1. Introduction
Haemonchus contortus and
Trichostrongylus colubriformis are considered to be the most important gastrointestinal nematodes of sheep in Brazil [
1,
2].
T. colubriformis causes disorders in the mucosa of the small intestine, which affect digestion and absorption of nutrients and leads to impairment of feed utilization [
3,
4,
5]. Blood-sucking
H. contortus parasitism leads to anemia, weakness and frequently deaths, particularly among young sheep and periparturient ewes [
6,
7]. Populations of both
H. contortus and
T. colubriformis with multidrug resistance are widespread [
8,
9,
10], which make it challenging to provide proper prophylaxis for parasitic infections and, in some circumstances, impractical to have sheep production under the existing grazing conditions.
Significant reductions in performance occur among young sheep that are mix-infected with
H. contortus and
T. colubriformis. Dorper lambs under conditions of serial artificial infection showed a 13.7% reduction in food consumption, 21.3% reduction in daily body weight gain and 16.6% reduction in cold carcass weight [
11]. A study on grazing lambs showed that supplemented animals had lower FECs compared with unsupplemented groups [
12].
Under a climate characterized by warm, rainy summers and dry winters, there is no seasonality in the transmission of
H. contortus and
T. colubriformis to sheep. Large worm burdens may develop even during periods of drought, due to the presence of infective larvae, which are able to survive despite the low environmental humidity [
1]. The infective larvae are considerably resilient, and can survive on pasture for periods of some months, provided that temperatures are not extreme and moisture is sufficient [
13].
Once an infective larva has been ingested by a host, its success in becoming established in the digestive tract depends on factors related to the host’s immune response. The optimal situation occurs when larvae are ingested by a naïve host. Conversely, in sheep that have developed immunity as a result of ongoing infections, establishment of larvae may be drastically reduced [
14,
15]. In this case, the infective larvae are often unable to settle in the mucosa and are eliminated. The development of the immune response is related with the release of epithelial-derived cytokines that activates a variety of innate immune cells that promote adaptive Th2 responses. Type 2 cytokines, either from innate or adaptive origin, activate effector mechanisms at the gut tissue. IL-5 and IL-9 recruit eosinophils and mast cells and IL-13 enhances the turnover of the epithelium. Goblet cell hyperplasia, augmented mucus production along with changes in its composition, production of resistin-like molecule b (RELM-b), and increased muscle contractility are common effector mechanisms against helminths driven by both IL-4 and IL-13. All these local changes favor parasite dislodging and clearance [
16].
As a way to evade the immune response of the host, the development of infective larvae that manage to become established becomes inhibited, usually when the larvae are at the early fourth stage (early-L4). This process of inhibition is called hypobiosis, dormancy or arrested development. In the case of
H. contortus, about 90% of hypobiotic larvae are recovered from the abomasal contents and washings, thereby suggesting that early-L4 may be only loosely attached to the abomasal mucosa, from which they may be dislodged during the processing of the abomasa for worm examination [
17].
Some evidence suggesting a connection between presence of adult worms and inhibition of development exists. Hypobiosis might involve a threshold of infection that is regulated by the adult worm population that has already become established in the gastrointestinal tract. It was found that greater numbers of inhibited
H. contortus larvae were present when a challenge was superimposed on extant infections, thus indicating that resident worms or a factor activated by their presence induced developmental arrest [
14,
18,
19].
Hypobiosis may also be triggered when the environmental conditions are adverse for development and survival of the free-living stages. Because the infective stage of
H. contortus is highly susceptible to winter conditions, hypobiosis is usually the major factor in overwinter survival in cold temperate zones, with arrested development of the majority of infective larvae ingested in autumn. Rapid development during short periods of high summer temperatures can lead to haemonchosis [
7].
There are also indications that hypobiosis can occur due to lack of moisture in markedly seasonal climates with long and hot dry seasons, in semi-arid regions, which are also adverse for development and survival of the free-living stages. Under such conditions, hypobiosis largely explains the common, though sporadic, outbreaks of haemonchosis. There have been instances when acute haemonchosis was reported during the dry season, even before the onset of the rains in semi-arid areas of Kenya. The source of this disease was attributed to resumption of the development of hypobiotic larvae [
17].
Although less frequent, there are also reports of inhibited development of
Trichostrongylus spp. While other trichostrongylids are inhibited as early-L4 larvae, Trichostrongylus spp. is found inhibited as L3 [
20,
21]. According to [
20], in the Netherlands, the main cause of inhibition of
Trichostrongylus spp. is presumably immunity of the adult sheep.
Because of the importance of hypobiosis in the epidemiology of parasitic gastroenteritis, the present experiment was carried out with the aim of determining, under controlled conditions of infection and nutrition, what influence four levels of dietary supplementation would have on the development of the immune response and on the biology of H. contortus and T. colubriformis, in Dorper lambs under serial artificial infections.
2. Materials and Methods
All the procedures involving animals in this study were conducted in accordance with international ethical standards and were approved by the local ethics committee on animal use (protocol number 78/2014-CEUA; FMVZ/UNESP).
2.1. Description of the experiment
Details of the experimental design of the present study were published previously by [
11]. Briefly, we used lambs raised in pens from birth with low exposure to helminth infection. On arrival at the University facilities, nematode fecal egg counts (FEC) by means of the McMaster technique [
22] demonstrated that two animals had 100 eggs per gram of feces (EPG) of
Strongyloides papillosus and neither of them was shedding strongylid eggs. However, through using the Willis method, which is more sensitive [
22], it was found that 11 animals were infected with strongylids and 42 were infected with
S. papillosus. For this reason all animals were dewormed before the beginning of the study. No nematode eggs were found in fecal examinations performed 14 days after the anthelmintic treatment.
The lambs were fed with one of the following four diets: Diet 1 - hay alone (7.4% metabolizable protein (MP) and 1861 kcal/kg of metabolizable energy (ME)); Diet 2 - proportions of 75% hay and 25% concentrate (8.8% MP and 2133 kcal/kg ME); Diet 3 - proportions of 50% hay and 50% concentrate (10.2% MP and 2418 kcal/kg ME); and Diet 4 - proportions of 25% hay and 75% concentrate (11.5% MP and 2726 kcal/kg ME). The experiment used a completely randomized 2 × 4 factorial design (infected or control × four diets) with 44 Dorper lambs, randomized to the eight treatments as follows:
• Diet 1 - Infected (n = 7) and Control (n = 4);
• Diet 2 - Infected (n = 7) and Control (n = 4);
• Diet 3 - Infected (n = 7) and Control (n = 4);
• Diet 4 - Infected (n = 7) and Control (n = 4).
One animal in the infected group that received Diet 4 was excluded from the experiment because it did not adapt to the diet and showed clinical signs of ruminal acidosis. For this reason, this group was made up of six lambs.
The diets for this experiment consisted of ground coast cross hay (
Cynodon dactylon) and concentrate formulated with ground corn, soybean meal, calcitic limestone, urea and ammonium chloride [
11]. Before the beginning of the serial infections, the animals were subjected to a period of adaptation to the diets over three weeks. The lambs were kept in individual pens (3 m2), where they had free access (
ad libitum) to the diets and water. At the time of the initial infection, the lambs were four months of age and had a mean body weight of 31.5 ± 3.24 kg.
2.2. Nematode isolates, production of infective larvae and serial infections
The
T. colubriformis isolate was obtained in 2003 from a sheep that was naturally infected with
Haemonchus spp. and
Trichostrongylus spp. In order to eliminate
Haemonchus and thus isolate the infective
T. colubriformis larvae, two treatments with organophosphate were carried out (June 12, 2003, and July 4, 2003), at a dose of 100 mg/kg body weight (Neguvon-Bayer), which caused elimination of the
Haemonchus infection [
23].
The
H. contortus isolate was obtained from two male lambs acquired in Pratânia, state of São Paulo, in May 2006 [
24]. Upon arrival at the UNESP facilities, the animals were treated with moxidectin (200 μg/kg, Cydectin NF*, 1% injectable solution, Fort Dodge®). On that day, the two lambs presented 1100 and 3200 EPG. In coprocultures, only
Haemonchus larvae were present. Seven days after treatment, the animals showed increases in EPG counts, respectively to 5600 and 7400 EPG, and coprocultures confirmed that the animals were only infected by
Haemonchus spp. Since the time of isolation, infective larvae (L3) of both isolates have been kept frozen in liquid nitrogen.
For production of the L3 used in the present trial, we used two donor lambs for each nematode species. These lambs were infected immediately after the preserved larvae had been thawed.
Each animal in the infected groups received 1,000 L3 of H. contortus and 1,000 L3 of T. colubriformis every three days, thus totaling 28,000 infective larvae of each species over the 12 weeks of the infection period.
2.3. Blood samples
Once a week, blood samples (5 mL) were collected by means of jugular vein puncture, into Vacutainer® tubes containing anticoagulant (EDTA). Blood eosinophils were quantified and the excess blood was centrifuged to collect plasma, which was stored at −20 °C for further immunological tests.
2.4. Worm examination
All the lambs were slaughtered at the end of the trial, four days after the last infection, for recovery and enumeration of parasites. After they had been euthanized, the abomasa and small intestines were frozen at -20 °C. Subsequently, after thawing, each organ was washed separately in saline solution and their contents fixed in 5% formaldehyde. The abomasal and small intestine contents were collected and the parasites present in a 10% subsample were counted, sexed and classified according to their stage of development and species [
22].
2.5. Enzyme-linked immunosorbent assay (ELISA)
The plasma levels of IgG antibodies against total antigens of
H. contortus and
T. colubriformis L3 were estimated using ELISA. The antigen production method was previously described by [
25], and the protocol for measuring the parasite-specific plasma IgG levels was as described by [
26], with the following modifications: plates were coated with 2.5 µg/ml of antigen and peroxidase conjugate diluted 1:20,000. The results were expressed as the percentage of the optical density value (OD) of the positive standard serum, by means of the following formula: % OD = [(OD mean of the tested serum − OD mean of blank)/(OD mean of the positive standard serum − OD mean of blank)] × 100 [
27].
2.6. Statistical analysis
The data were assessed using analysis of variance for the variables with just one measurement (EPG and worm counting). For the weekly measurements (eosinophils and IgG), repeated-measurement analysis using the general linear models (GLM) procedure of the Statistical Analysis System, version 9.4 [
28] was used. Diet and infection status were the classes evaluated. Averages were compared by means of Tukey’s test at a 5% significance level, and only significant interactions at this level were reported in the results. Data on worm counting were analyzed under log transformation (log (x+1)).
The association between the average number of worms and the chemical composition of the diets (metabolizable protein and metabolizable energy) was evaluated by means of linear regression.
3. Results
3.1. Haemonchus contortus
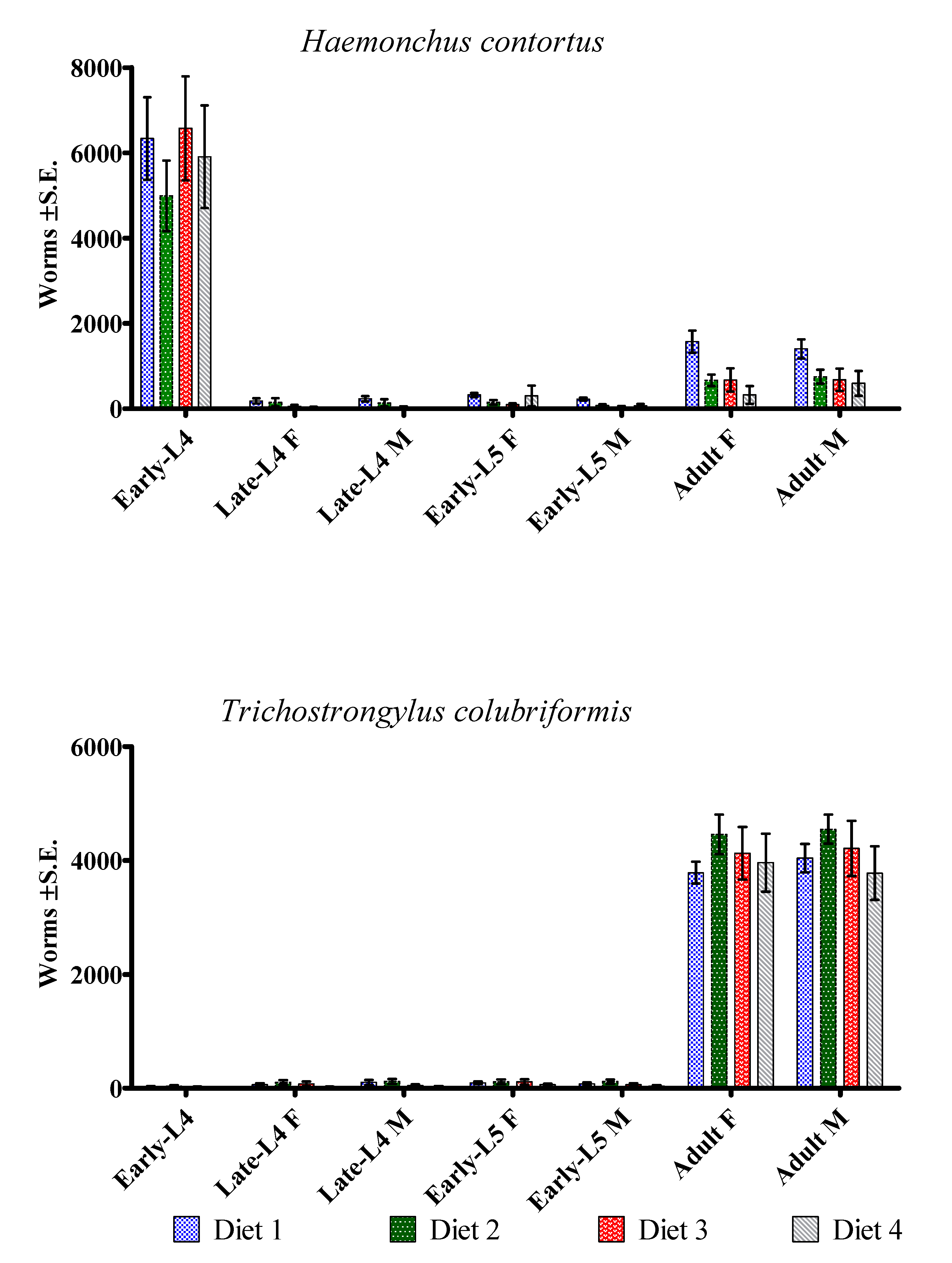
With regard to averages (with minimum-maximum values) for
H. contortus total worm counts, 10269 worms (6380-13240) were recorded in Diet 1; 6916 (530-9470) in Diet 2; 8153 (350- 13090) in Diet 3; and 7227 (830-12730) in Diet 4, which corresponded to mean (upper and lower confidence interval limits (95%)) rates of establishment , respectively, of 36.7% (29 - 44), 24.7% (15 – 34), 29.1% (15 – 43) and 25.9% (11 – 41) for the infective larvae (28000 L3) that were administered to the lambs during the trial. Within the
H. contortus worm population that became established, most specimens were found at the early-L4 stage (
Figure 1), which represented 59.5% (39.6 - 79.4), 75.8% (62.7 - 89.0), 83.3% (71.8 - 94.7) and 86.3% (69.3 - 103.2) of the total
Haemonchus worm burden in the Diet 1, 2, 3 and 4 groups, respectively. This result indicates that larval development of
H. contortus had become inhibited (hypobiosis). Averages of late-L4 males, early-L5 males and adult females were significantly lower in the Diet 4 in comparison with Diet 1 group (P<0.05).
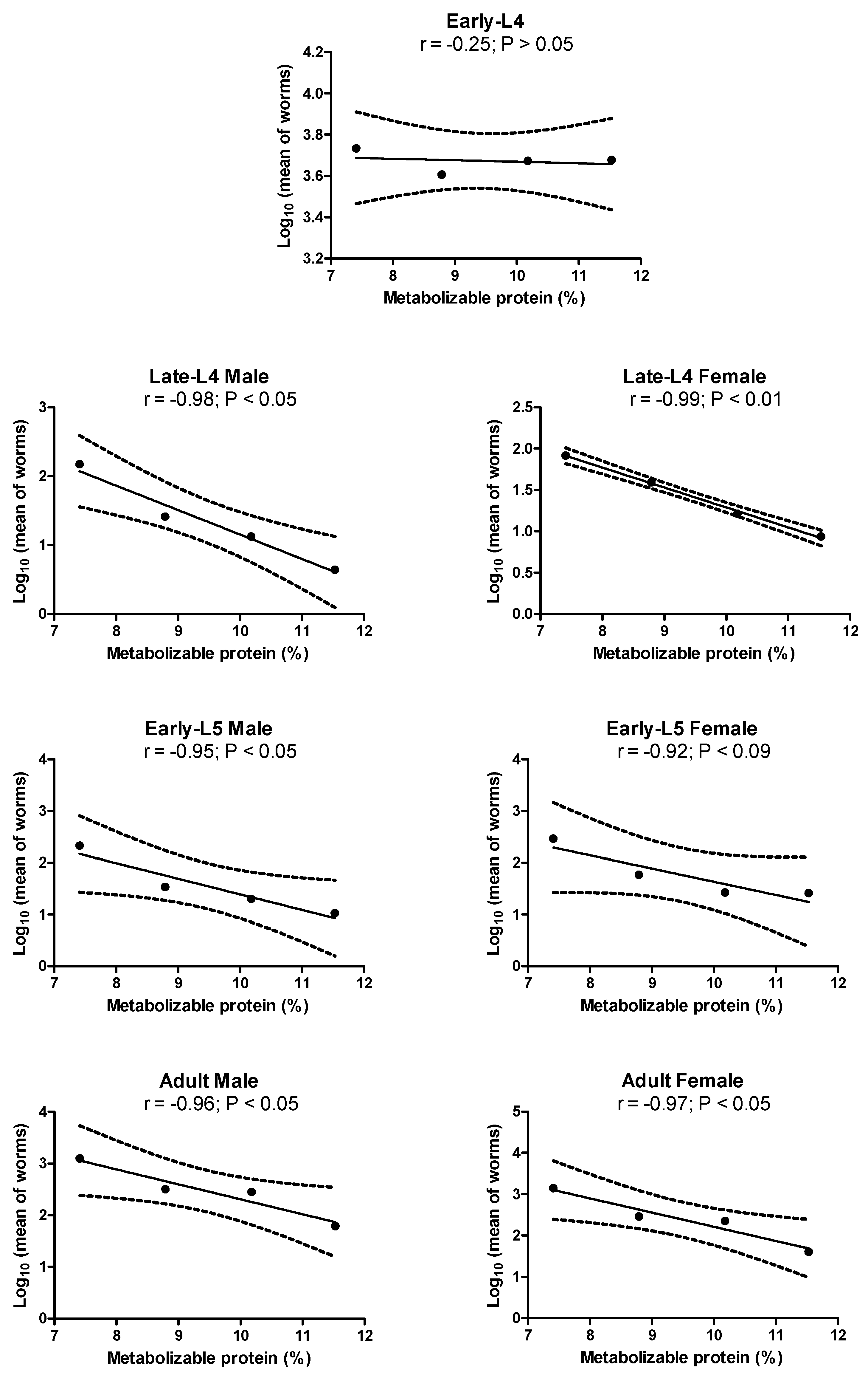
Figure 2 presents the results regarding the association between H. contortus worm counts and the levels of metabolizable protein (MP). There was no association between the level of MP in the diet and the early-L4 counts (r = -0.25; P > 0.05). Conversely, significant inverse associations were detected between MP and the other stages of development of Haemonchus with correlation coefficients ranging from -0.92 to -0.99. For example, the numbers of adults, i.e. the most pathogenic stage, corresponded to 10.6%, 5.1%, 4.8% and 3.3% of the numbers of L3 given, respectively, in diets 1, 2, 3 and 4. The correlation coefficients between metabolizable energy (ME) and the total number of H. contortus worms matched those obtained using MP. To avoid repetition, they are not presented here.
3.2. Trichostrongylus colubriformis
No group differences were detected regarding
T. colubriformis worm counting (P>0.05); and no significant correlations between worm counts and levels of MP or ME in the diets. The
T. colubriformis worm burdens were as follows: 8191 (6800-10490) in Diet 1; 9493 (6780-11830) in Diet 2; 8654 (4240-12230) in Diet 3; and 7897 (3320-9690) in Diet 4. These corresponded to establishment of 29.3% (25 - 33), 33.9% (28 - 40), 30.9% (22 - 40) and 28.2% (19 - 37), respectively, of the 28000 L3 specimens that were given to the lambs during the trial. Differently from
Haemonchus, most of the
T. colubriformis worms were found in the adult stage (
Figure 2) with percentages of 95.4%, 94.6%, 96.2% and 98.0% of the total worm burden in the groups with Diets 1, 2, 3 and 4, respectively. Therefore, there was no evidence of arrested development in
T. colubriformis.
The non-infected control lambs did not present worms at the end of the trial.
3.3. Immune response
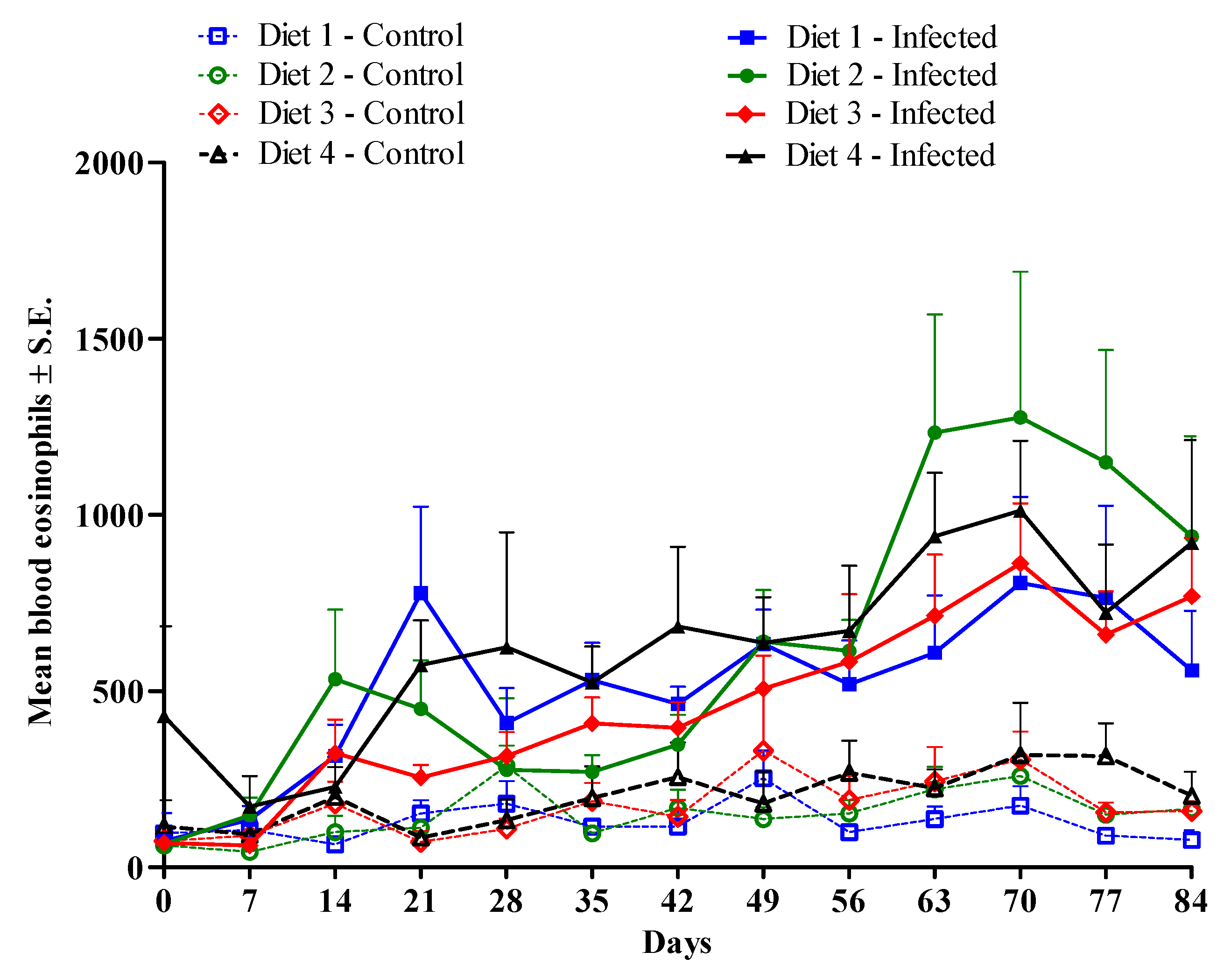
Infection had a significant effect on eosinophil counts (P < 0.001). The averages in the non-infected control groups remained below 350 eosinophils/µL of blood during the study, while the infected lambs showed increases in blood eosinophil counts (
Figure 3), with significant differences (P < 0.05) between infected and non-infected animals from day 14 until the end of the trial (
Figure 3). No significant diet * infection interaction and no diet effect were recorded in relation to blood eosinophils (P > 0.05).
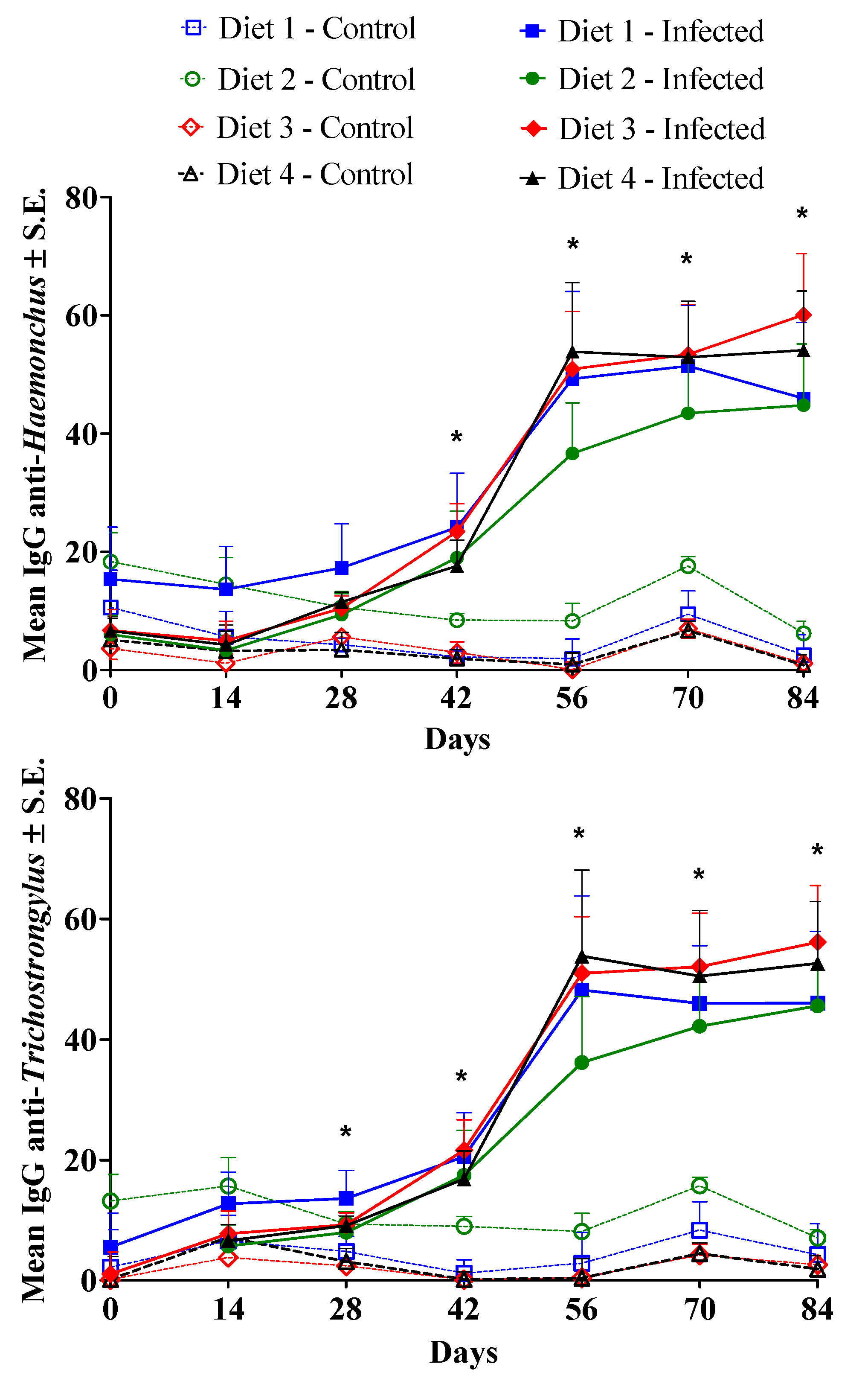
Significant time * infection interactions (P < 0.0001) occurred in relation to IgG values. Sharp increases in the averages for IgG anti-
H. contortus and anti-
T. colubriformis L3 antigens were observed, with significant infection effects (P < 0.05), starting at days 28 and 42 post-infection, respectively, for
T. colubriformis and
H. contortus, and continuing until the end of the study (
Figure 4). The IgG levels remained high and stable in the last three samplings from the infected animals. No diet effect was recorded (P > 0.05).
4. Discussion
An inverse relationship between the levels of nutrients in the diets (MP and ME) and the numbers of late-L4, early-L5 and adults of
H. contortus was observed based on the high negative correlation coefficient recorded between the average numbers of specimens of each stage of development and the level of metabolizable protein in the four diets. These results clearly showed that the quality of the diet had a significant impact on
H. contortus parasitism. This finding was in agreement with those of other studies, which also showed that resistance to nematodes was influenced by diet [
29], and particularly by the supply of metabolizable protein [
30]. There was an evident immune response to infection in the lambs of all diet groups, manifested by eosinophilia and production of anti-L3 immunoglobulins in comparison with the non-infected controls. These results are in agreement with studies conducted with infected and non-infected sheep demonstrating an important role of innate and adaptative response against helminth infection [
16]. However, even the animals fed only with hay were able to produce antibodies and inflammatory cells in numbers similar to those observed in the other supplemented groups. Therefore, it was not possible to detect any influence from the diets on these immunological variables.
Multiple pathways related to the immune response and tissue repair play critical roles in the resistance process. The type 2 immune response (Th2) has evolved to direct the wound-healing machinery not only to repairing and remodeling tissue but also to mediating the containment, destruction or expulsion of helminths [
31]. The Th2 response induces mast cell hyperplasia, eosinophilia and production of anti-parasite immunoglobulins, as demonstrated in the infected lambs of the present trial. Resistance has been correlated also with hemostasis, which is important for stopping bleeding, thereby deterring parasite feeding. This, in association with increased mucus production could accelerate parasite expulsion [
32]. Therefore, it is possible that with an increase in diet quality, parasites may find a less friendly environment in well-nourished lambs, which would lead to reduction of the numbers of larvae under development and the numbers of adult worms. In this context, animals kept on a nutrient-rich diet would be able to respond more efficiently, with greater capacity for clotting and for repairing tissue damage, thus reducing the harm caused by
H. contortus.
There was a marked difference between the species regarding the developmental stages at which the parasites were found. While we did not find evidence of hypobiosis involving
T. colubriformis, most of the
H. contortus parasite population was in the early fourth stage, thus indicating inhibition of development. In the present trial, in which infection was administered over a 12-week period, the early-L4 stage represented 56.4%, 67.2%, 78.4% and 77.4% of the total
Haemonchus worm burden, respectively, in the groups fed with Diets 1, 2, 3 and 4. In other studies conducted in our laboratory, a single infection administered to worm-free lambs, consisting of 4000 L3 of
H. contortus, resulted in the presence of adult worms almost entirely, in counts performed between 28 and 42 days post-infection [
15]. The opposite occurred when lambs acquired natural infections while grazing on contaminated pasture. In this case, the proportion of
H. contortus early-L4 larvae was relatively high [
1,
33].
Interestingly, in the animals of the same trials that were also infected with
T. colubriformis, virtually the entire population of
T. colubriformis recovered was made up of adults [
1,
33]. Similar results were reported in the United States, from St. Croix × Dorset lambs that were kept on contaminated pasture for eight weeks [
34]. Those lambs had parasite counts of 1535 adults and 2964 early-L4 larvae of
H. contortus. The same animals also had a high
T. colubriformis worm burden (17471 adult worms), but without evidence of inhibited development in this species. Our results, as well as those of [
19], indicated that in the case of
H. contortus, the existence of an adult population established in the abomasum is important for induction of hypobiosis. Similarly, some evidence suggesting a connection between the presence of adult worms and inhibition of development of
Ostertagia ostertagi in cattle exists [
35].
In the present experiment, as well as in other studies on T. colubriformis, adult worms predominated, regardless of the type of infection (natural or artificial) (3,34). Therefore, in lambs, the phenomenon of hypobiosis apparently does not occur in T. colubriformis, or it is far less important in comparison with its occurrence in H. contortus. However, (21) found L3 of T. colubriformis in a state of hypobiosis and drew attention to the difficulty in quantifying the parasites at this stage, which could lead to underestimating the real number of worms in hypobiosis.
In Brazil, investigations on the importance of hypobiosis in the epidemiology of parasitic gastroenteritis are scarce. It is likely that hypobiosis plays an important role in outbreaks that occur during the periparturient relaxation of immunity in ewes. Outbreaks of haemonchosis have also been reported in small ruminants during periods of prolonged drought, when it is unlikely that there is any source of environmental infection. In this case, the resumption of the development of hypobiotic larvae is possibly involved in these outbreaks. Therefore, the present results show that more attention should be given to hypobiosis in epidemiological studies and also to designing approaches for sustainable control of parasitic gastroenteritis. The efficacy of anthelmintic treatments against hypobiotic larvae is possibly reduced. A similar problem can occur with the vaccine against haemonchosis (Barbervax®), because the worms are affected when they ingest blood with antibodies produced by vaccinated sheep, which attach to the lining of the worm intestine, blocking digestion and starving the parasite. Because the larvae in hypobiosis are not feeding, they might survive. These issues need to be addressed in further studies.
5. Conclusions
As the amount of metabolizable energy and metabolizable protein in the diet of Dorper lambs increased, there were decreases in the recovery of H. contortus at late-L4, early-L5 and adult stages. However, there was no effect from the diet on the numbers of hypobiotic larvae (early-L4), which became the predominant stage in the H. contortus abomasal population at the end of 12 weeks of serial infections. In the case of T. colubriformis, the phenomenon of hypobiosis was not observed, and the diet had no influence on establishment of the parasite.
There was an evident immune response to infection in the lambs of all diet groups, manifested by eosinophilia and production of anti-L3 immunoglobulins in comparison with the non-infected controls, but it was not possible to detect any influence from the diets on these immunological variables.
Author Contributions
Conceptualization, N.C. and A.F.T.A.; methodology, N.C. and A.F.T.A.; validation, N.C. and A.F.T.A.; formal analysis, N.C. and A.F.T.A.; investigation, N.C., C.S.P., J.H.N. and A.F.T.A.; resources, A.F.T.A.; data curation, N.C. and A.F.T.A.; writing—original draft preparation, N.C.; writing—review and editing, A.F.T.A.; supervision, A.F.T.A.; project administration, N.C. and A.F.T.A.; funding acquisition, A.F.T.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) grant 2015/14751-3. The N.C. was funded by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), J.H.N. was funded by FAPESP (Project 2014/02961-0), and C.S.P. was funded by PIBIC-CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico). A.F.T.A. is a recipient of a fellowship from CNPq (303624/2021-3).
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Ethics Committee of Faculdade de Medicina Veterinária e Zootecnia/UNESP (protocol number 78/2014-CEUA; 9 May 2014).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is unavailable due to privacy or ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wilmsen, M.O.; Silva, B.F.; Bassetto, C.C.; Amarante, A.F.T. Gastrointestinal nematode infections in sheep raised in Botucatu, State of São Paulo, Brazil. Rev. Bras. Parasitol. Vet. 2014, 23, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Chagas, A.C.S.; Tupy, O.; Santos, I.B.; Esteves, S.N. Economic impact of gastrointestinal nematodes in Morada Nova sheep in Brazil. Rev. Bras. Parasitol. Vet. 2022, 31, e008722. [Google Scholar] [CrossRef] [PubMed]
- Cardia, D.F.F.; Rocha-Oliveira, R.A.; Tsunemi, M.H.; Amarante, A.F.T. Immune response and performance of growing Santa Ines lambs to artificial Trichostrongylus colubriformis infections. Vet. Parasitol. 2011, 182, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Kyriazakis, I.; Anderson, D.H.; Oldham, J.D.; Coop, R.L.; Jackson, F. Long-term subclinical infection with Trichostrongylus colubriformis: effects on food intake, diet selection and performance of growing lambs. Vet. Parasitol. 1996, 61, 297–313. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.A.; Bompadre, T.F.V.; Fernandes, E.A.N.; Katiki, L.M.; Mui, T.S.; Abdalla, A.L.; Louvandini, H. Computed tomography and radioactive 32P detected phosphorus impairment in metabolism, reduced bones density and animal performance caused by mixed infection of Haemonchus contortus and Trichostrongylus colubriformis in sheep. Vet. Parasitol. 2023, 315, 109887. [Google Scholar] [CrossRef]
- O’Sullivan, B.M.; Donald, A.D. Responses to infection with Haemonchus contortus and Trichostrongylus colubriformis in ewes of different reproductive status. Int. J. Parasitol. 1973, 3, 521–530. [Google Scholar] [CrossRef]
- Besier, R.B.; Kahn, L.P.; Sargison, N.D.; Van Wyk, J.A. The pathophysiology, ecology and epidemiology of Haemonchus contortus infection in small ruminants. Adv. Parasitol. 2016, 93, 95–143. [Google Scholar] [CrossRef]
- Salgado, J.A.; Santos, C.P. Overview of anthelmintic resistance of gastrointestinal nematodes of small ruminants in Brazil. Rev Bras Parasitol Vet. 2016, 25, 3–17. [Google Scholar] [CrossRef]
- Cintra, M.C.R.; Teixeira, V.N.; Nascimento, L.V.; Sotomaior, C.S. Lack of efficacy of monepantel against Trichostrongylus colubriformis in sheep in Brazil. Vet. Parasitol. 2016, 216, 4–6. [Google Scholar] [CrossRef]
- Gainza, Y.A.; Santos, I.B.; Figueiredo, A.; Santos, L.A.L.; Esteves, S.N.; Barioni-Junior, W.; Minho, A.P.; Chagas, A.C.S. Anthelmintic resistance of Haemonchus contortus from sheep flocks in Brazil: concordance of in vivo and in vitro (RESISTA-Test©) methods. Rev. Bras. Parasitol. Vet. 2021, 30, 025120. [Google Scholar] [CrossRef]
- Carvalho, N.; Neves, J.H.; Pennacchi, C.S.; Castilhos, A.M.; Amarante, A.F.T. Performance of lambs under four levels of dietary supplementation and artificially mix-infected with Haemonchus contortus and Trichostrongylus colubriformis. Rev. Bras. Parasitol. Vet. 2021, 30, 025420. [Google Scholar] [CrossRef] [PubMed]
- Louvandini, H.; Veloso, C.F.M.; Paludo, G.R.; Dell’ Porto, A.; Gennari, S.M.; McManus, C.M. Influence of protein supplementation on the resistance and resilience on young hair sheep naturally infected with gastrointestinal nematodes during rainy and dry seasons. Vet. Parasitol. 2006, 137, 103–111. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, L.J.; Walkden-Brown, S.W.; Kahn, L.P. Ecology of the free-living stages of major trichostrongylid parasites of sheep. Vet. Parasitol. 2006, 42, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.B. Observations on the self-cure reaction and other forms of immunological responsiveness against Haemonchus contortus in sheep. Int. J. Parasitol. 1983, 13, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.C.; Xavier, J.K.; Amarante, M.R.V.; Bassetto, C.C.; Amarante, A.F.T. Immune response to Haemonchus contortus and Haemonchus placei in sheep and its role on parasite specificity. Vet. Parasitol. 2014, 203, 127–138. [Google Scholar] [CrossRef]
- Cortés, A.; Muñoz-Antoli, C.; Esteban, J.G.; Toledo, R. Th2 and Th1 responses: Clear and hidden sides of immunity against intestinal helminths. Trends Parasitol. 2017, 33, 678–693. [Google Scholar] [CrossRef]
- Gatongi, P.M.; Prichard, R.K.; Ranjan, S.; Gathuma, J.M.; Munyua, W.K.; Cheruiyot, H.; Scott, M.E. Hypobiosis of Haemonchus contortus in natural infections of sheep and goats in a semi-arid area of Kenya. Vet. Parasitol. 1998, 77, 49–61. [Google Scholar] [CrossRef]
- Dineen, J.K.; Donald, A.D.; Wagland, B.M.; Offner, J. The dynamics of the host-parasite relationship: III. The response of sheep to primary infection with Haemonchus contortus. Parasitology 1965, 55, 515–525. [Google Scholar] [CrossRef]
- Barger, I.A.; Le Jambre, L.F.; Georgi, J.R.; Davies, H.I. Regulation of Haemonchus contortus populations in sheep exposed to continuous infection. Int. J. Parasitol. 1985, 15, 529–533. [Google Scholar] [CrossRef]
- Eysker, M. Inhibition of the development of Trichostrongylus spp. as third stage larvae in sheep. Vet. Parasitol. 1978, 4, 29–33. [Google Scholar] [CrossRef]
- Dobson, R.J.; Waller, P.J.; Donald, A.D. Population dynamics of Trichostrongylus colubriformis in sheep: the effect of host age on the establishment of infective larvae. Int. J. Parasitol. 1990, 20, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Gonçalves, PC. Manual para diagnóstico das helmintoses de ruminantes, 4th ed.; Japan International Cooperation Agency: Tokyo, Japan, 1998. [Google Scholar]
- Rocha, R.A. Sobrevivência e migração vertical de larvas infectantes de Trichostrongylus colubriformis em gramíneas, nas diferentes estações do ano. Doctoral Thesis, Universidade Estadual Paulista, Botucatu – SP, Brazil, 24 April 2006. Available online: http://hdl.handle.net/11449/104111 (accessed on 24 July 2023).
- Silva, B.F. Migração vertical das larvas infectantes de Haemonchus contortus em capim Braquiária (Brachiaria decumbens). Masters dissertation, Universidade Estadual Paulista, Botucatu-SP, Brazil, 2007. Available online: https://www2.ibb.unesp.br/posgrad/teses/bga_me_2007_bruna_silva.pdf (accessed on 24 July 2023).
- Amarante, A.F.T.; Susin, I.; Rocha, R.A.; Silva, M.B.; Mendes, C.Q.; Pires, A.V. Resistance of Santa Ines and crossbred ewes to naturally acquired gastrointestinal nematode infections. Vet. Parasitol. 2009, 165, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.F.; Bassetto, C.C.; Amarante, A.F.T. Immune responses in sheep naturally infected with Oestrus ovis (Diptera: Oestridae) and gastrointestinal nematodes. Vet. Parasitol. 2012, 190, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Kanobana, K.; Vervelde, L.; Van Der Veer, M.; Eysker, M.; Ploeger, H. Characterization of host responder types after a single Cooperia oncophora infection: kinetics of the systemic immune response. Parasite Immunol. 2001, 23, 641–653. [Google Scholar] [CrossRef]
- SAS Institute. SAS user’s guide. Version 9.4 [online]. Cary, NC: SAS Institute Inc.; 2016. Available online: https://support.sas.com/ (accessed on 24 July 2023).
- Melo, G.K.A.; Ítavo, C.C.B.F.; Monteiro, K.L.S.; Silva, J.A.; Silva, P.C.G.; Ítavo, L.C.V.; Borges, D.G.L.; Borges, F.A. Effect of creep-fed supplement on the susceptibility of pasture-grazed suckling lambs to gastrointestinal helminthes. Vet. Parasitol. 2017, 239, 26–30. [Google Scholar] [CrossRef]
- Abbott, E.M.; Parkins, J.J.; Holmes, P.H. The effect of dietary protein on the pathophysiology of acute ovine haemonchosis. Vet. Parasitol. 1986, 20, 291–306. [Google Scholar] [CrossRef]
- Faz-López, B.; Morales-Montor, J.; Terrazas, L.I. Role of macrophages in the repair process during the tissue migrating and resident helminth infections. BioMed. Res. Int. 2016, 2016, 8634603. [Google Scholar] [CrossRef]
- Benavides, M.V.; Sonstegard, T.S.; Kemp, S.; Mugambi, J.M.; Gibson, J.P.; Baker, R.L.; Hanotte, O.; Marshall, K.; Van Tassell, C. Identification of novel loci associated with gastrointestinal parasite resistance in a Red Maasai x Dorper backcross population. PLoS ONE 2015, 10, 0122797. [Google Scholar] [CrossRef]
- Albuquerque, A.C.A.; Bassetto, C.C.; Almeida, F.A.; Hildersley, K.A.; McNeilly, T.N.; Britton, C.; Amarante, A.F.T. Differences in immune responses to Haemonchus contortus infection in the susceptible Ile de France and the resistant Santa Ines sheep under different anthelmintic treatments regimens. Vet. Res. 2019, 50, 104–116. [Google Scholar] [CrossRef]
- Courtney, C.H.; Parker, C.F.; McClure, K.E.; Herd, R.P. Population dynamics of Haemonchus contortus and Trichostrongylus spp. in sheep. Int. J. Parasitol. 1983, 13, 557–560. [Google Scholar] [CrossRef]
- Michel, J.F. The phenomena of host resistance and the course of infection of Ostertagia ostertagi in calves. Parasitology 1963, 53, 63–84. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).