Submitted:
22 August 2023
Posted:
24 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
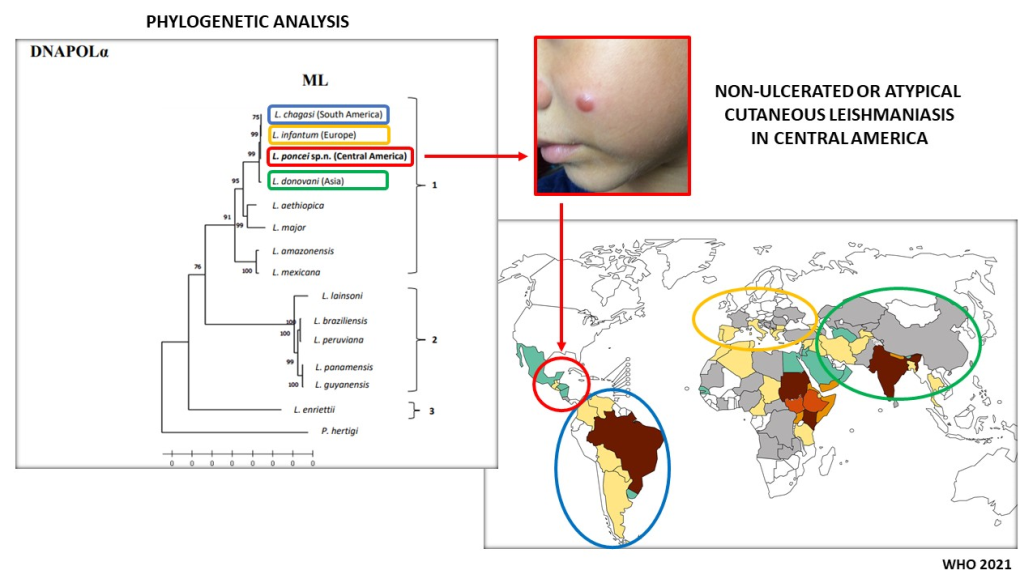
2. Integrative taxonomic analysis of the biological, epidemiological, clinical-immunological and molecular characters of the leishmanine parasite causing both NUCL and AVL in Honduras, Central America
2.1. Study area
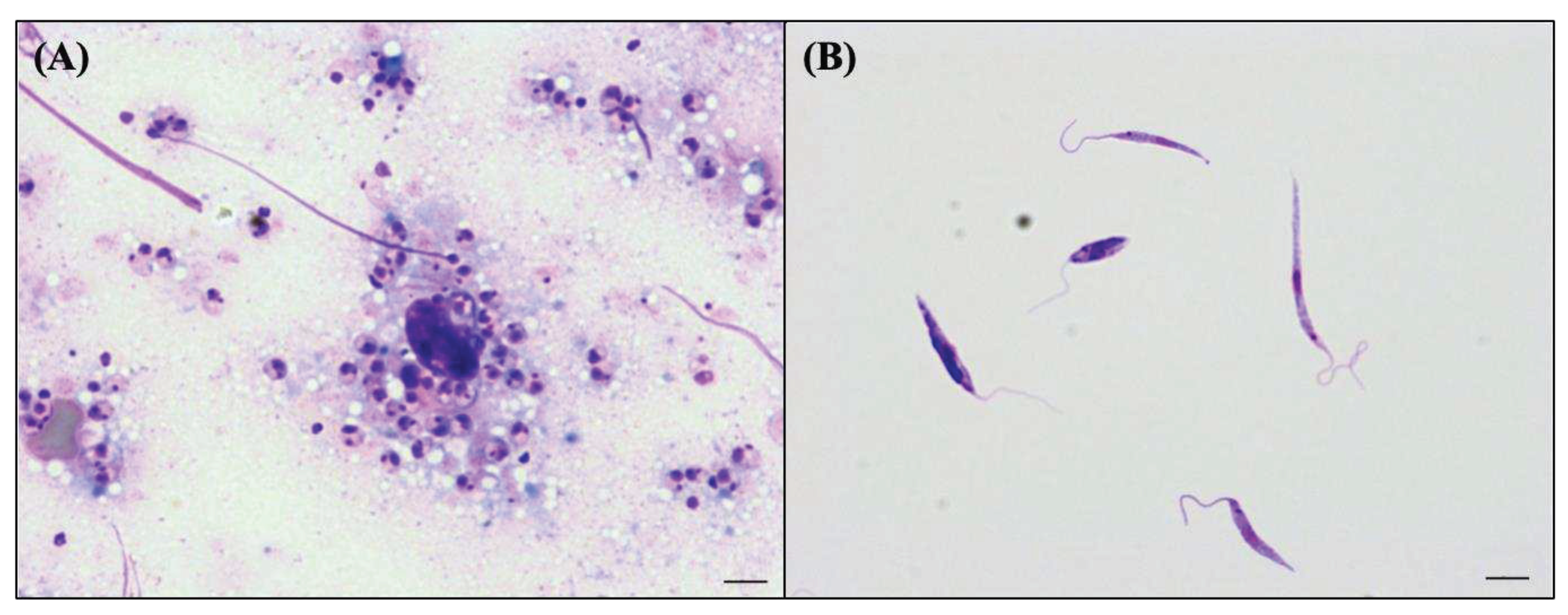
2.2. Parasite
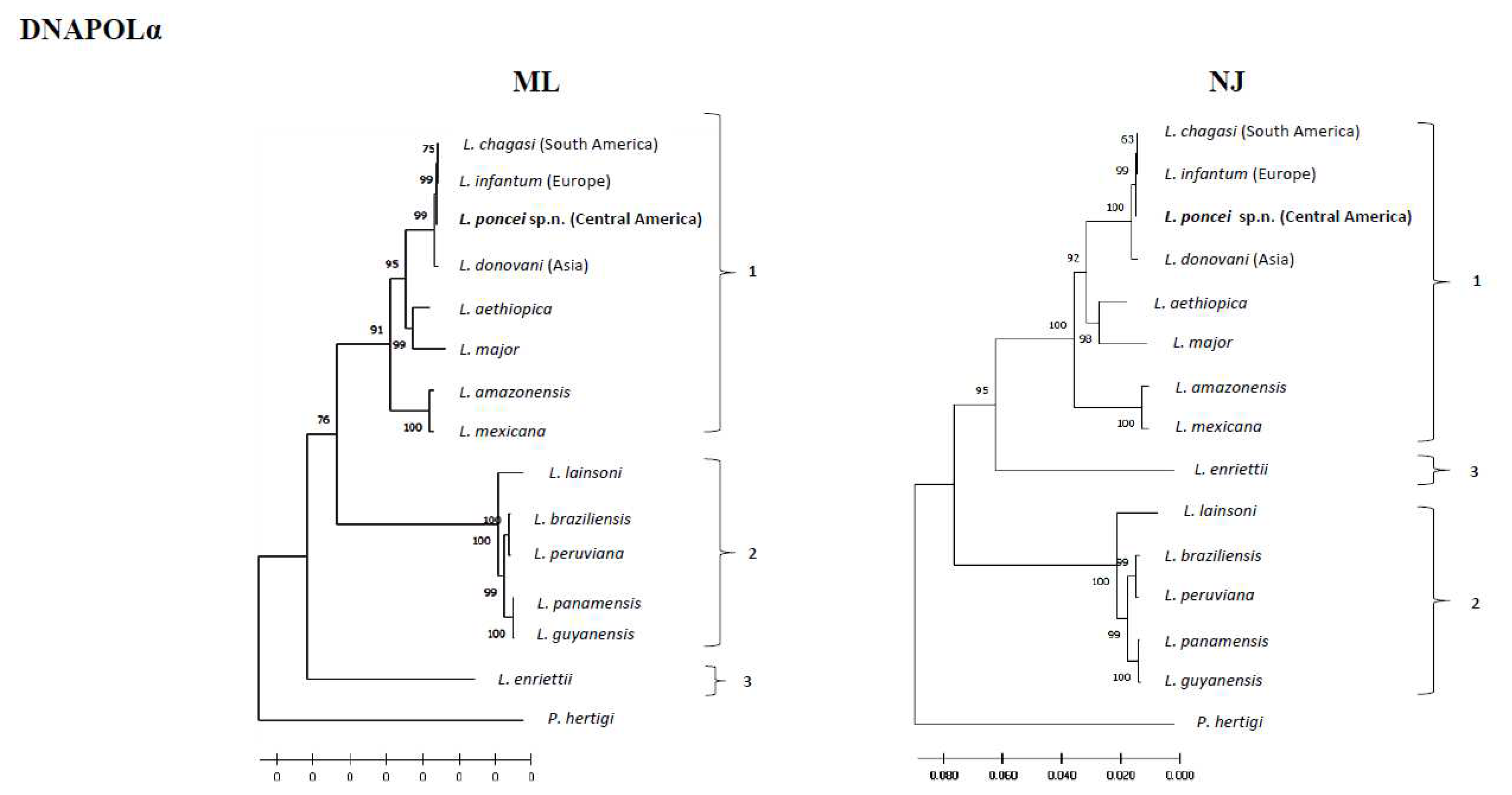
2.3. Genomic/phylogenetic analyses
2.4. Biological taxonomic characters of the Honduran parasite
2.5. Epidemiological taxonomic characters of infection of the Honduran parasite
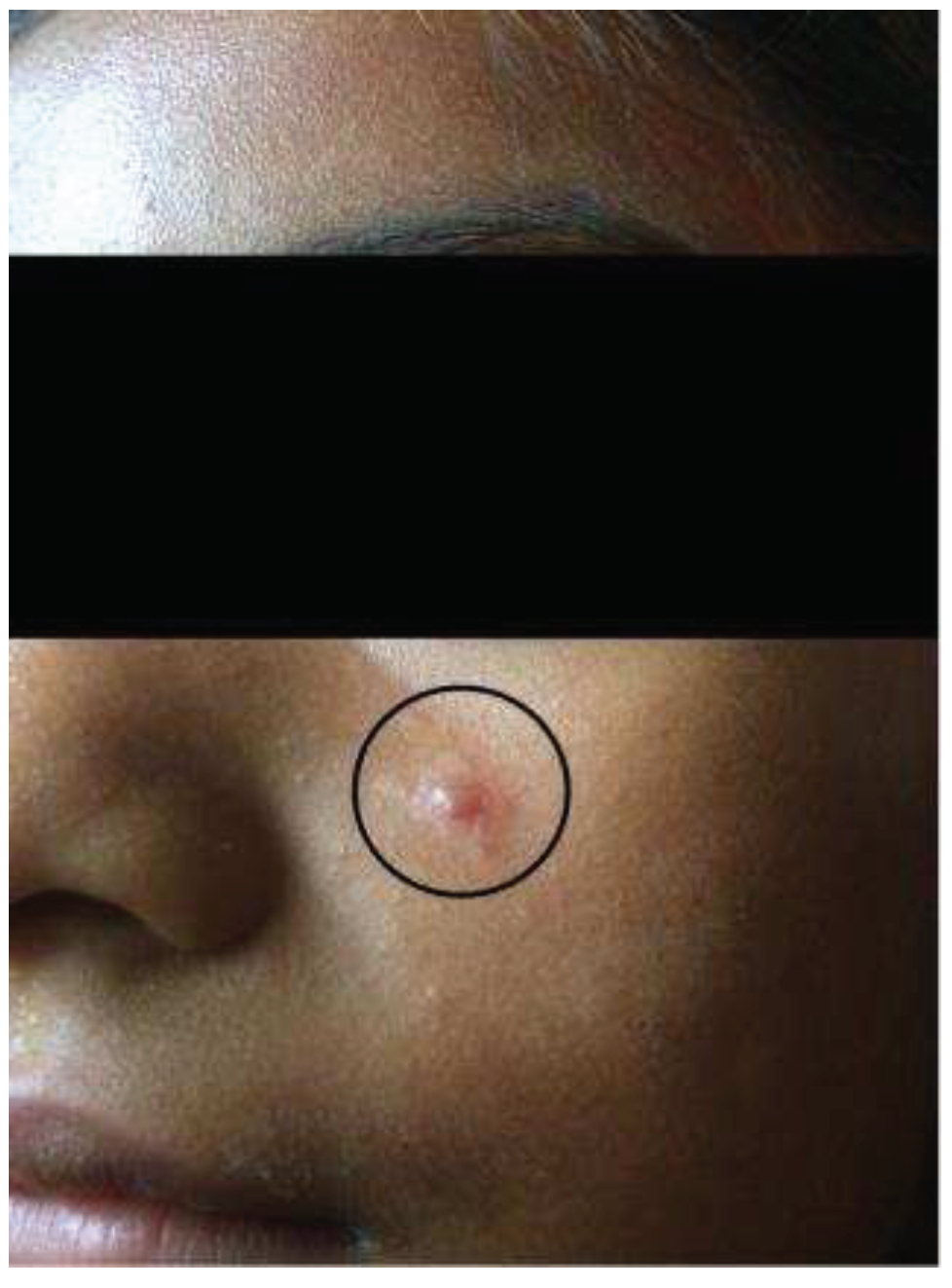
2.6. Clinical-immunological taxonomic characters of infection by the Honduran parasite
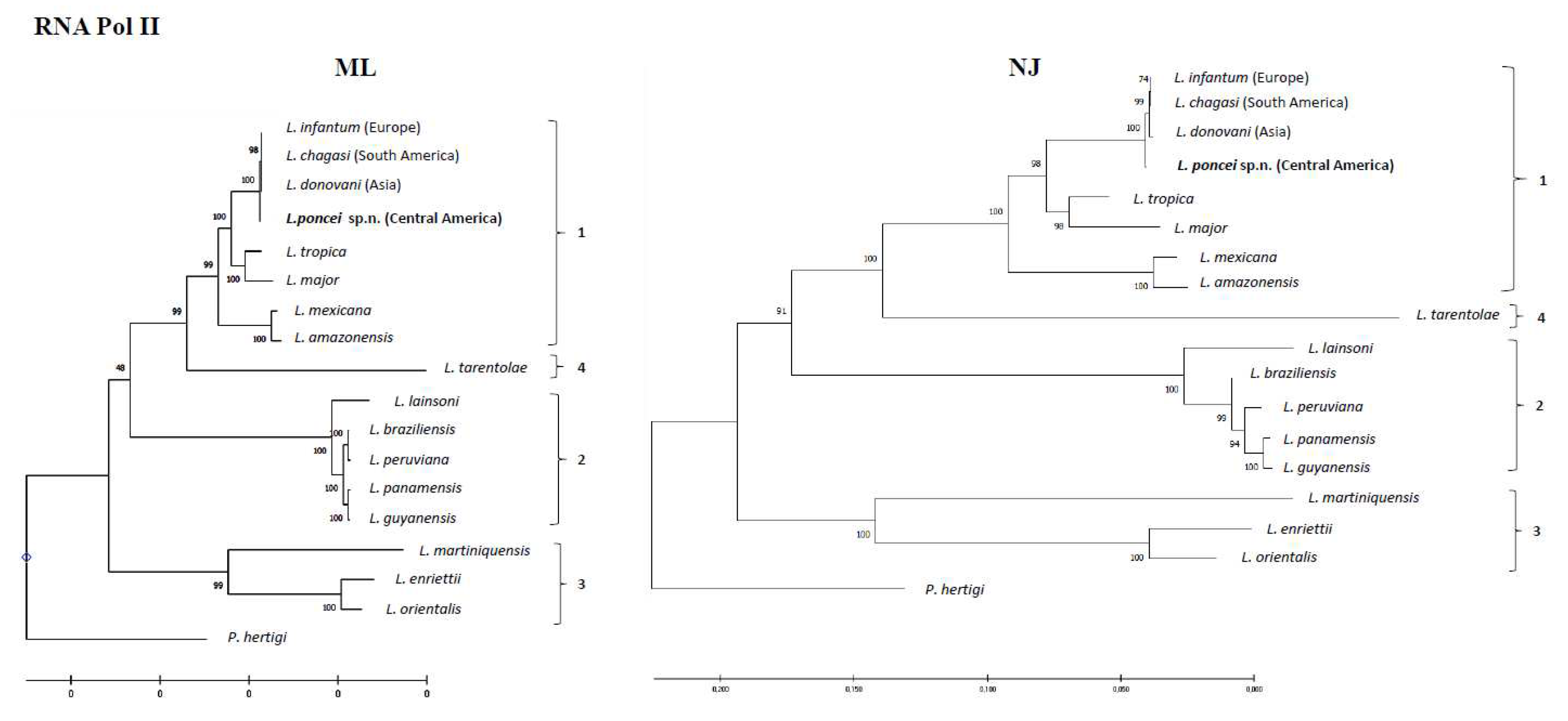
2.7. Molecular (genomic) taxonomic characters of the Honduran parasite
3. Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lainson: R.; Shaw, J.J. New World Leishmaniasis. In Topley & Wilson’s microbilogy & microbial infections: parasitology.; Hodder Arnold ASM Press, Ed.; London, 2005; pp. 313–349.
- Lainson, R. The Neotropical Leishmania Species: A Brief Historical Review of Their Discovery, Ecology and Taxonomy. Rev Panamazonica Saude 2010, 1, 13–32. [CrossRef]
- Silveira, F.T.; Corbett, C.E.P. Leishmania Chagasi Cunha & Chagas, 1937: Indigenous or Introduced? A Brief Review. Rev Panamazonica Saude 2010, 1, 143–147. [CrossRef]
- Silveira, F.T.; Junior, E.C.S.; Silvestre, R.V.; Costa-Martins, A.G.; Pinheiro, K. da C.; Ochoa, W.S.; Santos, T.V. dos; Ramos, P.K.; Casseb, S.; Silva, S.P. da; et al. Whole-Genome Sequencing of Leishmania Infantum Chagasi Isolates from Honduras and Brazil. Microbiol Resour Announc 2021, 10, 1–3. [CrossRef]
- Silveira, F.T.; Sousa Junior, E.C.; Silvestre, R.V.D.; Vasconcelos dos Santos, T.; Sosa-Ochoa, W.; Valeriano, C.Z.; Ramos, P.K.S.; Casseb, S.M.M.; Lima, L.V. do R.; Campos, M.B.; et al. Comparative Genomic Analyses of New and Old World Viscerotropic Leishmanine Parasites: Further Insights into the Origins of Visceral Leishmaniasis Agents. Microorganisms 2023, 11, 25. [CrossRef]
- Marcili, A.; Sperança, M.A.; da Costa, A.P.; Madeira, M. de F.; Soares, H.S.; Sanches, C. de O.C.C.; Acosta, I. da C.L.; Girotto, A.; Minervino, A.H.H.; Horta, M.C.; et al. Phylogenetic Relationships of Leishmania Species Based on Trypanosomatid Barcode (SSU RDNA) and GGAPDH Genes: Taxonomic Revision of Leishmania (L.) Infantum Chagasi in South America. Infection, Genetics and Evolution 2014, 25, 44–51. [CrossRef]
- Lainson, R.; Rangel, E.F. Lutzomyia Longipalpis and the Eco-Epidemiology of American Visceral Leishmaniasis, with Particular Reference to Brazil: A Review. Mem Inst Oswaldo Cruz 2005, 100, 811–827. [CrossRef]
- Silveira, F.T.; Lima, L.V. do R.; Santos, T.V. dos; Ramos, P.K.S.; Campos, M.B. Reviewing the Trajectory of American Visceral Leishmaniasis in Brazilian Amazon/Revendo a Trajetória Da Leishmaniose Visceral Americana Na Amazônia, Brasil: De Evandro Chagas Aos Dias Atuais. Rev Pan-Amaz Saude 2016, 15–22.
- Silveira, F.T.; Lainson, R.; de Souza, A.A.A.; Crescente, J.A.B.; Corbett, C.E.P. Leishmaniose Visceral Americana. In Medicina Tropical e Infectologia na Amazônia; Leão, R., Samauma, Eds.; Belem, Brazil, 2013; Vol. 2, pp. 1245–1274.
- Pearson, R.D.; Sousa, A. de Q. Clinical Spectrum of Leishmaniasis. Clinical Infectious Diseases 1996, 22, 1–13. [CrossRef]
- Furtado, R.R.; Alves, A.C.; Lima, L.V.R.; Vasconcelos Dos Santos, T.; Campos, M.B.; Ramos, P.K.S.; Gomes, C.M.C.; Laurenti, M.D.; da Matta, V.L.; Corbett, C.E.; et al. Visceral Leishmaniasis Urbanization in the Brazilian Amazon Is Supported by Significantly Higher Infection Transmission Rates Than in Rural Area. Microorganisms 2022, 10. [CrossRef]
- Ponce, C.; Ponce, E.; Morrison, A.; Cruz, A.; Neva, F. Leishmaniasis Cutânea Em Honduras Causada Por Leishmania Donovani Chagasi. XVI Congreso Centroamericano de Dermatología Y II Congreso Costarricense de Dermatología. Programa y Libro de Resúmenes, 1988, 73.
- Ponce, C.; Ponce, E.; Morrison, A.; Cruz, A.; Kreutzer, R.; McMahon-Pratt, D.; Neva, F. Leishmania Donovani Chagasi: New Clinical Variant of Cutaneous Leishmaniasis in Honduras. Lancet 1991, 337, 67–70. [CrossRef]
- Zeledón, R.; Hidalgo, H.; Víquez, A.; Urbina, A. Atypical Cutaneous Leishmaniasis in a Semiarid Region of North-West Costa Rica. Trans R Soc Trop Med Hyg 1989, 83, 786. [CrossRef]
- Noyes, H.; Chance, M.; Ponce, C.; Ponce, E.; Maingon, R. Leishmania Chagasi: Genotypically Similar Parasites from Honduras Cause Both Visceral and Cutaneous Leishmaniasis in Humans. Exp Parasitol 1997, 85, 264–273. [CrossRef]
- Vasconcelos, I. de A.; Sousa, A. de Q.; Vasconcelos, A.W.; Diógenes, M.J.; Momen, H.; Grimaldi Júnior, G.; Menezes, D.B.; Sleigh, A.C. Cutaneous Parasitism by Leishmania (Leishmania) Chagasi during South American Visceral Leishmaniasis. Bulletin de la Société de pathologie exotique 1933, 86, 101–105.
- Moura, C.R.L. de P.; Costa, C.H.N.; Moura, R. de D.; Braga, A.R.F.; Silva, V.C.; Costa, D.L. Cutaneous Parasitism in Patients with American Visceral Leishmaniasis in an Endemic Area. Rev Soc Bras Med Trop 2020, 53, e20190446. [CrossRef]
- Oliveira Neto, M.P.; Grimaldi, G.; Momen, H.; Pacheco, R.S.; Marzochi, M.C.; McMahon Pratt, D. Active Cutaneous Leishmaniasis in Brazil, Induced by Leishmania Donovani Chagasi. Mem Inst Oswaldo Cruz 1986, 81, 303–309.
- Lyra, M.R.; Pimentel, M.I.F.; Madeira, M. de F.; Antonio, L. de F.; Lyra, J.P. de M.; Fagundes, A.; Schubach, A. de O. First Report of Cutaneous Leishmaniasis Caused by Leishmania (Leishmania) Infantum Chagasi in an Urban Area of Rio de Janeiro, Brazil. Rev Inst Med Trop Sao Paulo 2015, 57, 451–454. [CrossRef]
- Sosa-Ochoa, W.; Zúniga, C.; Chaves, L.F.; Araujo Flores, G.V.; Sandoval Pacheco, C.M.; Ribeiro da Matta, V.L.; Pereira Corbett, C.E.; Tobias Silveira, F.; Dalastra Laurenti, M. Clinical and Immunological Features of Human Leishmania (L.) Infantum-Infection, Novel Insights Honduras, Central America. Pathogens 2020, 9, 554. [CrossRef]
- Cardoso, C.A.; Araujo, G. V.; Sandoval, C.M.; Nogueira, P.M.; Zúniga, C.; Sosa-Ochoa, W.H.; Laurenti, M.D.; Soares, R.P. Lipophosphoglycans from Dermotropic Leishmania Infantum Are More Pro-Inflammatory than Those from Viscerotropic Strains. Mem Inst Oswaldo Cruz 2020, 115, 1–6. [CrossRef]
- Araujo Flores, G. V.; Sandoval Pacheco, C.M.; Ferreira, A.F.; Tomokane, T.Y.; Nunes, J.B.; Colombo, F.A.; Sosa-Ochoa, W.H.; Zúniga, C.; Silveira, F.T.; Corbett, C.E.P.; et al. Leishmania (L.) Infantum Chagasi Isolated from Skin Lesions of Patients Affected by Non-Ulcerated Cutaneous Leishmaniasis Lead to Visceral Lesion in Hamsters. Parasitol Int 2023, 93, 102723. [CrossRef]
- Sosa-Ochoa, W.; Varela Amador, J.; Lozano-Sardaneta, Y.; Rodriguez Segura, G.; Zúniga Valeriano, C.; Araujo, G.V.; Sandoval Pacheco, C.M.; Laurenti, M.D.; Galvis-Ovallos, F. Detection of Leishmania Infantum DNA in Pintomyia Evansi and Lutzomyia Longipalpis in Honduras. Parasit Vectors 2020, 13, 593. [CrossRef]
- Segura, G.B.R.; Ochoa, W.H.S.; Matta, V.L.R.D.; Martínez, M.; Tercero, C.R.; Gonzalez, R.R.; Pacheco, C.M.S.; Flores, G.V.A.; Silveira, F.T.; Henriquez, M.M.R.; et al. Can Domestic Dogs Be Considered a Good Reservoir of Leishmania (L.) Infantum Chagasi in an Endemic Area of Nonulcerated Cutaneous Leishmaniasis in Southern Honduras? Rev Inst Med Trop Sao Paulo 2023, 65. [CrossRef]
- Araujo Flores, G.V.; Sandoval Pacheco, C.M.; Tomokane, T.Y.; Sosa Ochoa, W.; Zúniga Valeriano, C.; Castro Gomes, C.M.; Corbett, C.E.P.; Laurenti, M.D. Evaluation of Regulatory Immune Response in Skin Lesions of Patients Affected by Nonulcerated or Atypical Cutaneous Leishmaniasis in Honduras, Central America. Mediators Inflamm 2018, 2018, 1–7. [CrossRef]
- Araujo Flores, G.V.; Sandoval Pacheco, C.M.; Sosa Ochoa, W.; Castro Gomes, C.M.; Zúniga, C.; Corbett, C.P.; Laurenti, M.D. Th17 Lymphocytes in Atypical Cutaneous Leishmaniasis Caused by Leishmania (L.) Infantum Chagasi in Central America. Parasite Immunol 2020, 42, 1–6. [CrossRef]
- Sandoval Pacheco, C.M.; Araujo Flores, G.V.; Favero Ferreira, A.; Sosa Ochoa, W.; Ribeiro da Matta, V.L.; Zúniga Valeriano, C.; Pereira Corbett, C.E.; Dalastra Laurenti, M. Histopathological Features of Skin Lesions in Patients Affected by Non-Ulcerated or Atypical Cutaneous Leishmaniasis in Honduras, Central America. Int J Exp Pathol 2018, 99, 249–257. [CrossRef]
- Sandoval, C.; Araujo, G.; Sosa, W.; Avalos, S.; Silveira, F.; Corbett, C.; Zúniga, C.; Laurenti, M. In Situ Cellular Immune Response in Non-Ulcerated Skin Lesions Due to Leishmania (L.) Infantum Chagasi Infection. Journal of Venomous Animals and Toxins including Tropical Diseases 2021, 27, 1–8. [CrossRef]
- Sandoval Pacheco, C.M.; Araujo Flores, G. V.; Gonzalez, K.; De Castro Gomes, C.M.; Passero, L.F.D.; Tomokane, T.Y.; Sosa-Ochoa, W.; Zúniga, C.; Calzada, J.; Saldaña, A.; et al. Macrophage Polarization in the Skin Lesion Caused by Neotropical Species of Leishmania Sp. J Immunol Res 2021, 2021, 1–8. [CrossRef]
- Sandoval Pacheco, C.M.; Araujo Flores, G. V.; Ferreira, A.F.; da Matta, V.L.R.; de Castro Gomes, C.M.; Sosa-Ochoa, W.H.; Zúniga, C.; Silveira, F.T.; Corbett, C.E.P.; Laurenti, M.D. Role of Antigen-presenting Cells in Non-ulcerated Skin Lesions Caused by Leishmania (Leishmania) Infantum Chagasi. Parasite Immunol 2023, e12971. [CrossRef]
- Laurenti, M.D.; Sosa-Ochoa, W.; Araujo Flores, G.V.; Sandoval Pacheco, C.M.; Tomokane, T.Y.; Oliveira, L.M. da S.; Zúniga, C.; Silveira, F.T.; Corbett, C.E.P. Evaluation of Systemic Immunity in Atypical Cutaneous Leishmaniasis Caused by Leishmania (L.) Infantum Chagasi. Parasite Immunol 2021, 00, 1–6. [CrossRef]
- Lainson, R.; Shaw, J.J. Evolution, Classification and Geographical Distribution. In The leishmaniases in biology and medicine. Volume I. Biology and epidemiology; Wallace Peters, R.K.-K., Ed.; Academic Press: London, 1987; p. 120 ISBN 0-12-552101-4.
- Silveira, F.T.; Lainson, R.; Corbett, C.E. Clinical and Immunopathological Spectrum of American Cutaneous Leishmaniasis with Special Reference to the Disease in Amazonian Brazil: A Review. Mem Inst Oswaldo Cruz 2004, 99, 239–251. [CrossRef]
- Silveira, F.T.; Lainson, R.; De Castro Gomes, C.M.; Laurenti, M.D.; Corbett, C.E.P. Immunopathogenic Competences of Leishmania (V.) Braziliensis and L. (L.) Amazonensis in American Cutaneous Leishmaniasis. Parasite Immunol 2009, 31, 423–431. [CrossRef]
- T. Silveira, F.; B. Campos, M.; F. Müller, S.; K. Ramos, P.; V. Lima, L.; V. dos Santos, T.; Maria Gomes, C.; D. Laurenti, M.; Lucia da Matta, V.; Eduardo Corbett, C. From Biology to Disease: Importance of Species-Specific Leishmania Antigens from the Subgenera Viannia (L. Braziliensis) and Leishmania (L. Amazonensis) in the Pathogenesis of American Cutaneous Leishmaniasis . In Leishmania Parasites; IntechOpen: Croatia, 2023; pp. 1–29 ISBN 978-1-83768-311-6.
- Silveira, F.T. What Makes Mucosal and Anergic Diffuse Cutaneous Leishmaniases so Clinically and Immunopathogically Different? A Review in Brazil. Trans R Soc Trop Med Hyg 2019, 113, 505–516. [CrossRef]
- Giorgione, J.R.; Turco, S.J.; Epand, R.M. Transbilayer Inhibition of Protein Kinase C by the Lipophosphoglycan from Leishmania Donovani. Proc Natl Acad Sci U S A 1996, 93. [CrossRef]
- Späth, G.; Garraway, L.; Turco, S.; Beverley, S. The Role(s) of Lipophosphoglycan (LPG) in the Establishment of Leishmania Major Infections in Mammalian Hosts. Proc Natl Acad Sci U S A 2003, 100. [CrossRef]
- Sosa-Ochoa, W.; Morales Cortedano, X.; Argüello, S.; Zuniga, C.; Henríquez, J.; Mejía, R.; Mejía, A.; Araujo, G.; Sandoval, C.; Quan, D. Ecoepidemiología de La Leishmaniasis Cutánea No Ulcerada En Honduras. Revista Ciencia y Tecnología 2015, 115–128. [CrossRef]
- Mejía, Á.; Matamoros, G.; Fontecha, G.; Sosa-Ochoa, W. Bionomic Aspects of Lutzomyia Evansi and Lutzomyia Longipalpis, Proven Vectors of Leishmania Infantum in an Endemic Area of Non-Ulcerative Cutaneous Leishmaniasis in Honduras. Parasit Vectors 2018, 11. [CrossRef]
- Chaves, L.F.; Añez, N. Nestedness Patterns of Sand Fly (Diptera: Psychodidae) Species in a Neotropical Semi-Arid Environment. Acta Trop 2016, 153. [CrossRef]
- Travi, B.L.; Vélez, I.D.; Brutus, L.; Segura, I.; Jaramillo, C.; Montoya, J. Lutzomyia Evansi, an Alternate Vector of Leishmania Chagasi in a Colombian Focus of Visceral Leishmaniasis. Trans R Soc Trop Med Hyg 1990, 84, 676–677. [CrossRef]
- Travi, B.L.; Montoya, J.; Gallego, J.; Jaramillo, C.; Llano, R.; Velez, I.D. Bionomics of Lutzomyia Evansi (Diptera: Psychodidae) Vector of Visceral Leishmaniasis in Northern Colombia. J Med Entomol 1996, 33, 278–285. [CrossRef]
- Moreira, M.A.B.; Luvizotto, M.C.R.; Garcia, J.F.; Corbett, C.E.P.; Laurenti, M.D. Comparison of Parasitological, Immunological and Molecular Methods for the Diagnosis of Leishmaniasis in Dogs with Different Clinical Signs. Vet Parasitol 2007, 145, 245–252. [CrossRef]
- Silveira, F.T.; Carneiro, L.A.; Ramos, P.K.S.; Chagas, E.J.; Lima, L.V.R.; Campos, M.B.; Laurenti, M.D.; Gomes, C.M.C.; Corbett, C.E.P. A Cross-Sectional Study on Canine Leishmania (L.) Infantum Chagasi Infection in Amazonian Brazil Ratifies a Higher Prevalence of Specific IgG-Antibody Response than Delayed-Type Hypersensitivity in Symptomatic and Asymptomatic Dogs. Parasitol Res 2012, 111, 1513–1522. [CrossRef]
- Laurenti, M.D.; Rossi, C.N.; Matta, V.L.R. da; Tomokane, T.Y.; Corbett, C.E.P.; Secundino, N.F.C.; Pimenta, P.F.P.; Marcondes, M. Asymptomatic Dogs Are Highly Competent to Transmit Leishmania (Leishmania) Infantum Chagasi to the Natural Vector. Vet Parasitol 2013, 196, 296–300. [CrossRef]
- Aschar, M.; de Oliveira, E.T.B.; Laurenti, M.D.; Marcondes, M.; Tolezano, J.E.; Hiramoto, R.M.; Corbett, C.E.P.; da Matta, V.L.R. Value of the Oral Swab for the Molecular Diagnosis of Dogs in Different Stages of Infection with Leishmania Infantum. Vet Parasitol 2016, 225, 108–113. [CrossRef]
- Carneiro, L.A.; Lima, L.V.; Campos, M.B.; dos Santos, T.V.; Ramos, P.K.; Laurenti, M.D.; Silveira, F.T. Prevalence and Incidence of Canine Visceral Leishmaniasis and Its Clinical-Immunological Features in an Endemic Area of the Brazilian Amazon. Vet. Med. Sci. 2023, 1–12.
- Maurício, I.; Stothard, J.; Miles, M. The Strange Case of Leishmania Chagasi. Parasitol Today 2000, 16. [CrossRef]
- Lukes, J.; Mauricio, I.; Schönian, G.; Dujardin, J.; Soteriadou, K.; Dedet, J.; Kuhls, K.; Tintaya, K.; Jirků, M.; Chocholová, E.; et al. Evolutionary and Geographical History of the Leishmania Donovani Complex with a Revision of Current Taxonomy. Proc Natl Acad Sci U S A 2007, 104. [CrossRef]
- Leblois, R.; Kuhls, K.; François, O.; Schönian, G.; Wirth, T. Guns, Germs and Dogs: On the Origin of Leishmania Chagasi. Infect Genet Evol 2011, 11, 1091–1095. [CrossRef]
- Kuhls, K.; Alam, M.Z.; Cupolillo, E.; Ferreira, G.E.M.; Mauricio, I.L.; Oddone, R.; Feliciangeli, M.D.; Wirth, T.; Miles, M.A.; Schönian, G. Comparative Microsatellite Typing of New World Leishmania Infantum Reveals Low Heterogeneity among Populations and Its Recent Old World Origin. PLoS Negl Trop Dis 2011, 5, e1155. [CrossRef]
- Desbois, N.; Pratlong, F.; Quist, D.; Dedet, J.-P. Leishmania (Leishmania) Martiniquensis n. Sp. (Kinetoplastida: Trypanosomatidae), Description of the Parasite Responsible for Cutaneous Leishmaniasis in Martinique Island (French West Indies). Parasite 2014, 21. [CrossRef]
- Espinosa, O.A.; Serrano, M.G.; Camargo, E.P.; Teixeira, M.M.G.; Shaw, J.J. An Appraisal of the Taxonomy and Nomenclature of Trypanosomatids Presently Classified as Leishmania and Endotrypanum. Parasitology 2016, 145, 430–442. [CrossRef]
- Jariyapan, N.; Daroontum, T.; Jaiwong, K.; Chanmol, W.; Intakhan, N.; Sor-suwan, S.; Siriyasatien, P.; Somboon, P.; Bates, M.D.; Bates, P.A. Leishmania (Mundinia) Orientalis n. Sp. (Trypanosomatidae), a Parasite from Thailand Responsible for Localised Cutaneous Leishmaniasis. Parasit Vectors 2018, 11, 351. [CrossRef]
- Kwakye-Nuako, G.; Mosore, M.-T.; Boakye, D.; Bates, P.A. Description, Biology, and Medical Significance of Leishmania (Mundinia) Chancei n. Sp. (Kinetoplastea: Trypanosomatidae) from Ghana and Leishmania (Mundinia) Procaviensis n. Sp. (Kinetoplastea: Trypanosomatidae) from Namibia. Journal of Parasitology 2023, 109, 43–50. [CrossRef]
- Shaw, J.; Pratlong, F.; Floeter-Winter, L.; Ishikawa, E.; El Baidouri, F.; Ravel, C.; Dedet, J.-P. Characterization of Leishmania (Leishmania) Waltoni n.Sp. (Kinetoplastida: Trypanosomatidae), the Parasite Responsible for Diffuse Cutaneous Leishmaniasis in the Dominican Republic. Am J Trop Med Hyg 2015, 93, 552–558. [CrossRef]
- Castro, L.S.; França, A. de O.; Ferreira, E. de C.; Hans Filho, G.; Higa Júnior, M.G.; Gontijo, C.M.F.; Pereira, A.A.S.; Dorval, M.E.M.C.; PEREIRA, A.A.S.; DORVAL, M.E.M.C. Leishmania Infantum as a Causative Agent of Cutaneous Leishmaniasis in the State of Mato Grosso Do Sul, Brazil. Rev Inst Med Trop Sao Paulo 2016, 58, 23. [CrossRef]
- Lima, L.V.R.; Carneiro, L.A.; Campos, M.B.; Chagas, E.J.; Laurenti, M.D.; Corbett, C.E.P.; Lainson, R.; Silveira, F.T. Canine Visceral Leishmaniasis Due to Leishmania (L.) Infantum Chagasi in Amazonian Brazil: Comparison of the Parasite Density from the Skin, Lymph Node and Visceral Tissues between Symptomatic and Asymptomatic, Seropositive Dogs. Rev Inst Med Trop Sao Paulo 2010, 52, 259–266. [CrossRef]
- Butenko, A.; Kostygov, A.Y.; Sádlová, J.; Kleschenko, Y.; Bečvář, T.; Podešvová, L.; Macedo, D.H.; Žihala, D.; Lukeš, J.; Bates, P.A.; et al. Comparative Genomics of Leishmania (Mundinia). BMC Genomics 2019, 20, 726. [CrossRef]
- da Matta, V.L.R.; Gonçalves, A.N.; Gomes, C.M.C.; Chouman, I.H.; Ferreira, F.M.; Campos, M.B.; Lima, L. V; Vasconcelos Dos Santos, T.; Ramos, P.K.; Furtado, R.R.; et al. Gene Signatures of Symptomatic and Asymptomatic Clinical-Immunological Profiles of Human Infection by Leishmania (L.) Chagasi in Amazonian Brazil. Microorganisms 2023, 11. [CrossRef]
- Vasconcelos dos Santos, T.; Ramos, P.K.S.; da Silva, F.M.M.; Alves, A.C.; Lima, L.V.R.; Campos, M.B.; Furtado, R.R.; Silveira, F.T. Presence of Lutzomyia Longipalpis (Diptera: Psychodidae) and Natural Leishmania (L.) Infantum Chagasi-Infection in the Wild Rodent, Proechimys Sp. (Rodentia: Echimydae), in the “Serra Dos Carajás”, Southern of Pará State, Brazil. In Proceedings of the 6th World Congress of Leishmaniasis, Poster Section, Toledo, Span 2016.
- Moreno, E.S.; Sabioni, L.A.; Seixas, M.M.M. de; Souza Filho, J.A. de; Marcelino, A.P.; Shimabukuro, P.H.F. Evidence of a Sylvatic Enzootic Cycle of Leishmania Infantum in the State of Amapá, Brazil. Rev Soc Bras Med Trop 2020, 53, e20190169. [CrossRef]
- Kaufer, A.; Stark, D.; Ellis, J. Evolutionary Insight into the Trypanosomatidae Using Alignment-Free Phylogenomics of the Kinetoplast. Pathogens 2019, 8. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




