Submitted:
25 August 2023
Posted:
28 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Thermodynamic and crossover transition behaviors in 1-alkanols
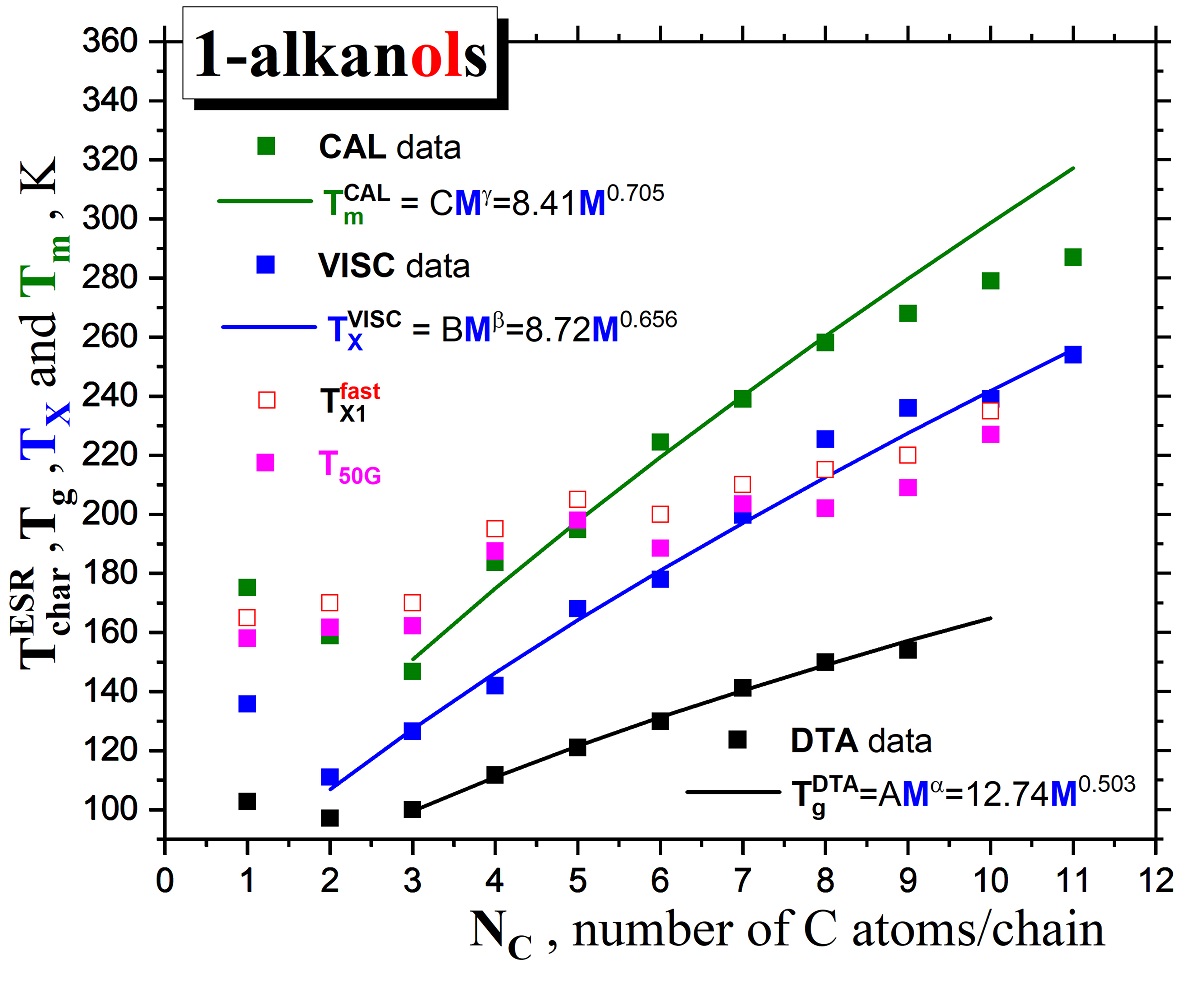
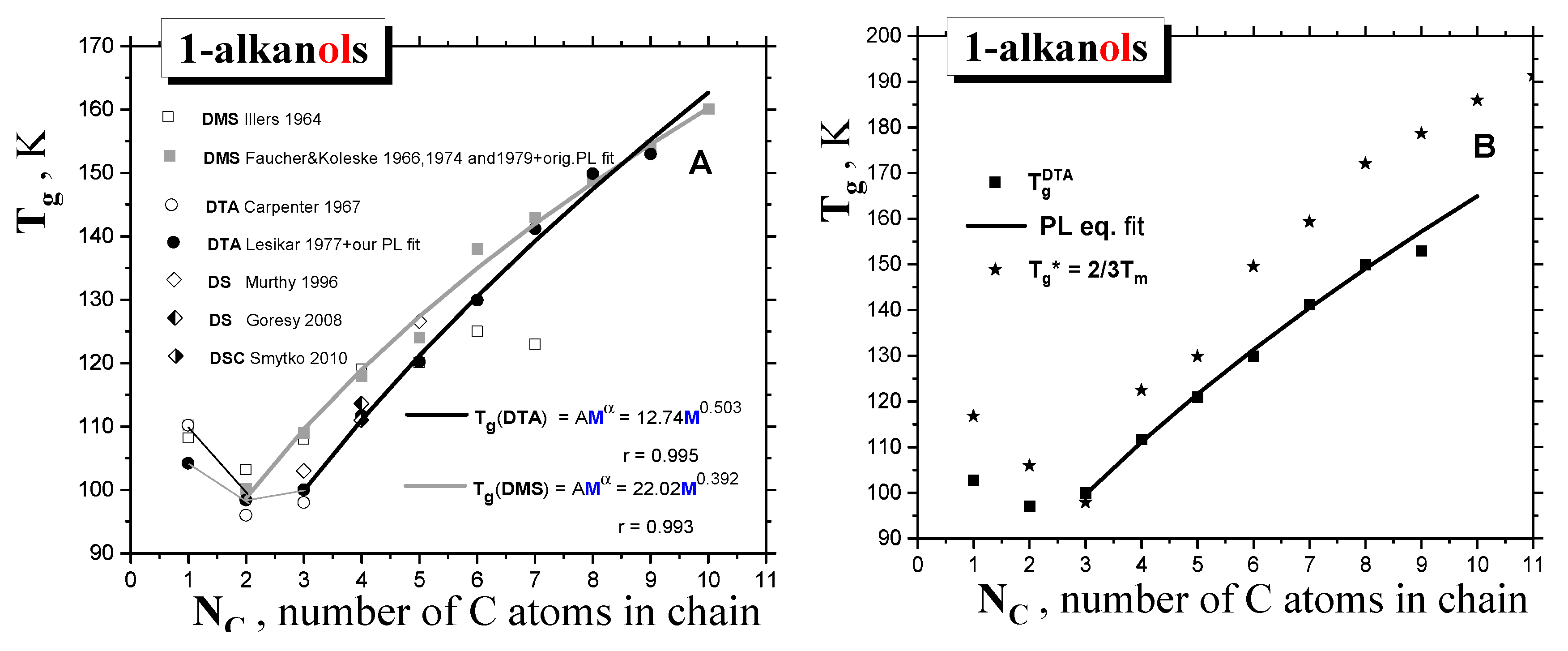
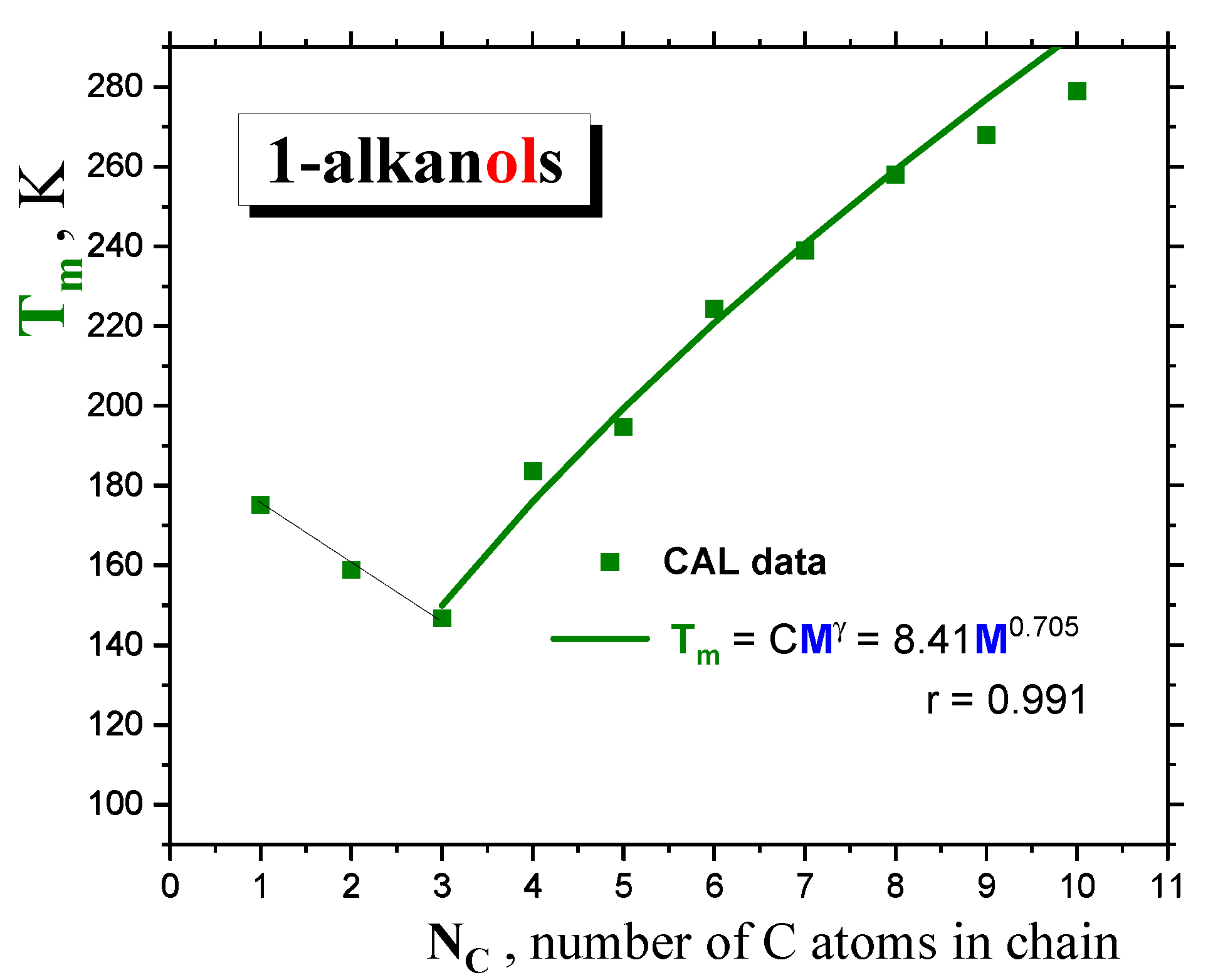
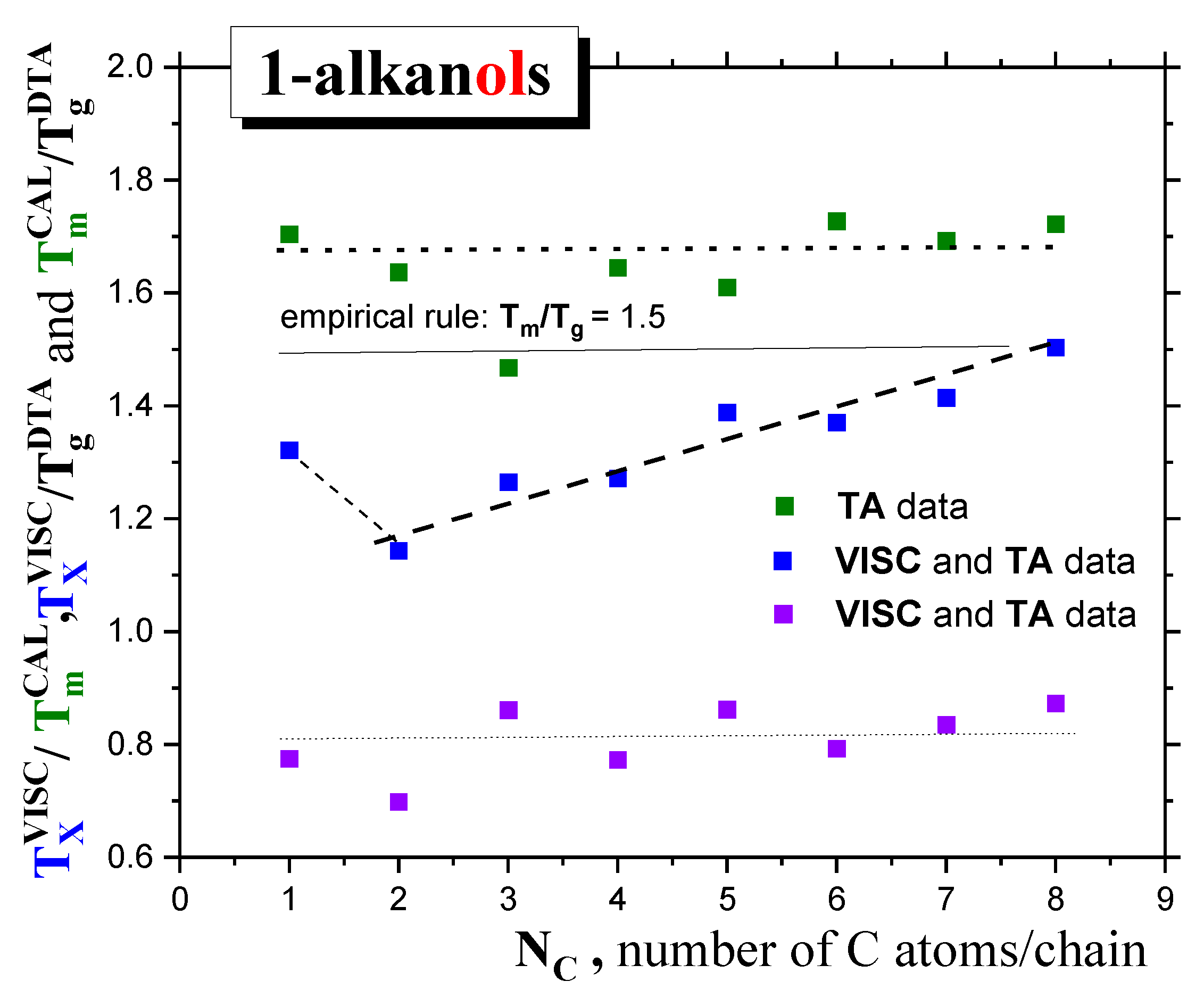
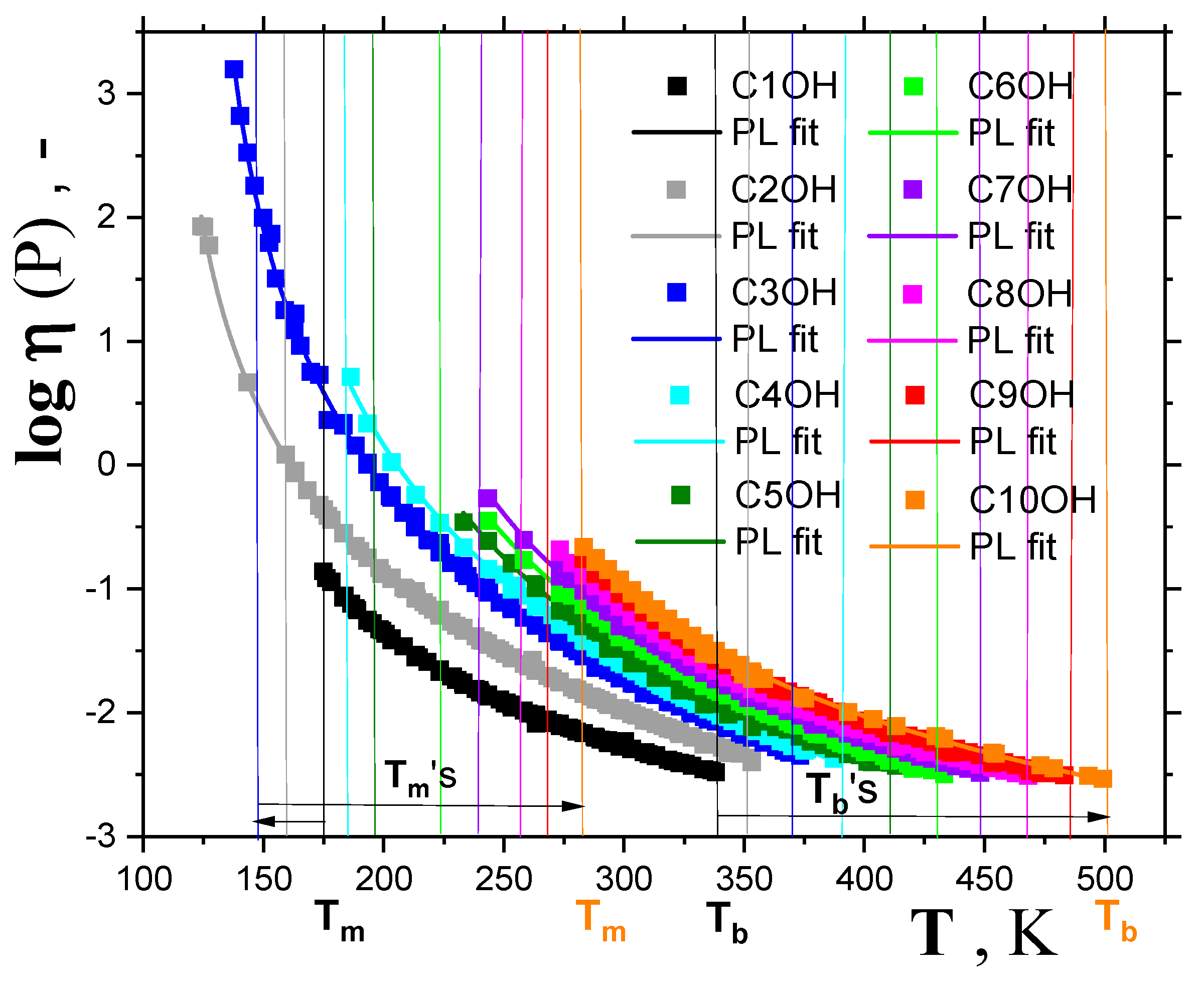
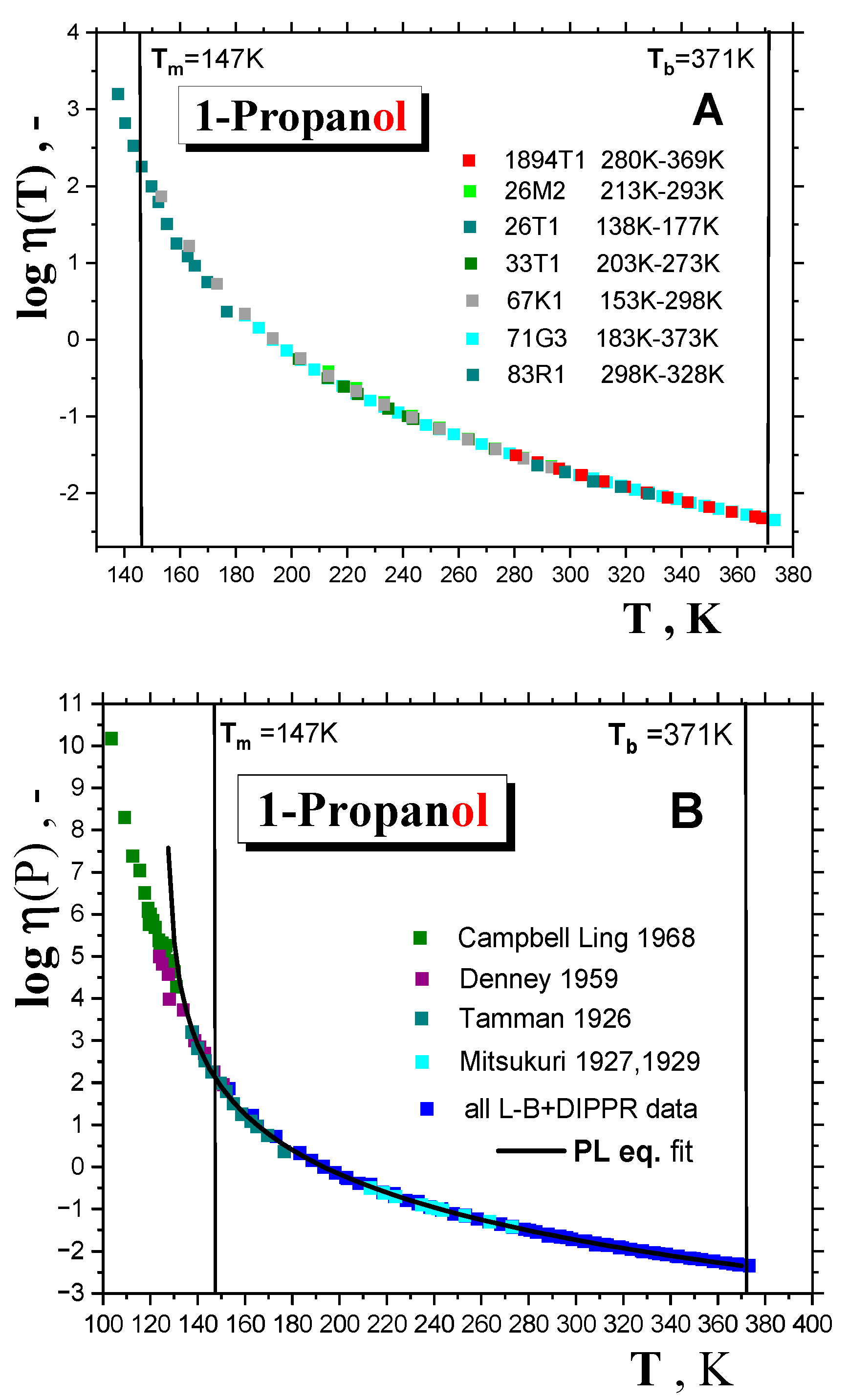
2.1.1. Thermodynamic transitions in 1-alkanols
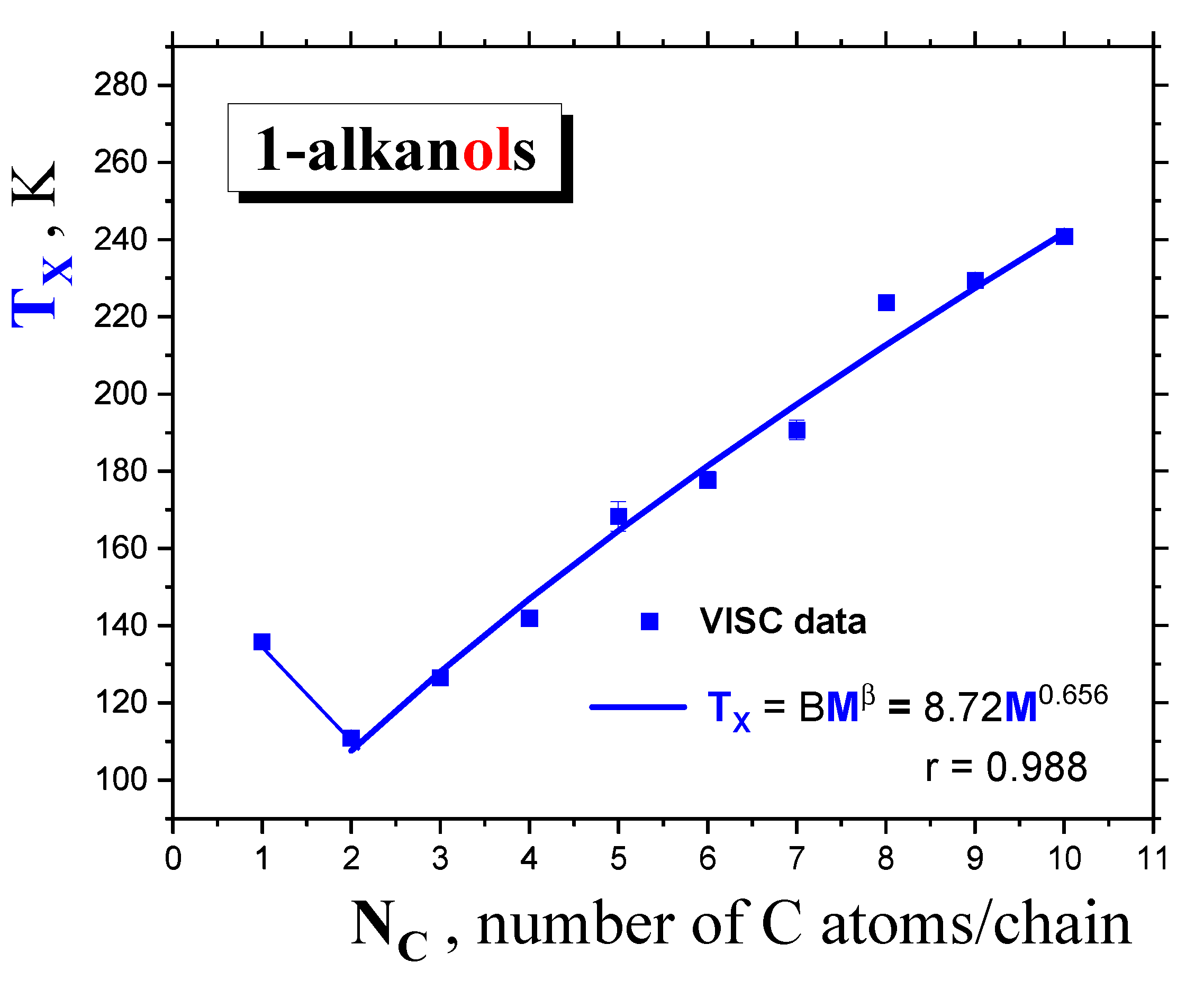
2.1.2. Dynamic crossovers in 1-alkanols
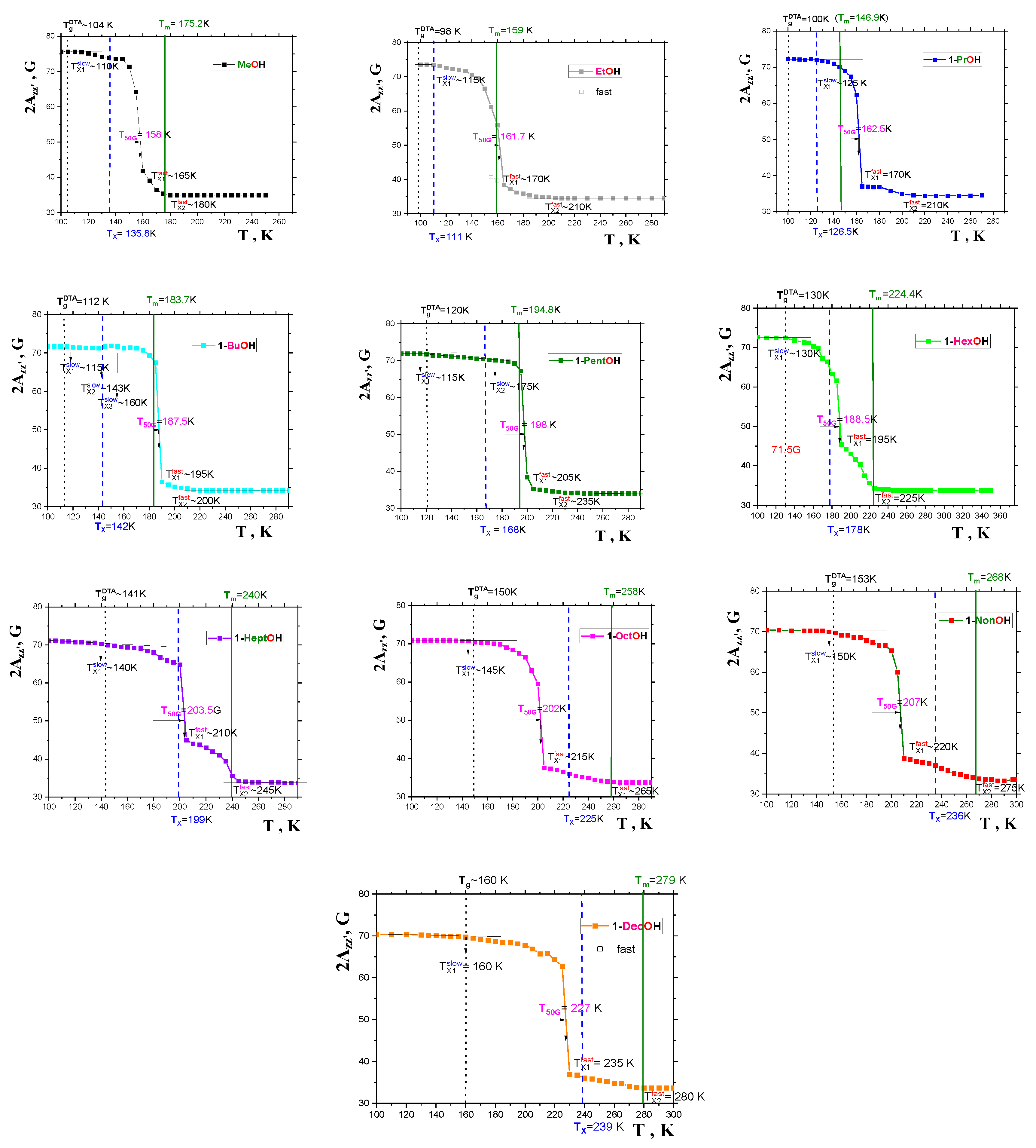
2.2. ESR data
2.2.1. General spectral and dynamic features
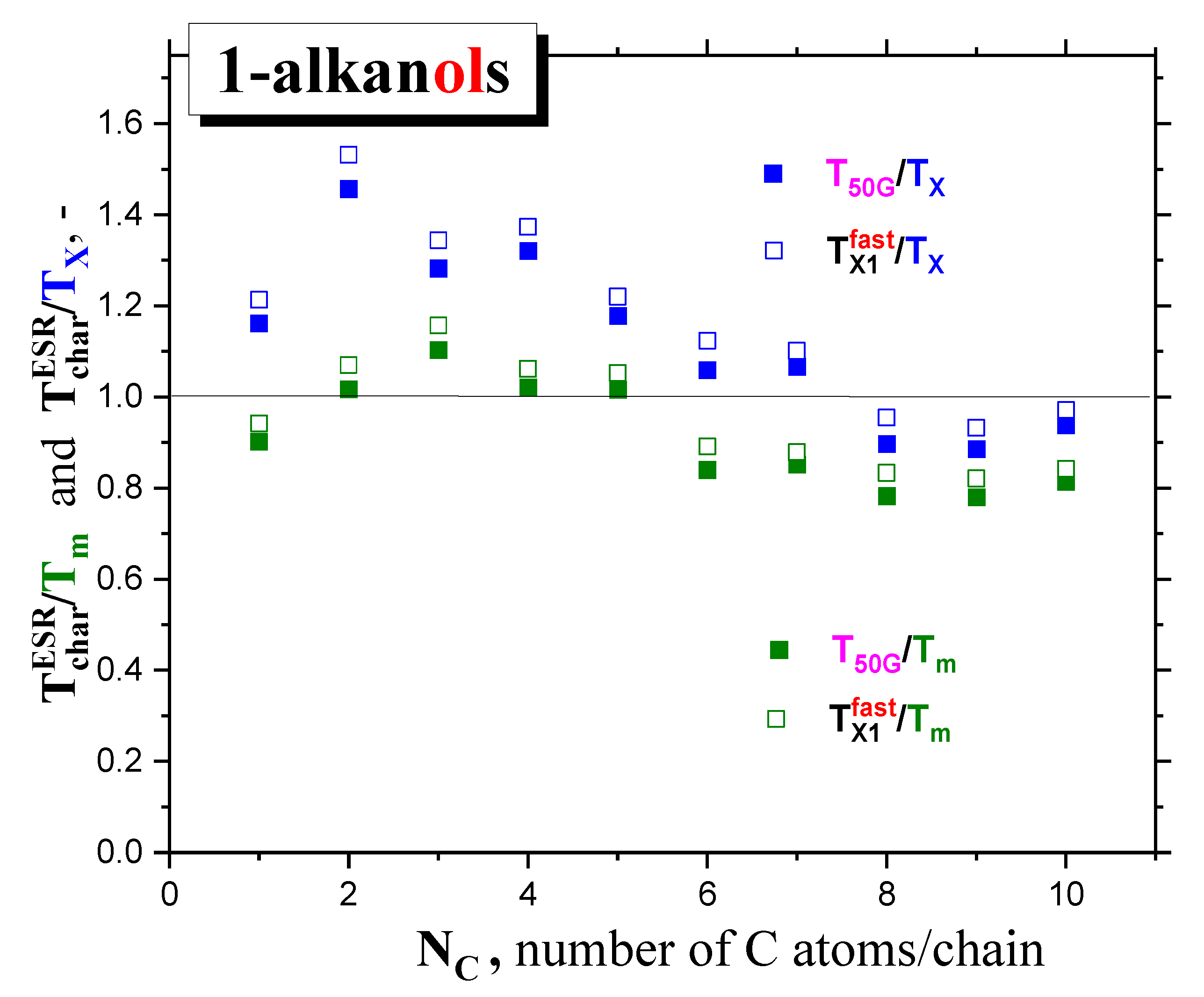
2.2.2. The mutual relationships of T50G and TX1fast with thermodynamic and dynamic transitions
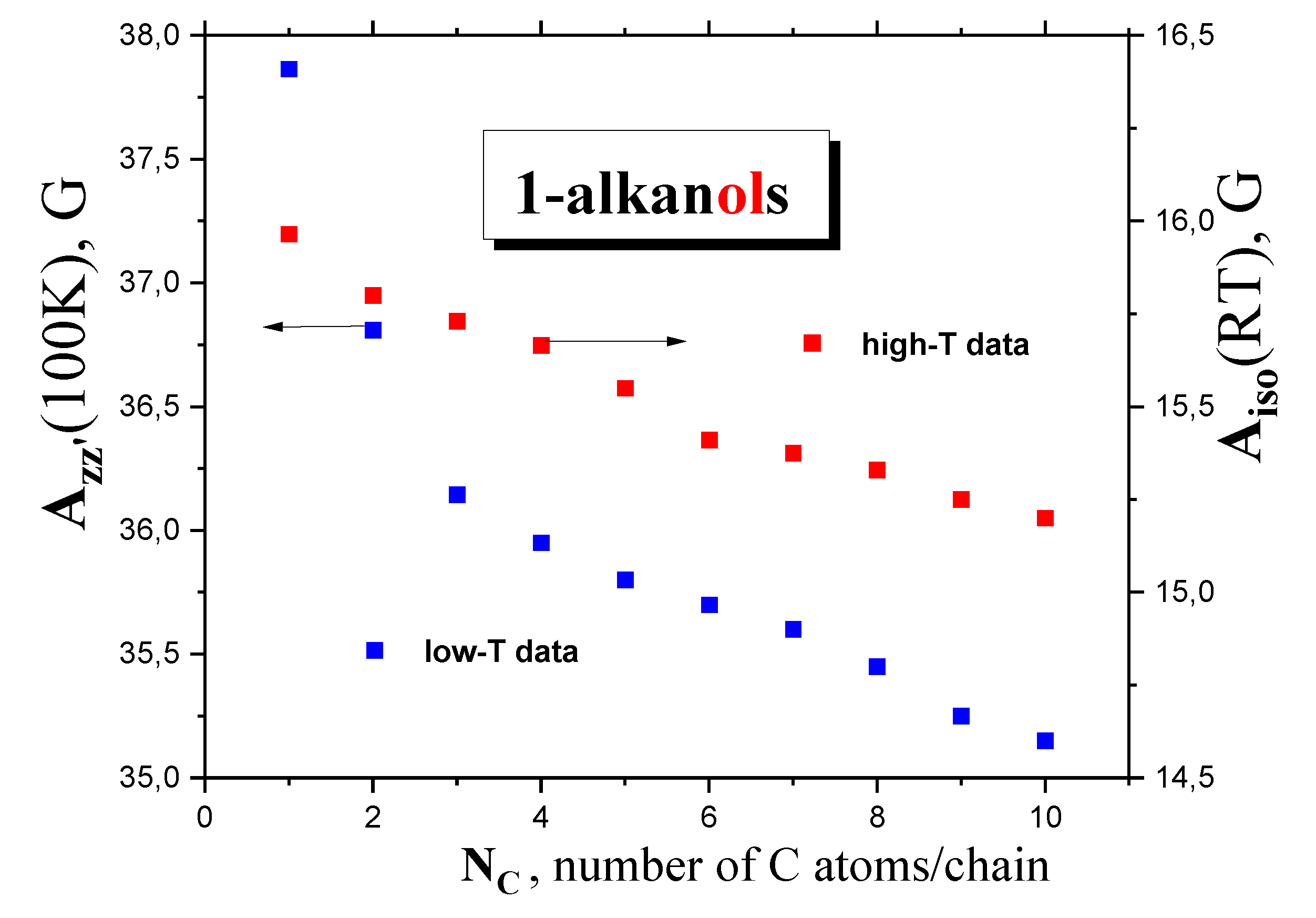
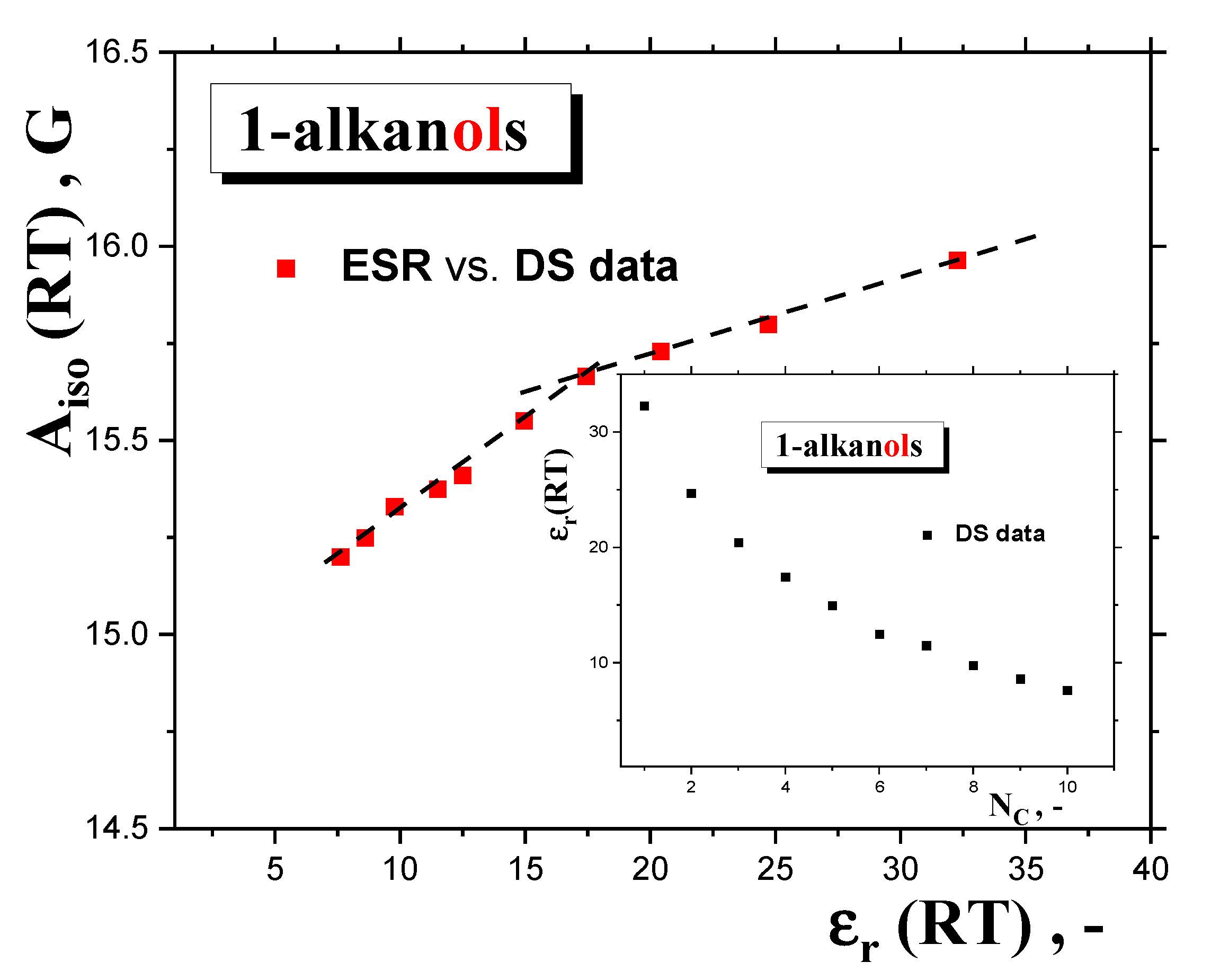
2.2.3. Isotropic and anisotropic hyperfine constants Aiso(RT), Azz‘(100 K) as a function of NC and polarity and proticity of 1-alkanols
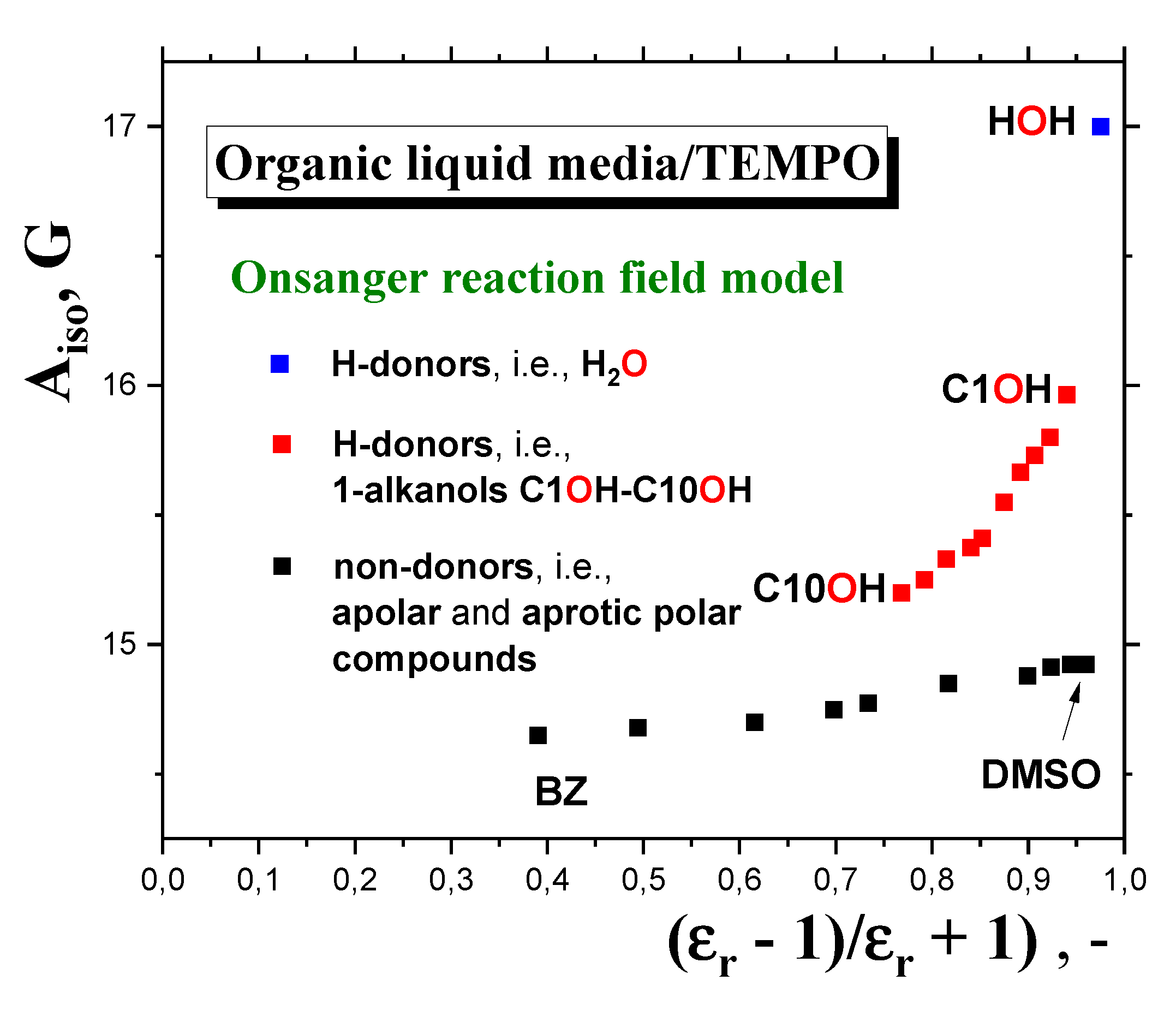
2.2.4. Connection of the main slow to fast motion transition of the spin probe TEMPO with the polarity and proticity and the thermodynamic and dynamic transition behavior of 1-alkanols.
3. Experimental
3.1. Materials and Methods
3.2. ESR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barlow, A.J.; Lamb, J.; Matheson, A.J. Viscous behavior of supercooled liquids. Proc.Roy.Soc.London,Ser.A 1966, 292, 322–342. [Google Scholar]
- Taborek, P.; Kleinman, R.N.; Bishop, D.J. Power law behavior in the viscosity of supercooled liquids. Phys.Rev.B 1986, 34, 1835–1840. [Google Scholar] [CrossRef]
- Stickel, F.; Fischer, E.W.; Richert, R. Dynamics of glass-forming liquids. I. Temperature-derivative analysis of dielectric relaxation data. J.Chem.Phys. 1995, 102, 6251–6257. [Google Scholar] [CrossRef]
- Stickel, F.; Fischer, E.W.; Richert, R. Dynamics of glass-forming liquids. II. Detailed comparison of dielectric relaxation, dc-conductivity and viscosity data. J.Chem.Phys. 1996, 104, 2043–2055. [Google Scholar] [CrossRef]
- Martinez-Garcia, J.C.; Martinez-Garcia, J.; Rzoska, S.; Huellinger, J. The new insight into dynamic crossover in glass forming liquids from the apparent enthalpy analysis. J.Chem.Phys. 2012, 137, 064501-8. [Google Scholar] [CrossRef]
- a. Leon, L.; Ngai, K.L. Rapidity of the Change of the Kohlrausch Exponent of the α-Relaxation of Glass-Forming Liquids at TB or Tβ and Consequence. J.Phys.Chem. B 1999, 103, 4045–4051; b. Ngai, K.L.; Roland, C.M. Development of cooperativity in the local segmental dynamics of poly(vinylacetate): synergy of thermodynamics and intermolecular coupling. Polymer 2002, 43, 567–573.
- Schönhals, A. Evidence for a universal crossover behavior of the dynamic glass transition. Europhys. Lett. 2001, 56, 815–521. [Google Scholar] [CrossRef]
- a. Johari, G.P.; Goldstein, M. Viscous Liquids and the Glass Transition. II. Secondary Relaxations in Glasses of Rigid Molecules. J. Chem.Phys. 1970, 53, 2372–2388; b. Beiner, M.; Huth, H.; Schröter, K. Crossover region of dynamic glass transition: general trends and individual aspects, J. Non-Cryst.Solids 2001, 279, 126–135; c. Mallamace, F.; Corsaro, C.; Leone,N.; Villari, V.; Micali, N.; Chen, S.H. On the ergodicity of supercooled molecular glass-forming liquids at the dynamic arrest: ortho-terphenyl case. Sci.Rep. 2014, 4, 3747.
- Mallamace, F.; Branca, C.; Corsaro, C.; Leone, N.; Spooren, J.; Chen, S.H.; Stanley, H.E. Transport properties of glass-forming liquids suggest that dynamic crossover temperature is as important as the glass transition temperature. Proc.Natl.Acad.Sci.U.S.A. 2010, 107, 22457–22462. [Google Scholar] [CrossRef]
- a. Roland, C.M. Characteristic relaxation times and their invariance to thermodynamic conditions. Soft Matter 2008,4,2316-2322; b. Roland, C.M. Relaxation Phenomena in Vitrifying Polymers and Molecular Liquids. Macromolecules 2010, 43, 7875–7890.
- Götze, W.; Sjögren, L. Relaxation processes in supercooled liquids, Rep.Progr.Phys., 1992, 55, 241–376; b. Götze, W. Recent tests of the mode-coupling theory for glassy dynamics. J.Phys.-Cond.Matter, 1999, 11, A1–A45; c. Götze, W. Complex Dynamics of Glass-Forming Liquids,:A mode coupling theory, Oxford Univ.Press, Oxford, 2009.
- Novikov, V.N.; Sokolov, A.P. Universality of the dynamic crossover in glass-forming liquids: A ‘‘magic’’ relaxation time. Phys. Rev.E 2003, 67, 031507. [Google Scholar] [CrossRef]
- a. Hyde, P.D.; Evert, T.E.; Cicerone, M.T.; Ediger, M.D. Rotational motion of molecular probes in orto-terphenyl and cis-poly-isoprene, J Non-Cryst.Solids 1991, 131-133, 42-47; b. Ediger, M.D. Spatially Heterogeneous Dynamics in Supercooled liquids. Annu. Rev.Phys.Chem. 2000, 51, 99–128.1, Todd E. 14. a. Andreozzi, L.; Schinoy, A.D.; Giordano, M.; Leporini, D. A study of the Debye–Stokes–Einstein law in supercooled fluids J. Phys.-Cond.Matter 1996, 8, 9605–9608; b. Andreozzi, L.;Faetti, M.; Giordano, M. Fractional Debye-Stokes-Einstein law and scaling of the rotational relaxation in molecular glass formers: linear and non-linear ESR studies. Rec.Res.Devel.Phys.Chem. 2001, 5, 219–254; c. Andreozzi, L.; Faetti, M.; Giordano, M.; Zulli, F. Length Scales and Dynamics in the Reorientational Relaxation of Tracers in molecular and Polymeric Glass Formers via ESR. J.Phys.Chem.B 2010, 114, 12833–12839.
- a. Bartoš, J.; Šauša, O.; Bandžuch, P.; Zrubcová, J.; Krištiak, J. Free volume factor in supercooled liquid dynamics .J. Non-Cryst. Solids 2002, 307–310, 417–425. b. Bartoš, J.; Šauša, O.; Krištiak, J.; Blochowicz, T.; Rössler, E. Free-volume microstructure of glycerol and its supercooled liquid-state dynamics. J.Phys.-Cond.Matter 2001,13, 11473–11484.
- Bartoš, J.; Corsaro, C.; Mallamace, D.; Švajdlenková, H.; Lukešová, M. ESR evidence of the dynamic crossover in the supercooled liquid states of a series of solid n-alkanes. Phys.Chem.Chem.Phys. 2018, 20, 11145–11151. [Google Scholar] [CrossRef]
- Ramos, M.A.; Talon, C.; Jimenez-Riobóo, R.J.; Vieira, S. Low-temperature specific heat of structural and orientational glasses of simple alcohols. J. Phys.-Cond.Matter 2003, 15, S1007. [Google Scholar] [CrossRef]
- Available online: http://webbooknistgov/chemistry/.
- Illers, K.H. Innere Rotationen in Festkörpern aus hoch- und niedrigmolekularen organischen Molekülen. Rheol. Acta 1964, 3, 183–193. [Google Scholar] [CrossRef]
- a. Faucher, J.A.; Koleske, J.V. Glass Transitions of Organic Compounds. 1.Lower Aliphatic Alcohols, Phys. Chem. Glasses 1966, 7, 202–208; b. Koleske, J.V.; Faucher, J.A. Glass Transitions of Organic Compounds. 2.Linear Aliphatic Alcohols, Phys.Chem. Glasses, 1974, 15, 65–67.
- Sugisaki, H.; Suga, H.; Seki, S. Calorimetric Study of the Glassy state.III. Novel Type Calorimeter for Study of Glassy State and heat Capacity of Glassy Methanol. Bull.Chem.Soc.Jpn. 1968, 41, 2586–2591. [Google Scholar] [CrossRef]
- Lesikar, A.V. On the self-association of the normal alcohols and the glass transition in alcohol-alcohol solutions. J.Solut.Chem. 1977, 6, 81–93. [Google Scholar] [CrossRef]
- Carpenter, M.R.; Davis, D.B.; Matheson, A.J. Measurement of the Glass Transition Temperature of Simple Liquids. J.Chem.Phys. 1967, 48, 2451–2454. [Google Scholar] [CrossRef]
- Haida, O.; Suga, H.; Seki, S. Calorimetric study of the glassy state XII. Plural glass transition phenomena of ethanol. J.Chem. Thermodyn. 1977, 9, 1133–1148; b. Brand, R.; Lunkenheimer, P.; Schneider, U.; Loidl,A. Excess wing in the dielectric loss of glass-forming ethanol: A relaxation process Phys.Rev. B 2000, 62, 8878–8883.
- Murthy, S.S.N. Experimental study of dielectric relaxation in supercooled alcohols and polyols. Mol.Phys. 1996, 97, 691–709. [Google Scholar] [CrossRef]
- Koleske, J.V.; Faucher, J.A. Glass Transitions of Organic Compounds. III. Cellulose Substrate Technique and Aliphatic Alcohols. Polym.Engn.Sci. 1979, 19, 716–721. [Google Scholar] [CrossRef]
- a. El Goresy,T.; Böhmer, B. Diluting the hydrogen bonds in viscous solutions of n-butanol with n-bromobutane: A dielectric study. J.Chem. Phys. 2008, 28, 154520 ; b. Bartoš, J.; Šauša, O.; Vyroubalová, M.; Maťko, I. Švajdlenková,H. Confined effects on Structural isomers in the MCM-41-SIL Matrix as Seen by extrinsic probes via PALS and ESR“ n-Butanol vs. tert-Butanol J.Phys. Chem. C 2021, 125, 15796–15811.
- Hassaine, M.; Jimenez-Rioboo, R.J.; Ramos, M.A.; Sharapova, I.V.; Koroyluk, O.A.; Krivchikov, A.I. Thermal properties and Brillouin scattering study of glass, crystal and „glacial“ state in n-butanol. J.Chem.Phys. 2009, 131, 174508. [Google Scholar] [CrossRef]
- Novikov, V.N.; Rössler, E.A. Correlation between glass transition temperature and molecular mass in non-polymeric and polymer glass formers. Polymer 2013, 54, 6987–699113. [Google Scholar] [CrossRef]
- Kauzmann, W. The nature of the glassy state and the behavior of liquids at low temperature. Chem.Rev. 1948, 43, 219–256. [Google Scholar] [CrossRef]
- Boyer, R. The relation of transition temperatures to chemical structure in high polymers. Rubber Chem.Technol. 1963, 36, 1303–1421. [Google Scholar] [CrossRef]
- Sakka, S.; McKenzie, J.D. Relation between apparent glass transition temperature and liquidus temperature for inorganic glasses. J. Non-Cryst. Solids 1971, 6, 145–162. [Google Scholar] [CrossRef]
- Landolt-Börnstein - Group IV Physical Chemistry, Pure Organic Liquids, Subvolume B ‘Pure Organic Liquids’ of Volume 18 Viscosity of Pure Organic Liquids and Binary Liquid Mixtures’ of Landolt-Börnstein - Group IV Physical Chemistry.
- R. Rowley, DIPPR Data Compilation of Pure Chemical Properties, Design Institute for Physical Properties, 2010 (Ref Type: Electronic Citation).
- Barrera, M.C.; Jorge, M. A Polarization-Consistent Model for Alcohols to Predict Solvatation Free Energies. J.Chem.Info & Modelling 2020, 60, 1352–1367. [Google Scholar]
- Tamman, G.; Hesse, W.Z. Die Abhängigkeit der Viskosität von der Temperatur die unterkühlten Flüssigkeiten. Zeitsch.Anorg. Allgem.Chem. 1926, 156, 245–257. [Google Scholar] [CrossRef]
- Denney, D.J. Viscosities of some undercooled liquid alkylhalides. J.Chem.Phys. 1959, 30, 159–162. [Google Scholar] [CrossRef]
- Campbell Ling, A.A.C.; Willard, J.E. Viscosities of some organic glasses used as trapping matrixes. J.Phys.Chem. 1968, 72, 1918–1923. [Google Scholar] [CrossRef]
- Mitsukuri, S.; Tonomura, T. Proc. Imper Academy of Japan 1927, 3, 155 and 1929, 5, 23.
- Krakoviack, V.; Alba-Simionesco, C.; Krauzman, M. Study of the depolarized light scattering spectra of supercooled liquids by a simple mode-coupling model. J.Chem.Phys. 1997, 107, 3417–3427. [Google Scholar] [CrossRef]
- Bartoš, J.; Švajdlenková, H.; Šauša, O.; Lukešová, M.; Ehlers, D.; Michl, M.; Lunkenheimer, P.; Loidl, A. Molecular probe dynamics and free volume in organic glass-formers and their relationships to structural relaxation:1-propanol. J.Phys.-Cond.Matt 2016, 28, 015101. [Google Scholar] [CrossRef]
- Dodd, G.H.; Barratt, M.D.; Rayner, L. Spin probes for binding site polarity. FEBS 1970, 8, 286–288. [Google Scholar] [CrossRef] [PubMed]
- Jolicoeur, C.; Friedman, H.L. Hydrophobic nitroxide radicals as probes to investigate the hydrophobic interaction. J.Sol. Chem. 1974, 3, 15–43. [Google Scholar] [CrossRef]
- Al-Bala, R.D. Bates, Jr. R.D. Medium Effects on ESR Spectra in Studies of Hydrogen-Bonded Transient Solvent-Solute Complexes. J.Magn.Res. 1987, 73, 78–89. [Google Scholar] [CrossRef]
- Laleveé, J.; Allonas, X.; Jacques, P. Electronic distribution and solvatochromism of investigation a model radical 2,2,6,6-tetramethyl piperidine N – oxyl: TEMPO through TD-DFT calculation including PCM solvatation. J.Mol.Struct.Theochem. 2006, 767, 143–147. [Google Scholar] [CrossRef]
- Kawamura, T.; Matsunami, S.; Yonezawa, T. Solvent Effects on the g-value of Di-t-butyl Nitric oxide. Bull.Chem.Soc.Jpn. 1967, 40, 1111–1115. [Google Scholar] [CrossRef]
- Griffith, O.H.; Dehlinger, P.J.; Van, S.P. Shape of the Hydrophobic Barrier of Phospholipid Bilayers (Evidence for Water Penetration in Biological Membranes). J.Membrane Biol. 1974, 15, 159–192. [Google Scholar] [CrossRef]
- Krinichnyi, V.I.; Grinberg, O.Y., Bogatyrenko, V.R.; Likhtenshtein, G.I.; Lebedev, Y.S. Biophysics 1985, 30, 233.
- Ondar, M.A.; Grinberg, O.Y.; Dubinskii, A.A.; Lebedev, Y.S. Sov.J.Chem.Phys. 1985, 3, 781.
- Owenius, R.; Engström, M.R.; Lindgren, M. Influence of Solventr Polarity and Hydrogen Bonding on the EPR parameters of a Nitroxide Spin label Studied by 9-GHZ and 95-GHz EPR Spectroscopy and DFT calculations. J.Phys.Chem. 2001, 105, 10967–10977. [Google Scholar] [CrossRef]
- Seelig, J.; Limacher, H.; Bader, P. Molecular Architecture of Liquid Crystalline Bilayer. J.Amer.Chem Soc. 1972, 94, 6364–6371. [Google Scholar] [CrossRef]
- Onsanger, L. Electric Moments of Molecules in Liquids. J.Amer.Chem.Soc. 1936, 58, 1486–1493. [Google Scholar] [CrossRef]
- Böttcher, C.J.F. Theory of Electric Polarisation; Elsevier: New York, N. Y., Amsterdam, 1952; p. 70.
- Rabold, G.P.; Spin-Probe Studies.II. Applications to Polymer Characterization. J.Polym.Sci. A 1969, 7, 1203–1223. [Google Scholar] [CrossRef]
- Bartoš, J.; Švajdlenková, H.; Zaleski, R.; Edelmann, M.; Lukešová, M. Spin probe dynamics in relation to free volume in crystalline organics by means of ESR and PALS: n-Hexadecane. Physica B Cond.Mat. 2013, 430, 99–105. [Google Scholar] [CrossRef]
- Švajdlenková, H.; Iskrová, M.; Šauša, O.; Dlubek, G.; Krištiak, J.; Bartoš, J. The Spin probe Dynamics and the Free Volume in a series of Amorphous Polymer Glass-Formers. Macromol.Symp. 2011, 305, 108–115. [Google Scholar] [CrossRef]
- Švajdlenková, H.; Arrese-Igor, S.; Nógellová, Z.; Alegría, A.; Bartoš, J. Molecular dynamic heterogeneity in relation to free volume and relaxation dynamics in organic glass-formers: oligomeric cis-1,4-poly(isoprene). Phys.Chem.Chem.Phys. 2017, 19, 15215–15226. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





