Submitted:
21 August 2023
Posted:
25 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. CTLI mortality and amputation rate
3. Limb Salvage and mortality
4. Medical management improving survival in CLTI and limb salvage
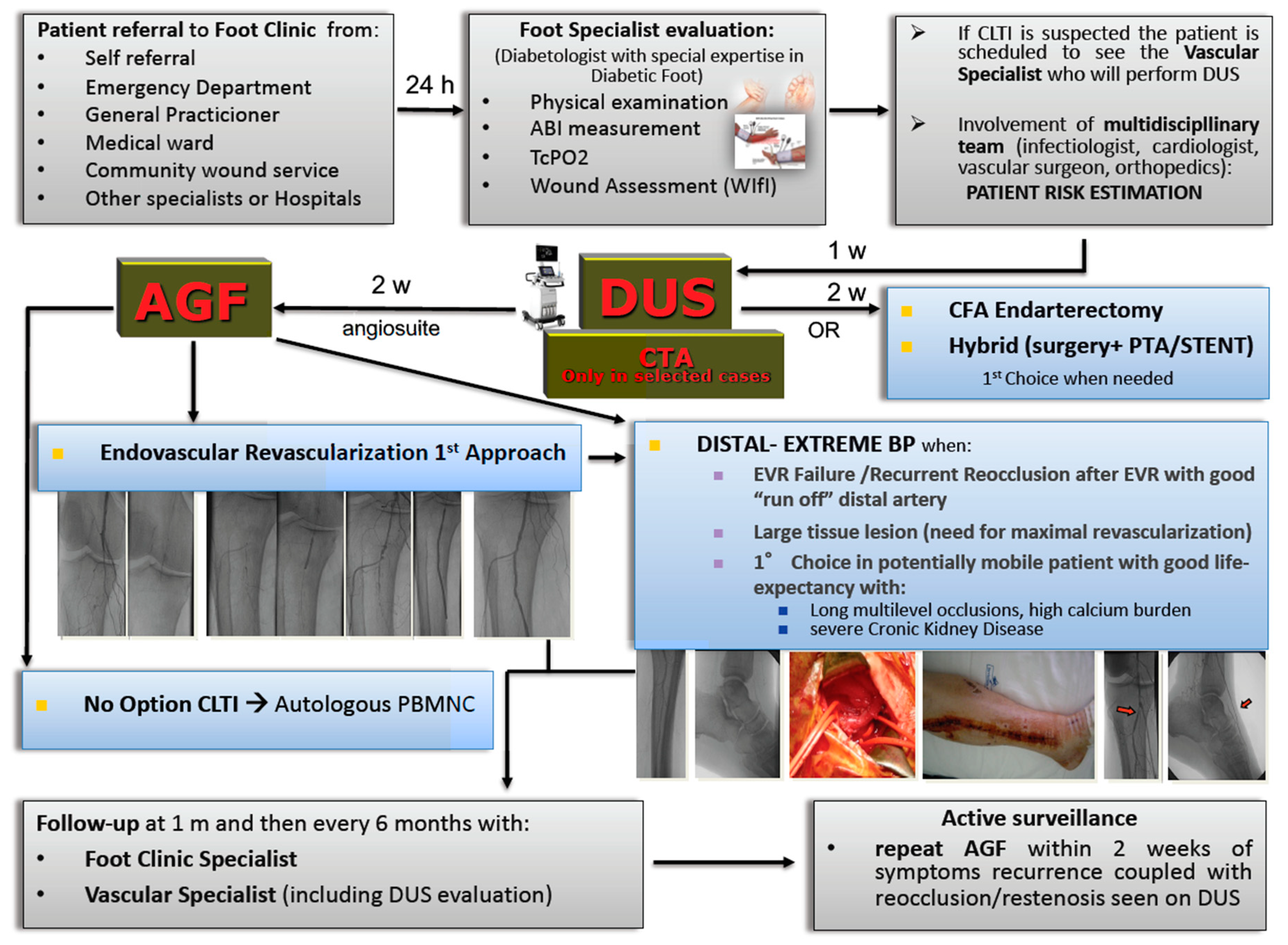
5. The need for Fast Track team-based management for optimal CLTI care
- (1)
- Clinical: prevent major amputation (above the ankle) and obtain LS keeping plantar standing despite the need for minor (below the ankle) amputations
- (2)
- Technical: obtain direct flow at least on one tibial artery
6. The impact of Paclitaxel-Eluting Devices (PED) on Mortality
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fowkes, F.G.R.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 2018, 138, 271–81. [Google Scholar] [CrossRef]
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.B.; Suresh, K.R.; Murad, M.H.; et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg 2019, 69, 3S–125S. [Google Scholar] [CrossRef]
- Mohammedi, K.; Woodward, M.; Hirakawa, Y.; Zoungas, S.; Colagiuri, S.; Hamet, P.; Harrap, S.; Poulter, N.; Matthews, D.R.; Marre, M.; et al. Presentations of major peripheral arterial disease and risk of major outcomes in patients with type 2 diabetes: Results from the ADVANCE-ON study. Cardiovasc Diabetol 2016, 15, 129. [Google Scholar] [CrossRef]
- Mustapha, J.A.; Katzen, B.T.; Neville, R.F.; Lookstein, R.A.; Zeller, T.; Miller, L.E.; Jaff, M.R. Disease burden and clinical outcomes following initial diagnosis of critical limb ischemia in the Medicare population. JACC Cardiovasc Interv 2018, 11, 1011–1012. [Google Scholar] [CrossRef]
- Abu Dabrh, A.M.; Steffen, M.W.; Undavalli, C.; Asi, N.; Wang, Z.; Elamin, M.B.; Conte, M.S.; Murad, M.H. The natural history of untreated severe or critical limb ischemia. J Vasc Surg 2015, 62, 1642–51. [Google Scholar] [CrossRef]
- Van Haelst, S.T.W.; Koopman, C.; Den Ruijter, H.M.; Moll, F.L.; Visseren, F.L.; Vaartjes, I.; de Borst, G.J. Cardiovascular and all-cause mortality in patients with intermittent claudication and critical limb ischaemia. Br J Surg.
- Mustapha, J.A.; Katzen, B.T.; Neville, R.F.; Lookstein, R.A; Zeller, T.; Miller, L.E.; Driver, V.R.; and JAFF, M.R. Critical Limb Ischemia: a threat to life and limb. Endovascular Today, 20 May.
- Ward, R.; Dunn, J.; Clavijo, L.; Shavelle, D.; Rowe, V.; Woo, K. Outcomes of critical limb ischemia in an urban, safety net hospital population with high WIfI amputation scores. Ann Vasc Surg 2017, 38, 84–9. [Google Scholar] [CrossRef]
- Duff, S.; Mafilios, M.S.; Bhounsule, P.; Hasegawa, J.T. The burden of critical limb ischemia: a review of recent literature. Vasc Health Risk Manag 2019, 15, 187–208. [Google Scholar] [CrossRef]
- Marston, W.A.; Davies, S.W.; Armstrong, B.; Farber, M.A.; Mendes, R.C.; Fulton, J.J.; Keagy, B.A. Natural history of limbs with arterial insufficiency and chronic ulceration treated without revascularization. J Vasc Surg 2006, 44, 108–14. [Google Scholar] [CrossRef]
- Verwer, M.C.; Wijnand, J.G.J.; Teraa, M.; Verhaar, M.C.; de Borst, G.J. Long Term Survival and Limb Salvage in Patients With Non-Revascularisable Chronic Limb Threatening Ischaemia. Eur J Vasc Endovasc Surg 2021, 62, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Meloni, M.; Izzo, V.; Da Ros, V.; Morosetti, D.; Stefanini, M.; Brocco, E.; Giurato, L.; Gandini, R.; Uccioli, L. Characteristics and Outcome for Persons with Diabetic Foot Ulcer and No-Option Critical Limb Ischemia. J Clin Med 2020, 9, 3745. [Google Scholar] [CrossRef]
- Klaphake, S.; de Leur, K.; Mulder, P.G.; Ho, G.H.; de Groot, H.G.; Veen, E.J.; Verhagen, H.J.; van der Laan, L. Mortality after major amputation in elderly patients with critical limb ischemia. Clin Interv Aging 2017, 12, 1985–92. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.P.; Eidt, J.F.; Capps, C.; Kirtley, L.; Moursi, M.M. Major lower extremity amputations at a Veterans Affairs hospital. Am J Surg 2003, 186, 449–54. [Google Scholar] [CrossRef]
- Ferraresi, R.; Mauri, G.; Losurdo, F.; Troisi, N.; Brancaccio, D.; Caravaggi, C.; Neri, L. BAD transmission and SAD distribution: A new scenario for critical limb ischemia. J Cardiovasc Surg 2018, 59, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Almasri, J.; Adusumalli, J.; Asi, N.; Lakis, S.; Alsawas, M.; Prokop, L.J.; Bradbury, A.; Kolh, P.; Conte, M.S.; Murad, M.H. A systematic review and meta-analysis of revascularization outcomes of infrainguinal chronic limb-threatening ischemia. J Vasc Surg 2018, 68, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Stella, J.; Engelbertz, C.; Gebauer, K.; Hassu, J.; Meyborg, M.; Freisinger, E.; Malyar, N.M. Outcome of patients with chronic limb-threatening ischemia with and without revascularization. Vasa 2020, 49, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Martelli, E.; Zamboni, M.; Sotgiu, G.; Saderi, L.; Federici, M.; Sangiorgi, G.M.; Puci, M.V.; Martelli, A.R.; Messina, T.; Frigatti, P.; et al. Sex-Related Differences and Factors Associated with Peri-Procedural and 1 Year Mortality in Chronic Limb-Threatening Ischemia Patients from the CLIMATE Italian Registry. J Pers Med 2023, 13, 316. [Google Scholar] [CrossRef]
- Armstrong, E.J.; Wu, J.; Singh, J.D.; Dawson, D.L.; Pevec, W.C.; Amsterdam, E.A.; Laird, J.R. Smoking cessation is associated with decreased mortality and improved amputation-free survival among patients with symptomatic peripheral artery disease. J Vasc Surg 2014, 60, 1565–1571. [Google Scholar] [CrossRef]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; Carlo, M.D.; Debus, S.; et al. 2017 ESC guidelines on the diagnosis and treatment of Peripheral Arterial Diseases in collaboration with the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–821. [Google Scholar] [CrossRef]
- Hung, J.; Timaran, D.A.; Modrall, J.G.; Ahn, C.; Timaran, C.H; Kirkwood, M.L.; Baig, M.S.; Valentine, R.J. Optimal medical therapy predicts amputation-free survival in chronic critical limb ischemia. J Vasc Surg 2013, 58, 972–980. [Google Scholar]
- Anand, S.S.; Bosch, J.; Eikelboom, J.W.; Connolly, S.J.; Diaz, R.; Widimsky, P.; Aboyans, V.; Alings, M.; Kakkar, A.K.; Keltai, K.; et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: An international, randomised, double-blind, placebo-controlled trial. Lancet 2018, 391, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Bonaca, M.P.; Bauersachs, R.M.; Anand, S.S.; Debus, E.S.; Nehler, M.R.; Patel, M.R.; Fanelli, F.; Capell, W.H.; Diao, L.; Jaeger, N.; et al. Rivaroxaban in peripheral artery disease after revascularization. N. Engl. J. Med. 2020, 382, 1994–2004. [Google Scholar] [CrossRef] [PubMed]
- Nickinson, A.T.O.; Bridgwood, B.; Houghton, J.S.M.; Nduwayo, S.; Pepper, C.; Payne, T.; Bown, M.J.; Davies, R.S.M.; Sayers, R.D. A systematic review investigating the identification, causes, and outcomes of delays in the management of chronic limb-threatening ischemia and diabetic foot ulceration. J Vasc Surg 2020, 71, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Goodney, P.P.; Holman, K.; Henke, P.K.; Travis, L.L.; Dimick, J.B.; Stukel, T.A.; Fisher, E.S.; Birkmeyer, J.D. Regional intensity of vascular care and lower extremity amputation rates. J Vasc Surg, 1471; -9. [Google Scholar]
- Alexandrescu, V.; Hubermont, G.; Coessens, V.; Philips, Y.; Guillaumie, B.; Ngongang, C.; Vincent, G.; Azdad, K.; Ledent, G.; De Marre, C.; Macoir, C. Why a multidisciplinary team may represent a key factor for lowering the inferior limb loss rate in diabetic neuro-ischaemic wounds: application in a departmental institution. Acta Chir Belg 2009, 109, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Liistro, F.; Porto, I.; Angioli, P.; Ducci, K.; Falsini, G.; Grotti, S.; Ricci, L.; Ventoruzzo, G.; Turini, F.; Bellandi, G.; et al. A Team-Based Strategy for Optimal CLI Care. Endovascular Today 2014, 5, 35–40. [Google Scholar]
- Caetano, A.P.; Conde Vasco, I.; Veloso Gomes, F.; Costa, N.V.; Luz, J.H.; Spaepen, E.; Formiga, A.; Coimbra, É. ; Neves, J.; Bilhim, T. Successful Revascularization has a Significant Impact on Limb Salvage Rate and Wound Healing for Patients with Diabetic Foot Ulcers: Single-Centre Retrospective Analysis with a Multidisciplinary Approach. Cardiovasc Intervent Radiol 2020, 43, 1449–1459. [Google Scholar] [CrossRef]
- Rigato, M.; Monami, M.; Fadini, G.P. Autologous Cell Therapy for Peripheral Arterial Disease: Systematic Review and Meta- Analysis of Randomized, Nonrandomized, and Noncontrolled Studies. Circ Res 2017, 120, 1326–1340. [Google Scholar] [CrossRef]
- Scatena, A.; Petruzzi, P.; Maioli, F.; Lucaroni, F.; Ambrosone, C.; Ventoruzzo, G.; Liistro, F.; Tacconi, D.; Di Filippi, M.; Attempati, N.; et al. Autologous Peripheral Blood Mononuclear Cells for Limb Salvage in Diabetic Foot Patients with No-Option Critical Limb Ischemia. J Clin Med 2021, 10(10), 2213. [Google Scholar] [CrossRef]
- Liistro, F.; Porto, I.; Angioli, P.; Grotti, S.; Ricci, L.; Ducci, K.; Falsini, G.; Ventoruzzo, G.; Turini, F.; Bellandi, G.; et al. Drug-eluting balloon in peripheral intervention for below the knee angioplasty evaluation (DEBATE-BTK): a randomized trial in diabetic patients with critical limb ischemia. Circulation 2013, 128, 615–621. [Google Scholar] [CrossRef]
- Liistro, F.; Grotti, S.; Porto, I.; Angioli, P.; Ricci, L.; Ducci, K.; Falsini, G.; Ventoruzzo, G.; Turini, F.; Bellandi, G.; et al. Drug-eluting balloon in peripheral intervention for the superficial femoral artery: the DEBATE-SFA randomized trial (drug eluting balloon in peripheral intervention for the superficial femoral artery). JACC Cardiovasc Interv 2013, 6, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Liistro, F.; Angioli, P.; Porto, I.; Ricci, L.; Ducci, K.; Grotti, S.; Falsini, G.; Ventoruzzo, G.; Turini, F.; Bellandi, G.; et al. Paclitaxel-eluting balloon vs. standard angioplasty to reduce recurrent restenosis in diabetic patients with in-stent restenosis of the superficial femoral and proximal popliteal arteries: the DEBATE- ISR study. J Endovasc Ther 2014, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liistro, F.; Angioli, P.; Porto, I.; Ducci, K.; Falsini, G.; Ventoruzzo, G.; Ricci, L.; Scatena, A.; Grotti, S.; Bolognese, L. Drug-eluting balloon versus drug-eluting stent for complex femoropopliteal arterial lesions: The drastico study. J Am Coll Cardiol 2019, 74, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Liistro, F.; Angioli, P.; Ventoruzzo, G.; Ducci, K.; Reccia, M.R.; Ricci, L.; Falsini, G.; Scatena, A.; Pieroni, M.; Bolognese, L. Randomized Controlled Trial of Acotec Drug-Eluting Balloon Versus Plain Balloon for Below-the-Knee Angioplasty. JACC Cardiovasc Interv, 2277; 13. [Google Scholar]
- Liistro, F.; Angioli, P.; Reccia, M.R.; Ducci, K.; Falsini, G.; Pieroni, M.; Ventoruzzo, G.; Scatena, A.; Bolognese, L. Long-term mortality in patients undergoing lower-limb revascularization with Paclitaxel eluting devices. Int J Cardiol 2021, 339, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.N.; Armstrong, E.J.; Aronow, H.D.; Gigliotti, O.S.; Jaff, M.R.; Klein, A.J.; Parikh, S.A.; Prasad, A.; Rosenfield, K.; Shishehbor, M.H.; et al. SCAI consensus guidelines for device selection in femoral-popliteal arterial interventions. Catheter Cardiovasc Interv 2018, 92, 124–140. [Google Scholar] [CrossRef]
- Katsanos, K.; Spiliopoulos, S.; Kitrou, P.; Krokidis, M.; Karnabatidis, D. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: A systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc 2018, 7, e011245. [Google Scholar] [CrossRef]
- Schneider, P.A.; Laird, J.R.; Doros, G.; Gao, Q.; Ansel, G.; Brodmann, M.; Micari, A.; Shishehbor, M.H.; Tepe, G.; Zeller, T. Mortality Not Correlated With Paclitaxel Exposure: An Independent Patient-Level Meta-Analysis of a Drug-Coated Balloon. J Am Coll Cardiol 2019, 73, 2550–2563. [Google Scholar] [CrossRef]
- Freisinger, E.; Koeppe, J.; Gerss, J.; Goerlich, D.; Malyar, N.M.; Marschall, U.; Faldum, A.; Reinecke, H. Mortality after use of paclitaxel-based devices in peripheral arteries: a real-world safety analysis. Eur Heart J, 3732. [Google Scholar]
- Dake, M.D.; Ansel, G.M.; Bosiers, M.; Holden, A.; Iida, O.; Jaff, M.R.; Lottes, A.E.; O'Leary, E.E.; Saunders, A.T.; Schermerhorn, M.; et al. Paclitaxel-Coated Zilver PTX Drug-Eluting Stent Treatment Does Not Result in Increased Long-Term All-Cause Mortality Compared to Uncoated Devices. Cardiovasc Intervent Radiol 2020, 43, 8–19. [Google Scholar] [CrossRef]
- Secemsky, E.A.; Kundi, H.; Weinberg, I.; Jaff, M.R.; Krawisz, A.; Parikh, S.A.; Beckman, J.A.; Mustapha, J.; Rosenfield, K.; Yeh, R.W. Association of Survival With Femoropopliteal Artery Revascularization With Drug-Coated Devices. JAMA Cardiol 2019, 4, 332–340. [Google Scholar] [CrossRef]
- Hess, C.N.; Patel, M.R.; Bauersachs, R.M.; Anand, S.S.; Debus, E.S.; Nehler, M.R.; Fanelli, F.; Yeh, R.W.; Secemsky, E.A.; Beckman, J.A.; et al. Safety and Effectiveness of Paclitaxel Drug-Coated Devices in Peripheral Artery Revascularization: Insights From VOYAGER PAD. J Am Coll Cardiol 2021, 78(18), 1768–1778. [Google Scholar] [CrossRef]
- Liistro, F.; Angioli, P.; Reccia, M.R.; Ducci, K.; Falsini, G.; Pieroni, M.; Ventoruzzo, G.; Scatena, A.; Bolognese, L. Long-term mortality in patients undergoing lower-limb revascularization with Paclitaxel eluting devices. Int J Cardiol 2021, 339, 150–157. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




