Submitted:
24 August 2023
Posted:
24 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Effects of Nitrogen on Plants
3. Effects of Nitrogen on Rapeseed Yield
3.1. Effects of Nitrogen on Photosynthetic Assimilation of Rapeseed
3.2. Nitrogen Affects the Substance Distribution of Rapeseed
3.3. Molecular Mechanism of Nitrogen Affecting Rapeseed Yield
| Genes | Plants | Functions |
|---|---|---|
|
LBD gene family (LBD37, LBD38, LBD39) |
Arabidopsis | LBD transcription factors inhibition of anthocyanin biosynthesis and nitrogen availability signal [65] |
|
NRT1.1; NRT2.1; NRT2 |
Arabidopsis | Nitrate transport [66,67,68,69] |
| MYB | Soybean; Sugarcane ; Foxtail millet |
Regulate nitrate transporters [70,71,72] |
| NLP7 | Arabidopsis | Regulation of nitrate assimilation [73] |
| Nitrate sensors and transcriptional activators initiate nitrate-mediated transcriptome signaling [74] | ||
| NLP5 | Maize | Regulation of nitrate assimilation [75] |
| CLC gene family | Arabidopsis | Regulation of nitrate transport [76,77] |
| CBL, CIPK8 | Arabidopsis | Regulation of nitrate transport and assimilation [78] |
|
AMT1.3; AMT2.1; AMT2.2; AMT2.3; AMT3.1; AMT3.2; |
Rice | Regulation of ammonium salt absorption and transport [79] |
| SWEETs | Maize; Arabidopsis |
Sucrose transport; the expression affected by nitrogen [55,80,81] |
| SUTs | Arabidopsis; Maize |
Sucrose transport; expression is affected by nitrogen [55,82] |
| NAS1 /NAP1 | Soybean | Regulating the flow distribution of PEP to maintain carbon and nitrogen balance [83] |
| PBF1 | Maize | Regulate carbon and nitrogen metabolism in a nitrogen-dependent manner [5] |
| ANR1 | Arabidopsis; Chrysanthemum |
ANR1-mediated auxin response [9,84] |
| DNR1 | Rice | DNR1-mediated auxin response [63] |
| GRF4 | Rice | Regulation of nitrogen absorption, assimilation, and transport [64] |
| DREB1C | Rice | Regulation of nitrogen utilization; Ethylene-mediated [62] |
4. Effects of Nitrogen on the Oil Content of Rapeseed
4.1. Nitrogen Affects the Synthesis of Lipids and Proteins in Rapeseed
4.2. Molecular Mechanism of Nitrogen Level Affecting Lipid Synthesis in Rapeseed
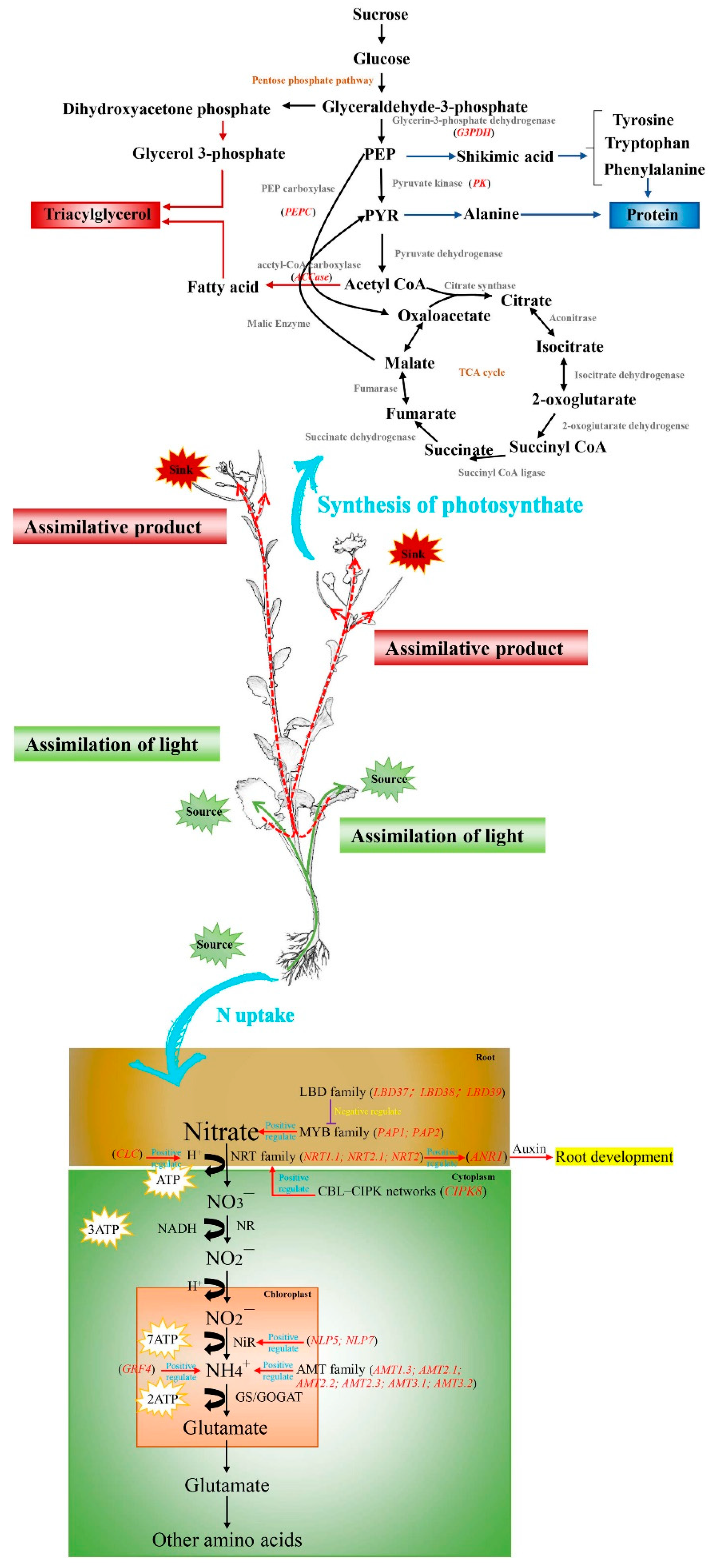
5. Discussion and Expectation
Acknowledgments
References
- Yan, G.; Chen, B.; Xu, K.; Gao, G.; Lv, P.; Wu, X.; Li, F.; Li, J. , Differential gene expression profiles in developing seeds of Brassica napus L. under different nitrogen application levels. Acta Agron. Sin 2013, 38, 2052–2060. [Google Scholar] [CrossRef]
- Wang, H.; Yin, Y. , Analysis and strategy for oil crop industry in China. Chin. J. Oil Crop Sci 2014, 36, 414–421. [Google Scholar]
- Stahl, A.; Vollrath, P.; Samans, B.; Frisch, M.; Wittkop, B.; Snowdon, R. J. , Effect of breeding on nitrogen use efficiency-associated traits in oilseed rape. J Exp Bot 2019, 70, 1969–1986. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, J.; Gu, H.; Lu, Z.; Liao, S.; Li, X.; Cong, R.; Ren, T.; Lu, J. Effects of application of nitrogen on seed yield and quality of winter oilseed rape (Brassica napus L.). Acta Agron. Sin 2023, 2002-2011.
- Ning, L.; Wang, Y.; Shi, X.; Zhou, L.; Ge, M.; Liang, S.; Wu, Y.; Zhang, T.; Zhao, H. , Nitrogen-dependent binding of the transcription factor PBF1 contributes to the balance of protein and carbohydrate storage in maize endosperm. Plant Cell 2023, 35, 409–434. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Cheng, B.; Xu, K.; Gao, G.; Lv, P.; Wu, X.; Li, F.; Li, J. , Response of ACCase, DGAT2 and PEPC genes in developing seeds of Brassica napus to different nitrogen levels. Plant Nutr. Fert. Sci 2012, 18, 1370–1377. [Google Scholar]
- Zhou, N. The research on the nitrogen's effect on rapeseed yield and quality and relevant analysis. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2005. [Google Scholar]
- Huang, S.; Sun, G.; Jin, J.; He, P.; Wang, X.; Zhang, G.; Xie, J.; Zhang, K. , The effect of nitrogen levels on grain yield, protein, amino acid and fatty acid of high-oil maize. Chin. Agric. Sci 2004, 37, 250–255. [Google Scholar]
- Zhang, H.; Forde, B. G. , An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science 1998, 279, 407–409. [Google Scholar] [CrossRef]
- Zhai, J.; Xue, J.; Zhang, Y.; Zhang, G.; Shen, D.; Wang, Q.; Liu, C.; Li, S. , Effect of nitrogen application rate on lodging resistance of spring maize stalks under integrated irrigation with water and fertilizer. J. Maize Sci 2021, 29, 137–144. [Google Scholar]
- Ni, F. Effect of different agronomic measures on carbon-nitrogen metabolism and oil accumulation in rapeseed pod. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2018. [Google Scholar]
- Remans, T.; Nacry, P.; Pervent, M.; Girin, T.; Tillard, P.; Lepetit, M.; Gojon, A. , A central role for the nitrate transporter NRT2.1 in the integrated morphological and physiological responses of the root system to nitrogen limitation in Arabidopsis. Plant Physiol 2006, 140, 909–921. [Google Scholar] [CrossRef]
- Guan, C. , High-quality rapeseed physiological ecology and modern cultivation technology. first ed.; China Threegorges Press: Beijing, 2006. [Google Scholar]
- Pan, R. , Plant physiology. seventh ed.; Higher Education Press: Beijing, 2012. [Google Scholar]
- Zhang, X.; He, S.; Wang, W.; Guo, W.; Yu, L. , Effect of different nitrogen level on nitrogen metaboli characteristic, grain yield, and quality of strong gluten spring wheat. J. Triticeae Crops 2006, 130–133. [Google Scholar]
- Khan, S.; Anwar, S.; Kuai, J.; Noman, A.; Shahid, M.; Din, M.; Ali, A.; Zhou, G. , Alteration in yield and oil quality traits of winter rapeseed by lodging at different planting density and nitrogen rates. Sci Rep 2018, 8, 634. [Google Scholar] [CrossRef] [PubMed]
- Akhatar, J.; Singh, M. P.; Sharma, A.; Kaur, H.; Kaur, N.; Sharma, S.; Bharti, B.; Sardana, V. K.; Banga, S. S. , Association mapping of seed quality traits under varying conditions of nitrogen application in Brassica juncea L. czern & coss. Front Plant Sci 2020, 11, 744. [Google Scholar]
- Tang, W. Effects of nitrate and ammonium supply ratios on growth, physiology and gene expression of oilseed rape (Brassica napus). Ph.D. Thesis, Hunan Agricultural University, Changsha, China, 2019. [Google Scholar]
- Wu, Y.; Ma, N.; Huang, X.; Peng, H.; Li, Z.; Niu, Y.; Zhang, C. , Effect of nitrogen fertilizer on agronomic traits, yield, quality and nitrogen use efficiency in Brassica napus of "ZhongShuang 11" under Different Densities. J. Sichuan Agric. Univ 2014, 32, 260–264+282. [Google Scholar]
- Zhu, S.; Li, Y.; Yu, C.; Xie, L.; Hu, X.; Zhang, S.; Liao, X.; Liao, X.; Che, Z. , Effects of planting density and nitrogen application rate on rapeseed yield and nitrogen use efficiency. Chin. J. Oil Crop Sci. 2013, 35, 179–184. [Google Scholar]
- Plaxton, W. C. In Annual plant reviews volume 22 control of primary metabolism in plants, 2006.
- Zhou, X.; Fei, Z.; Thannhauser, T. W.; Li, L. , Transcriptome analysis of ectopic chloroplast development in green curd cauliflower (Brassica oleracea L. var. botrytis). BMC Plant Biol 2011, 11, 169. [Google Scholar] [CrossRef] [PubMed]
- Urban, L.; Aarrouf, J.; Bidel, L. P. R. , Assessing the effects of water deficit on photosynthesis using parameters derived from measurements of leaf gas exchange and of chlorophyll a fluorescence. Front Plant Sci 2017, 8, 2068. [Google Scholar] [CrossRef] [PubMed]
- Michelet, L.; Zaffagnini, M.; Morisse, S.; Sparla, F.; Pérez-Pérez, M. E.; Francia, F.; Danon, A.; Marchand, C. H.; Fermani, S.; Trost, P.; Lemaire, S. D. , Redox regulation of the Calvin-Benson cycle: something old, something new. Front Plant Sci 2013, 4, 470. [Google Scholar] [CrossRef]
- Chapman, S. P.; Trindade dos Santos, M.; Johnson, G. N.; Kritz, M. V.; Schwartz, J.-M. , Cyclic decomposition explains a photosynthetic down regulation for Chlamydomonas reinhardtii. Biosystems 2017, 162, 119–127. [Google Scholar] [CrossRef]
- Liu, T. Mechanisms underlying the effects of nitrogen supply on leaf nitrogen-forms partitioning and photosynthetic nitrogen use efficiency of Brassica napus. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2018. [Google Scholar]
- Xu, H. Nitrogen diagnosis of oilseed rape at different growth stages and leaf positions using chlorophyll fluorescence techniques. Ph.D. Thesis, Zhejiang university, Hangzhou, China, 2020. [Google Scholar]
- Bi, H.; Liu, P.; Jiang, Z.; Ai, X. , Overexpression of the rubisco activase gene improves growth and low temperature and weak light tolerance in Cucumis sativus. Physiol Plant 2017, 161, 224–234. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, H.; Song, J.; Wei, S.; Zhang, Y.; Wang, Q. , Effects of nitrogen fertilizer rate on the chlorophyll content and quality of kidney bean. Crops 2013, 81–87. [Google Scholar]
- Zhang, R. Effect of urea application rate on yield and quality of different soybeans. Ph.D. Thesis, Shenyang Agricultural University, Shenyang, China, 2008. [Google Scholar]
- Xiong, D.; Liu, X.; Liu, L.; Douthe, C.; Li, Y.; Peng, S.; Huang, J. , Rapid responses of mesophyll conductance to changes of CO2 concentration, temperature and irradiance are affected by N supplements in rice. Plant Cell Environ 2015, 38, 2541–2550. [Google Scholar] [CrossRef] [PubMed]
- Bloom, A. J. , Energetics of nitrogen acquisition. In Annual Plant Reviews Volume 42, 2010; pp 63-81.
- Yu, S.; Lo, S.; Ho, T. D. , Source-sink communication: regulated by hormone, nutrient, and stress cross-signaling. Trends Plant Sci 2015, 20, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Baxter, I. R.; Lahner, B.; Reinders, A.; Salt, D. E.; Ward, J. M. , Arabidopsis NPCC6/NaKR1 is a phloem mobile metal binding protein necessary for phloem function and root meristem maintenance. Plant Cell 2010, 22, 3963–3979. [Google Scholar] [CrossRef]
- Tilsner, J.; Kassner, N.; Struck, C.; Lohaus, G. , Amino acid contents and transport in oilseed rape (Brassica napus L.) under different nitrogen conditions. Planta 2005, 221, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Masclaux-Daubresse, C.; Reisdorf-Cren, M.; Orsel, M. , Leaf nitrogen remobilisation for plant development and grain filling. Plant Biol 2008, 10 (Suppl. 1), 23–36. [Google Scholar] [CrossRef]
- Osorio, S.; Ruan, Y.; Fernie, A. R. , An update on source-to-sink carbon partitioning in tomato. Front Plant Sci 2014, 5, 516. [Google Scholar] [CrossRef]
- Gomes, E. N.; Moterle, D.; Biasi, L. A.; Koehler, H. S.; Kanis, L. A.; Deschamps, C. , Plant densities and harvesting times on productive and physiological aspects of Stevia rebaudiana Bertoni grown in southern Brazil. An. Acad. Bras. Cienc 2018, 90, 3249–3264. [Google Scholar] [CrossRef]
- Cholewa, E.; Griffith, M. , The unusual vascular structure of the corm of eriophorum vaginatum: implications for efficient retranslocation of nutrients. J Exp Bot 2004, 55, 731–741. [Google Scholar] [CrossRef]
- Yu, S. M.; Lo, S. F.; Ho, T. D. , Source-sink communication: Regulated by hormone, nutrient, and stress cross-signaling. Trends Plant Sci 2015, 20, 844–857. [Google Scholar] [CrossRef]
- Crafts-Brandner, S.; Hölzer, R.; Feller, U. , Influence of nitrogen deficiency on senescence and the amounts of RNA and proteins in wheat leaves. Physiol. Plant 1998, 102, 192–200. [Google Scholar] [CrossRef]
- Uhart, S. A.; Andrade, F. H. , Nitrogen and carbon accumulation and remobilization during grain filling in maize under different source/sink ratios. Crop Sci 1995, 35. [Google Scholar] [CrossRef]
- Cao, L.; Wu, X.; Yang, R.; Tian, Y.; Chen, X.; Chen, B.; Li, Y.; Gao, Y. , Differences of nitrogen status between different N-uptake-efficiency rapeseed (Brassica napus L.) cultivars. Acta Agron 2013, 38, 887–895. [Google Scholar] [CrossRef]
- Lam, H.; Coschigano, K. T.; Oliveira, I. C.; Melo-Oliveira, R.; Coruzzi, G. , The molecular-genetics of nitrogen assimilation into amino acids in higher plants. Annu Rev Plant Physiol Plant Mol Biol 1996, 47, 569–593. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cheng, Y.; Chen, K.; Tsay, Y. , Nitrate transport, signaling, and use efficiency. Annu Rev Plant Biol 2018, 69, 85–122. [Google Scholar] [CrossRef]
- Xing, J.; Cao, X.; Zhang, M.; Wei, X.; Zhang, J.; Wan, X. , Plant nitrogen availability and crosstalk with phytohormones signalings and their biotechnology breeding application in crops. Plant Biotechnol J 2022, 1320–1342. [Google Scholar]
- Khademi, S. O. C., Joseph 3rd; Remis, J.; Robles-Colmenares, Y.; Miercke, L. J. W.; Stroud, R. M. , Mechanism of ammonia transport by Amt/MEP/Rh: structure of AmtB at 1.3.5 angstrom. Science 2004, 305, 1587–1594. [Google Scholar] [CrossRef]
- Garai, S.; Tripathy, B. C. , Alleviation of nitrogen and sulfur deficiency and enhancement of photosynthesis in Arabidopsis thaliana by overexpression of uroporphyrinogen III methyltransferase (UPM1). Front Plant Sci 2018, 8. [Google Scholar]
- Meng, S.; Wang, S.; Quan, J.; Su, W.; Lian, C.; Wang, D.; Xia, X.; Yin, W. , Distinct carbon and nitrogen metabolism of two contrasting poplar species in response to different N supply levels. Int J Mol Sci 2018, 19, 2302. [Google Scholar] [CrossRef]
- Lea, P. J.; Azevedo, R. A. , Nitrogen use efficiency. 1. Uptake of nitrogen from the soil. Ann App Biol 2006, 149, 243–247. [Google Scholar] [CrossRef]
- Navarro-León, E.; Barrameda-Medina, Y.; Lentini, M.; Esposito, S.; Ruiz, J. M.; Blasco, B. , Comparative study of Zn deficiency in L. sativa and B. oleracea plants: NH4+assimilation and nitrogen derived protective compounds. Plant Sci 2016, 248, 8–16. [Google Scholar] [CrossRef]
- Tang, W.; He, X.; Qian, L.; Wang, F.; Zhang, Z.; Sun, C.; Lin, L.; Guan, C. , Comparative transcriptome analysis in oilseed rape (Brassica napus) reveals distinct gene expression details between nitrate and ammonium nutrition. Genes (Basel) 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Tang, L.; Zhang, W.; Cao, W.; Zhu, Y. , Dynamic analysis on response of dry matter accumulation and partitioning to nitrogen fertilizer in wheat cultivars with different plant types. Acta Agron. Sin 2009, 35, 2258–2265. [Google Scholar]
- Song, X.; Liu, Q.; Song, H.; Guan, C.; Rong, x.; Wang, J.; Wang, S. , Changes of soluble sugar and free amino acids in stem and leaf and their effects on yield of rapeseed. Acta Agric. Boreali- Occident. Sin 2010, 19, 187–191. [Google Scholar]
- Zhao, Y.; Ning, P.; Feng, X.; Ren, H.; Cui, M.; Yang, L. , Characterization of stem nodes associated with carbon partitioning in maize in response to nitrogen availability. Int J Mol Sci 2022, 23, 4389. [Google Scholar] [CrossRef]
- Tang, X.; Guan, C. , Advances in the regulation of carbon, nitrogen and fat metabolism in crop yield and quality. J. Hunan Agric. Univ 1997, 96–106. [Google Scholar]
- Tang, X.; Guan, C. , Effects of nitrogen application on enzymatic activities of rape and its relationship with yield and quality. Chin. J. Oil Crop Sci 2001, 33–38. [Google Scholar]
- Mao, C.; He, J.; Liu, L.; Deng, Q.; Yao, X.; Liu, C.; Qiao, Y.; Li, P.; Ming, F. , OsNAC2 integrates auxin and cytokinin pathways to modulate rice root development. Plant Biotechnol J 2020, 18, 429–442. [Google Scholar] [CrossRef]
- Wu, K.; Wang, S.; Song, W.; Zhang, J.; Wang, Y.; Liu, Q.; Yu, J.; Ye, Y.; Li, S.; Chen, J.; Zhao, Y.; Wang, J.; Wu, X.; Wang, M.; Zhang, Y.; Liu, B.; Wu, Y.; Harberd, N. P.; Fu, X. , Enhanced sustainable green revolution yield via nitrogen-responsive chromatin modulation in rice. Science 2020, 367, eaaz2046. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, K.; Harberd, N. P.; Fu, X. , Green Revolution DELLAs: From translational reinitiation to future sustainable agriculture. Mol. Plant 2021, 14, 547–549. [Google Scholar] [CrossRef]
- Nunes-Nesi, A.; Fernie, A. R.; Stitt, M. , Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol. Plant 2010, 3, 973–996. [Google Scholar] [CrossRef]
- Wei, S.; Li, X.; Lu, Z.; Zhang, H.; Ye, X.; Zhou, Y.; Li, J.; Yan, Y.; Pei, H.; Duan, F.; Wang, D.; Chen, S.; Wang, P.; Zhang, C.; Shang, L.; Zhou, Y.; Yan, P.; Zhao, M.; Huang, J.; Bock, R.; Qian, Q.; Zhou, W. , A transcriptional regulator that boosts grain yields and shortens the growth duration of rice. Science 2022, 377, eabi8455. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, L.; Shen, C.; Ji, Z.; Zhang, H.; Zhang, T.; Li, Y.; Yu, J.; Yang, N.; He, Y.; Tian, Y.; Wu, K.; Wu, J.; Harberd, N. P.; Zhao, Y.; Fu, X.; Wang, S.; Li, S. , Natural allelic variation in a modulator of auxin homeostasis improves grain yield and nitrogen use efficiency in rice. The Plant Cell 2021, 33, 566–580. [Google Scholar]
- Li, S.; Tian, Y.; Wu, K.; Ye, Y.; Yu, J.; Zhang, J.; Liu, Q.; Hu, M.; Li, H.; Tong, Y.; Harberd, N. P.; Fu, X. , Modulating plant growth-metabolism coordination for sustainable agriculture. Nature 2018, 560, 595–600. [Google Scholar] [PubMed]
- Rubin, G.; Tohge, T.; Matsuda, F.; Saito, K.; Scheible, W.-R. , Members of the LBD family of transcription factors repress anthocyanin synthesis and affect additional nitrogen responses in Arabidopsis. The Plant cell 2009, 21, 3567–3584. [Google Scholar] [PubMed]
- Ho, C.; Lin, S.; Hu, H.; Tsay, Y. , CHL1 functions as a nitrate sensor in plants. Cell 2009, 138, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Tsay, Y.; Schroeder, J. I.; Feldmann, K. A.; Crawford, N. M. , The herbicide sensitivity gene CHL1 of Arabidopsis encodes a nitrate-inducible nitrate transporter. Cell 1993, 72, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Little, D. Y.; Rao, H.; Oliva, S.; Daniel-Vedele, F.; Krapp, A.; Malamy, J. E. , The putative high-affinity nitrate transporter NRT2.1 represses lateral root initiation in response to nutritional cues. PNAS 2005, 102, 13693–13698. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Okamoto, M.; Crawford, N. M.; Siddiqi, M. Y.; Glass, A. D. M. , Dissection of the AtNRT2.1: AtNRT2.2 inducible high-affinity nitrate transporter gene cluster. Plant Physiol 2007, 143, 425–433. [Google Scholar] [CrossRef]
- Miyake, K.; Ito, T.; Senda, M.; Ishikawa, R.; Harada, T.; Niizeki, M.; Akada, S. , Isolation of a subfamily of genes for R2R3-MYB transcription factors showing up-regulated expression under nitrogen nutrient-limited conditions. Plant Mol Biol 2003, 53, 237–245. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, S.; Su, Y.; Lin, Z.; Guo, J.; Li, M.; Wang, Z.; Que, Y.; Xu, L. , Transcripts and low nitrogen tolerance: regulatory and metabolic pathways in sugarcane under low nitrogen stress. Environ. Exp. Bot 2019, 163, 97–111. [Google Scholar]
- Qian, D. Q.; Ting, W. X.; Qin, H. L.; Xin, Q.; Hao, G. L.; Ya, X. W.; Shi, X. Z.; Bin, Z. Y.; Qing, J. G.; Min, D. X.; Hong, M. D.; Zhi, M. Y.; Ming, C. , MYB-like transcription factor SiMYB42 from foxtail millet (Setaria italica L.) enhances Arabidopsis tolerance to low-nitrogen stress. Hereditas 2018, 40. [Google Scholar]
- Castaings, L.; Camargo, A.; Pocholle, D.; Gaudon, V.; Texier, Y.; Boutet-Mercey, S.; Taconnat, L.; Renou, J.-P.; Daniel-Vedele, F.; Fernandez, E.; Meyer, C.; Krapp, A. , The nodule inception-like protein 7 modulates nitrate sensing and metabolism in Arabidopsis. Plant J. Cell Mol. Biol 2009, 57, 426–435. [Google Scholar]
- Liu, K.; Liu, M.; Lin, Z.; Wang, Z.; Chen, B.; Liu, C.; Guo, A.; Konishi, M.; Yanagisawa, S.; Wagner, G.; Sheen, J. , NIN-like protein 7 transcription factor is a plant nitrate sensor. Science 2022, 377, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Wang, Y.; Liu, Y.; Jiang, L.; He, B.; Ning, L.; Du, H.; Lv, Y.; Zhou, L.; Lin, F.; Zhang, T.; Liang, S.; Lu, H.; Zhao, H. , The NIN-like protein 5 (ZmNLP5) transcription factor is involved in modulating the nitrogen response in maize. Plant J 2020, 102, 353–368. [Google Scholar] [CrossRef]
- Harada, H.; Kuromori, T.; Hirayama, T.; Shinozaki, K.; Leigh, R. A. , Quantitative trait loci analysis of nitrate storage in Arabidopsis leading to an investigation of the contribution of the anion channel gene, AtCLC-c, to variation in nitrate levels. J Exp Bot 2004, 55, 2005–2014. [Google Scholar] [CrossRef]
- De Angeli, A.; Monachello, D.; Ephritikhine, G.; Frachisse, J.-M.; Thomine, S.; Gambale, F.; Barbier-Brygoo, H. , CLC-mediated anion transport in plant cells. P Philos. Trans.: Biol. Sci 2009, 364, 195–201. [Google Scholar]
- Hu, H.; Wang, Y.; Tsay, Y. , AtCIPK8, a CBL-interacting protein kinase, regulates the low-affinity phase of the primary nitrate response. Plant J. Cell Mol. Biol 2009, 57, 264–278. [Google Scholar] [CrossRef]
- Wu, X.; Ding, C.; Baerson, S. R.; Lian, F.; Lin, X.; Zhang, L.; Wu, C.; Hwang, S.; Zeng, R.; Song, Y. , The roles of jasmonate signalling in nitrogen uptake and allocation in rice (Oryza sativa L. ). Plant Cell Environ 2019, 42, 659–672. [Google Scholar]
- Ning, P.; Yang, L.; Li, C.; Fritschi, F. B. , Post-silking carbon partitioning under nitrogen deficiency revealed sink limitation of grain yield in maize. J Exp Bot 2018, 69, 1707–1719. [Google Scholar]
- Chen, H.; Huh, J.; Yu, Y.; Ho, L.; Chen, L.; Tholl, D.; Frommer, W. B.; Guo, W. , The Arabidopsis vacuolar sugar transporter SWEET2 limits carbon sequestration from roots and restricts Pythium infection. Plant J. Cell Mol. Biol 2015, 83, 1046–1058. [Google Scholar]
- Slewinski, T. L.; Meeley, R.; Braun, D. M. , Sucrose transporter1 functions in phloem loading in maize leaves. J Exp Bot 2009, 60, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Xiao, H.; Peng, Y.; Wang, J.; Lv, Q.; Wang, X. , Phosphoenolpyruvate reallocation links nitrogen fixation rates to root nodule energy state. Science 2022, 378, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Yu, J.; Duan, X.; Wang, J.; Zhang, Q.; Gu, K.; Hu, D.; Zheng, C. , The MADS transcription factor CmANR1 positively modulates root system development by directly regulating CmPIN2 in chrysanthemum. Hortic Res 2018, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Rathke, G. W.; Christen, O.; Diepenbrock, W. , Effects of nitrogen source and rate on productivity and quality of winter oilseed rape (Brassica napus L.) grown in different crop rotations. Field Crops Res. 2005, 94, 103–113. [Google Scholar] [CrossRef]
- Chen, J.; Lang, C.; Hu, Z.; Liu, Z.; Huang, R. , Antisense PEP gene regulates to ratio of protein and lipid content in Brassica napus seeds. J. Agric. Biotechnol 1999, 7, 316–320. [Google Scholar]
- Sangwan R S, Singh N, Plaxton W C. Phosphoenolpyruvate carboxylase activity and concentration in the endosperm of developing and germinating castor oil seeds. Plant Physiol 1992, 99(2): 445-9.
- Yu, S.; Du, S.; Yuan, J.; Hu, Y. , Fatty acid profile in the seeds and seed tissues of Paeonia L. species as new oil plant resources. Sci Rep 2016, 6, 26944. [Google Scholar] [CrossRef]
- Tang, M.; Guschina, I. A.; O'Hara, P.; Slabas, A. R.; Quant, P. A.; Fawcett, T.; Harwood, J. L. , Metabolic control analysis of developing oilseed rape (Brassica napus cv Westar) embryos shows that lipid assembly exerts significant control over oil accumulation. New Phytol 2012, 196, 414–426. [Google Scholar] [CrossRef]
- Nikolau, B. J.; Ohlrogge, J. B.; Wurtele, E. S. , Plant biotin-containing carboxylases. Arch. Biochem. Biophys 2003, 414, 211–222. [Google Scholar] [CrossRef]
- Chao, H.; Wang, H.; Wang, X.; Guo, L.; Gu, J.; Zhao, W.; Li, B.; Chen, D.; Raboanatahiry, N.; Li, M. , Genetic dissection of seed oil and protein content and identification of networks associated with oil content in Brassica napus. Sci Rep 2017, 7, 46295. [Google Scholar] [CrossRef]
- Champigny, M.-L.; Foyer, C. , Nitrate activation of cytosolic protein kinases diverts photosynthetic carbon from sucrose to amino acid biosynthesis: basis for a new concept. Plant Physiol 1992, 100, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, F. Cloning and functional analysis of key ACCase and PEPCase genes in lipid biosynthesis of rape seeds (Brasscia napus L.). Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2012. [Google Scholar]
- Bian, Z.; Chen, G.; Zhou, Q.; Lv, X.; Zhu, J.; Sun, L. , Cloning of a lipid synthesis-related gene DGAT2 from sunflower and transformation into tobacco. J. Shihezi Univ 2016, 34, 624–631. [Google Scholar]
- Mazzarino, R. C.; Baresova, V.; Zikánová, M.; Duval, N.; Wilkinson, T. G., 2nd; Patterson, D.; Vacano, G. N. , The CRISPR-Cas9 crADSL HeLa transcriptome: A first step in establishing a model for ADSL deficiency and SAICAR accumulation. MGM Rep 2019, 21, 100512. [Google Scholar] [CrossRef]
- Koga, Y.; Konishi, K.; Kobayashi, A.; Kanaya, S.; Takano, K. , Anaerobic glycerol-3-phosphate dehydrogenase complex from hyperthermophilic archaeon Thermococcus kodakarensis KOD1. J Biosci Bioeng 2019, 127, 679–685. [Google Scholar] [CrossRef]
- Zhou, X.; Fu, S. , Response of G3PDH and PK genes in Brassica napus L. to nitrogen fertilizer. J. Integr Agric 2019, 35, 110–114. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





