Submitted:
21 August 2023
Posted:
22 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
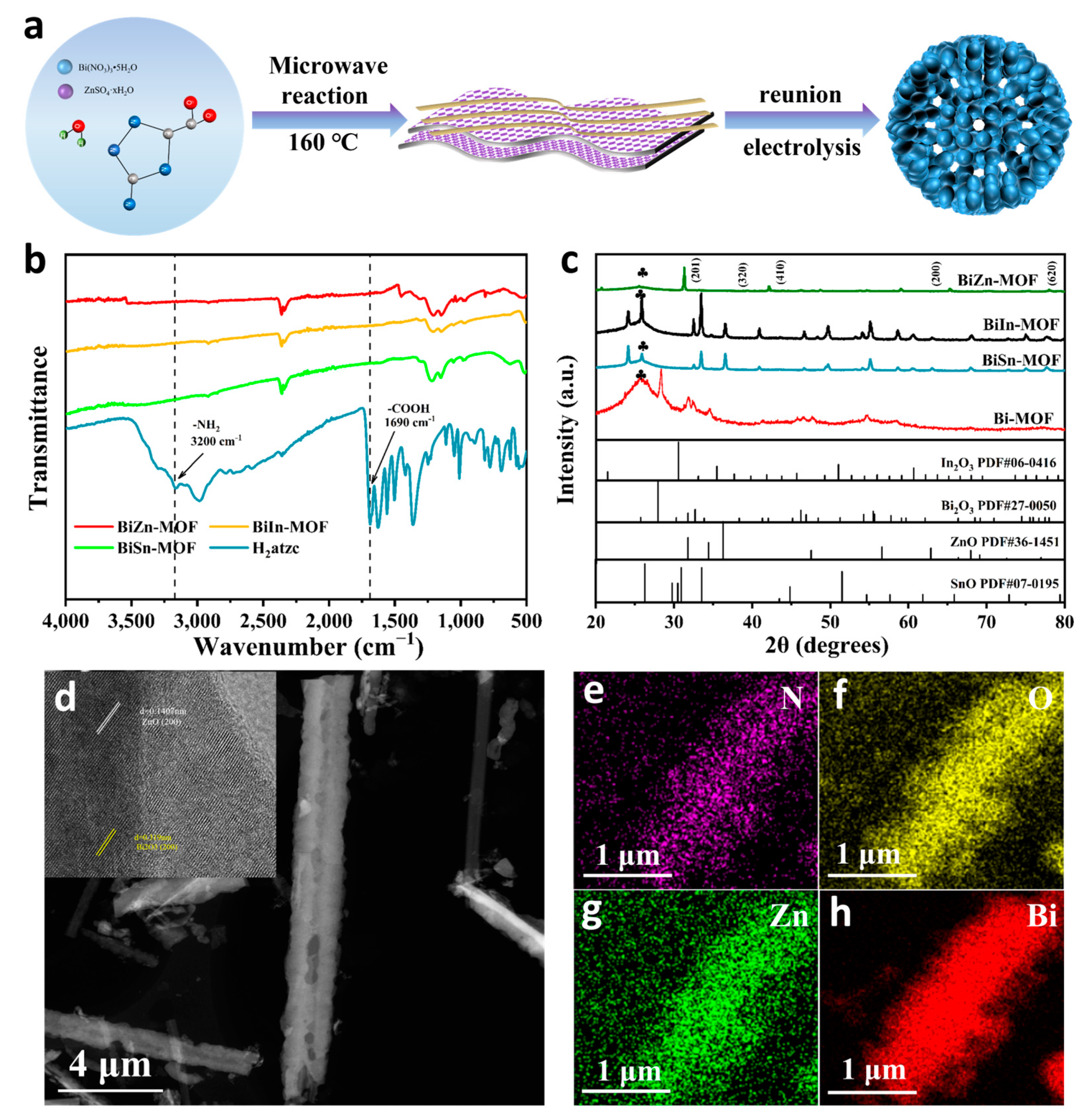
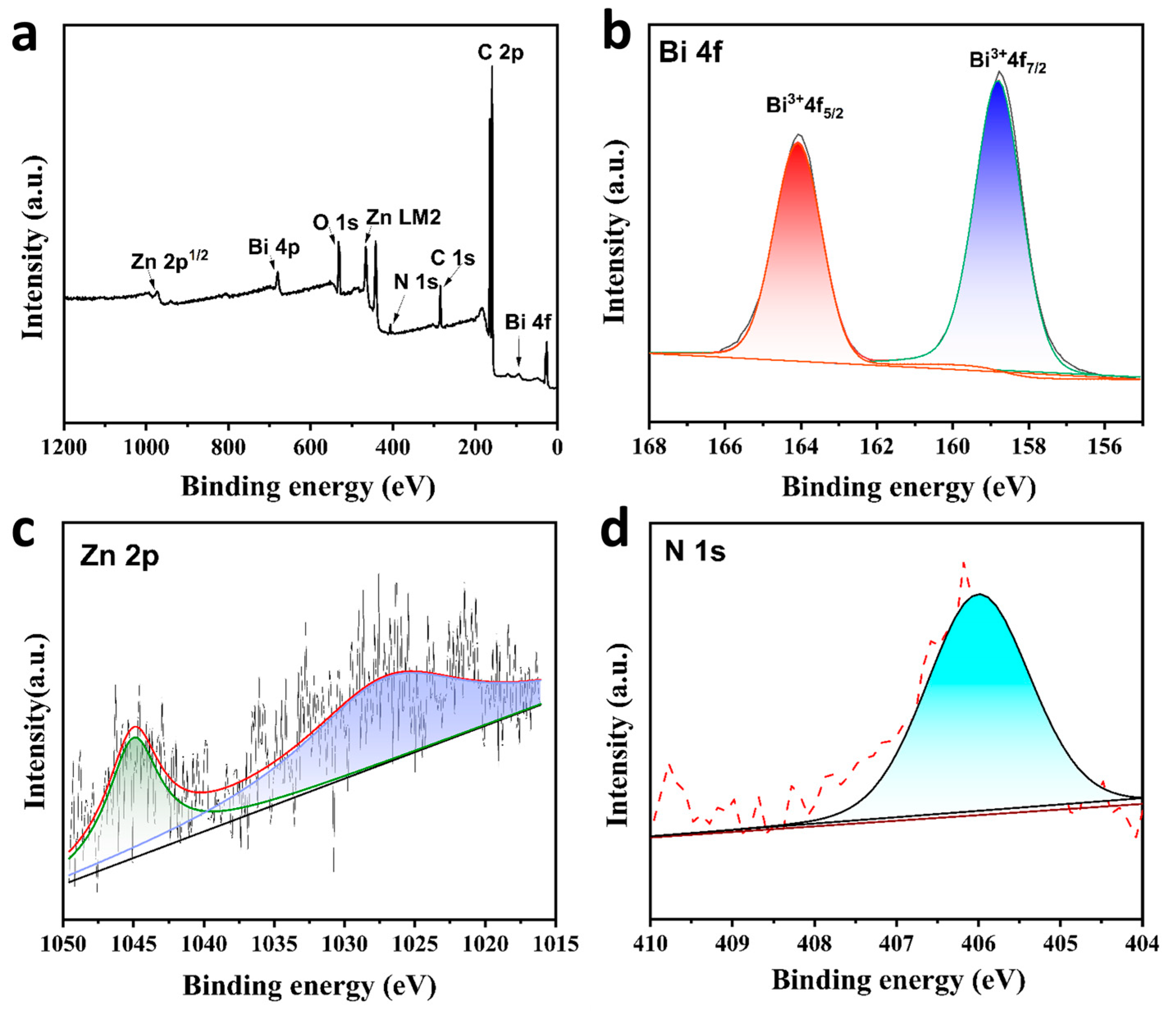
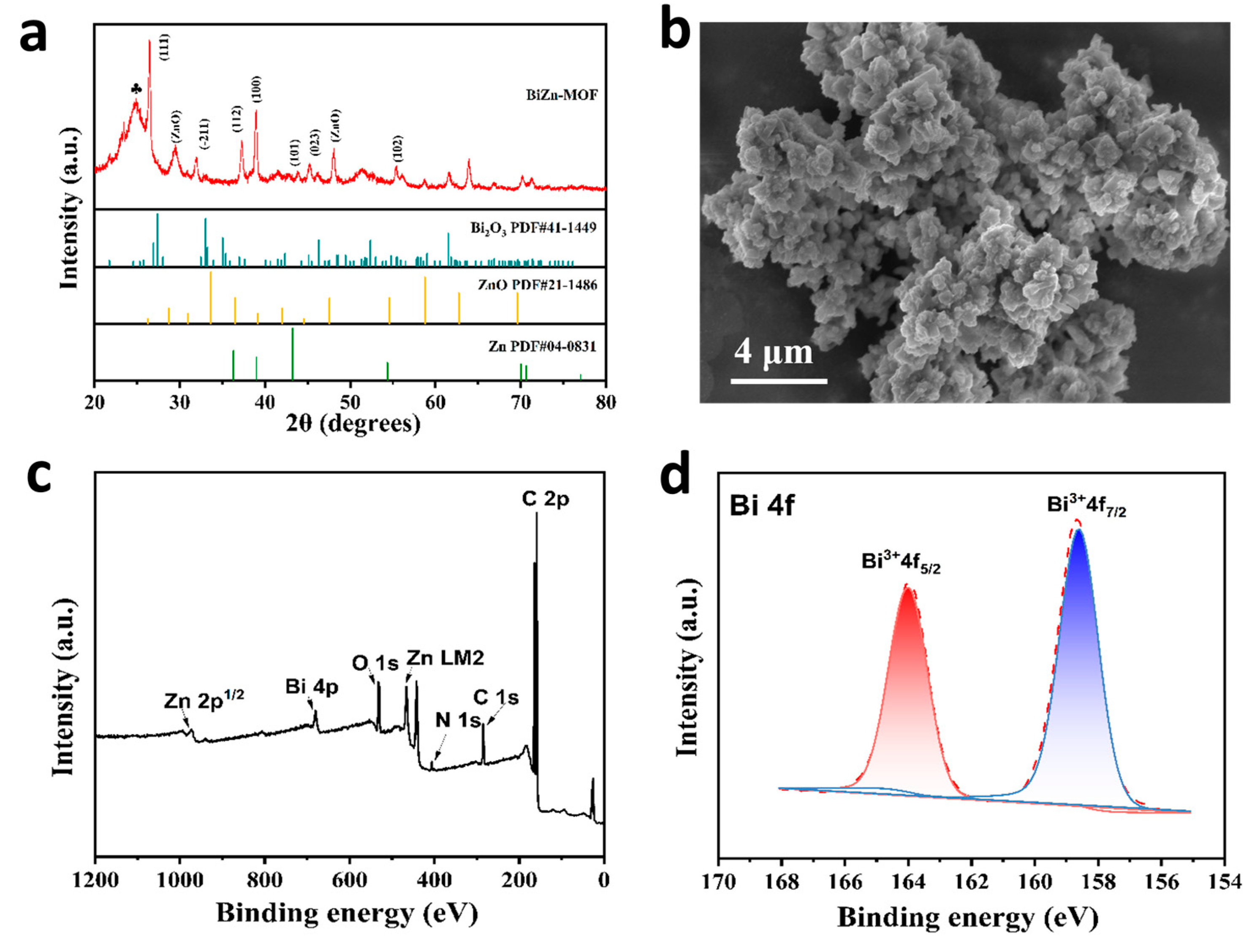
2.1. Morphology and Structure of Bimetallic MOF
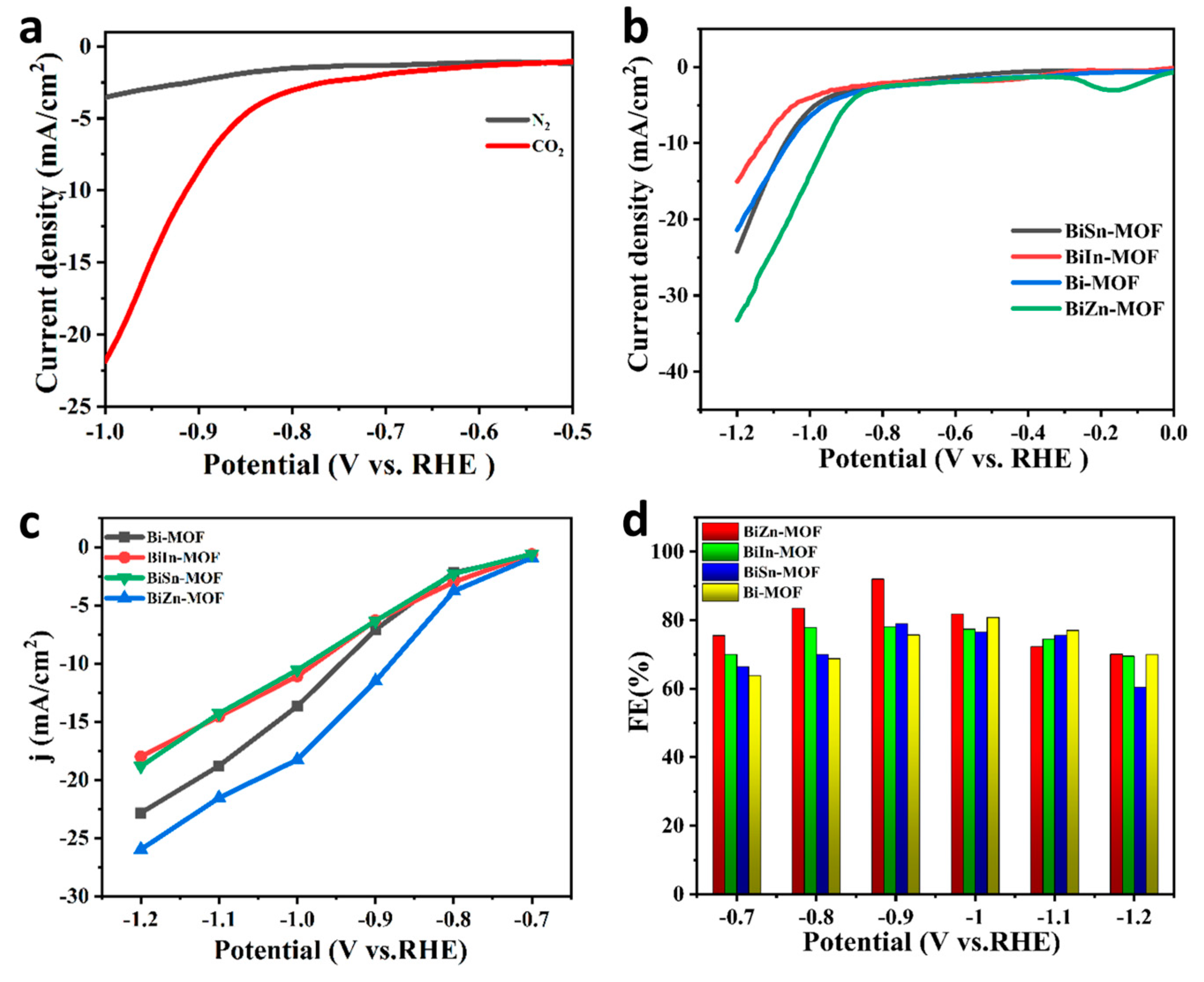
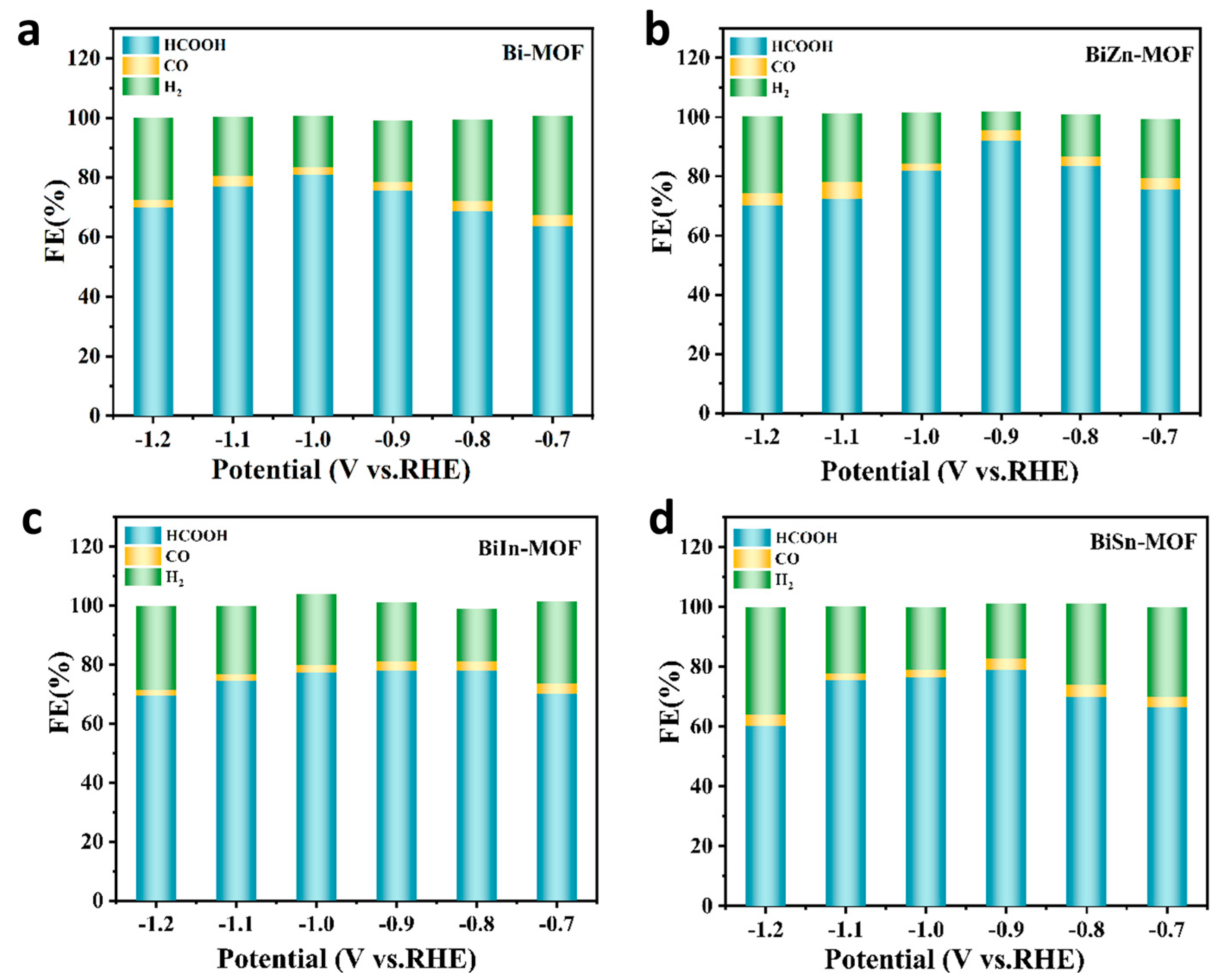
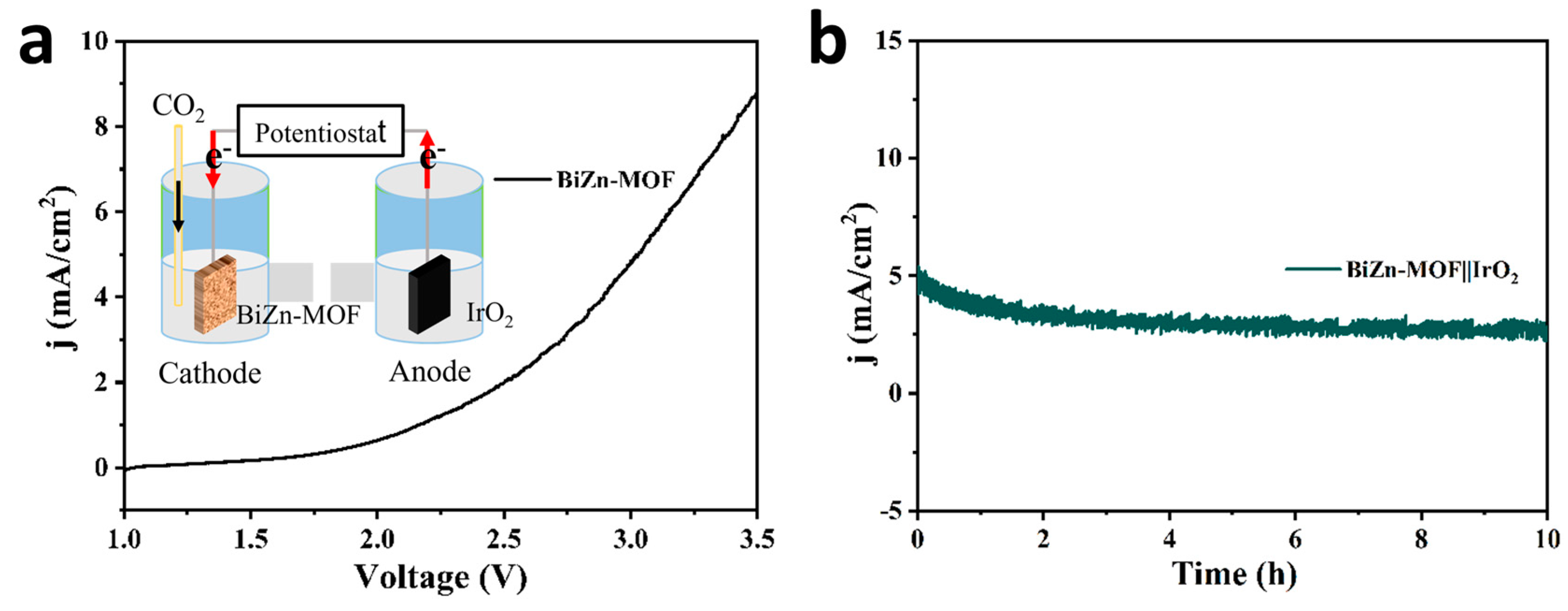
2.2. Electrocatalytic Performance of CO2RR
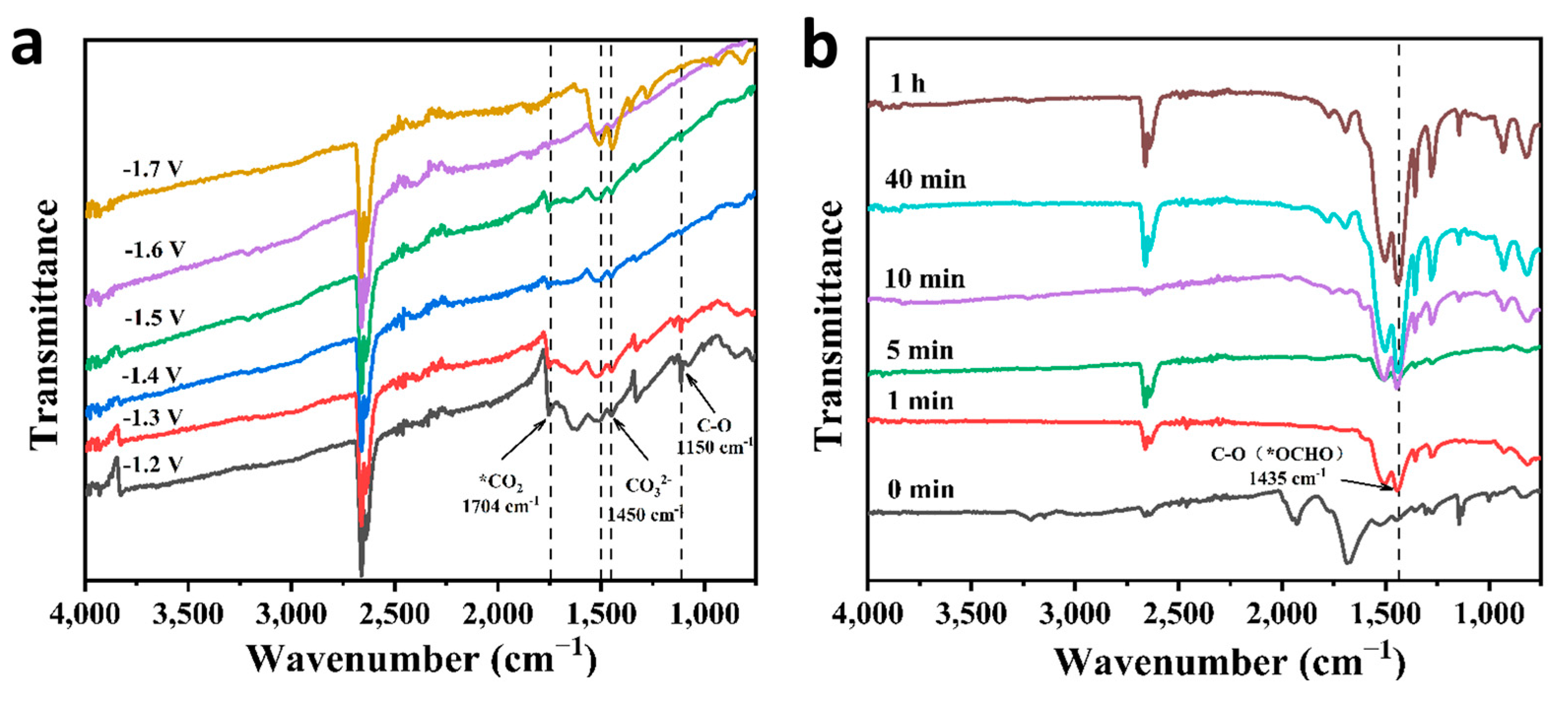
2.3. Catalytic Mechanism
3. Materials and Methods
3.1. Synthesis of Bimetallic MOF Catalysts
3.2. Electrochemical Measurements
3.3. Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernández-Torres, M.J.; Dednam, W.; Caballero, J.A. Economic and environmental assessment of directly converting CO2 into a gasoline fuel. Energy Conversion and Management. 2022, 252, 115115. [Google Scholar] [CrossRef]
- Ma, W.; He, X.; Wang, W.; Xie, S.; Zhang, Q.; Wang, Y. Electrocatalytic reduction of CO2 and CO to multi-carbon compounds over Cu-based catalysts. Chemical Society Reviews. 2021, 50, 12897–12914. [Google Scholar] [CrossRef]
- Kang, D.; Byun, J.; Han, J. Evaluating the environmental impacts of formic acid production from CO2: catalytic hydrogenation vs. electrocatalytic reduction. Green Chemistry. 2021, 23, 9470–9478. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, Q.; Wu, C. Rapid and scalable synthesis of bismuth dendrites on copper mesh as a high-performance cathode for electroreduction of CO2 to formate. Journal of CO2 Utilization. 2020, 36, 96–104. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Luo, W.; Sherrell, P.C.; Chen, J.; Yang, J. Heterogeneous single-atom catalysts for electrochemical CO2 reduction reaction. Advanced Materials. 2020, 32, 2001848. [Google Scholar] [CrossRef]
- Li, J.; Zhu, M.; Han, Y.F. Recent advances in electrochemical CO2 reduction on indium-based catalysts. ChemCatChem. 2021, 13, 514–531. [Google Scholar] [CrossRef]
- Yan, L.; Wu, Z.; Li, C.; Wang, J. Sb-doped SnS2 nanosheets enhance electrochemical reduction of carbon dioxide to formate. Journal of Industrial and Engineering Chemistry. 2023, 123, 33–40. [Google Scholar] [CrossRef]
- Ai, L.; Ng, S.-F.; Ong, W.-J. Carbon dioxide electroreduction into formic acid and ethylene: a review. Environmental Chemistry Letters. 2022, 20, 3555–3612. [Google Scholar] [CrossRef]
- Pavesi, D.; Ali, F.S.; Anastasiadou, D.; Kallio, T.; Figueiredo, M.; Gruter, G.-J. M.; Koper, M.T.; Schouten, K.J. P. CO2 electroreduction on bimetallic Pd–In nanoparticles. Catalysis Science & Technology. 2020, 10, 4264–4270. [Google Scholar]
- Yang, C.; Hu, Y.; Li, S.; Huang, Q.; Peng, J. Self-Supporting Bi–Sb Bimetallic Nanoleaf for Electrochemical Synthesis of Formate by Highly Selective CO2 Reduction. ACS Applied Materials & Interfaces. 2023, 15, 6942–6950. [Google Scholar]
- Wang, Y.; Cao, L.; Libretto, N.J.; Li, X.; Li, C.; Wan, Y.; He, C.; Lee, J.; Gregg, J.; Zong, H. Ensemble effect in bimetallic electrocatalysts for CO2 reduction. Journal of the American Chemical Society. 2019, 141, 16635–16642. [Google Scholar] [CrossRef]
- Han, N.; Ding, P.; He, L.; Li, Y.; Li, Y. Promises of main group metal–based nanostructured materials for electrochemical CO2 reduction to formate. Advanced Energy Materials. 2020, 10, 1902338. [Google Scholar] [CrossRef]
- Peng, L.; Wang, Y.; Wang, Y.; Xu, N.; Lou, W.; Liu, P.; Cai, D.; Huang, H.; Qiao, J. Separated growth of Bi-Cu bimetallic electrocatalysts on defective copper foam for highly converting CO2 to formate with alkaline anion-exchange membrane beyond KHCO3 electrolyte. Applied Catalysis B: Environmental. 2021, 288, 120003. [Google Scholar] [CrossRef]
- Wang, M.; Liu, S.; Chen, B.; Tian, F.; Peng, C. Synergistic geometric and electronic effects in Bi–Cu bimetallic catalysts for CO2 electroreduction to formate over a wide potential window. ACS Sustainable Chemistry & Engineering. 2022, 10, 5693–5701. [Google Scholar]
- Allioux, F.-M.; Merhebi, S.; Ghasemian, M.B.; Tang, J.; Merenda, A.; Abbasi, R.; Mayyas, M.; Daeneke, T.; O’Mullane, A.P.; Daiyan, R. Bi–Sn catalytic foam governed by nanometallurgy of liquid metals. Nano letters. 2020, 20, 4403–4409. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Wang, H.; Yang, X.; Huang, Y.; Kou, C.; Jing, T.; Chen, S.; Wang, Z.; Liu, Y.; Liang, H. Metal-organic framework derived dual-metal sites for electroreduction of carbon dioxide to HCOOH. Applied Catalysis B: Environmental. 2022, 311, 121377. [Google Scholar] [CrossRef]
- He, C.; Zhang, Y.; Zhang, Y.; Zhao, L.; Yuan, L.P.; Zhang, J.; Ma, J.; Hu, J.S. Molecular evidence for metallic cobalt boosting CO2 electroreduction on pyridinic nitrogen. Angewandte Chemie. 2020, 132, 4944–4949. [Google Scholar] [CrossRef]
- Wang, K.; Li, Y.; Xie, L.-H.; Li, X.; Li, J.-R. Construction and application of base-stable MOFs: a critical review. Chemical Society Reviews. 2022, 51, 6417–6441. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.; Wan, J.; Yu, C. MOF-on-MOF hybrids: Synthesis and applications. Coordination Chemistry Reviews. 2021, 432, 213743. [Google Scholar] [CrossRef]
- Ye, Z.; Jiang, Y.; Li, L.; Wu, F.; Chen, R. Rational design of MOF-based materials for next-generation rechargeable batteries. Nano-Micro Letters. 2021, 13, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Luo, J.; Wan, K.; Plessers, D.; Sels, B.; Song, J.; Chen, L.; Zhang, T.; Tang, P.; Morante, J.R. From rational design of a new bimetallic MOF family with tunable linkers to OER catalysts. Journal of Materials Chemistry A. 2019, 7, 1616–1628. [Google Scholar] [CrossRef]
- Du, C.; Wang, X.; Chen, W.; Feng, S.; Wen, J.; Wu, Y. CO2 transformation to multicarbon products by photocatalysis and electrocatalysis. Materials Today Advances. 2020, 6, 100071. [Google Scholar] [CrossRef]
- Guan, X.; Gao, W.; Jiang, Q. Design of bimetallic atomic catalysts for CO2 reduction based on an effective descriptor. Journal of Materials Chemistry A. 2021, 9, 4770–4780. [Google Scholar] [CrossRef]
- Zhong, H.; Ghorbani-Asl, M.; Ly, K.H.; Zhang, J.; Ge, J.; Wang, M.; Liao, Z.; Makarov, D.; Zschech, E.; Brunner, E. Synergistic electroreduction of carbon dioxide to carbon monoxide on bimetallic layered conjugated metal-organic frameworks. Nature communications. 2020, 11, 1409. [Google Scholar] [CrossRef] [PubMed]
- Zu, X.; Zhao, Y.; Li, X.; Chen, R.; Shao, W.; Wang, Z.; Hu, J.; Zhu, J.; Pan, Y.; Sun, Y. Ultrastable and efficient visible-light-driven CO2 reduction triggered by regenerative oxygen-vacancies in Bi2O2CO3 nanosheets. Angewandte Chemie International Edition. 2021, 60, 13840–13846. [Google Scholar] [CrossRef]
- Xing, Y.; Kong, X.; Guo, X.; Liu, Y.; Li, Q.; Zhang, Y.; Sheng, Y.; Yang, X.; Geng, Z.; Zeng, J. Bi@ Sn core–shell structure with compressive strain boosts the electroreduction of CO2 into formic acid. Advanced Science. 2020, 7, 1902989. [Google Scholar] [CrossRef]
- Yang, W.; Dastafkan, K.; Jia, C.; Zhao, C. Design of electrocatalysts and electrochemical cells for carbon dioxide reduction reactions. Advanced Materials Technologies. 2018, 3, 1700377. [Google Scholar] [CrossRef]
- Kwon, I.S.; Debela, T.T.; Kwak, I.H.; Seo, H.W.; Park, K.; Kim, D.; Yoo, S.J.; Kim, J.-G.; Park, J.; Kang, H.S. Selective electrochemical reduction of carbon dioxide to formic acid using indium–zinc bimetallic nanocrystals. Journal of Materials Chemistry A. 2019, 7, 22879–22883. [Google Scholar] [CrossRef]
- Jiang, H.; Zhao, Y.; Wang, L.; Kong, Y.; Li, F.; Li, P. Electrochemical CO2 reduction to formate on Tin cathode: Influence of anode materials. Journal of CO2 Utilization. 2018, 26, 408–414. [Google Scholar] [CrossRef]
- Hai, G.; Xue, X.; Feng, S.; Ma, Y.; Huang, X. High-Throughput Computational Screening of Metal–Organic Frameworks as High-Performance Electrocatalysts for CO2RR. ACS Catalysis. 2022, 12, 15271–15281. [Google Scholar] [CrossRef]
- Fernández-Caso, K.; Díaz-Sainz, G.; Alvarez-Guerra, M.; Irabien, A. Electroreduction of CO2: advances in the continuous production of formic acid and formate. ACS Energy Letters. 2023, 8, 1992–2024. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Q.; Wang, H.; Tan, X.; Shi, F.; Xiong, C.; Man, N.; Hu, H.; Liu, G.; Jiang, J. Bi–Zn codoping in GeTe synergistically enhances band convergence and phonon scattering for high thermoelectric performance. Journal of Materials Chemistry A. 2020, 8, 21642–21648. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




