in cereals. Frontiers in Microbiology 2018, 9, 1794. [Google Scholar] [CrossRef] [PubMed]
- Broughton, W.J.; Zhang, F.; Perret, X.; Staehelin, C. Signals exchanged between legumes and Rhizobium: agricultural uses and perspectives. Plant and Soil 2003, 252, 129–137. [Google Scholar] [CrossRef]
- Checcucci, A.; Marchetti, M. The rhizosphere talk show: The rhizobia on stage. Frontiers in Agronomy 2020, 2, 591494. [Google Scholar] [CrossRef]
- Hesselbjerg Christensen, J. Climate phenomena and their relevance for future regional climate change Climate Change 2013: The Physical Science Basis ed TF Stocker et al. Cambridge: Cambridge University Press: 2013.
- Christiansen, H.H.; Etzelmüller, B.; Isaksen, K.; Juliussen, H.; Farbrot, H.; Humlum, O.; Johansson, M.; Ingeman-Nielsen, T.; Kristensen, L.; Hjort, J. The thermal state of permafrost in the nordic area during the international polar year 2007–2009. Permafrost and Periglacial Processes 2010, 21, 156–181. [Google Scholar] [CrossRef]
- Romanovsky, V.E.; Drozdov, D.; Oberman, N.G.; Malkova, G.; Kholodov, A.L.; Marchenko, S.; Moskalenko, N.G.; Sergeev, D.; Ukraintseva, N.; Abramov, A. Thermal state of permafrost in Russia. Permafrost and Periglacial Processes 2010, 21, 136–155. [Google Scholar] [CrossRef]
- Grosse, G.; Goetz, S.; McGuire, A.D.; Romanovsky, V.E.; Schuur, E.A. Changing permafrost in a warming world and feedbacks to the Earth system. Environmental Research Letters 2016, 11, 040201. [Google Scholar] [CrossRef]
- Harden, J.W.; Koven, C.D.; Ping, C.L.; Hugelius, G.; David McGuire, A.; Camill, P.; Jorgenson, T.; Kuhry, P.; Michaelson, G.J.; O'Donnell, J.A. Field information links permafrost carbon to physical vulnerabilities of thawing. Geophysical Research Letters 2012, 39. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Philosophical Transactions of the Royal Society B: Biological Sciences 2013, 368, 20130122. [Google Scholar] [CrossRef]
- Kuypers, M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nature Reviews Microbiology 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Weidner, S.; Pühler, A.; Küster, H. Genomics insights into symbiotic nitrogen fixation. Current opinion in biotechnology 2003, 14, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.; Kennedy, C.; Kavanagh, E.; Goldberg, R.B.; Hanau, R. Nitrogen fixation gene (nifL) involved in oxygen regulation of nitrogenase synthesis in K. peumoniae. Nature 1981, 290, 424–426. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.; Kahn, D. Genetic regulation of biological nitrogen fixation. Nature Reviews Microbiology 2004, 2, 621–631. [Google Scholar] [CrossRef]
- Burgess, B.K.; Lowe, D.J. Mechanism of molybdenum nitrogenase. Chemical reviews 1996, 96, 2983–3012. [Google Scholar] [CrossRef]
- Bjornsson, R.; Delgado-Jaime, M.U.; Lima, F.A.; Sippel, D.; Schlesier, J.; Weyhermüller, T.; Einsle, O.; Neese, F.; DeBeer, S. Molybdenum L-Edge XAS Spectra of MoFe Nitrogenase. Zeitschrift für anorganische und allgemeine Chemie 2015, 641, 65–71. [Google Scholar] [CrossRef]
- Setubal, J.C.; Dos Santos, P.; Goldman, B.S.; Ertesvåg, H.; Espin, G.; Rubio, L.M.; Valla, S.; Almeida, N.F.; Balasubramanian, D.; Cromes, L. Genome sequence of Azotobacter vinelandii, an obligate aerobe specialized to support diverse anaerobic metabolic processes. Journal of bacteriology 2009, 191, 4534–4545. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Yang, J.; Dou, Y.; Chen, M.; Ping, S.; Peng, J.; Lu, W.; Zhang, W.; Yao, Z.; Li, H. Nitrogen fixation island and rhizosphere competence traits in the genome of root-associated Pseudomonas stutzeri A1501. Proceedings of the National Academy of Sciences 2008, 105, 7564–7569. [Google Scholar] [CrossRef] [PubMed]
- Lalucat, J.; Bennasar, A.; Bosch, R.; García-Valdés, E.; Palleroni, N.J. Biology of Pseudomonas stutzeri. Microbiology and molecular biology reviews 2006, 70, 510–547. [Google Scholar] [CrossRef]
- Dingler, C.; Kuhla, J.; Wassink, H.; Oelze, J. Levels and activities of nitrogenase proteins in Azotobacter vinelandii grown at different dissolved oxygen concentrations. Journal of bacteriology 1988, 170, 2148–2152. [Google Scholar] [CrossRef]
- Desnoues, N.; Lin, M.; Guo, X.; Ma, L.; Carreño-Lopez, R.; Elmerich, C. Nitrogen fixation genetics and regulation in a Pseudomonas stutzeri strain associated with rice. Microbiology 2003, 149, 2251–2262. [Google Scholar] [CrossRef]
- Oelze, J. Respiratory protection of nitrogenase in Azotobacter species: is a widely held hypothesis unequivocally supported by experimental evidence? FEMS microbiology reviews 2000, 24, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Curatti, L.; Brown, C.S.; Ludden, P.W.; Rubio, L.M. Genes required for rapid expression of nitrogenase activity in Azotobacter vinelandii. Proceedings of the National Academy of Sciences 2005, 102, 6291–6296. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.; Poole, R.; Yates, M.; Kennedy, C. Cloning and mutagenesis of genes encoding the cytochrome bd terminal oxidase complex in Azotobacter vinelandii: mutants deficient in the cytochrome d complex are unable to fix nitrogen in air. Journal of bacteriology 1990, 172, 6010–6019. [Google Scholar] [CrossRef] [PubMed]
- Dedysh, S.N.; Ricke, P.; Liesack, W. NifH and NifD phylogenies: an evolutionary basis for understanding nitrogen fixation capabilities of methanotrophic bacteria. Microbiology 2004, 150, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.; Siefert, J.L.; Staples, C.R.; Blankenship, R.E. The natural history of nitrogen fixation. Molecular biology and evolution 2004, 21, 541–554. [Google Scholar] [CrossRef]
- Henson, B.J.; Watson, L.E.; Barnum, S.R. The evolutionary history of nitrogen fixation, as assessed by nif D. Journal of molecular evolution 2004, 58, 390–399. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




