Submitted:
19 August 2023
Posted:
23 August 2023
You are already at the latest version
Abstract
Keywords:
Introduction
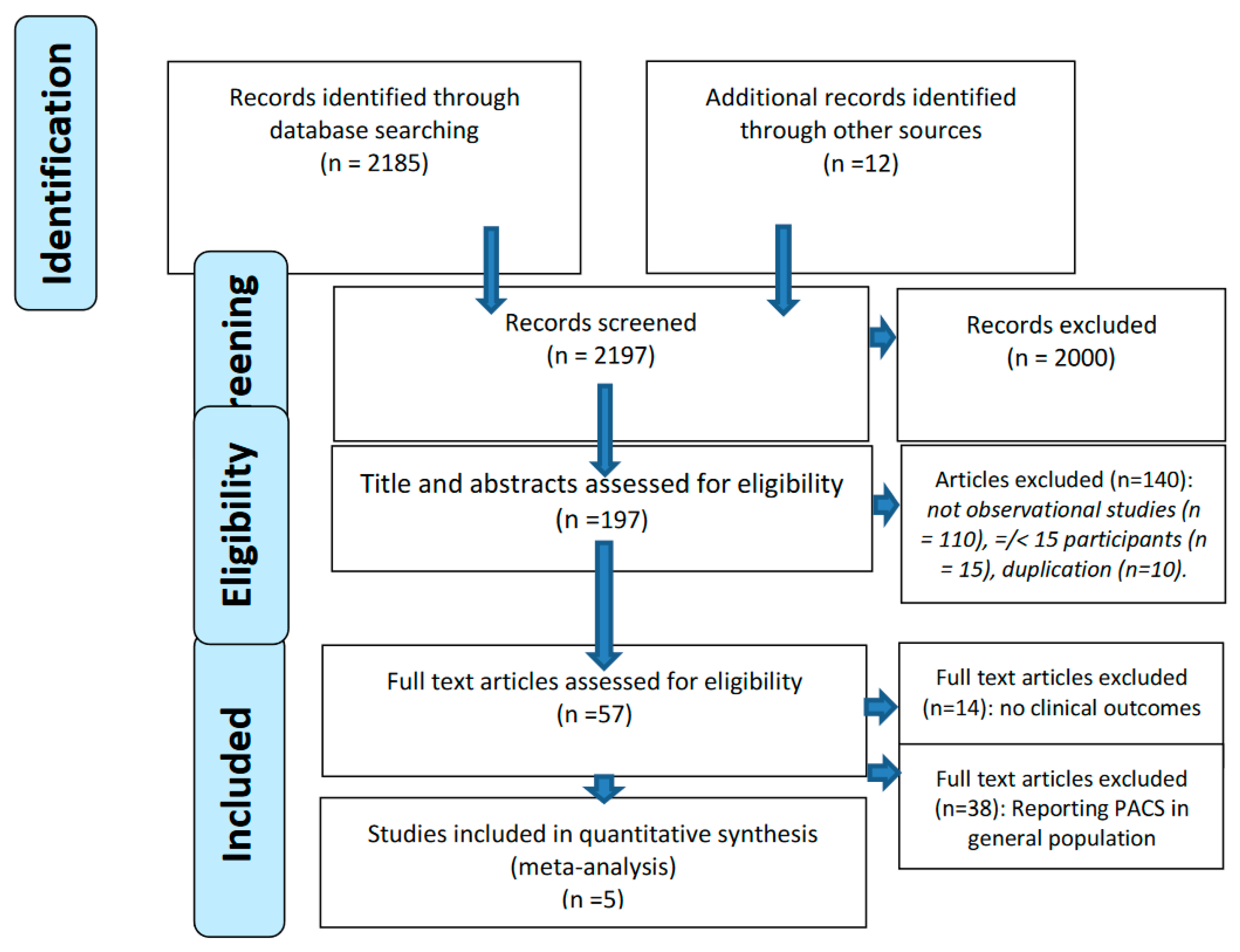
Methods
Search terms and criteria for inclusion
Data extraction
Outcome measures
Quality assessment
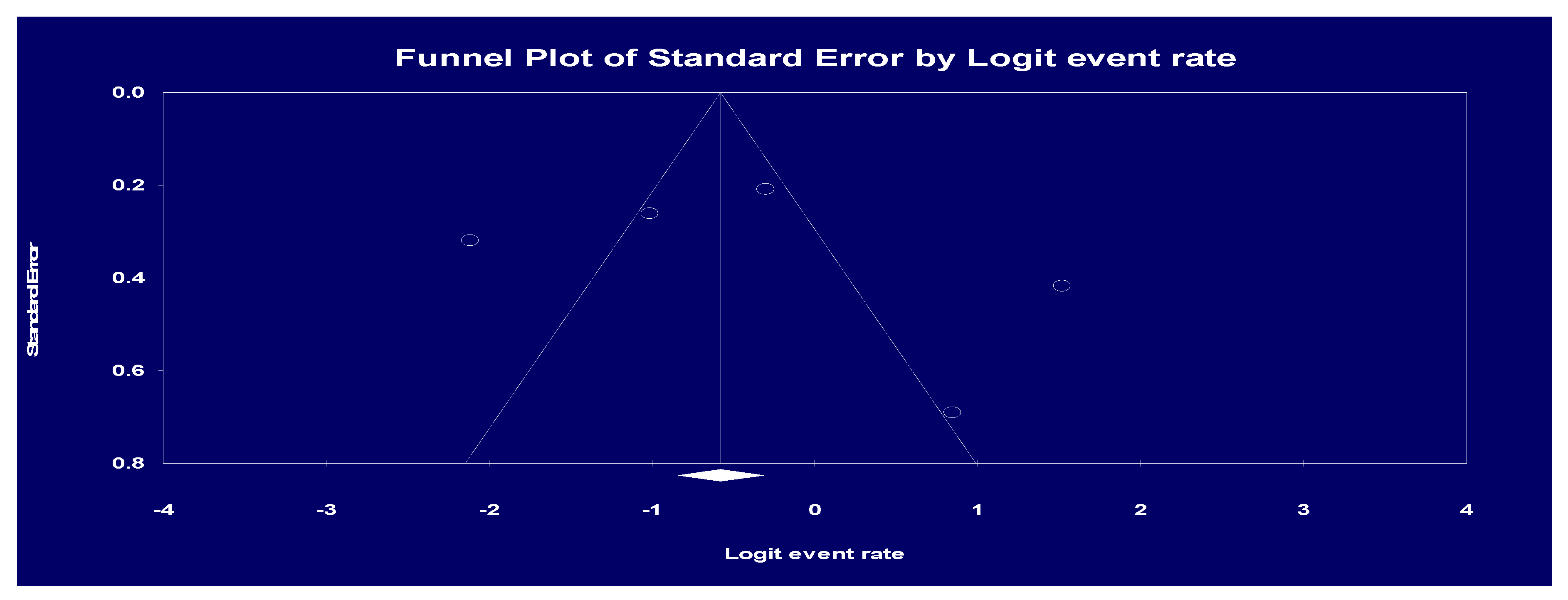
Statistical analysis
Results
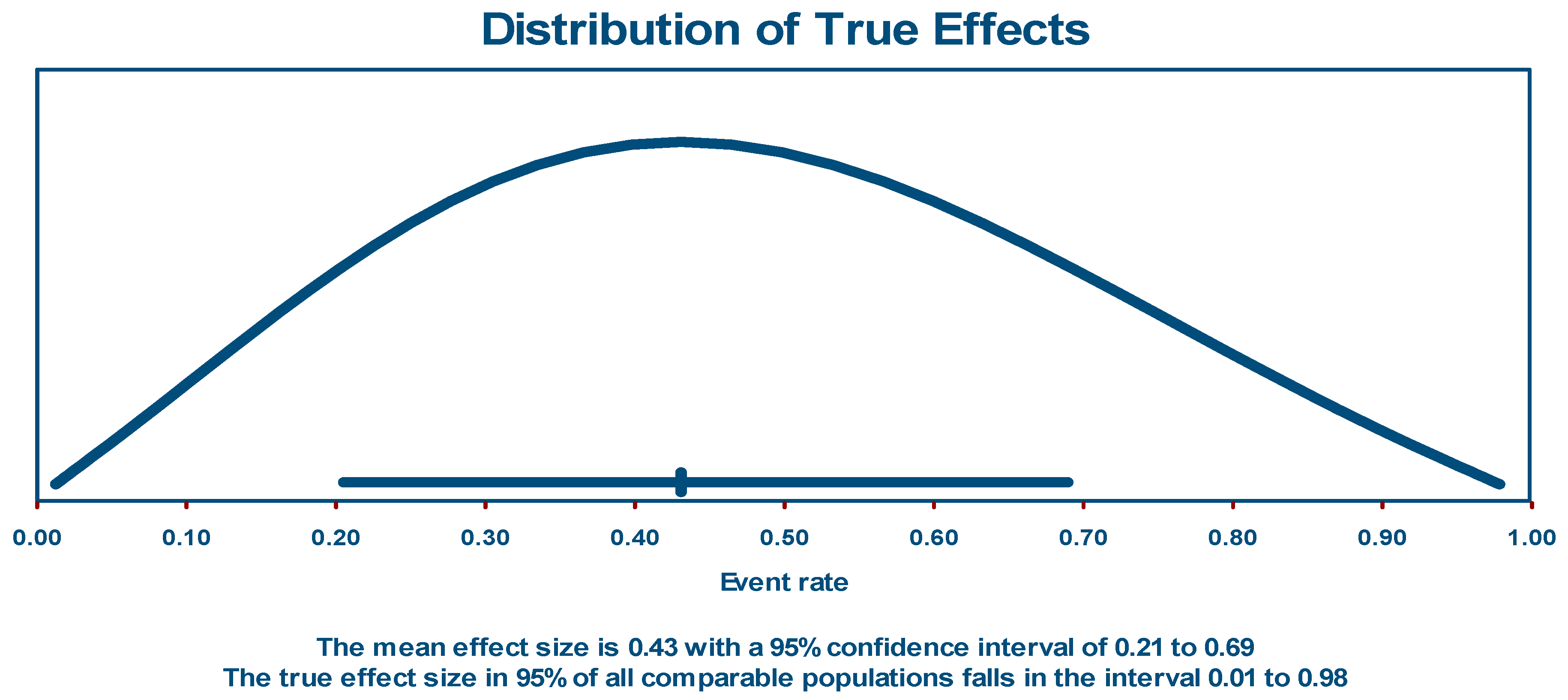
Post-acute COVID-19 sequalae event rate in PLHIV
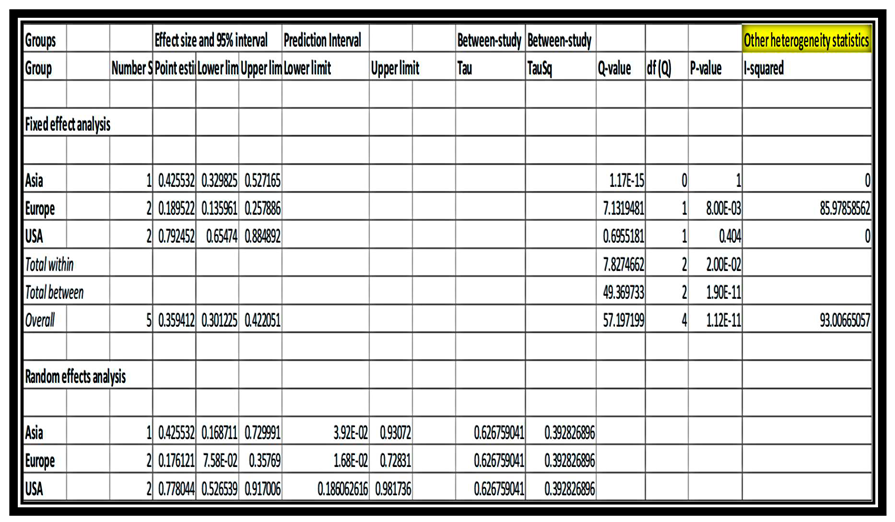
Sub-group and meta-regression analysis
Discussion
Author Contributions
Funding statement
Ethical review statement
References
- Sisó-Almirall A, Brito-Zerón P, Ferrín LC, et al. Long covid-19: Proposed primary care clinical guidelines for diagnosis and disease management. Int J Environ Res Public Health. 2021;18(8). [CrossRef]
- Amenta EM, Spallone A, Rodriguez-Barradas MC, Sahly HME, Atmar RL, Kulkarni PA. Postacute covid-19: An overview and approach to classification. Open Forum Infect Dis. 2020;7(12). [CrossRef]
- Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28(3). [CrossRef]
- Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022;10(5). [CrossRef]
- Mancini DM, Brunjes DL, Lala A, Trivieri MG, Contreras JP, Natelson BH. Use of Cardiopulmonary Stress Testing for Patients With Unexplained Dyspnea Post–Coronavirus Disease. JACC Hear Fail. 2021;9(12). [CrossRef]
- Rando HM, Bennett TD, Byrd JB, et al. Challenges in defining Long COVID: Striking differences across literature, Electronic Health Records, and patient-reported information. medRxiv Prepr Serv Heal Sci. Published online 2021. [CrossRef]
- Blomberg B, Mohn KGI, Brokstad KA, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021;27(9). [CrossRef]
- Galeotti C, Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat Rev Rheumatol. 2020;16(8). [CrossRef]
- Ramakrishnan RK, Kashour T, Hamid Q, Halwani R, Tleyjeh IM. Unraveling the Mystery Surrounding Post-Acute Sequelae of COVID-19. Front Immunol. 2021;12. [CrossRef]
- Vu T, McGill SC. An Overview of Post–COVID-19 Condition (Long COVID). Can J Heal Technol. 2021;1(9). [CrossRef]
- Posada-Vergara MP, Alzate-ángel JC, Martínez-Buitrago E. Covid-19 and hiv [Covid-19 y vih]. Colomb Med. 2020;51(2).
- Lesko CR, Bengtson AM. HIV and COVID-19: Intersecting Epidemics with Many Unknowns. Am J Epidemiol. 2021;190(1). [CrossRef]
- Massarvva, T. Clinical outcomes of COVID-19 amongst HIV patients: A systematic literature review. Epidemiol Health. 2021;43. [CrossRef]
- Pujari S, Gaikwad S, Chitalikar A, Dabhade D, Joshi K, Bele V. Long-coronavirus disease among people living with HIV in western India: An observational study. Immunity, Inflamm Dis. 2021;9(3). [CrossRef]
- Garg M, Maralakunte M, Garg S, et al. The conundrum of ‘long-covid-19ʹ: A narrative review. Int J Gen Med. 2021;14. [CrossRef]
- Tawfik GM, Dila KAS, Mohamed MYF, et al. A step by step guide for conducting a systematic review and meta-analysis with simulation data. Trop Med Health. 2019;47(1). [CrossRef]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372. [CrossRef]
- Bagias C, Sukumar N, Weldeselassie Y, Oyebode O, Saravanan P. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Int J Environ Res Public Health. 2021;18(4).
- Schreck N, Piepho HP, Schlather M. Best prediction of the additive genomic variance in random-effects models. Genetics. 2019;213(2). [CrossRef]
- Suurmond R, van Rhee H, Hak T. Introduction, comparison, and validation of Meta-Essentials: A free and simple tool for meta-analysis. Res Synth Methods. 2017;8(4). [CrossRef]
- Cochrane. Review Manager (RevMan) [Computer program]. Version 5.4. Copenhagen: The Nordic Cochrane Centre. Cochrane Collab. Published online 2020.
- Peluso MJ, Spinelli MA, Deveau TM, et al. Postacute sequelae and adaptive immune responses in people with HIV recovering from SARS-COV-2 infection. AIDS. 2022;36(12). [CrossRef]
- Mazzitelli M, Trunfio M, Sasset L, et al. Factors associated with post-acute COVID-19 syndrome in a cohort of PLWH. HIV Med. 2021;22(SUPPL 3).
- Kingery JR, Safford MM, Martin P, et al. Health Status, Persistent Symptoms, and Effort Intolerance One Year After Acute COVID-19 Infection. J Gen Intern Med. 2022;37(5). [CrossRef]
- Mazzitelli M, Trunfio M, Sasset L, et al. Factors Associated with Severe COVID-19 and Post-Acute COVID-19 Syndrome in a Cohort of People Living with HIV on Antiretroviral Treatment and with Undetectable HIV RNA. Viruses. 2022;14(3). [CrossRef]
- Goel N, Goyal N, Kumar R. Clinico-radiological evaluation of post COVID-19 at a tertiary pulmonary care centre in Delhi, India. Monaldi Arch chest Dis = Arch Monaldi per le Mal del torace. 2021;91(3). [CrossRef]
- Mahmud R, Rahman MM, Rassel MA, et al. Post-COVID-19 syndrome among symptomatic COVID-19 patients: A prospective cohort study in a tertiary care center of Bangladesh. PLoS One. 2021;16(4 April). [CrossRef]
- Khodeir MM, Shabana HA, Rasheed Z, et al. COVID-19: Post-recovery long-term symptoms among patients in Saudi Arabia. PLoS One. 2021;16(12 December). [CrossRef]
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223). [CrossRef]
- Kayaaslan B, Eser F, Kalem AK, et al. Post-COVID syndrome: A single-center questionnaire study on 1007 participants recovered from COVID-19. J Med Virol. 2021;93(12). [CrossRef]
- Maestre-Muñiz MM, Arias Á, Mata-Vázquez E, et al. Long-term outcomes of patients with coronavirus disease 2019 at one year after hospital discharge. J Clin Med. 2021;10(13). [CrossRef]
- Moreno-Pérez O, Merino E, Leon-Ramirez JM, et al. Post-acute COVID-19 syndrome. Incidence and risk factors: A Mediterranean cohort study. J Infect. 2021;82(3). [CrossRef]
- Venturelli S, Benatti S V., Casati M, et al. Surviving COVID-19 in Bergamo Province: A post-Acute outpatient re-evaluation. Epidemiol Infect. Published online 2021. [CrossRef]
- Hirschtick JL, Titus AR, Slocum E, et al. Population-Based Estimates of Post-acute Sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection (PASC) Prevalence and Characteristics. Clin Infect Dis. 2021;73(11). [CrossRef]
- Boscolo-Rizzo P, Guida F, Polesel J, et al. Sequelae in adults at 12 months after mild-to-moderate coronavirus disease 2019 (COVID-19). Int Forum Allergy Rhinol. 2021;11(12). [CrossRef]
- Petersen MS, Kristiansen MF, Hanusson KD, et al. Long COVID in the Faroe Islands: A Longitudinal Study among Nonhospitalized Patients. Clin Infect Dis. 2021;73(11). [CrossRef]
- Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in Adults at 6 Months after COVID-19 Infection. JAMA Netw Open. 2021;4(2). [CrossRef]
- Elkan M, Dvir A, Zaidenstein R, et al. Patient-reported outcome measures after hospitalization during the covid-19 pandemic: A survey among covid-19 and non-covid-19 patients. Int J Gen Med. 2021;14. [CrossRef]
- Maamar M, Artime A, Pariente E, et al. Post-COVID-19 syndrome, low-grade inflammation and inflammatory markers: a cross-sectional study. Curr Med Res Opin. 2022;38(6). [CrossRef]
- Tleyjeh IM, Saddik B, AlSwaidan N, et al. Prevalence and predictors of Post-Acute COVID-19 Syndrome (PACS) after hospital discharge: A cohort study with 4 months median follow-up. PLoS One. 2021;16(12 December). [CrossRef]
- Wanga V, Chevinsky JR, Dimitrov L V., et al. Long-Term Symptoms Among Adults Tested for SARS-CoV-2 — United States, January 2020-April 2021. MMWR Recomm Reports. 2021;70(36). [CrossRef]
- Ogoina D, James HI, Ogoinja SZ. Post-discharge symptoms among hospitalized covid-19 patients in Nigeria: A single-center study. Am J Trop Med Hyg. 2021;105(3). [CrossRef]
- Lucas C, Wong P, Klein J, et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584(7821). [CrossRef]
- Dryden MTG, Mudara C, Vika C, et al. Post COVID-19 Condition in South Africa: 3-Month Follow-Up after Hospitalisation with SARS-CoV-2. SSRN Electron J. Published online 2022. [CrossRef]
- Bell ML, Catalfamo CJ, Farland L V., et al. Post-acute sequelae of COVID-19 in a non-hospitalized cohort: Results from the Arizona CoVHORT. PLoS One. 2021;16(8 August). [CrossRef]
- Becker C, Beck K, Zumbrunn S, et al. Long COVID 1 year after hospitalisation for COVID-19: a prospective bicentric cohort study. Swiss Med Wkly. 2021;151(41-42). [CrossRef]
- Wang G, Cao K, Liu K, et al. Kynurenic acid, an IDO metabolite, controls TSG-6-mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ. 2018;25(7). [CrossRef]
- Günster C, Busse R, Spoden M, et al. 6-month mortality and readmissions of hospitalized COVID-19 patients: A nationwide cohort study of 8,679 patients in Germany. PLoS One. 2021;16(8 August). [CrossRef]
- Arnold DT, Hamilton FW, Milne A, et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: Results from a prospective UK cohort. Thorax. 2021;76(4). [CrossRef]
- Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270). [CrossRef]
- Ayoubkhani D, Khunti K, Nafilyan V, et al. Post-covid syndrome in individuals admitted to hospital with covid-19: Retrospective cohort study. BMJ. 2021;372. [CrossRef]
- Osikomaiya B, Erinoso O, Wright KO, et al. ‘Long COVID’: persistent COVID-19 symptoms in survivors managed in Lagos State, Nigeria. BMC Infect Dis. 2021;21(1). [CrossRef]
- Somani SS, Richter F, Fuster V, et al. Characterization of Patients Who Return to Hospital Following Discharge from Hospitalization for COVID-19. J Gen Intern Med. 2020;35(10). [CrossRef]
- Sigfrid L, Drake TM, Pauley E, et al. Long Covid in adults discharged from UK hospitals after Covid-19: A prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg Heal - Eur. 2021;8. [CrossRef]
- Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021;18(9). [CrossRef]
- Wynberg E, van Willigen HDG, Dijkstra M, et al. Evolution of Coronavirus Disease 2019 (COVID-19) Symptoms During the First 12 Months After Illness Onset. Clin Infect Dis. 2022;75(1). [CrossRef]
- Osmanov IM, Spiridonova E, Bobkova P, et al. Risk factors for long covid in previously hospitalised children using the ISARIC Global follow-up protocol: A prospective cohort study. Eur Respir J. 2022;59(2). [CrossRef]
- Pereira C, Harris BHL, Di Giovannantonio M, et al. The Association Between Antibody Response to Severe Acute Respiratory Syndrome Coronavirus 2 Infection and Post-COVID-19 Syndrome in Healthcare Workers. J Infect Dis. 2021;223(10). [CrossRef]
- Fernández-de-las-Peñas C, Guijarro C, Plaza-Canteli S, Hernández-Barrera V, Torres-Macho J. Prevalence of Post-COVID-19 Cough One Year After SARS-CoV-2 Infection: A Multicenter Study. Lung. 2021;199(3). [CrossRef]
- Abdelrahman MM, Abd-Elrahman NM, Bakheet TM. Persistence of symptoms after improvement of acute COVID19 infection, a longitudinal study. J Med Virol. 2021;93(10). [CrossRef]
- Perlis RH, Green J, Santillana M, et al. Persistence of symptoms up to 10 months following acute COVID-19 illness. medRxiv Prepr Serv Heal Sci. Published online 2021. [CrossRef]
- Leijte WT, Wagemaker NMM, van Kraaij TDA, et al. [Mortality and re-admission after hospitalization with COVID-19]. Ned Tijdschr Geneeskd. 2020;164.
- Pinato DJ, Tabernero J, Bower M, et al. Prevalence and impact of COVID-19 sequelae on treatment and survival of patients with cancer who recovered from SARS-CoV-2 infection: evidence from the OnCovid retrospective, multicentre registry study. Lancet Oncol. 2021;22(12). [CrossRef]
- P. S, Madhavan S, Pandurangan V. Prevalence, Pattern and Functional Outcome of Post COVID-19 Syndrome in Older Adults. Cureus. Published online 2021. [CrossRef]
- Guarin G, Lo KB, Bhargav R, et al. Factors associated with hospital readmissions among patients with COVID-19: A single-center experience. J Med Virol. 2021;93(9). [CrossRef]
- Zayet S, Zahra H, Royer PY, et al. Post-COVID-19 syndrome: Nine months after SARS-CoV-2 infection in a cohort of 354 patients: Data from the first wave of COVID-19 in nord franche-comté hospital, France. Microorganisms. 2021;9(8). [CrossRef]
- Myall KJ, Mukherjee B, Castanheira AM, et al. Persistent post–COVID-19 interstitial lung disease: An observational study of corticosteroid treatment. Ann Am Thorac Soc. 2021;18(5). [CrossRef]
- Naik S, Haldar SN, Soneja M, et al. Post COVID-19 sequelae: A prospective observational study from Northern India. Drug Discov Ther. 2021;15(5). [CrossRef]
- Peghin M, Palese A, Venturini M, et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin Microbiol Infect. 2021;27(10). [CrossRef]
- Menges D, Ballouz T, Anagnostopoulos A, et al. Burden of post-COVID-19 syndrome and implications for healthcare service planning: A population-based cohort study. PLoS One. 2021;16(7 July). [CrossRef]
- Peluso MJ, Hellmuth J, Chow FC. Central Nervous System Effects of COVID-19 in People with HIV Infection. Curr HIV/AIDS Rep. 2021;18(6). [CrossRef]
- Robineau O, Wiernik E, Lemogne C, et al. Persistent symptoms after the first wave of COVID-19 in relation to SARS-CoV-2 serology and experience of acute symptoms: A nested survey in a population-based cohort. Lancet Reg Heal - Eur. 2022;17. [CrossRef]
- Yendewa G, Perez JA, Patil N, McComsey GA. HIV Infection is Associated with Higher Risk of Post-Acute Sequelae of SARS-CoV-2 (PASC) However Vaccination is Protective. SSRN Electron J. Published online 2022. [CrossRef]
- Peluso MJ, Deveau TM, Munter SE, et al. Chronic viral coinfections differentially affect the likelihood of developing long COVID. J Clin Invest. 2023;133(3). [CrossRef]
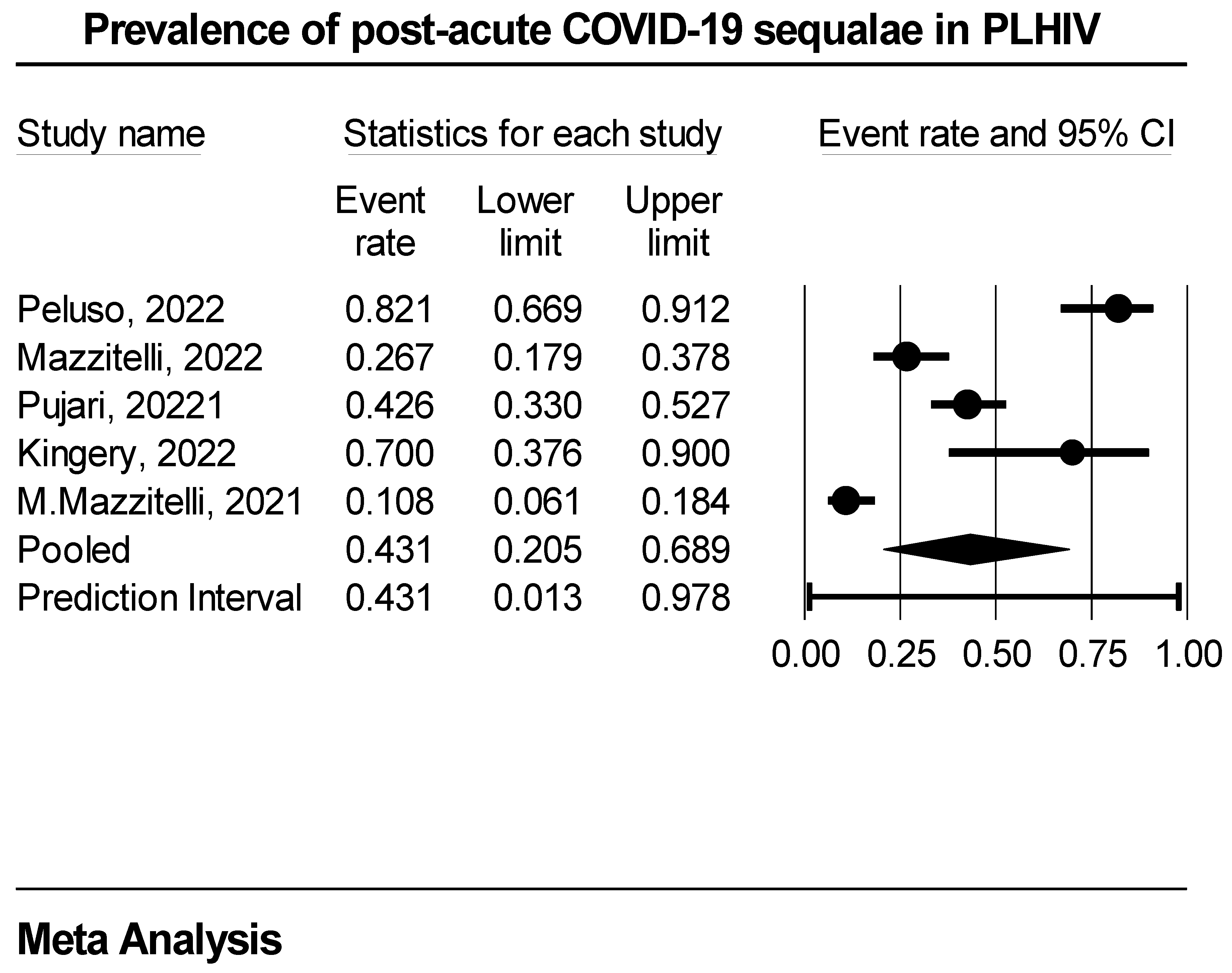
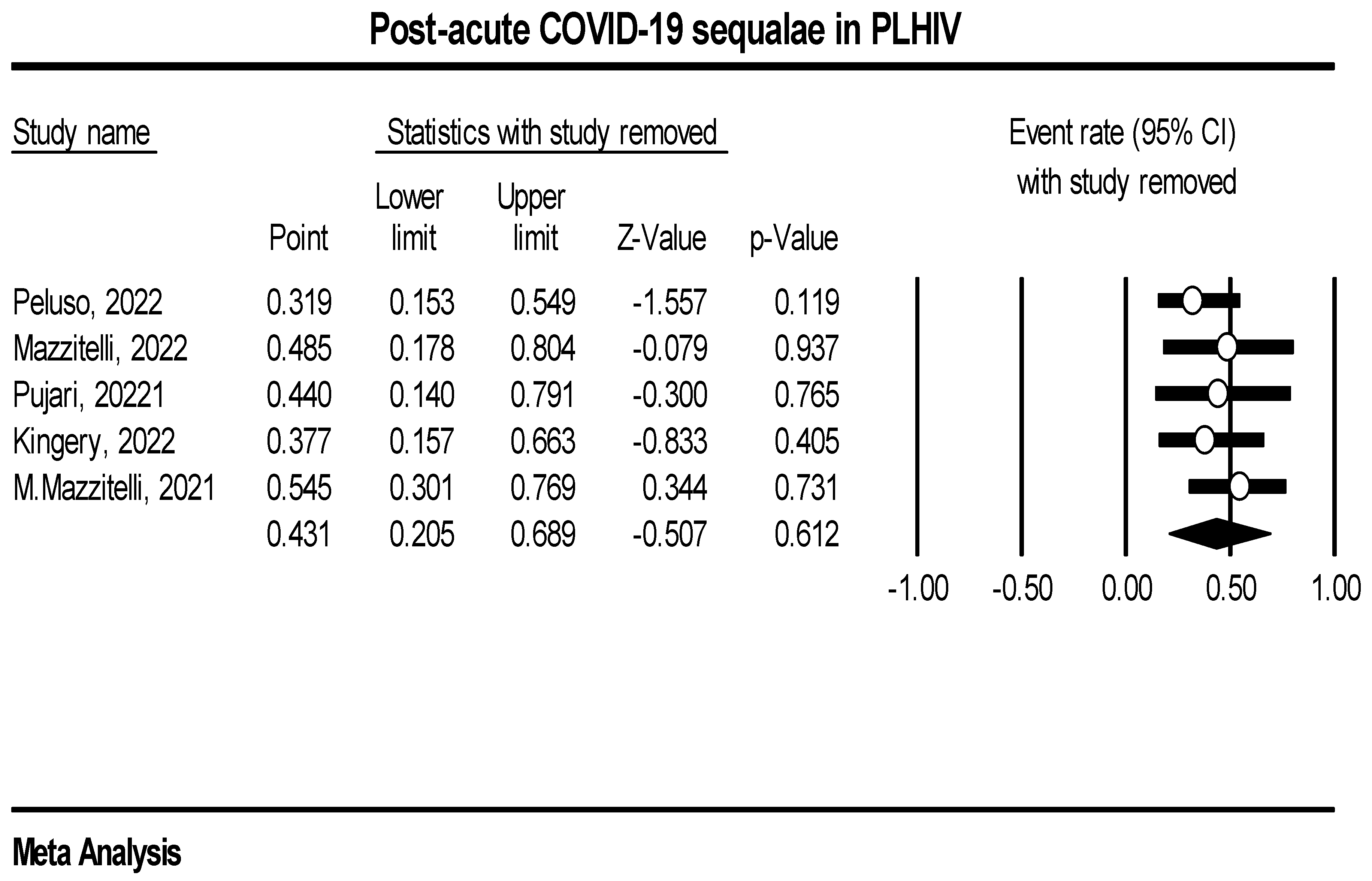
| Study/Author | Region | Study Design | Study Setting | Average time to PACS diagnosis (in months) | |
| 1. | Peluso, 2022 | USA | Prospective | Single | 4 |
| 2. | Mazzitelli, 2022 | Europe | Prospective | Multicenter | 6 |
| 3. | Pujari, 2022 | Asia | Prospective | Single | 4 |
| 4. | Kingery, 2022 | USA | Retrospective | Single | 12 |
| 5. | Mazzitelli, 2022 | Europe | Prospective | Single | 1 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





