1. Introduction
Home hospitalization is already considered as a viable alternative for conventional in-hospital stay worldwide. The Hospital-at-home (HAH) model provides the delivery of acute care for patients suffering from several medical conditions including community acquired pneumonia (both bacterial and viral as in the case of COVID-19 pneumonia), urinary tract infections (of both lower and upper urinary tracts), soft tissue infections and exacerbations of chronic diseases including congestive heart failure and chronic obstructive pulmonary disease (COPD) [
1,
2,
3,
4]. Previous studies have shown not only satisfactory health outcomes in the home-hospitalization setting in the aforementioned diagnoses [
5,
6], but also cost reduction for the health care system, as well as increased patient satisfaction levels when compared to usual care [
3,
6].
Electrolyte abnormalities are common in acute patients, especially in those with many background comorbidities. For example, hyponatremia is common amongst cancer patients, suffering from both solid tumors and hematologic malignancies, and is associated with poor clinical outcomes [
7]. Hyponatremia is also common amongst patients presenting with COPD exacerbations, associated with worse prognosis, increased risk for readmissions and death [
8]. Patients suffering from pneumonia are also prone to develop hyponatremia, mainly as a consequence of the Syndrome of Inappropriate Andi-Diuretic Hormone Secretion (SIADH). Here also, hyponatremia is associated with significantly worse clinical outcomes [
9]. Hyperkalemia is common amongst end-stage renal failure patients, and is considered as a life-threatening complication, associated with catastrophic anomalies of the cardiac conduction system [
10,
11]. Acute kidney injury which is very common amongst acute patients [
12], giving rise to myriad electrolyte imbalances, is associated with increased risk of cardiac arrythmias, even beyond those directly inflicted by derangements of potassium [
13]. Hypomagnesemia is also common amongst acutely ill patients and is associated with worse clinical outcomes in general [
14] and increased risk of cardiac arrythmia in particular [
15]. Hypophosphatemia, either associated with sepsis or refeeding syndrome is also associated with increased incidence of muscle weakness, respiratory failure, lethargy, state of confusion and arrhythmias [
16,
17].
In light of the above, it is only reasonable to assume that the incidence of electrolyte abnormalities in the HAH setting is higher than reported. Although not included in the primary indications for HAH, the accumulated evidence regarding in-hospital patients makes it of the utmost importance to monitor electrolytes during home hospitalization as well as electrocardiographic (ECG) changes potentially arising from such anomalies. For example, potassium is an essential electrolyte used for maintenance of cardiac cells’ resting membrane potential and its derangements might lead to recognizable changes in ECG. These changes include peaked T waves and QRS prolongation [
18]. Monitoring the dynamics of QRS prolongation for example is established as an essential tool for clinicians, mainly in the setting of remote monitoring [
19,
20]. Our basic assumption is that the incidence of electrolytes’ imbalances and resultant risk of arrythmia in the HAH setting is still unknown.
In a yet unpublished research results [
21], the authors appreciated the rate of electrolyte abnormalities amongst our HAH patients reaching up to 80% of patients. During meticulous monitoring of HAH acutely ill patients, there are other, potentially life-threatening anomalies, necessitating ECG monitoring – such as those associated with covert myocardial damage during acute illness (also known as type 2 acute myocardial infarction, ascertained by increased levels of blood troponin, reaching a level of 30% in our HAH patients’ populations). Such findings, resembling electrolyte abnormalities, necessitate frequent ECG monitoring at patients’ homes [
22].
The above findings guided us to pursue an ECG technology that would enable once or twice daily ECG recording, potentially done by the patients themselves or their family members, that would be transmitted as a telemedicine input to our headquarter clinic. This would not be feasible using the legacy, 12-lead ECG machines we use in-hospital.
The Kardiamobile 6L ECG (supplementary Figure S1) is a portable ECG monitor that has shown promising results in its ability to monitor heart rate, atrioventricular (AV) conduction, as well as enabling selected interval duration measurements [
23]. In Sheba-Beyond, we established a HAH service utilized for acute-care patients as an alternative for in-hospital stay of acutely ill patients. We already published previous case reports regarding the safety and feasibility of this model [
24].
The aim of this study was to evaluate the reliability of the Kardiamobile portable 6-lead ECG device in comparison to a standard (legacy) 12-lead ECG in the monitoring of HAH patients. The main purpose of this comparative evaluation was to provide a research-based validation for the device's potential to identify specific arrhythmias and enable measurements of basic ECG intervals, as means for early detection of conduction derangements, and/or myocardial damage associated with electrolyte abnormalities, and covert myocardial damage in the HAH setting.
2. Methods
Study design
This was a prospective, comparative, open study. The study protocol was approved by the institutional review board at the Chaim Sheba Medical Center (IRB approval # SMC-23-3023) prior to initiation. All participants provided informed consent before enrollment in this study.
We prospectively recruited consecutive patients admitted to the Sheba Beyond, virtual hospital, in the HAH department, during a 3-month duration. We included adult patients that were hospitalized in the HAH settings for miscellaneous clinical indications and were hemodynamically stable (Systolic blood pressure above 110mmHg, diastolic blood pressure above 50mmHg, without new onset arrythmia upon admission and without rapid, chronic atrial flutter or fibrillation). Exclusion criteria were age younger than 18 years, patients with acute respiratory insufficiency (patients with room-air hypoxemia below 94% were supplemented with an oxygen generator), patients with decreased or fluctuating consciousness and those that were hemodynamically unstable.
Upon enrollment, demographic information, medical history, and baseline characteristics of the patients during hospitalization were collected to a datasheet from their electronic medical record.
Each patient underwent a 12-lead ECG recording using the legacy device as part of the routine patient's medical admission. The legacy ECG was obtained by a trained medical staff member, adhering to the standardized guidelines, at the emergency room or at an internal medicine department – prior to patient’s transportation to their homes. For patients admitted directly at their homes the first ECG was already done at their homes. The included patients went through a subsequent additional ECG recording, by the Kardiamobile portable 6-lead ECG upon admission. The Kardiamobile ECG was also recorded by a trained medical staff member. The device was placed as per the manufacturer's instructions, to maintain consistency in the placement and quality of the recordings.
The researchers assessed the records of both ECG modalities for the following parameters: Basic ECG/rhythm diagnoses: including Normal Sinus Rhythm, Sinus Tachycardia (over 100 BPM, Sinus Bradycardia (lower than 60 BPM), Atrial Fibrillation, Atrial Flutter, 1st Degree AV Block, 2nd Degree AV Block, Complete AV Block, and Sinus Arrhythmia. The ECG predominant intervals (PR, RR, and QT) were measured either automatically by the 12-lead legacy device or calculated from the printed output of the study device by the researchers. The comparison between devices was done using a binary classification for each parameter, as being either normal or abnormal. Normal values used were 0.12 - 0.2 and 0.6 - 1.2 seconds for PR and RR intervals respectively. For corrected QT (QTc), the normal values used were 0.35-0.45 and 0.36-0.46 seconds for men and women respectively.
Extracted data, from patients’ electronic medical records, included laboratory results and patient characteristics. Blood levels of sodium, potassium, calcium, magnesium, troponin, and D-dimer (taken routinely, as part of our HAH service, during hospitalization on clinical grounds) were recorded, as well as the following demographics: age, gender, weight, height, main complaint, length of hospitalization, background diagnoses, medications and clinical outcomes of the index hospitalization.
Statistical analysis
Categorical variables were described as frequencies and percentages. The distribution of continuous variables was tested by a histogram and a Q-Q plot. Continuous variables that were distributed normally are presented as mean ± standard deviations, and those that do not are described as median ± IQR [inter-quartile range].
Assessment of the level of agreement of diagnoses between devices
We compared the degree of agreement and disagreement of the research, 6-lead device (Kardiamobile) with the standard 12-lead ECG (legacy) with regards to nine of the options that are included in the automatic read-out of the legacy device (Normal Sinus Rhythm, Sinus Tachycardia, Sinus Bradycardia, Atrial Fibrillation, Atrial Flutter, 1st Degree AV Block, 2nd Degree AV Block, Complete AV Block, & Sinus Arrhythmia). Not all diagnoses and intervals are automatically calculated and reported by the 6-lead device and some were diagnosed/calculated by the researchers, as intended by the manufacturer’ instructions.
When comparing the overall agreement between devices and methods, we translated each diagnosis and interval to a binary parameter, either normal or pathologic. We appreciated the level of consensus between devices by calculating the Cohen’s Kappa coefficient for inter-rater reliability (Ϗ), which subtracts the likelihood of random agreement from the overall agreement [
25,
26] and classify the level of agreement (
Table 1).
Results
Data regarding ECG results of the Kardiamobile 6-lead were taken from home visits of
50 consecutive, eligible patients that were hospitalized in the Sheba-Beyond HAH service between 30.4.2023 and 17.07.2023.
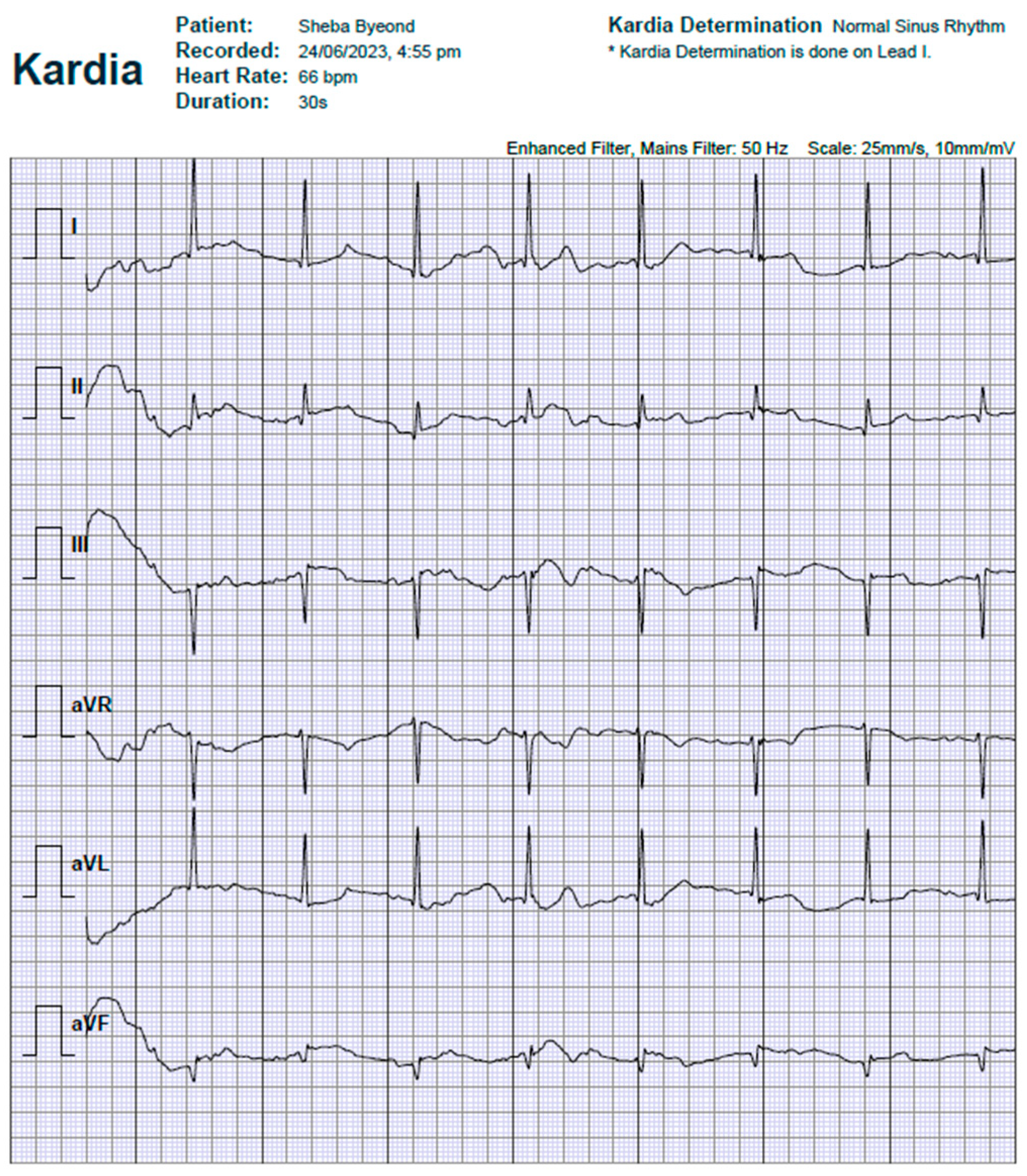
Figure 1 show a typical output of the research, 6-lead ECG as automatically generated in both the patient’s smartphone application and Sheba Beyond clinical monitoring office
The demographic characteristics of our study cohort population are present at
Table 2. The data taken was of the four possible automated results provided by the device as well as researchers’ interpretations of the results for diagnoses that were not provided automatically. Additionally, the 6-lead device’s intervals were manually calculated as that data was not provided in the automatic read-out. These results were compared to the standard Legacy 12-lead ECG device.
The median age of patients was 80 years (IQR = 14) and 54% of the patients were males. 14% of our patients were active smokers during their study participation. The average length of stay in HAH was 3.42 days (not significantly different from our hospitals’ average length of in-hospital stay). The baseline prevalence of cardiovascular disease was 76%, pulmonary disease affected 22% of the patients at baseline. Additionally, 52% of the patients used antiarrhythmic drugs (which included medications from the four classes), while 20% used anticonvulsant drugs (which included the classic antiepileptic drugs as well as certain benzodiazepines, pregabalin, and gabapentin) for associated comorbidities.
Abnormal D-dimer levels were observed in 33 out of 50 patients, accounting for 66% of the total patient population, with a mean of 1439 ± 2021 ng/ml (normal values are up to 500 ng/ml). 12 patients (24%) had abnormal troponin values (mean of 13.98 ± 31 ng/L, normal value of this high-sensitivity troponin kit is up to 12 ng/L) and were classified as suffering from occult myocardial damage, as part of a Type 2 Acute Myocardial Infarction, since none of our patients presented with ST-elevations nor significant ST-depressions during their HAH stay.
In total, 26 (52%) patients were flagged as having electrolyte anomalies (
Table 3). Among them, there were 13 cases of hyponatremia with a mean blood concentration of 137.5 ± 5.25 mg/dL (normal values are in the range of 136 to 148 mg/dL). There were no cases of hypernatremia. There were 3 cases of hyperkalemia (normal values are in the range of 3.5 – 5.2 meq/L), and 3 cases of hypokalemia, with a potassium mean blood concentration of 4.43 ± 0.6 mg/dL. There were 9 cases of hypomagnesemia (mean blood concentration of 2.03 ± 0.23 mg/dL, normal range of values is 1.9 – 2.7 mg/dL)) and no cases of hypermagnesemia. Additionally, there were 4 cases of hyperphosphatemia (mean blood concentration of 3.25 ± 0.51 mg/dl, normal range of values is 2.0 – 4.0 mg/dL) and no cases of hypophosphatemia. The mean blood calcium concentration was 9.072, no cases of calcium abnormalities were observed (normal range of values is 8.1 – 10.4 mg/dL).
Determining the extent of agreement between devices
We assessed the level of agreement between the researched 6-lead device with the standard 12-lead ECG (legacy) with regards to the nine options that are included in the automatic read-out of the legacy device.
Table 4 displays the obtained results.
Regarding level of agreement relating to ECG diagnoses, we found a level of 94.5% cases of raw agreement (refers to the proportions of cases where there was complete agreement between the two methods) between devices, when compared directly. The calculation of Cohen’s Kappa for inter-rater reliability (Ϗ), which subtracts the likelihood of random agreement from the overall agreement, equaled 0.72, which is classified as substantial agreement between devices.
Regarding level of agreement relating to ECG intervals: during comparison of the devices, agreement was measured with relation to the following intervals: PR, RR, and QT. Since QTc is derived from QT and RR it was not added into the calculation of agreement between the devices. The overall rate of raw agreement between the devices was 78.5% and the calculated Ϗ was 0.42, which corresponds to a moderate level of agreement.
The overall compliance of the staff members to the operation of the 6-lead ECG device was very good and all patients who gave their consent also fully complied with participation. There were no cases of consent withdrawal during this study. Also, there were no cases in which technical difficulties interrupted with completion of study measurements and observations. No safety breeches were documented during this study and no side effects were related to either the legacy or the research ECG machines.
Discussion
The potential and actual benefits of HAH are well established. Already, in a 2005 Cochrane review, in which 22 trials were included, evaluating early discharge to hospital at home scenarios, it was shown that although the financial expected benefits were not met, several clinical scenarios gained a shortened length of hospital stay so acute-care, in-hospital beds were saved [
27]. Ever since, accumulated experience in HAH settings established, worldwide, with many HAH services promoting clear criteria for safe and effective management of HAH patients [
28,
29].
Increasing the safety belt for HAH patients is a worldwide challenge and demand. Future tasks for the HAH world would include maintaining patients in their home environment without jeopardizing their health [
30,
31,
32,
33] bearing in mind that novel infrastructures and technologies, such as IoT (Internet of Things) must be evaluated and adopted [
34,
35] for this purpose. Nevertheless, current HAH practices still do not include reliable, validated means for patients’ deterioration detection and prediction [
36]. Several previous publications addressed these pressing issues in several clinical settings such as surgical, and post-discharge patients [
37,
38]. Therefore, reliable, accessible and simple ways for arrythmia detection (amongst other physiologic parameters indicative for imminent patients’ deterioration) are necessary in order for the HAH world to proceed to its next steps of evolution.
A major task facing the global community of HAH is the task of moving acutely ill patients from the in-hospital environment to the HAH settings. Only such shifts will truly enable future expansion of the global HAH project and reduce in-hospital excessive morbidity and mortality. Acutely ill patients necessitate close monitoring (albeit not necessarily continuous monitoring) in order to accurately predict imminent patients’ deterioration and enable timely in-hospital evacuation. Current published studies on HAH patients tend to focus on financial and reimbursement issues rather than acute-patients’ safety aspects [
39].
Rapid, unexpected patients’ deterioration would be, in most scenarios, a result of cardiac arrhythmias. Indeed, in-hospital preparedness focus on clinical scenarios such as “witnessed cardiac arrest”. While most evidence exist regarding the fate of these patients in the post resuscitation scheme [
40], such cases could be anticipated in patients suffering from myocardial damage and/or serum electrolytes anomalies and could be reflected by changes in their basic ECG rhythm and patterns [
41]. Previous publications relate to older age and prolonged hospitalizations as risk factors for arrythmia development, as is the case for hospitalized COVID-19 patients [
42], nevertheless, no previous publications addressed this critical issue in the HAH settings.
In the current study, aiming at future easy, accessible and reliable means for home-based ECG recordings, we compared the outputs of two ECG devices with intention of validating a mobile, simple usage, 6-lead ECG device. The aim of the study was to enable future routine usage of such devices in the HAH setting whenever periodic, even twice daily ECG tracing would be appropriate – mainly for patients diagnosed as suffering from electrolyte disturbances and/or myocardial damage. We compared these devices in the true, clinical field of HAH patients. Assessment was done both directly (raw agreement rates) and by calculating the degree of agreement using the kappa coefficient. Our results show an acceptable level of agreement for the measurable parameters including major rhythm and arrythmia diagnoses and measurements of the most predominant ECG conduction intervals. It is important to emphasize that we did not record, nor compared any ECG variables directly assessing potential myocardial ischemia (e.g., ST segments’ elevation or depressions, T waves’ anomalies). Such direct assessment and diagnoses relating to the spectrum of acute coronary syndromes should rely only on a full, 12-lead ECG recordings unavailable by the 6-lead research device. Nevertheless, it is reasonable to assume that some levels of myocardial damage might lead to arrythmias.
The proportion of patients in this study who were predisposed to myocardial damage and arrhythmia was high: we showed that there was a substantial amount of patients suffering from background diagnoses relating to the cardiovascular system and the respiratory system. Also, we found a significant portion of patients had electrolyte disturbances and/or medical treatments (either anti-arrhythmic drugs and anti-epileptic drugs) which might initiate rhythm anomalies.
The usage of 6-lead ECG as the research device, in this study was performed by researchers, during their clinical work in the HAH field. The overall compliance and comfort of usage was high, with no notable side effects reported.
Conclusion
Using a mobile, 6-Lead ECG device in the setting of HAH was found to be safe and bearing satisfying agreement levels with a legacy, 12-lead ECG device, enabling quick, accessible arrythmia detection in this setting. This is applicable also for high-risk population of patients, including those suffering from electrolyte anomalies and patients with increased levels of blood troponin, indicative for possible occult myocardial damage. We conclude that similar devices should be used in the HAH settings, enabling incorporation of more complex patients in this realm. The authors recommend further study of such devices used by the patients themselves. This should be investigated in a larger, prospective study. Until such prospective studies are done, we recommend all healthcare professionals engaging the HAH realm to adopt and incorporate into their routines and guidelines both serum electrolytes’ measurements and 6-lead ECG recordings.
Study limitations
This was a single-center study in a population of pre-defined patients (e.g., hemodynamically stable). Therefore, our findings should be related to similar patients’ populations and not to diverse ethnicities. The research device was handled by the clinical staff and not by the patients themselves. Therefore, it cannot be concluded that such devices can be easily operated by the patients themselves. Future, larger studies in diverse HAH patients’ populations are warranted.
References
- Jeppesen, E.; Brurberg, K.G.; E Vist, G.; A Wedzicha, J.; Wright, J.J.; Greenstone, M.; Walters, J.A. Hospital at home for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2012, CD003573. [Google Scholar] [CrossRef] [PubMed]
- Casteli, C.P.M.; Mbemba, G.I.C.; Dumont, S.; Dallaire, C.; Juneau, L.; Martin, E.; Laferrière, M.-C.; Gagnon, M.-P. Indicators of home-based hospitalization model and strategies for its implementation: a systematic review of reviews. Syst. Rev. 2020, 9, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Shepperd, S.; Iliffe, S. Hospital at home versus in-patient hospital care. Cochrane Database of Systematic Reviews 2005. [Google Scholar]
- Conley, J.; O’Brien, C.W.; Leff, B.A.; Bolen, S.; Zulman, D. Alternative Strategies to Inpatient Hospitalization for Acute Medical Conditions: A Systematic Review. JAMA Intern Med. 2016, 176, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Hecimovic, A.; Matijasevic, V.; Frost, S.A. Characteristics and outcomes of patients receiving Hospital at Home Services in the South West of Sydney. BMC Heal. Serv. Res. 2020, 20, 1–5. [Google Scholar] [CrossRef]
- Ko, S.Q.; Goh, J.; Tay, Y.K.; Nashi, N.; Hooi, B.M.; Luo, N.; Kuan, W.S.; Soong, J.T.; Chan, D.; Lai, Y.F.; et al. Treating acutely ill patients at home: Data from Singapore. Ann. Acad. Med. Singap. 2022, 51, 392–399. [Google Scholar] [CrossRef]
- Mc Donald, D.; Sherlock, M.; Thompson, C.J. Hyponatraemia and the syndrome of inappropriate antidiuresis (SIAD) in cancer. Endocr. Oncol. 2022, 2, R78–R89. [Google Scholar] [CrossRef]
- Xiao, M.; Wang, X.; Wang, H.; Du, F.; Yao, Y.; Wang, X.; Wang, J.; Yang, J.; Xiong, W.; Wang, Q.; et al. Risk factors for hyponatremia in acute exacerbation chronic obstructive pulmonary disease (AECOPD): a multicenter cross-sectional study. BMC Pulm. Med. 2023, 23, 39. [Google Scholar] [CrossRef]
- Nair, V.; Niederman, M.S.; Masani, N.; Fishbane, S. Hyponatremia in community-acquired pneumonia. Am J Nephrol. 2007, 27, 184–190. [Google Scholar] [CrossRef]
- Rafique, Z.; Hoang, B.; Mesbah, H.; Pappal, R.; Peacock, F.W.; Juarez-Vela, R.; Szarpak, L.; Kuo, D.C. Hyperkalemia and Electrocardiogram Manifestations in End-Stage Renal Disease. Int. J. Environ. Res. Public Health 2022, 19, 16140. [Google Scholar] [CrossRef]
- Humphrey, T.; Davids, M.R.; Chothia, M.-Y.; Pecoits-Filho, R.; Pollock, C.; James, G. How common is hyperkalaemia? A systematic review and meta-analysis of the prevalence and incidence of hyperkalaemia reported in observational studies. Clin. Kidney J. 2021, 15, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Szeto, C. Perspectives on acute kidney injury strategy: Hong Kong. Nephrology 2018, 23, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Genovesi, S.; Regolisti, G.; Burlacu, A.; Covic, A.; Combe, C.; Mitra, S.; Basile, C.; Bartolucci, C. ; The EuDial Working Group of ERA The conundrum of the complex relationship between acute kidney injury and cardiac arrhythmias. Nephrol. Dial. Transplant. 2022, 38, 1097–1112. [Google Scholar] [CrossRef] [PubMed]
- Talwar, V.; Gonuguntla, V.; Krishna, B.; Srinivasan, G. Correlation of Serum Magnesium Levels with Clinical Outcome: A Prospective Observational Study in Critically Ill Patients Admitted to a Tertiary Care ICU in India. Indian J. Crit. Care Med. 2023, 27, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Ergün, B.; Ergan, B.; Küçük, M.; Yakar, M.N.; Öztürk, M.C.; Aydemir, F.D.; et al. Is serum magnesium level associated with atrial fibrillation in the mixed medical/surgical intensive care unit setting? Magnes Res 2022, 35, 96–107. [Google Scholar] [CrossRef]
- Measrbghch, S.S. Hypophosphatemia in critically ill patients. J Crit Care. 2013, 28, 536. [Google Scholar]
- Pistolesi, V.; Zeppilli, L.; Fiaccadori, E.; Regolisti, G.; Tritapepe, L.; Morabito, S. Hypophosphatemia in critically ill patients with acute kidney injury on renal replacement therapies. J. Nephrol. 2019, 32, 895–908. [Google Scholar] [CrossRef]
- Balcı, A.K.; Koksal, O.; Kose, A.; Armagan, E.; Ozdemir, F.; Inal, T.; Oner, N. General characteristics of patients with electrolyte imbalance admitted to emergency department. World J. Emerg. Med. 2013, 4, 113–116. [Google Scholar] [CrossRef]
- Castelletti, S.; Winkel, B.G.; Schwartz, P.J. Remote Monitoring of the QT Interval and Emerging Indications for Arrhythmia Prevention. Card. Electrophysiol. Clin. 2021, 13, 523–530. [Google Scholar] [CrossRef]
- Hnatkova, K.; Malik, M. Sources of QTc variability: Implications for effective ECG monitoring in clinical practice. Ann. Noninvasive Electrocardiol. 2019, 25, e12730. [Google Scholar] [CrossRef]
- (17) (PDF) Patients with Electrolyte Disturbances Can Be Safely and Effectively Treated in a Hospital-at-home, Telemedicine Controlled Environment [Internet]. Available online: https://www.researchgate.net/publication/371240739_Patients_with_Electrolyte_Disturbances_Can_Be_Safely_and_Effectively_Treated_in_a_Hospital-at-home_Telemedicine_Controlled_Environment (accessed on 19 August 2023).
- Pillai, B.; Trikkur, S.; Farooque, U.; Ramakrishnan, D.; Kakkra, J.J.; Kashyap, G.; Lalwani, C.; Mani, A.B.; Vishwanath, J. Type II Myocardial Infarction: Predisposing Factors, Precipitating Elements, and Outcomes. Cureus 2020, 12, e9254. [Google Scholar] [CrossRef] [PubMed]
- Koltowski, L.; Balsam, P.; Glłowczynska, R.; Rokicki, J.K.; Peller, M.; Maksym, J.; Blicharz, L.; Maciejewski, K.; Niedziela, M.; Opolski, G.; et al. Kardia Mobile applicability in clinical practice: A comparison of Kardia Mobile and standard 12-lead electrocardiogram records in 100 consecutive patients of a tertiary cardiovascular care center. Cardiol. J. 2021, 28, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Barkai, G.; Amir, H.; Dulberg, O.; Itelman, E.; Gez, G.; Carmon, T.; Merhav, L.; Zigler, S.; Atamne, A.; Pinhasov, O.; et al. “Staying at Home”: A pivotal trial of telemedicine-based internal medicine hospitalization at a nursing home. Digit. Health 2022, 8. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2012, 22, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Shepperd, S.; Iliffe, S. Hospital at home versus in-patient hospital care. Cochrane Database of Systematic Reviews 2005. [Google Scholar]
- Hospital at Home. 2020. Available online: www.healthcareimprovementscotland.org (accessed on 18 August 2023).
- Knight, T.; Lasserson, D. Hospital at home for acute medical illness: The 21st century acute medical unit for a changing population. J. Intern. Med. 2021, 291, 438–457. [Google Scholar] [CrossRef]
- Arsenault-Lapierre, G.; Henein, M.; Gaid, D.; Le Berre, M.; Gore, G.; Vedel, I. Hospital-at-Home Interventions vs In-Hospital Stay for Patients with Chronic Disease Who Present to the Emergency Department: A Systematic Review and Meta-analysis. JAMA Netw Open 2021, 4, e2111568. [Google Scholar] [CrossRef]
- Caplan, G.A.; Sulaiman, N.S.; Mangin, D.A.; Aimonino Ricauda, N.; Wilson, A.D.; Barclay, L. A meta-analysis of “hospital in the home”. Medical Journal of Australia 2012, 197, 512–519. [Google Scholar] [CrossRef]
- Sitammagari, K.; Murphy, S.; Kowalkowski, M.; Chou, S.-H.; Sullivan, M.; Taylor, S.; Kearns, J.; Batchelor, T.; Rivet, C.; Hole, C.; et al. Insights From Rapid Deployment of a “Virtual Hospital” as Standard Care During the COVID-19 Pandemic. Ann. Intern. Med. 2021, 174, 192–199. [Google Scholar] [CrossRef]
- Leff, B.; DeCherrie, L.V.; Montalto, M.; Levine, D.M. A research agenda for hospital at home. J. Am. Geriatr. Soc. 2022, 70, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Abril-Jiménez, P.; Merino-Barbancho, B.; Fico, G.; Martín Guirado, J.C.; Vera-Muñoz, C.; Mallo, I.; et al. Evaluating IoT-Based Services to Support Patient Empowerment in Digital Home Hospitalization Services. Sensors 2023, 23, 1744. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, D.; Conley, J. The Next Frontier of Remote Patient Monitoring: Hospital at Home. J Med Internet Res. 2023, 25. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.D.; Good, N.M.; Fatehi, F.; Khanna, S.; Campbell, V.; Conway, R.; Sullivan, C.; Staib, A.; Joyce, C.; Cook, D. Predicting Patient Deterioration: A Review of Tools in the Digital Hospital Setting. J. Med Internet Res. 2021, 23, e28209. [Google Scholar] [CrossRef] [PubMed]
- Breteler, M.J.M.; Numan, L.; Ruurda, J.P.; van Hillegersberg, R.; van der Horst, S.; Dohmen, D.A.J.; van Rossum, M.C.; Kalkman, C.J. Wireless Remote Home Monitoring of Vital Signs in Patients Discharged Early After Esophagectomy: Observational Feasibility Study. JMIR Perioper. Med. 2020, 3, e21705. [Google Scholar] [CrossRef] [PubMed]
- Leenen, J.P.L.; Ardesch, V.; Patijn, G. Remote Home Monitoring of Continuous Vital Sign Measurements by Wearables in Patients Discharged After Colorectal Surgery: Observational Feasibility Study. JMIR Perioper. Med. 2023, 6, e45113. [Google Scholar] [CrossRef]
- Levine, D.M.; Ouchi, K.; Blanchfield, B.; Saenz, A.; Burke, K.; Paz, M.; et al. Hospital-Level Care at Home for Acutely Ill Adults: A Randomized Controlled Trial. Ann Intern Med. 2019, 172, 77–85. [Google Scholar] [CrossRef]
- Carrick, R.T.; Park, J.G.; McGinnes, H.L.; Lundquist, C.; Brown, K.D.; Janes, W.A.; Wessler, B.S.; Kent, D.M. Clinical Predictive Models of Sudden Cardiac Arrest: A Survey of the Current Science and Analysis of Model Performances. J. Am. Hear. Assoc. 2020, 9, 17625. [Google Scholar] [CrossRef] [PubMed]
- Panchal, A.R.; Bartos, J.A.; Cabañas, J.G.; Donnino, M.W.; Drennan, I.R.; Hirsch, K.G.; Kudenchuk, P.J.; Kurz, M.C.; Lavonas, E.J.; Morley, P.T.; et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2020, 142, S366–S468. [Google Scholar] [CrossRef]
- Zylla, M.M.; Merle, U.; Vey, J.A.; Korosoglou, G.; Hofmann, E.; Müller, M.; Herth, F.; Schmidt, W.; Blessing, E.; Göggelmann, C.; et al. Predictors and Prognostic Implications of Cardiac Arrhythmias in Patients Hospitalized for COVID-19. J. Clin. Med. 2021, 10, 133. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).