Submitted:
24 August 2023
Posted:
25 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Optimization of variable conditions for SFE
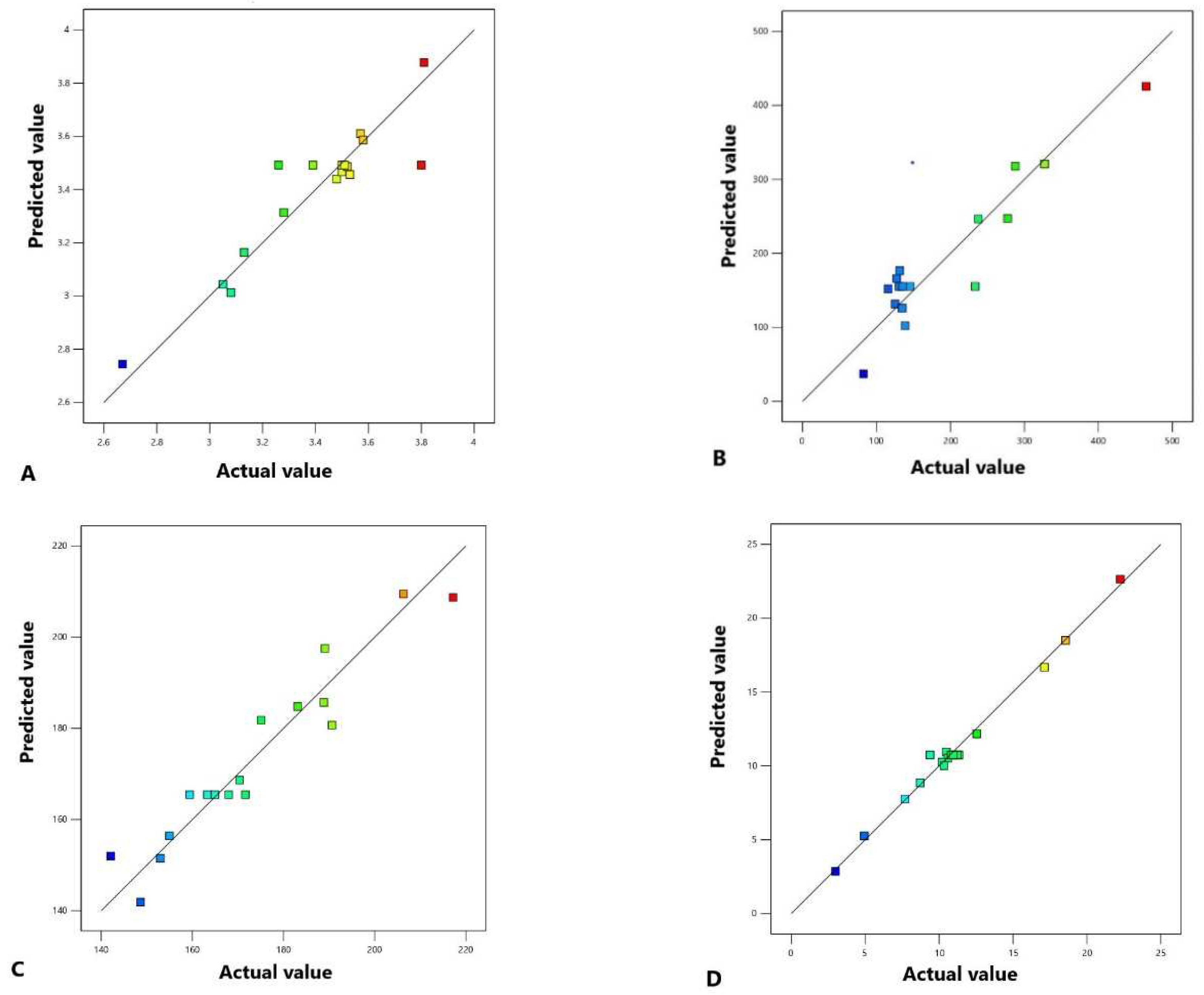
2.2. Effects of SFE conditions on the percentage extraction yield
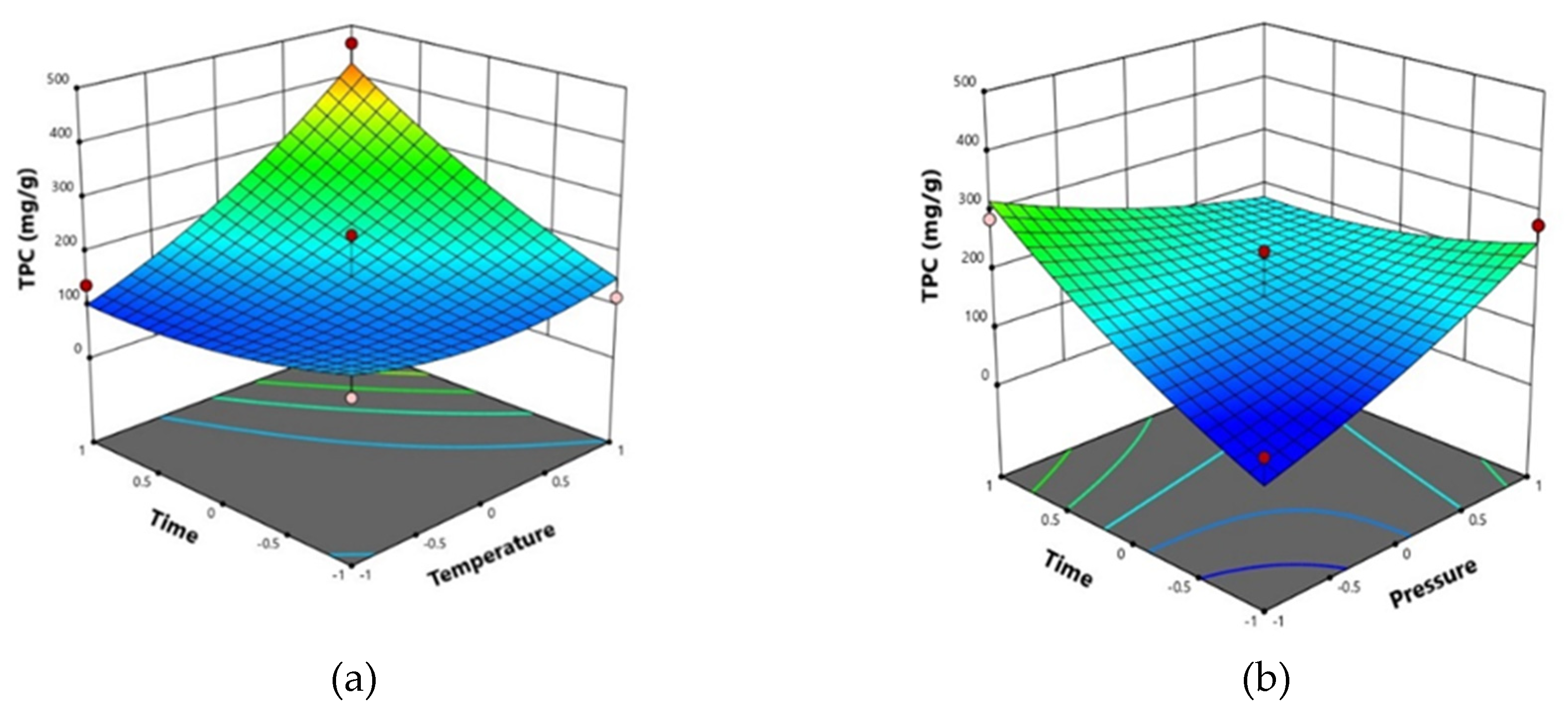
2.3. Effects of SFE conditions on total phenolic content (TPC)
2.4. Effects of SFE conditions on total flavonoid content (TFC)
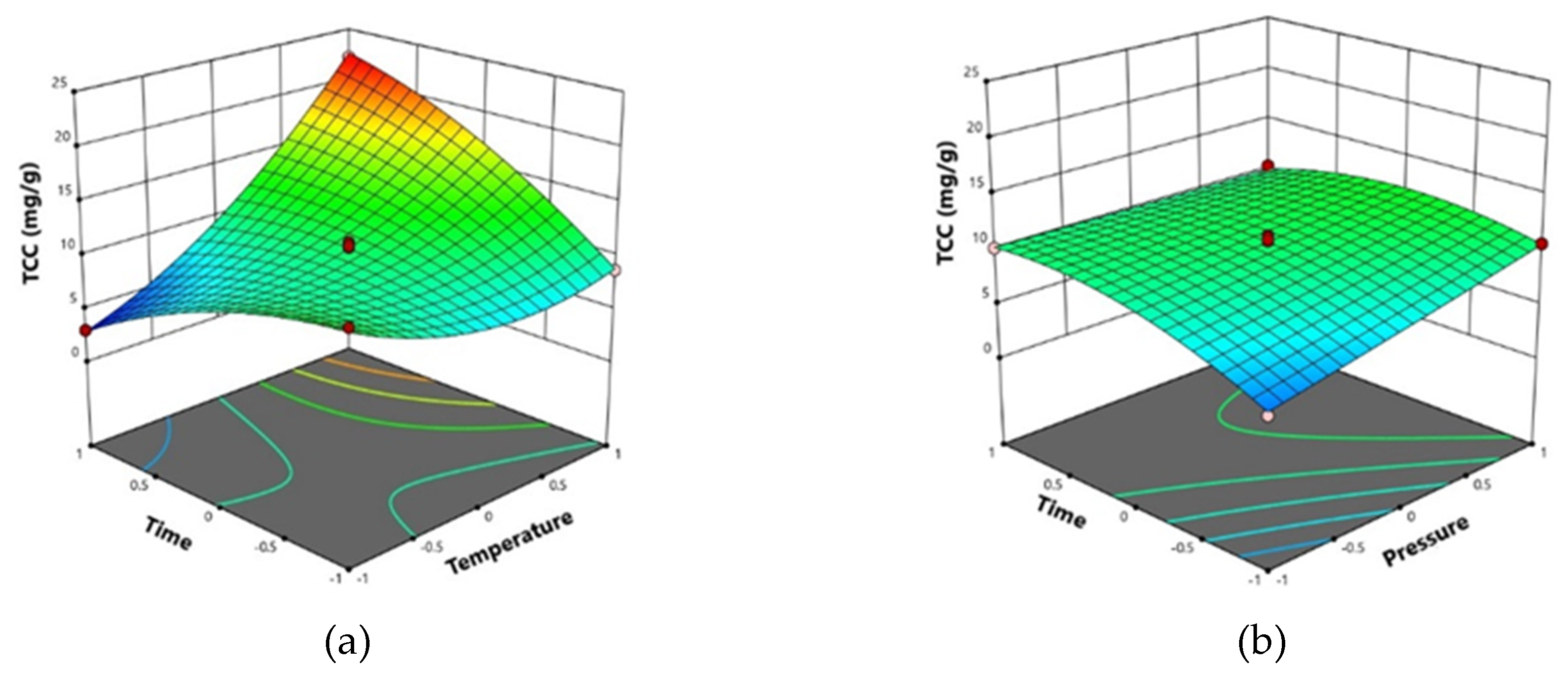
2.5. Effects of SFE conditions on total carotenoid content (TCC)
2.6. Effects of SFE conditions on total anthocyanin content (TAC)
3. Discussion
4. Materials and Methods
4.1. Plant materials
4.2. Chemicals and reagents
4.3. Experimental design
4.4. Supercritical fluid extraction (SFE) for KBD formula
4.5. Determination of the extraction yield
4.6. Analysis of phytochemical content in KBD extract
4.6.1. Determination of the total phenolic content (TPC)
4.6.2. Determination of the total flavonoid content (TFC)
4.6.3. Determination of the total carotenoid content (TCC)
4.6.4. Determination of the total anthocyanin content (TAC)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ngamkhae, N.; Monthakantirat, O.; Chulikhit, Y.; Boonyarat, C.; Khamphukdee, C.; Maneenat, J.; Kwankhao, P.; Pitiporn, S.; Daodee, S. Optimized Extraction Method for Kleeb Bua Daeng Formula with the Aid of the Experimental Design. J. Chem. 2021, 2021, 1457729. [Google Scholar] [CrossRef]
- Chheng, C.; Waiwut, P.; Plekratoke, K.; Chulikhit, Y.; Daodee, S.; Monthakantirat, O.; Pitiporn, S.; Musigavong, N.; Kwankhao, P.; Boonyarat, C. Multitarget Activities of Kleeb Bua Daeng, a Thai Traditional Herbal Formula, Against Alzheimer’s Disease. Pharmaceuticals 2020, 13, 1–15. [Google Scholar] [CrossRef]
- Maneenet, J.; Monthakantirat, O.; Daodee, S.; Boonyarat, C.; Chotritthirong, Y.; Kwankhao, P.; Pitiporn, S.; Awale, S.; Chulikhit, Y. Merging the Multi-Target Effects of Kleeb Bua Daeng, a Thai Traditional Herbal Formula in Unpredictable Chronic Mild Stress-Induced Depression. Pharmaceuticals 2021, 14, 1–17. [Google Scholar] [CrossRef]
- Musigavong, N.; Boonyarat, C.; Chulikhit, Y.; Monthakantirat, O.; Limudomporn, M.; Pitiporn, S.; Kwankhao, P.; Daodee, S. Efficacy and Safety of Kleeb Bua Daeng Formula in Mild Cognitive Impairment Patients: A Phase I Randomized, Double-Blind, Placebo-Controlled Trial. Evidence-based complementary and alternative medicine. eCAM 2022, 2022, 1148112. [Google Scholar] [CrossRef]
- Maneenet, J.; Daodee, S.; Monthakantirat, O.; Boonyarat, C.; Khamphukdee, C.; Kwankhao, P.; Pitiporn, S.; Awale, S.; Chulikhit, Y.; Kijjoa, A. Kleeb Bua Daeng, a Thai Traditional Herbal Formula, Ameliorated Unpredictable Chronic Mild Stress-Induced Cognitive Impairment in ICR Mice. Molecules 2019, 24, 4587. [Google Scholar] [CrossRef]
- Deng, J.; Chen, S.; Yin, X.; Wang, K.; Liu, Y.; Li, S.; Yang, P. Systematic qualitative and quantitative assessment of anthocyanins, flavones and flavonols in the petals of 108 lotus (Nelumbo nucifera) cultivars. Food Chem. 2013, 139, 307–312. [Google Scholar] [CrossRef]
- Intui, K.; Nuchniyom, P.; Laoung-On, J.; Jaikang, C.; Quiggins, R.; Sudwan, P. Neuroprotective Effect of White Nelumbo nucifera Gaertn. Petal Tea in Rats Poisoned with Mancozeb. Foods 2023, 12, 2175. [Google Scholar] [CrossRef]
- Maneenet, J.; Omar, A.M.; Sun, S.; Kim, M.J.; Daodee, S.; Monthakantirat, O.; Boonyarat, C.; Chulikhit, Y.; Awale, S. Benzylisoquinoline alkaloids from Nelumbo nucifera Gaertn. petals with antiausterity activities against the HeLa human cervical cancer cell line. Z Naturforsch C J Biosci. 2021, 76, 401–406. [Google Scholar] [CrossRef]
- Deng, Y.; Sriwiriyajan, S.; Tedasen, A.; Hiransai, P.; Graidist, P. Anti-cancer effects of Piper nigrum via inducing multiple molecular signaling in vivo and in vitro. J. Ethnopharmacol. 2016, 188, 87–95. [Google Scholar] [CrossRef]
- Hritcu, L.; Noumedem, J.A.; Cioanca, O.; Hancianu, M.; Postu, P.; Mihasan, M. Anxiolytic and antidepressant profile of the methanolic extract of Piper nigrum fruits in beta-amyloid (1-42) rat model of Alzheimer’s disease. Behav Brain Funct. 2015, 11, 1–13. [Google Scholar] [CrossRef]
- Shityakov, S.; Bigdelian, E.; Hussein, A.A.; Hussain, M.B.; Tripathi, Y.C.; Khan, M.U.; Shariati, M.A. Phytochemical and pharmacological attributes of piperine: A bioactive ingredient of black pepper. Eur. J. Med. Chem. 2019, 176, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Mojel, R.; Li, G. Physicochemical properties of black pepper (Piper nigrum) starch,” Carbohydr. Polym. 2018, 181, 986–993. [Google Scholar] [CrossRef]
- Azis, H.A.; Taher, M.; Ahmed, A.S.; Sulaiman, W.M.A.W.; Susanti, D. In vitro and In vivo wound healing studies of methanolic fraction of Centella asiatica extract. S. Afr. J. Bot. 2017, 108, 163–174. [Google Scholar] [CrossRef]
- Park, J.H.; Choi, J.Y.; Son, D.J.; Park, E.K.; Song, M.J.; Hellstrom, M.; Hong, J.T. Anti-inflammatory effect of titrated extract of Centella asiatica in phthalic anhydride-induced allergic dermatitis animal model,” Int. J. Mol. Sci. 2017, 18, 1–14. [Google Scholar] [CrossRef]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S.; Rupasinghe, H.P.V. Response surface optimization for recovery of polyphenols and carotenoids from leaves of Centella asiatica using an ethanol-based solvent system. Food Sci. Nutr. 2019, 7, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Panja, P. Green extraction methods of food polyphenols from vegetable materials. Curr. Opin. Food Sci. 2018, 23, 173–182. [Google Scholar] [CrossRef]
- Manjare, S.D.; Dhingra, K. Supercritical fluids in separation and purification: A review. Mater. Sci. En. Tech. 2019, 2, 463–484. [Google Scholar] [CrossRef]
- Budisa, N.; Schulze-Makuch, D. Supercritical carbon dioxide and its potential as a life-sustaining solvent in a planetary environment. Life 2014, 4, 331–340. [Google Scholar] [CrossRef]
- Wrona, O.; Rafińska, K.; Możeński, C.; Buszewski, B. Supercritical Fluid Extraction of Bioactive Compounds from Plant Materials. J. AOAC Int. 2017, 100, 1624–1635. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; dos Santos, W.N.L. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef]
- Ghadiri, K.; Raofie, F.; Davoodi, A. Response surface methodology for optimization of supercritical fluid extraction of orange peel essential oil. Pharm. Biomed. Res. 2020, 6, 303–312. [Google Scholar] [CrossRef]
- Ghafoor, K.; Park, J.; Choi, Y.H. Optimization of supercritical fluid extraction of bioactive compounds from grape (Vitis labrusca B.) peel by using response surface methodology. Innov. Food Sci. Emerg. Technol. 2010, 11, 485–490. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waskiewicz, A. Recent advances in supercritical fluid extraction of natural bioactive compounds from natural plant materials. Molecules. 2020, 25, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Ngamkhae, N.; Monthakantirat, O.; Chulikhit, Y.; Boonyarat, C.; Khamphukdee, C.; Maneenat, J.; Kwankhao, P.; Pitiporn, S.; Daodee, S. Optimization of extraction method for Kleeb Bua Daeng formula and comparison between ultrasound-assisted and microwave-assisted extraction. J. Appl. Res. Med. Aromat. Plants. 2022, 28, 1–11. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Extraction of phenolic compounds from lime peel waste using ultrasonic-assisted and microwave-Assisted extractions. Food Biosci. 2019, 28, 66–73. [Google Scholar] [CrossRef]
- Li, J.; Zu, Y.-G.; Fu, Y.-J.; Yang, Y.-C.; Li, S.-M.; Li, Z.-N.; Wink, M. Optimization of microwave-assisted extraction of triterpene saponins from defatted residue of yellow horn (Xanthoceras sorbifolia Bunge.) kernel and evaluation of its antioxidant activity. Innov. Food Sci. Emerg. Technol. 2010, 11, 637–643. [Google Scholar] [CrossRef]
- Hannachi, H.; Benmoussa, H.; Saadaoui, E.; Saanoun, I.; Negri, N.; Elfalleh, W. Optimization of ultrasound and microwave-assisted extraction of phenolic compounds from olive leaves by response surface methodology. Res. J. Biotechnol. 2019, 14, 28–37. [Google Scholar]
- Dahmoune, F.; Spigno, G.; Moussi, K.; Remini, H.; Cherbal, A.; Madani, K. Pistacia lentiscus leaves as a source of phenolic compounds: microwave-assisted extraction optimized and compared with ultrasound-assisted and conventional solvent extraction. Ind. Crop. Prod. 2014, 61, 31–40. [Google Scholar] [CrossRef]
- Orio, L.; Alexandru, L.; Cravotto, G.; Mantegna, S.; Barge, A. UAE, MAE, SFE-CO2 and classical methods for the extraction of Mitragyna speciosa leaves. Ultrason. Sonochem. 2012, 19, 591–595. [Google Scholar] [CrossRef]
- Majid, A.; Phull, A.R.; Khaskheli, A.H.; Abbasi, S.; Sirohi, M.H.; Ahmed, I.; Ujjan, S.H.; Jokhio, I.A.; Ahmed, W. Applications, and opportunities of supercritical fluid extraction in food processing technologies: A review. Int. J. Advances Appl. Sci. 2019, 6, 99–103. [Google Scholar] [CrossRef]
- Espinosa-Pardo, A.F.; Nakajima, M.V.; Macedo, A.G.; Macedo, A.J.; Martinez, J. Extraction of phenolic compounds from dry and fermented orange pomace using supercritical CO2 and co-solvents. Food Biopro. Process. 2017, 101, 1–10. [Google Scholar] [CrossRef]
- Maneenet, J.; Daodee, S.; Monthakantirat, O.; Boonyarat, C.; Khamphukdee, C.; Kwankhao, P.; Pitiporn, S.; Awale, S.; Chulikhit, Y.; Kijjoa, A. Kleeb Bua Daeng, a Thai Traditional Herbal Formula, Ameliorated Unpredictable Chronic Mild Stress-Induced Cognitive Impairment in ICR Mice. Molecules. 2019, 24, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Moon, S.H.; Keum, Y.S. An updated review on use of tomato pomace and crustacean processing waste to recover commercially vital carotenoids. Food Res. Int. 2018, 108, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Sodeifiana, G.; Sajadiana, A.S.; HOnarvarc, B. Mathematical Modelling for the Extraction of Oil from Dracocephalum kotschyi Seeds in Supercritical Fluid Extraction. Nat. Prod. Res. 2017, 32, 795–803. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1–21. [Google Scholar] [CrossRef]
- Das, A.K.; Mandal, V.; Mandal, S.C. A brief understanding of process optimisation in microwave-assisted extraction of botanical materials: options and opportunities with chemometric tools. Phytochem. Anal. 2014, 25, 1–12. [Google Scholar] [CrossRef]
- Khamphukdee, C.; Chulikhit, Y.; Monthakantirat, O.; Maneenet, J.; Boonyarat, C.; Daodee, S. Phytochemical constituents and antioxidative activity of Schleichera oleosa fruit. Trop. J. Nat. Prod. Res. 2021, 5, 1445–1449. [Google Scholar] [CrossRef]

| Run order | Extraction variables | Response | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| temperature (°C) | pressure (bar) |
time(min) | Percentage extraction yield (%) | TPC (mg GAE/g extract) |
TFC (mg QE/g extract) |
TCC (mg β-CE/g extract) |
|||||
| Experimental | Predicted | Experimental | Predicted | Experimental | Predicted | Experimental | Predicted | ||||
| 1 | -1 (30) | 0 (250) | 1 (90) | 3.28 | 3.31 | 138.78 | 102.31 | 206.30 | 209.48 | 2.99 | 2.86 |
| 2 | 0 (45) | 0 (250) | 0 (60) | 3.26 | 3.49 | 135.33 | 155.38 | 164.97 | 165.46 | 9.39 | 10.74 |
| 3 | 0 (45) | 0 (250) | 0 (60) | 3.51 | 3.49 | 130.45 | 155.38 | 167.97 | 165.46 | 10.95 | 10.74 |
| 4 | 0 (45) | 1 (300) | 1 (90) | 3.53 | 3.46 | 131.45 | 176.63 | 175.08 | 181.82 | 10.33 | 10.01 |
| 5 | -1 (30) | 1 (300) | 0 (60) | 3.57 | 3.61 | 134.78 | 126.07 | 190.63 | 180.72 | 10.5 | 10.95 |
| 6 | 0 (45) | 0 (250) | 0 (60) | 3.39 | 3.49 | 145.36 | 155.38 | 159.41 | 165.46 | 11.34 | 10.74 |
| 7 | -1 (30) | 0 (250) | -1 (30) | 3.05 | 3.04 | 127.22 | 166.00 | 189.08 | 197.53 | 12.55 | 12.17 |
| 8 | -1 (30) | -1 (200) | 0 (60) | 3.08 | 3.01 | 125.22 | 131.62 | 170.37 | 168.66 | 7.70 | 7.76 |
| 9 | 0 (45) | -1 (200) | -1 (30) | 2.67 | 2.74 | 82.33 | 37.15 | 148.63 | 141.89 | 4.94 | 5.26 |
| 10 | 1 (60) | -1 (200) | 0 (60) | 3.48 | 3.44 | 237.67 | 246.38 | 142.08 | 151.99 | 17.13 | 16.68 |
| 11 | 1 (60) | 1 (300) | 0 (60) | 3.81 | 3.88 | 327.22 | 320.82 | 183.08 | 184.79 | 18.56 | 18.50 |
| 12 | 1 (60) | 0 (250) | -1 (30) | 3.50 | 3.47 | 115.56 | 152.03 | 188.86 | 185.68 | 8.72 | 8.85 |
| 13 | 0 (45) | 0 (250) | 0 (60) | 3.50 | 3.49 | 233.44 | 155.38 | 171.63 | 165.46 | 10.81 | 10.74 |
| 14 | 1 (60) | 0 (250) | 1 (90) | 3.58 | 3.59 | 464.56 | 425.78 | 217.19 | 208.74 | 22.26 | 22.64 |
| 15 | 0 (45) | -1 (200) | 1 (90) | 3.13 | 3.16 | 287.78 | 317.85 | 152.97 | 151.51 | 10.19 | 10.26 |
| 16 | 0 (45) | 1 (300) | -1 (30) | 3.52 | 3.49 | 277.33 | 247.26 | 154.97 | 156.43 | 10.59 | 10.52 |
| 17 | 0 (45) | 0 (250) | 0 (60) | 3.80 | 3.49 | 132.33 | 155.38 | 163.3 | 165.46 | 11.21 | 10.74 |
| Source | DF | Percentage extraction yield (%) | TPC (mg GAE/g extract) |
TFC (mg QE/g extract) |
TCC (mg β-CE/g extract) |
||||
|---|---|---|---|---|---|---|---|---|---|
| F-value | p-value | F-value | p-value | F-value | p-value | F-value | p-value | ||
| Model | 9 | 4.72 | 0.0265* | 5.66 | 0.0162* | 8.67 | 0.0047* | 77.70 | < 0.0001* |
| A | 1 | 9.03 | 0.0198* | 17.16 | 0.0043* | 1.01 | 0.3474 | 277.81 | < 0.0001* |
| B | 1 | 20.02 | 0.0029* | 0.8500 | 0.3872 | 12.89 | 0.0089* | 25.72 | 0.0014* |
| C | 1 | 2.84 | 0.1357 | 7.90 | 0.0261* | 7.85 | 0.0265* | 20.61 | 0.0027* |
| AB | 1 | 0.2392 | 0.6397 | 0.5730 | 0.4738 | 1.38 | 0.2789 | 0.9617 | 0.3594 |
| AC | 1 | 0.2102 | 0.6605 | 10.20 | 0.0152* | 0.3953 | 0.5495 | 273.41 | < 0.0001* |
| BC | 1 | 1.89 | 0.2114 | 11.05 | 0.0127* | 0.7964 | 0.4018 | 15.56 | 0.0056 |
| A2 | 1 | 0.6959 | 0.4317 | 1.73 | 0.2304 | 31.75 | 0.0008* | 61.73 | 0.0001* |
| B2 | 1 | 0.8501 | 0.3872 | 0.4367 | 0.5299 | 17.83 | 0.0039* | 0.0280 | 0.8718 |
| C2 | 1 | 6.68 | 0.0363* | 0.7516 | 0.4147 | 6.10 | 0.0428* | 27.51 | 0.0012* |
| R2 | 0.8584 | 0.8792 | 0.9177 | 0.9901 | |||||
| Adj-R2 | 0.6764 | 0.7239 | 0.8119 | 0.9773 | |||||
| Factor | Symbol | Levels | ||
|---|---|---|---|---|
| -1 | 0 | 1 | ||
| Extraction temperature (°C) | A | 30 | 45 | 60 |
| Extraction pressure (bar) | B | 200 | 250 | 300 |
| Extraction time (min) | C | 30 | 60 | 90 |
| Run order | Extraction variables | ||
|---|---|---|---|
| Extraction temperature (°C) | Extraction pressure (bar) | Extraction time (min) | |
| 1 | 30 | 250 | 90 |
| 2 | 45 | 250 | 60 |
| 3 | 45 | 250 | 60 |
| 4 | 45 | 300 | 90 |
| 5 | 30 | 300 | 60 |
| 6 | 45 | 250 | 60 |
| 7 | 30 | 250 | 30 |
| 8 | 30 | 200 | 60 |
| 9 | 45 | 200 | 30 |
| 10 | 60 | 200 | 60 |
| 11 | 60 | 300 | 60 |
| 12 | 60 | 250 | 30 |
| 13 | 45 | 250 | 60 |
| 14 | 60 | 250 | 90 |
| 15 | 45 | 200 | 90 |
| 16 | 45 | 300 | 30 |
| 17 | 45 | 250 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





