1. Introduction
Recent studies in schizophrenia pointed to increased inflammatory activity and related mitochondrial dysfunction leading to increased levels of both C - reactive protein (CRP), leukocyte ratios (neutrophil/lymphocyte and monocyte/lymphocyte) and lactate [1-2]. The downstream effects of these inflammatory markers are decreased brain perfusion, reduced ATPase and mitochondrial activity, and increased cognitive and behavioral deficits.
The evidence of brain dysfunction has additional support from post-mortem studies (study on animals) revealing increased levels of lactate in dorsolateral prefrontal cortex, independent from drug use and mitochondrial mutation [
3]. On the other hand, regular physical exercise in addition to weight loss also contributes to increased metabolism, cell mitosis, mitochondrial activity, neurotrophic factor production, neuroplasticity and quality of life, and decreased oxidative stress [
4]. Such sequence of events can benefit patients with schizophrenia since they have less functional capacity and more frequent sedentary lifestyle, obesity, cardiomyopathy and metabolic diseases [
5]. Since physical exercise has the potential to revert this metabolic dysregulation and to induce better outcome in schizophrenia, the study measured the effect of two different physical interventions over metabolic markers. The clinical trial included Aerobic Intervention (AI), which activates oxidative and mitochondrial machinery, and Functional Intervention (FI), which mobilizes oxidative and mitochondrial machinery through glycolytic route. One group received AI and the other FI, and lactate, CK and CRP levels of both were compared with those in Healthy controls receiving the same interventions.
2. Materials and Methods
2.1. Design
Clinical trial of two physical interventions (aerobic physical intervention [AI] and functional physical intervention [FI]) were assigned to two groups of stable outpatients with diagnosis of schizophrenia and of healthy controls. Patients were under regular care in Public Facilities (AI group at Psychosocial Attention Center [CAPS] of Camaquã, RS, and FI at Hospital de Clínicas de Porto Alegre), and controls were paired by sex, age, social class (following classification of IBGE – Brazilian Institute of Geography and Statistics).
Patients must be stable for at least three months and under regular treatment. Psychiatric diagnosis was established after a three-step procedure consisting of: (1) clinical observation in at least three different evaluations; (2) a family interview; and (3) review of their medical records. A registered Psychiatrist performed all assessments. Patients must meet the following inclusion criteria: (a) Diagnostic of Schizophrenia by the Diagnostic and Statistical Manual of Mental Disorders [
6] of schizophrenia; (b) age between 18 and 65 years; (c) clinically stable under drug treatment for at least 3 months; (d) not involved in other physical activity programs during the intervention. Exclusion criteria were defined by the presence of any of 4 items: a. Suicide risk confirmed by direct contact with the patient and family; b. pregnancy or women of reproductive age not using a contraceptive method; and c. not agreeing to the study after full explanation. Controls were selected through specific social networks, and were submitted to direct interview to discharge any major mental illness, memory loss, psychosis (delusions and/or hallucinations), depression, mania, generalized anxiety disorder, or obsessive–compulsive disorder; and paired with patients by sex, age (3 years older or younger), social class; exclusion criteria were the same as those applied for patients with SCZ.
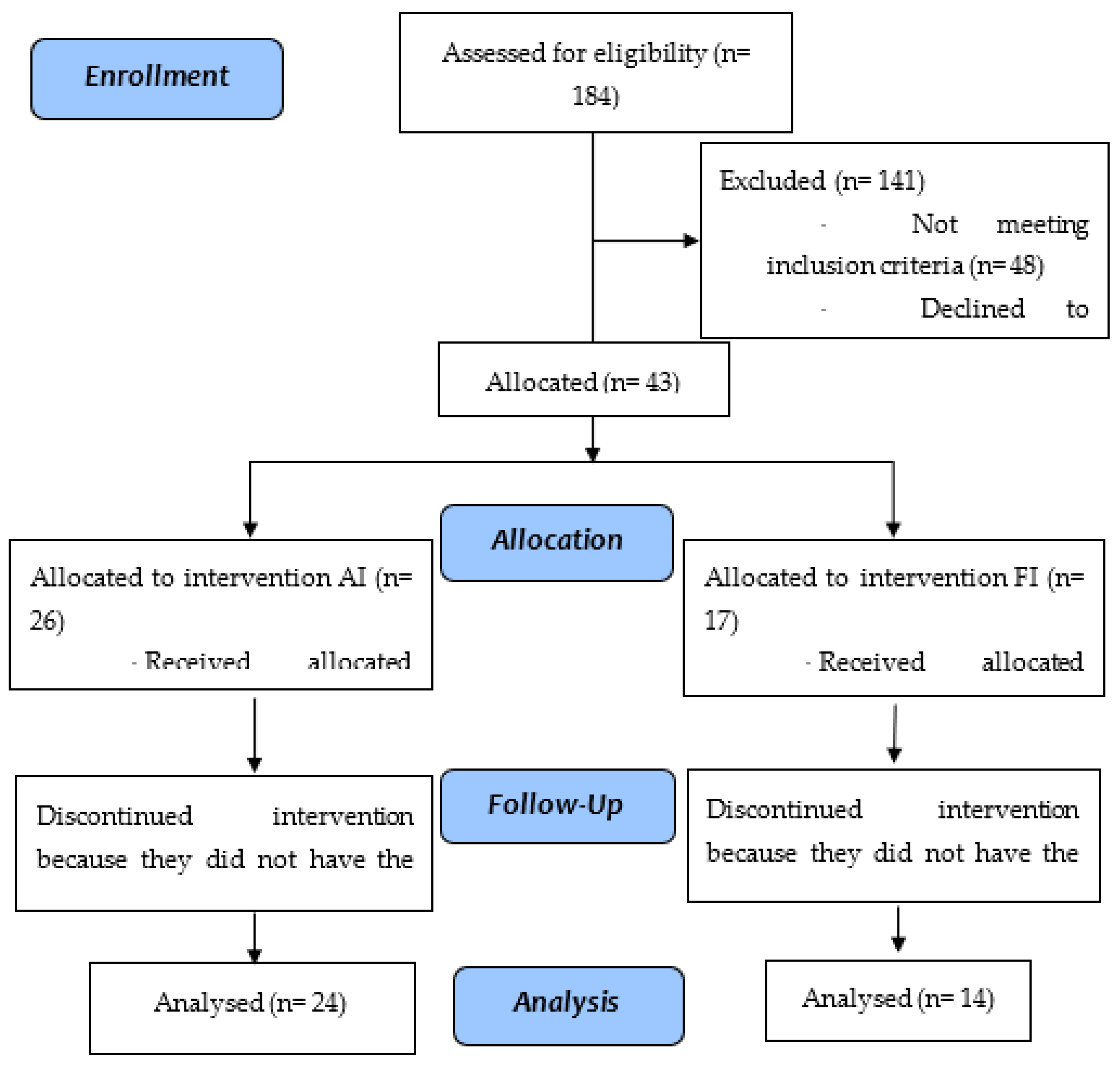
Figure 1.
- Study flowchart: aerobic intervention (AI) and functional intervention (FI) in patients with schizophrenia.
Figure 1.
- Study flowchart: aerobic intervention (AI) and functional intervention (FI) in patients with schizophrenia.
Controls were recruited through specific social networks, and paired by sex, age (3 years older or younger) and social class. Absence of any major mental illness was defined by direct interview questioning lifetime experiences of memory loss, psychosis - delusions and or hallucinations, depression, mania, generalized anxiety disorder and obsessive–compulsive symptoms. Exclusion criteria were the same as those applied for patients with SCZ.
2.2. Ethics
The study received the Brazilian Research Registry nº 43408615.7.0000.5327, registered in the Brazilian Registry of Clinical Trials (ReBEC), under nº RBR-2h2hjy and approved (#150066) by the Research Ethics Committee of Hospital de Clínicas de Porto Alegre (HCPA). Patients and their legal guardians provided written informed consent after reading and understanding the intervention program and their rights.
2.3. Clinical Assessment
After patient and control recruitment subjects received clinical and physical assessment by trained physiotherapists blinded to the type of intervention (Aerobic or Functional) before and after the 3-month intervention.
2.4. Blood collection
HCPA Clinical Pathology Service collected blood samples at the as per routine laboratory practice. High sensitivity C-reactive protein (hs-CRP) was assayed by turbidimetric immunoassay (reference range: < 3.0 mg/L = low/moderate risk; ≥ 3.0 mg/L = high risk), Lactate by colorimetric enzyme (reference range: 0,5-2,2 mmol/L) and Creatine Kinase (CK) by UV Enzyme (reference range: 0-170 U/L).
2.5. Physical Intervention
The physical intervention for cases and controls followed an initial assessment that took place after the consent form was read and signed. The aerobic or functional physical intervention program lasted 12 weeks in healthy cases and controls. Patients continued with regular clinical treatment in addition to standardized activity, and after completion of the intervention program, revaluation was performed using all the tests and questionnaires mentioned.
Aerobic protocol was adapted from previous studies in schizophrenia [
4], and Functional Protocol was adjusted to specific physical profile of patients [
7]. Both groups (patients and controls) performed similar exercises with the same frequency and duration.
2.6. Statistical Analyses
Kolmogorov-Smirnov test assessed normality of data distribution. Quantitative variables with a normal distribution were presented as means and standard deviations, while variables with an asymmetric distribution were presented as medians and interquartile ranges. Student’s paired t-test/independent t-test or the Wilcoxon test/Mann-Whitney test were used for comparison of normal and asymmetric variables, respectively. Comparisons between three or more groups were performed by the ANOVA test followed by Bonferroni. Categorical variables were presented as frequencies and analyzed using the Pearson chi-square test or the Fisher exact test. The main outcome measure was assessed using the generalized linear model (GLM) analysis (gamma distribution) and confounding factors were determined based on statistical criteria (association with either study factor and the outcome with p significance level ≤ 0.2). Significance level was p ≤ 0.05. The analysis of effect/size was used to evaluate the magnitude of the difference derived from GLM. The data were processed and analyzed in SPSS Statistics 22.0.
3. Results
Five of 43 individuals with SCZ who started the exercise protocol were excluded for not having the minimum required frequency (80%, 5 absences over 24 appointments were allowed), whereas in the control group, 49 started, and 11 were excluded for the same reason. Thus, the final sample numbers in both groups was 38 individuals, of which 24 from each group performed the aerobic intervention, and 14 from each group performed the functional intervention. This division of interventions was not randomized but was instead decided upon for convenience. The sociodemographic and clinical data of the samples are shown in
Table 1, where we can observe that the groups were homogeneous for sex, weight, and BMI, showing statistical differences only in age and height.
Table 2 shows the results of the biomarkers for both groups (cases and controls), compared in two moments (before and after interventions), taking into account the type of physical exercise performed (aerobic or functional).
3.1. Lactate
Both cases (p < 0.001) and controls (p = 0.005) showed increased lactate values after intervention, regardless the type. There was a significant difference between the pre and post moments in the intragroup comparison in the cases for both Aerobic (p < 0.001) and Functional (p < 0.001) intervention, with an increase in both. Both patients and controls receiving aerobic intervention increased lactate levels (cases p = 0.001; controls p = 0.026). In contrast, only cases receiving functional intervention had increased levels (p = 0.009). Controls differed before and after interventions (p = 0.003 and 0.002 respectively). Again, controls receiving aerobic intervention had higher titers in both times.
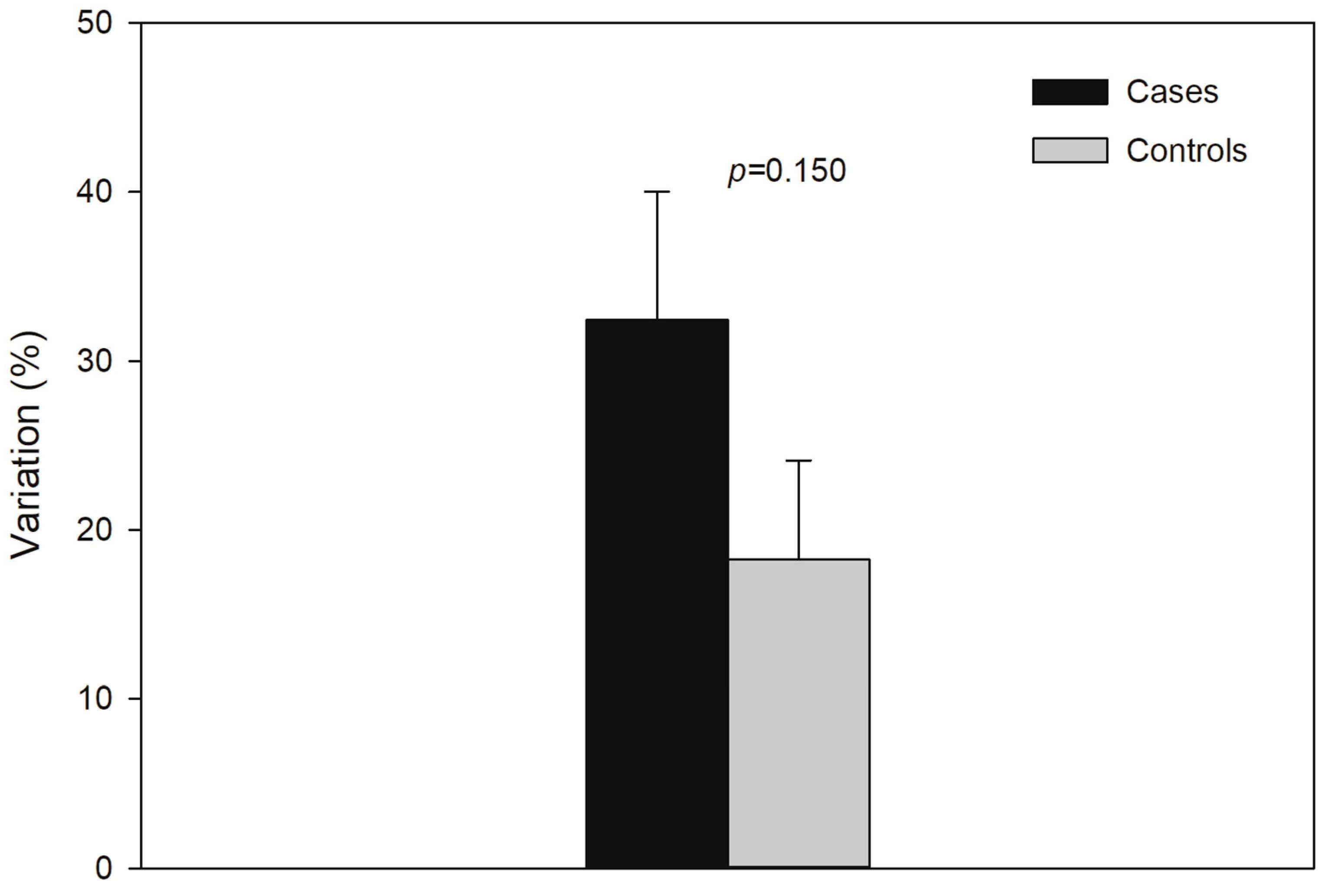
Figure 2 displays Percent (%) variation of lactate from baseline. There was a trend (p > 0.05) of higher lactate increase in controls.
3.2. CK
On the other hand, baseline CK was higher with significant increase in cases receiving AI (p = 0,042).
3.3. CRP
Both groups experienced CRP decrease after FI (p = 0.042), and in the control group there was a decrease regardless of the intervention (p = 0.016) and between the interventions regardless of the groups (p = 0.009). usCRP was different before intervention (p = 0.008).
4. Discussion
Patients diagnosed with schizophrenia receiving FI showed smaller increases of selected biomarkers compared to those under AI. FI might be less stressful to both cases and controls, which could be a protective factor for sedentary individuals under stressful activity: lactate usually increases until the organism performs the necessary oxidative adaptations, with changes in the number and shape of mitochondria. In the case of schizophrenia, this could be associated with reduced glia and oligodendrocyte mitochondria density and associated energy dysfunction. The study identified increased basal lactate in patients, with significant increase only after AI. This finding deserves special consideration, since it does not agree with other studies [
8] that found no difference in basal lactate levels between schizophrenia patients and controls, although they observed greater rise in lactate in the schizophrenia group during exercise that persisted after exercise.
The finding of increased basal lactate in schizophrenia is in line with other studies with this population [
9] of increased oxidative metabolism. The additional increase observed only after AI may reveal one additional step of the metabolic disturbance in schizophrenia, again in line with previous findings of decreased glutathione and lactate after exercise [
10], and of mitochondrial dysfunction at rest and after AI [
11].
Postmortem studies in schizophrenia [
9] also detected increased brain levels of lactate and pH in dorsolateral prefrontal cortex, frontal cortex, striatum, hippocampus, and cerebellum, whereas the Anterior limb of internal capsule (ALIC) had decreased lactate. These regional differences of brain lactate may be associated with different mitochondria activity according to tissue type [
12], and may explain bioenergetic differences in subjects with physical and mental disorders under different physical activity. In the case of schizophrenia, this could be associated with reduced glia and oligodendrocyte mitochondria density and associated energy dysfunction. In this perspective, the observed lactate differences in schizophrenia could be a consequence of previous mitochondrial dysfunction (either herd or acquired) generating chronic low level brain hypoxia, decreased astrocyte metabolism and downstream increased lactate levels [
13]. One explanation for the observed lactate increase in AI could fit with post-mortem evidence [
14] of glial and oligodendrocyte changes and consequent reduced myelination. The energetic process associated with mitochondrial dysfunction, increased lactate could lead to reduced synaptic plasticity, and neurotransmitter production and explain the observed cognitive deficits in schizophrenia [
3]. Despite several questions about lactate still deserve better explanation in schizophrenia, increased anaerobic state, decreased tissue perfusion, adrenaline release and sodium/potassium, and pump dysfunction could also contribute to consequent lactate increase and ATPase activity. Although additional information is needed about lactate (production, utilization and removal, particularly in the brain, where it is used for metabolic processes), altered lactate in schizophrenia provides evidence of energy imbalance and different energy and ATP production and utilization by different organs [
15]. In schizophrenia, chronic hypoxia, mitochondrial dysfunction, increased oxidative process would contribute to positive, negative and cognitive symptoms.
The observed bioenergetic cell changes have been previously described in other mental disorders such asdepression [
16] and bipolar disorder [
17] where mitochondrial function and shape end up undergoing changes, leading to an increase in oxidative stress and inflammation, as described in schizophrenia.
Lactate is an important substrate for skeletal muscle contraction and, in non-pathological situations, increases during and after physical exercise and is associated with good brain functioning [
18], as shown in the control group of this study. Recent animal studies detected a substance called Lac-Phe resulting from lactate and phenylalanine induced by physical exercise [
19] with important effect over feeding behavior. This effect raised the assumption of specific brain receptors activated by Lac-Phe, pointing to additional roles of lactate in the regulation of behavior.
The finding of different metabolic and oxidative processes in schizophrenia, allied to limited effect of neuroleptic drugs, calls attention to different treatment with non-pharmacological strategies in schizophrenia demonstrating an impact on mitochondrial dysfunction. This is the case of Systemic Photobiomodulation (PBM), which already demonstrated effect correcting mitochondrial dysfunction involving creatine kinase, lactate, troponin, C-reactive protein and Cytochrome C oxidase alterations in schizophrenia [
7]. Additionally, there is evidence of PBM increasing vascular flow and neural plasticity, through light absorption by ion channels, Ca
2+ release and resulting transcription factor activation, increased gene expression, increased neuronal metabolism and increased neuronal resistance to hypoxia [
20].
The relatively small sample size in both groups, especially in the postural group, was a limitation of this study. The absence of a control group with schizophrenia not performing physical activity also contributes to uncertainty. In addition, the study collected samples in two moments, before and after intervention, not allowing the measurement of changes over time. Future studies may include more subjects with weekly analyses of expanded blood markers, with additional measurements of behavior (feeding, activity, cognition) to perform this monitoring.
Results and additional evidence in patients, healthy subjects and animal models show that PBM by laser light therapy (LLT) deserves special attention in schizophrenia due to its potential for correcting basic metabolic dysfunction, in parallel to residual symptom improvement in schizophrenia. Additional PBM studies using these markers and outcome measures are essential to strengthen these initial findings of LLT in schizophrenia.
Author Contributions
"Conceptualization, Cristiano and Szortyka.; Methodology, Cristiano and Szortyka; Software, Cristiano and Szortyka; Validation, Cristiano and Szortyka, Formal Analysis, Cristiano and Abreu ; Investigation, Cristiano and Szortyka; Resources, Cristiano and Szortyka; Data Curation, Cristiano, Abreu and Szortyka Writing – Original Draft Preparation, Cristiano; Writing – Review & Editing, Cristiano and Abreu; Visualization, Abreu; Supervision, Abreu; Project Administration, Cristiano and Szortyka; Funding Acquisition, Cristiano, Abreu and Szortyka”.
Acknowledgments
We would like to thank the participants of this study and the researchers who collected the data.
Role of the funding source
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES, number 88887.653567/2021-00) and data collection and processing was supported by the Fund for Research Incentive (FIPE) – HCPA (number 150066).
Declaration of competing interest
The authors declare that they have no competing financial interests that could have appeared to influence the research reported in this paper.
Data availability
Data will be made available on request.
References
- Calì, C.; Tauffenberger, A.; Maistretti, P. The strategic location of glycogen and lactate: from body energy reserve to brain plasticity. Front Cell Neurosci 2019, 13, 82. [Google Scholar] [CrossRef]
- Proia, P.; Liegro, C.; Schiera, G.; Fricano, A.; Liegro, I. Lactate as a metabolite and a regulator in the central nervous system. Int J Mol Sci 2016, 17, 1450. [Google Scholar] [CrossRef]
- Sullivan, C.; Mielnik, C.; Funk, A.; O’Donovan, S.M.; Bentea, E.; Pletnikov, M.; et al. Measurement of lactate levels in postmortem brain, iPSCs, and animal models of schizophrenia. Sci Rep 2010, 9, 5087. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Cotter, J.; Elliott, R.; French, P.; Yung, A.R. A systematic review and meta-analysis of exercise interventions in schizophrenia patients. Psychol Med 2015, 45, 1343–61. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Küstne, R.B.; Martín, C.; Pastor, L. Prevalence of psychotic disorders and its association with methodological issues. A systematic review and meta-analyses. PLoS One 2018, 13, e0195687. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5); American Psychiatric Association: Arlington, VA, 2013. [Google Scholar]
- Cristiano, V.B.; Szortyka, M.F.V.; Lobato, M.I.; Ceresér, K.M.; Belmonte-de-Abreu, P. Postural changes in different stages of schizophrenia is associated with inflammation and pain: a cross-sectional observational study. Int J Psychiatry Clin Pract 2017, 21, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Valiente-Pallejà, A.; Torrell, H.; Alonso, Y.; Vilella, E.; Muntané, G.; Martorell, L. Increased blood lactate levels during exercise and mitochondrial DNA alterations converge on mitochondrial dysfunction in schizophrenia. Schizophr Res 2020, 220, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Pruett, B.S.; Meador-Woodruff, J.H. Evidence for altered energy metabolism, increased lactate, and decreased pH in schizophrenia brain: a focused review and meta-analysis of human postmortem and magnetic resonance spectroscopy studies. Schizophr Res 2020, 223, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Looney, J.M.; Childs, H.M. The Lactic Acid and Glutathione Contents of the Blood of Schizophrenic Patients. J Clin Invest 1934, 13, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Berk, M. The many roads to mitochondrial dysfunction in neuroimmune and neuropsychiatric disorders. BMC Med 2015, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Glancy, B.; Kane, D.A.; Kavazis, A.N.; Goodwin, M.L.; Willis, W.T.; Gladden, L.B. Mitochondrial lactate metabolism: history and implications for exercise and disease. J Physiol 2021, 599, 863–888. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.F.S.; Brito, M.D.; Yuzawa, J.M.C.; Rosenstock, T.R. Mitochondrial dysfunction and changes in high-energy compounds in diferent cellular models associated to hypoxia: implication to schizophrenia. Sci Rep 2019, 9, 18049. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.C. Mitochondrial dysfunction in schizophrenia: with a focus on postmortem studies. Mitochondrion 2021, 56, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Canever, L.; Oliveira, L.; D’Altoé De Luca, R.; Correa, P.T.; De BFraga, D.; Matos, M.P.; et al. A rodent model of schizophrenia reveals increase in creatine kinase activity with associated behavior changes. Oxid Med Cell Longev 2010, 3, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Sgumpp, A.M.; Behnke, A.; Bach, A.M.; Piller, S.; Boeck, C.; Rojas, R.; et al. Mitochondrial bioenergetics in leukocytes and oxidative stress in blood serum of mild to moderately depressed women. Mitochondrion 2021, 58, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Scaini, G.; Andrews, T.; Lima, C.N.C.; Benevenuto, D.; Streck, E.; Quevedo, J. Mitochondrial dysfunction as a critical event in the pathophysiology of bipolar disorder. Mitochondrion 2021, 57, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Tsukamoto, H.; Takenaka, S.; Olesen, N.D.; Petersen, L.G.; Sorensen, H.; et al. Maintained exercise-enhanced brain executive function related to cerebral lactate metabolism in men. FASEB J 2018, 32, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Li, V.L.; He, Y.; Contrepois, K.; Liu, H.; Kim, J.T.; Wiggenhorn, A.L.; et al. An exercise-inducible metabolite that suppresses feeding and obesity. Nature, 2022, 606, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Salehpour, F.; Mahmoudi, J.; Kamari, F.; Sadigh-Eteghad, S.; Rasta, S.H.; Hamblin, M.R. Brain Photobiomodulation Therapy: a narrative review. Mol Neurobiol 2018, 55, 6601–6636. [Google Scholar] [CrossRef] [PubMed]
Short Biography of Authors
Viviane Batista Cristiano
Born in Porto Alegre, Rio Grande do Sul, Viviane studied at Centro Universitário Metodista IPA((2006-2010), she completed a post graduation course in Sports Physiotherapy at Unisc, (2012-2014), becoming a specialist member of the National Sports and physical activity Physiotherapy Society (SONAFE) (2015).
She lives in Porto Alegre, Rio Grande do Sul, where she owns a private physiotherapy clinic.
She is a Post graduatio professor in Sports Physiotherapy at Uniguaçu and at Unijui. Besides that, she teaches at Laser Therapy courses, eletrotherapy, miofascial release and dry needling. She also worked as a volunteer at the 2016 Olimpic Games in Rio.
Michele Fonseca Szortyka
Graduated in Physiotherapy from Centro Universitário Metodista IPA (2006-2010). Master degree in the Program in Psychiatry and Behavioral Sciences, Federal University of Rio Grande do Sul - UFRGS (2012-2014). PhD by Program in Psychiatry and Behavioral Sciences, Federal University of Rio Grande do Sul - UFRGS (2016-2021), with a CAPES scholarship in these periods.She lives in Camaquã, Rio Grande do Sul, where she has an office and provides clinical care. She has articles published in the field of psychiatry
Paulo Silva Belmonte de Abreu
Born in Camaquã, Rio Grande do Sul, Paulo studied in public schools from primary school to higher education. Medical School at UFRGS(1972-1977), medical residency in psychiatry at PUCRS(1978-1981), specialized in TEM(Higher Education Methods and Techniques) at PUCRS(1985). Master´s Degree in Health Science sat the John Hopkins University School of Hygiene and Public Health, Baltimore, USA(1986-1987). Doctor´s degree in Medical Science at UFRGS with a CAPES doctorate sandwich at John Hopkins University School of Hygiene and Public Health(1995) and post doctorate in Molecular Biology at UFRGS(2000 -2001). Master´s degree in Health Sciences at Minho University, Portugal(2010). Currently living in Porto Alegre, Rio Grande do Sul and full professor at the Forensic and Psychiatry Department of Medicine at UFRGS, CNPq researcher 1C, Quotations 2060, number of academic studies 124 Factor H(*), 25 (web of Science), Quotations 4065 89 Factor H = 33 i1 = 89, 4065 quotations (Google Scholar), index H= 28(Research Gate); 2 times post graduation psychiatry program coordenator at UFRGS(2006-2008), 2 times coordenator at the UFRGS Board(CONSUN), 2 times at CEPE(Comissão de ensino e pesquisa – Teaching and Research Commission, Teaching Vacancy Commission, Unit Board member(UFRGS), head of the psychiatry department at UFRGS Medical School, from 2013 to 2017. Head of the psychiatry servisse at HCPA for two managements(2010-2013), member of the editorial board of the psychiatry magazine in RS and neurobiology magazine, Brazilian magazine psychiatry reviewer, Temas Magazine, Clinical Psychiatry magazine, Trends in Psychiatry, Epidemiology Brazilian Magazine, Archives of general Psychiatry and Biological Psychiatry, Psychiatry Medical Residency Program Coordenator, assistant physician at HCPA, leading a schizophrenia program(PRODESQ), and standing member of the Sulriograndense Medicine Academy, Celular Therapy Center Teams, , Translational Psychiatry Institute and Neuromodulation Group. Contemplated by the Universal call of CNPq 14/2013 adn Universal of CNPq, MctCT Infra and ProSUs.Two times presidente of the Transcranial Brazilian Association of magnetic stimulation(ABEMT). Current presidente of ABECER(Ass Brasilera de Estimulação Cerebral – Brazilian Association of Brain Stimulation), AGAFAPE(Associação Gaucha de Pacientes Esquizofênicos)Honorary Member, Member of the trauma studies Commission, Psychiatry Brazilian Association, National Health Department Consultant for Guidelines and Treatment with exceptional drugs since 2003 and member of the Neuromodulation Group and of the Interventionist Psychiatry Department of Moinhos de Vento Hospital. 41 years of medicine with emphasis in Psychiatry, working specially with the following topics: Schizophrenia, Psychoses and insanity, ttention déficit and Inteventionist Psychiatry. Participates in international collaborative studies in the determination of genetic bases in psychoses and attention disorders, of psychoses animal models and new therapeutic methods in Psychiatry through stem cells, brain stimulation through eletricity, magnetic pulses, photonics , deep implants, ablative surgery, , neuro protection and neurogenesis through neurotrophin regulation and oxidative stress reduction, along with other researchers from UFRGS, UFRJ, USP, Unifesp, University of Braga, Portugal and University of Toronto, with national(CNPq, CAPES, FAPERGS, FIPE-HCPA)and international(Stanley Foundation, Grices-Portugal, IBRO – International, Brain Foundation and University of Toronto)funding. His main goal is the development and spread of methods to identify, diagnose and treat in a more effective way mental disorders and decrease the side effects of these disorders in individuals, families and Society.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).