Submitted:
23 August 2023
Posted:
25 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
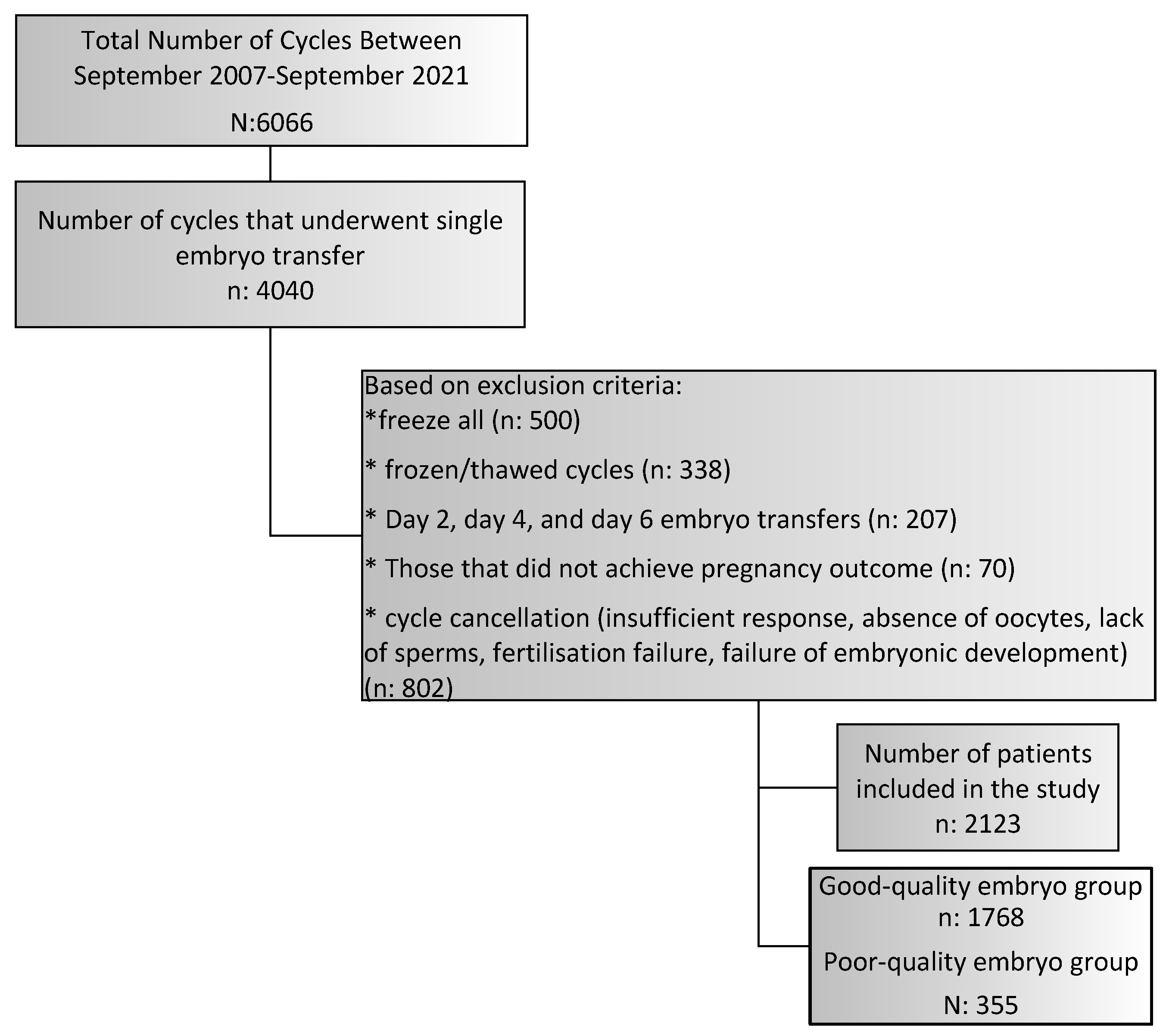
2.1. Study Design
2.2. Ovarian Stimulation, Intracytoplasmic Sperm Injection (ICSI), and Embryo Transfer Procedures
2.3. Assessment of Embryo Development
2.4. Pregnancy Outcomes
2.5. Statistical Method
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Fragouli, E.; Alfarawati, S.; Spath, K.; Wells, D. Morphological and cytogenetic assessment of cleavage and blastocyst stage embryos. Mol Hum Reprod. 2014, 20, 117–26. [Google Scholar] [CrossRef] [PubMed]
- Hardarson, T.; Caisander, G.; Sjögren, A.; Hanson, C.; Hamberger, L.; Lundin, K. A morphological and chromosomal study of blastocysts developing from morphologically suboptimal human pre-embryos compared with control blastocysts. Hum Reprod. 2003, 18, 399–407. [Google Scholar] [CrossRef]
- Alfarawati, S.; Fragouli, E.; Colls, P.; Stevens, J.; Gutieérrez-Mateo, C.; Schoolcraft, W.B. , et al. The relationship between blastocyst morphology, chromosomal abnormality, and embryo gender. Fertil Steril. 2011, 95, 520–4. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, A. , et al. Should we transfer poor quality embryos? Fertil Res Pract. 2020, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Oron, G.; Son, W.Y.; Buckett, W.; Tulandi, T.; Holzer, H. The association between embryo quality and perinatal outcome of singletons born after single embryo transfers: a pilot study. Hum Reprod. 2014, 29, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lian, Y.; Li, M.; Chen, L.; Liu, P.; Qiao, J. Does IVF cleavage stage embryo quality affect pregnancy complications and neonatal outcomes in singleton gestations after double embryo transfers? J Assist Reprod Genet. 2014, 31, 1635–1641. [Google Scholar] [CrossRef]
- Akamine, K.; Mekaru, K.; Gibo, K.; Nagata, C.; Oishi, S.; Miyagi, M.; Heshiki, C.; Kinjo, T.; Masamoto, H.; Aoki, Y. Comparative study of obstetric and neonatal outcomes of live births between poor- and good-quality embryo transfers. Reprod Med Biol. 2018, 17, 188–194. [Google Scholar] [CrossRef]
- Li, M.; Yin, M.; Wu, L.; Yan, Z.; Lyu, Q.; Yan, Z.; Li, B. Pregnancy and neonatal outcomes of morphologically grade CC blastocysts: are they of clinical value? Arch Gynecol Obstet. 2020, 302, 1511–1521. [Google Scholar] [CrossRef]
- Baczkowski, T.; Kurzawa, R.; Głabowski, W. Methods of embryo scoring in in vitro fertilization. Reprod Biol. 2004, 4, 5–22. [Google Scholar]
- Rehman, K.S.; Bukulmez, O.; Langley, M.; Carr, B.R.; Nackley, A.C.; Doody, K.M.; Doody, K.J. Late stages of embryo progression are a much better predictor of clinical pregnancy than early cleavage in intracytoplasmic sperm injection and in vitro fertilization cycles with blastocyst-stage transfer. Fertil. Steril. 2007, 87, 1041–1052. [Google Scholar] [CrossRef]
- Van den Abbeel, E.; Balaban, B.; Ziebe, S.; Lundin, K.; Cuesta, M.J.; Klein, B.M.; Helmgaard, L.; Arce, J.C. Association between blastocyst morphology and outcome of single-blastocyst transfer. Reprod. Biomed. Online 2013, 27, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Glujovsky, D.; Blake, D.; Farquhar, C.; Bardach, A. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst. Rev. 2012, 7, CD002118. [Google Scholar]
- Guerif, F.; Lemseffer, M.; Blanchard, M.; Royere, D. Top quality embryos at day 2: a prerequisite for single blastocyst transfer? An observational cohort study in women under 36. J. Assist. Reprod. Genet. 2009, 26, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Rjinders, P.M.; Jansen, C.A.M. The predictive value of day 3 embryo morphology regarding blastocyst formation, pregnancy and implantation rate after day 5 transfer following in vitro fertilization or intracytoplasmic sperm injection. Hum Reprod 1998, 13, 2869–73. [Google Scholar] [CrossRef] [PubMed]
- Balaban, B.; Urman, B.; Alatas, C.; Mercan, R.; Aksoy, S.; Isiklar, A. Blastocyst-stage transfer of poor-quality cleavage-stage embryos results in higher implantation rates. Fertil Steril. 2001, 75, 514–8. [Google Scholar] [CrossRef] [PubMed]
- Kemper, J.M.; Liu, Y.; Afnan, M.; Hammond, E.R.; Morbeck, D.E.; Mol, B.W.J. Should we look for a low-grade threshold for blastocyst transfer? A scoping review. Reprod Biomed Online. 2021, 42, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Sunkara, S.K.; Rittenberg, V.; Raine-Fenning, N.; et al. Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Hum Reprod. 2011, 26, 1768–1774. [Google Scholar] [CrossRef]
- Drakopoulos, P.; Blockeel, C.; Stoop, D.; et al. Conventional ovarian stimulation and single embryo transfer for IVF/ICSI. How many oocytes do we need to maximize cumulative live birth rates after utilization of all fresh and frozen embryos? Hum Reprod. 2016, 31, 370–376. [Google Scholar] [CrossRef]
- Drakopoulos, P.; Errázuriz, J.; Santos-Ribeiro, S.; Tournaye, H.; Vaiarelli, A.; Pluchino, N.; Blockeel, C.; Polyzos, N.P. Cumulative live birth rates in in-vitro fertilization. Minerva Ginecol. 2019, 71, 207–210. [Google Scholar] [CrossRef]
- Huber, M.; Hadziosmanovic, N.; Berglund, L.; Holte, J. Using the ovarian sensitivity index to define poor, normal, and high response after controlled ovarian hyperstimulation in the long gonadotropin-releasing hormoneagonist protocol: suggestions for a new principle to solve an old problem. Fertil Steril 2013, 100, 1270–6. [Google Scholar] [CrossRef]
- Lee, S.T.; Kim, T.M.; Cho, M.Y.; Moon, S.Y.; Han, J.Y.; Lim, J.M. Development of a hamster superovulation program and adverse effects of gonadotropins on microfilament formation during oocyte development. Fertil Steril 2005, 83 (Suppl. 1), 1264–74. [Google Scholar] [CrossRef]
- Wilson, J.M.; Jones, A.L.; Moore, K.; Looney, C.R.; Bondioli, K.R. Superovulation of cattle with a recombinant-DNA bovine follicle stimulating hormone. Anim Reprod Sci 1993, 33, 71–82. [Google Scholar] [CrossRef]
- Mapletoft, R.J.; Steward, K.B.; Adams, G.P. Recent advances in the superovulation in cattle. Reprod Nutr Dev 2003, 42, 601–11. [Google Scholar] [CrossRef]
- Barati, F.; Niasari-Naslaji, A.; Bolourchi, M.; Sarhaddi, F.; Razavi, K.; Naghzali, E.; et al. Superovulatory response of Sistani cattle to three different doses of FSH during winter and summer. Theriogenology 2006, 66, 1149–55. [Google Scholar] [CrossRef]
- Souza, A.H.; Sartori, R.; Guenther, J.N.; Caraviello, D.; Monson, R.; Wiltbank, M. Effect of semen source and dose of FSH on superovulatory response and embryo production in Holstein heifers. Anim Reprod 2007, 4, 70–6. [Google Scholar]
- Roberts, R.; Iatropoulou, A.; Ciantar, D.; Stark, J.; Becker, D.L.; Franks, S.; et al. Follicle- stimulating hormone affects metaphase I chromosome alignment and increases aneuploidy in mouse oocytes matured in vitro. Biol Reprod 2005, 72, 107–18. [Google Scholar] [CrossRef] [PubMed]
- Scheetz, D.; Folger, J.K.; Smith, G.W.; Ireland, J.J. Granulosa cells are refractory to FSH action in individuals with a low antral follicle count. Reprod Fertil Dev 2012, 24, 327–36. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.D. Models of luteinization. Biol Reprod 2000, 63, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Haaf, T.; Hahn, A.; Lambrecht, A.; Grossmann, B.; Schwaab, E.; Khanaga, O.; et al. A high oocyte yield for intracytoplasmic sperm injection treatment is associated with an increased chromosome error rate. Fertil Steril 2009, 91, 733–8. [Google Scholar] [CrossRef]
- Gianaroli, L.; Magli, M.C.; Cavallini, G.; Crippa, A.; Capoti, A.; Resta, S.; et al. Predicting aneuploidy in human oocytes: key factors which affect the meiotic process. Hum Reprod 2010, 25, 2374–86. [Google Scholar] [CrossRef]
- Ata, B.; Kaplan, B.; Danzer, H.; Glassner, M.; Opsahl, M.; Tan, S.L. , et al. Array CGH analysis shows that aneuploidy is not related to the number of embryos generated. Reprod Biomed Online 2012, 24, 614–20. [Google Scholar] [CrossRef]
- Labarta, E.; Bosch, E.; Mercader, A.; Alam_a, P.; Mateu, E.; Pellicer, A. Relationship between ovarian response and number of euploid embryos in oocyte donor cycles. Fertil Steril 2012, 98, S282. [Google Scholar] [CrossRef]
- Hohmann, F.P.; Macklon, N.S.; Fauser, B.C. A randomized comparison of two ovarian stimulation protocols with gonadotropin-releasing hormone (GnRH) antagonist cotreatment for in vitro fertilization commencing recombinant follicle-stimulating hormone on cycle day 2 or 5 with the standard long GnRH agonist protocol. J Clin Endocrinol Metab 2003, 88, 166–73. [Google Scholar] [PubMed]
- Baart, E.B.; Martini, E.; Eijkemans, M.J.; Van Opstal, D.; Beckers, N.G.; Verhoeff, A.; et al. Milder ovarian stimulation for in-vitro fertilization reduces aneuploidy in the human preimplantation embryo: a randomized controlled trial. Hum Reprod 2007, 22, 980–8. [Google Scholar] [CrossRef] [PubMed]
- Baker, V.L.; Brown, M.B.; Luke, B.; Smith, G.W.; Ireland, J.J. Gonadotropin dose is negatively correlated with live birth rate: analysis of more than 650,000 assisted reproductive technology cycles. Fertil Steril. 2015, 104, 1145–52. [Google Scholar] [CrossRef]
- Horcajadas, J.A.; Minguez, P.; Dopazo, J.; Esteban, F.J.; Dominguez, F.; Giudice, L.C.; et al. Controlled ovarian stimulation induces a functional genomic delay of the endometrium with potential clinical implications. J Clin Endocrinol Metab 2008, 93, 4500–10. [Google Scholar] [CrossRef]
- Andersen, C.Y.; Ezcurra, D. Human steroidogenesis: implications for controlled ovarian stimulation with exogenous gonadotropins. Reprod Biol Endocrinol 2014, 12, 128. [Google Scholar] [CrossRef]
| Good Quality n: 1,768 |
Poor Quality n: 355 |
P value | ||
| Female age (years) | 29.4±4.5 | 31.0±5.3 | <0.001* | |
| Body mass index (kg/m2) | 26.2±5.0 | 26.1±5.1 | 0.703* | |
| Number of cycles | 1.5±0.8 | 1.7±1.0 | <0.001* | |
| Basal D3 FSH (IU/L) | 8.2±21.2 | 8.8±6.1 | <0.001* | |
| Basal D3 E2 (pg/mL) | 48.1±32.4 | 50.6±34.6 | 0.021* | |
| AMH (ng/mL) | 3.0±3.5 | 2.7±3.0 | 0.376* | |
| Duration of infertility (months) | 62.1±43.4 | 63.5±46.9 | 0.906* | |
| Numbers of total antral follicle | 14.0±8.4 | 12.4±8.2 | <0.001* | |
| Male age (years) | 32.7±4.9 | 34.5±7.3 | <0.001* | |
| Total progressive motile sperm count (n) | 22,133.4±41,165.3 | 24,006.2±48,095.5 | 0.336* | |
| Sperm morphology: normal rate (%) | 8.2±4.3 | 6.7±7.5 | 0.806* | |
| Indication of treatment (%) | ||||
| Male factor | 733 (41.5) | 140 (39.4) | 0.480** | |
| Tubal factor | 127 (7.2) | 19 (5.4) | 0.259*** | |
| Diminished ovarian reserve | 243 (29.3) | 71 (35.9) | 0.072** | |
| Advanced female age | 56 (3.2) | 26 (7.3) | <0.001*** | |
| Hormonoovulatory dysfunction | 313 (17.7) | 57 (16.1) | 0.455** | |
| Unexplained infertility | 552 (31.2) | 90 (25.4) | 0.028** | |
| Severe pelvic adhesion | 11 (0.6) | 2 (0.6) | 1,000† | |
| Endometriosis | 102 (5.8) | 23 (6.5) | 0.604** | |
| Gnrh protocol (%) | ||||
| Microdose flare-up | 99 (5.6) | 16 (4.5) | 0.462** | |
| Antagonist | 984 (55.7) | 209 (58.9) | ||
| Long luteal | 685 (38.7) | 130 (36.6) | ||
| Duration of ovarian stimulation (n) | 9.9±1.7 | 10.0±1.8 | 0.441* | |
| Total gonadotropin dose (IU) | 2,187.1±897.0 | 2,414.8±1,038.2 | <0.001* | |
| Trigger (%) | ||||
| Hcg | 1,585 (89.6) | 322 (90.7) | 0.549** | |
| Agonist | 183 (10.4) | 33 (9.3) | ||
| Trigger Day | E2 level (pg/mL) | 2,433.7±1,629.4 | 2,197.3±1,470.2 | 0.027* |
| >=17mm follicle (n) | 3.3±2.6 | 3.3±2.6 | 0.671* | |
| 15-17mm follicle (n) | 3.8±3.1 | 3.3±2.7 | 0.001* | |
| 10-14mm follicle (n) | 6.0±4.8 | 4.9±4.2 | <0.001* | |
| Endometrial thickness (mm) | 10.1±2.0 | 9.8±2.1 | 0.072* | |
| Number of oocytes retrieved | 11.9±7.3 | 10.3±6.7 | <0.001* | |
| Number of mature oocytes | 8.9±5.6 | 7.6±5.3 | <0.001* | |
| Number of oocytes used for ICSI | 9.4±5.7 | 8.2±5.5 | <0.001* | |
| Oocyte quality index | 5.1±0.8 | 5.0±0.8 | 0.001* | |
| Numbers of 2PN | 4.8±3.6 | 3.6±2.7 | <0.001* | |
| Blastocyst transfer (n) | 0.33±0.48 | 0.33±0.49 | 0.980* | |
| Embryo transfer Day (%) | ||||
| Day 3 embryo transfers | 1,055 (59.7) | 221 (62.3) | 0.365** | |
| Day 5 embryo transfers | 713 (40.3) | 134 (37.7) | ||
| Good Quality n: 1,768 |
Poor Quality n: 355 |
P value | |
| No pregnancy | 992 (56.1) | 283 (79.7) | <0.001 |
| Biochemical pregnancy | 99 (5.6) | 19 (5.4) | NS |
| Clinical pregnancy | 677 (38.3) | 53 (14.9) | <0.001 |
| Miscarriage | 136 (7.7) | 17 (4.8) | NS |
| Live birth | 541 (30.6) | 36 (10.1) | <0.001 |
| Live Birth per Clinical Pregnancy | 541/677 (79.9) | 36/53 (67.9) | <0.001 |
| Miscarriage per Clinical Pregnancy | 136/677 (20) | 17/53 (32) | <0.001 |
| Day 3 Transfers (n: 1,376) |
Day 5 Transfers (n: 847) |
P value | ||
| Good quality | No pregnancy | 655 (62.1) | 337 (47.3) | <0.001* |
| Biochemical pregnancy | 52 (4.9) | 47 (6.6) | NS* | |
| Clinical pregnancy | 348 (33) | 329 (46.1) | <0.001* | |
| Miscarriage | 73 (6.9) | 63 (8.8) | NS* | |
| Live birth | 275 (26.1) | 266 (37.3) | <0.001* | |
| Live Birth per Clinical Pregnancy | 275/348 (79) | 266/329 (80.8) | NS* | |
| Miscarriage per Clinical Pregnancy | 73/348 (20.9) | 63/329 (19.1) | NS* | |
| Poor quality | No pregnancy | 180 (81.4) | 103 (76.9) | NS** |
| Biochemical pregnancy | 9 (4.1) | 10 (7.5) | NS** | |
| Clinical pregnancy | 32 (14.5) | 21 (15.7) | NS** | |
| Miscarriage | 8 (3.6) | 9 (6.7) | NS** | |
| Live birth | 24 (10.9) | 12 (9) | NS** | |
| Live Birth per Clinical Pregnancy | 24/32 (75) | 12/21 (57.1) | <0.001** | |
| Miscarriage per Clinical Pregnancy | 8/32 (25) | 9/21 (42.8) | <0.001** | |
| hCG POSITIVITY | LIVE BIRTH | ||||
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Indication of treatment (%) | |||||
| Male factor | 0.972 | 0.915 | 1.109 | 0.773 | |
| Tubal factor | 0.447 | 0.289 | 0.478 | 0.479 | |
| Diminished ovarian reserve | 0.871 | 0.690 | 0.96 | 0.920 | |
| Age-related infertility | 0.308 | 0.116 | 3.243 | 0.116 | |
| Hormonoovulatory dysfunction | 0.945 | 0.874 | 0.951 | 0.916 | |
| Unexplained infertility | 0.977 | 0.939 | 0.826 | 0.649 | |
| Severe pelvic adhesion | 3.972 | 0.332 | 0.252 | 0.332 | |
| Endometriosis | 1.099 | 0.857 | 0.835 | 0.813 | |
| Female age (years) | 0.976 | 0.339 | 0.945 | 0.111 | |
| Body Mass Index (kg/m2) | 1.024 | 0.363 | 1.048 | 0.158 | |
| Numbers of cycles | 0.906 | 0.484 | 0.932 | 0.704 | |
| Basal D3 FSH (IU/L) | 0.963 | 0.274 | 0.934 | 0.211 | |
| Basal D3 E2 (pg/mL) | 0.995 | 0.368 | 0.998 | 0.715 | |
| AMH (ng/mL) | 1.001 | 0.988 | 1.073 | 0.321 | |
| Duration of infertility (months) | 1 | 0.904 | 0.997 | 0.497 | |
| Numbers of total antral follicle | 1.015 | 0.352 | 1.022 | 0.280 | |
| Male age (years) | 0.986 | 0.486 | 0.97 | 0.279 | |
| Total progressive motile sperm count | 1 | 0.928 | 1 | 0.663 | |
| Sperm morphology: normal rate | 1.01 | 0.655 | 1.039 | 0.133 | |
| Protocol (Reference: long luteal) | |||||
| Microdose flare-up | 1.112 | 0.655 | 1.835 | 0.440 | |
| GnRH antagonist | 0.955 | 0.646 | 1.012 | 0.966 | |
| Duration of ovarian stimulation | 0.936 | 0.383 | 0.903 | 0.330 | |
| Total gonadotropin dose (IU) | 0.997 | 0.049 | 0.995 | 0.024 | |
| Trigger | 1.064 | 0.889 | 1.676 | 0.321 | |
| Trigger day | E2 level (pg/mL) | 1 | 0.376 | 1 | 0.423 |
| >=17 mm follicle | 1.062 | 0.223 | 0.987 | 0.853 | |
| 15-17mm follicle | 1.012 | 0.809 | 0.936 | 0.351 | |
| 10-14mm follicle | 1.043 | 0.150 | 1.043 | 0.269 | |
| Endometrial thickness (mm) | 1.103 | 0.155 | 1.072 | 0.453 | |
| Numbers of oocyte retrieved | 1.032 | 0.090 | 1.035 | 0.152 | |
| Numbers of mature oocyte | 1.037 | 0.120 | 1.035 | 0.256 | |
| Number of oocytes used for ICSI | 1.036 | 0.122 | 1.032 | 0.295 | |
| Oocyte quality index | 0.905 | 0.537 | 0.995 | 0.980 | |
| Numbers of 2PN | 1.416 | 0.045 | 1.097 | 0.111 | |
| Blastocyst transfer | 1.006 | 0.983 | 0.628 | 0.268 | |
| Transfer Day | 1.149 | 0.299 | 0.899 | 0.565 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





