Submitted:
25 August 2023
Posted:
28 August 2023
You are already at the latest version
Abstract
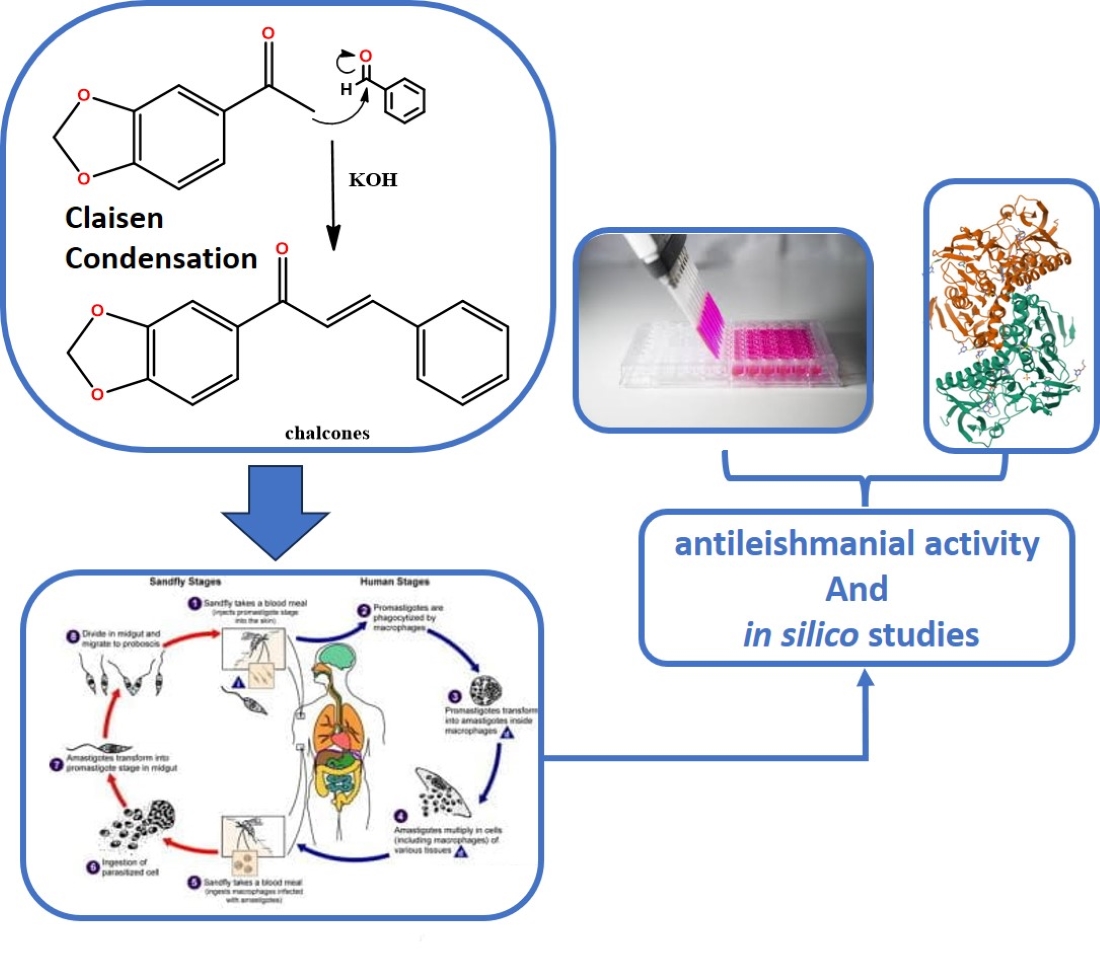
Keywords:
1. Introduction
2. Results
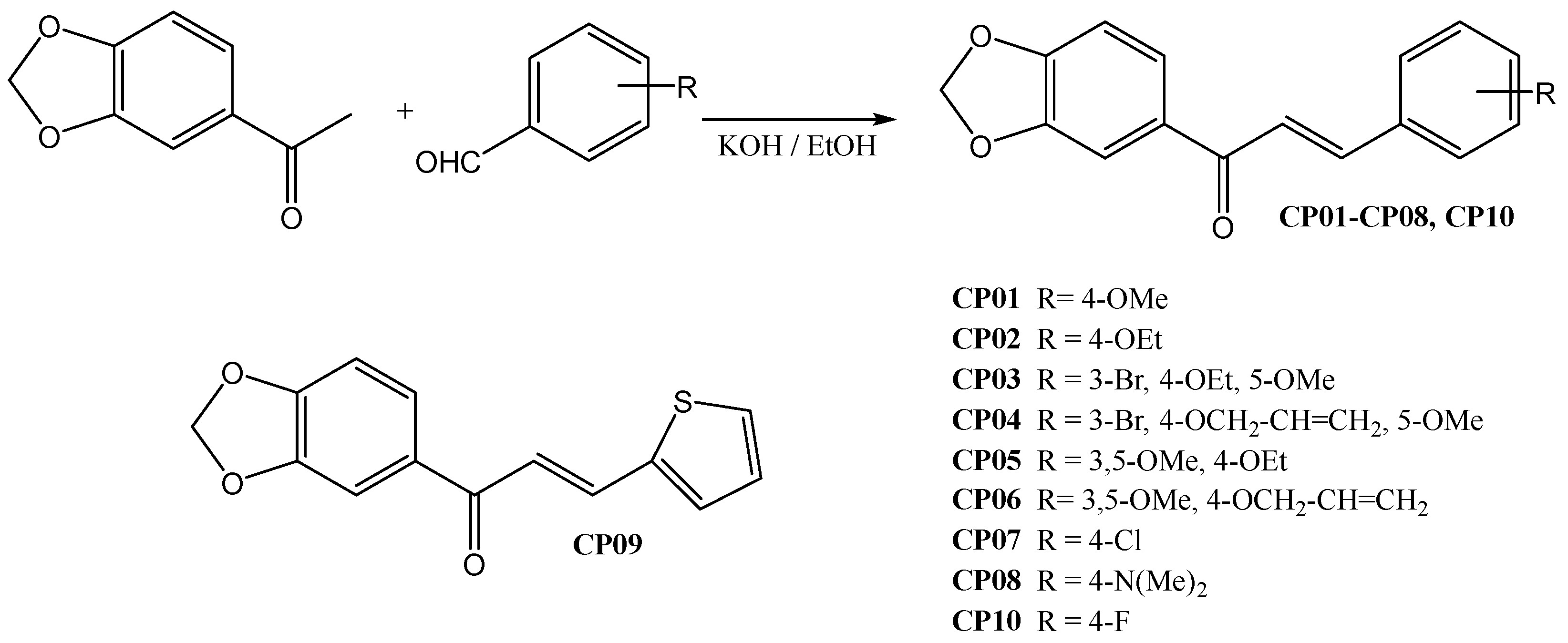
2.1. Chemistry
2.2. Anti-Leishmania activity
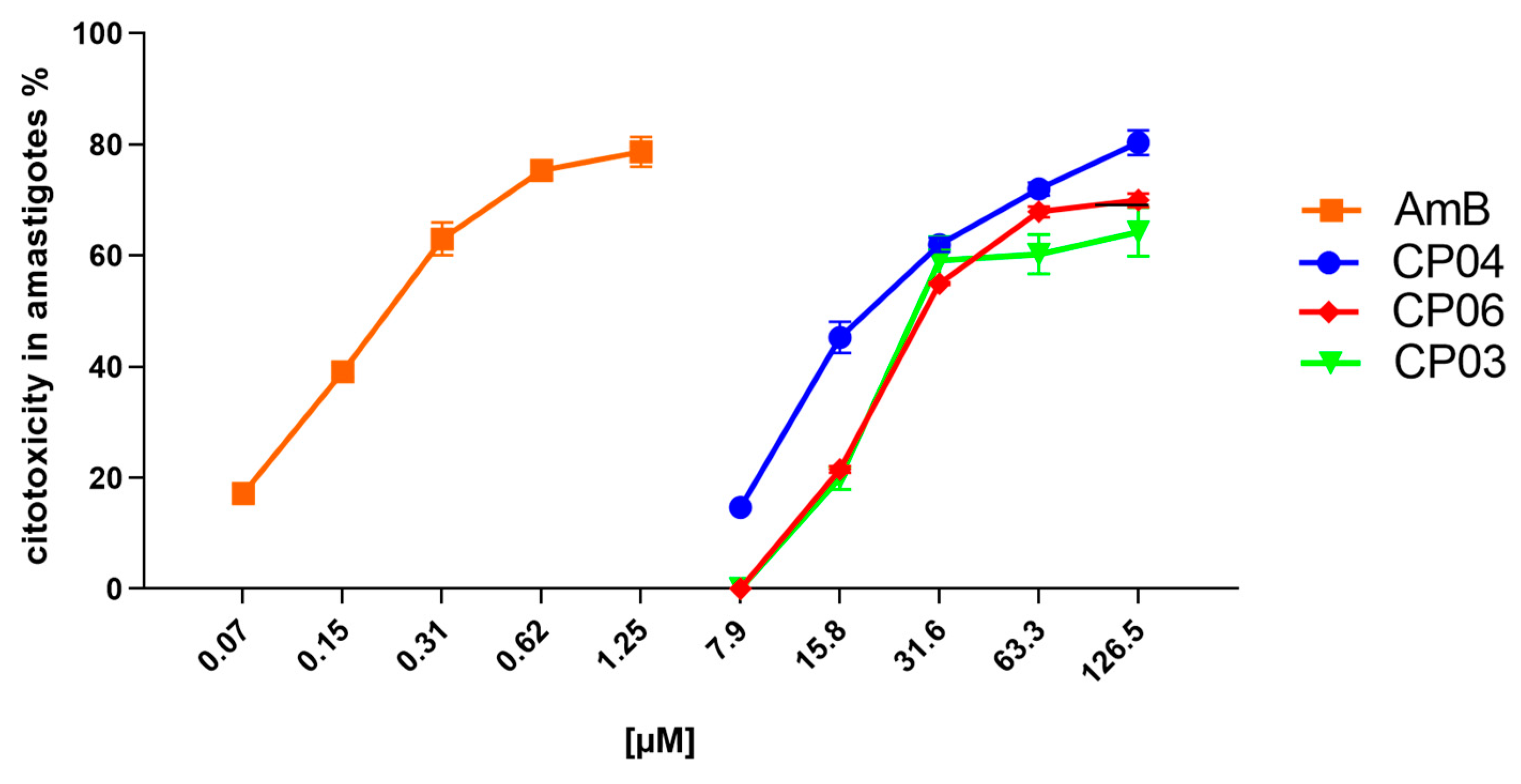
2.3. Cytotoxicity evaluation
2.4. Computational chemistry
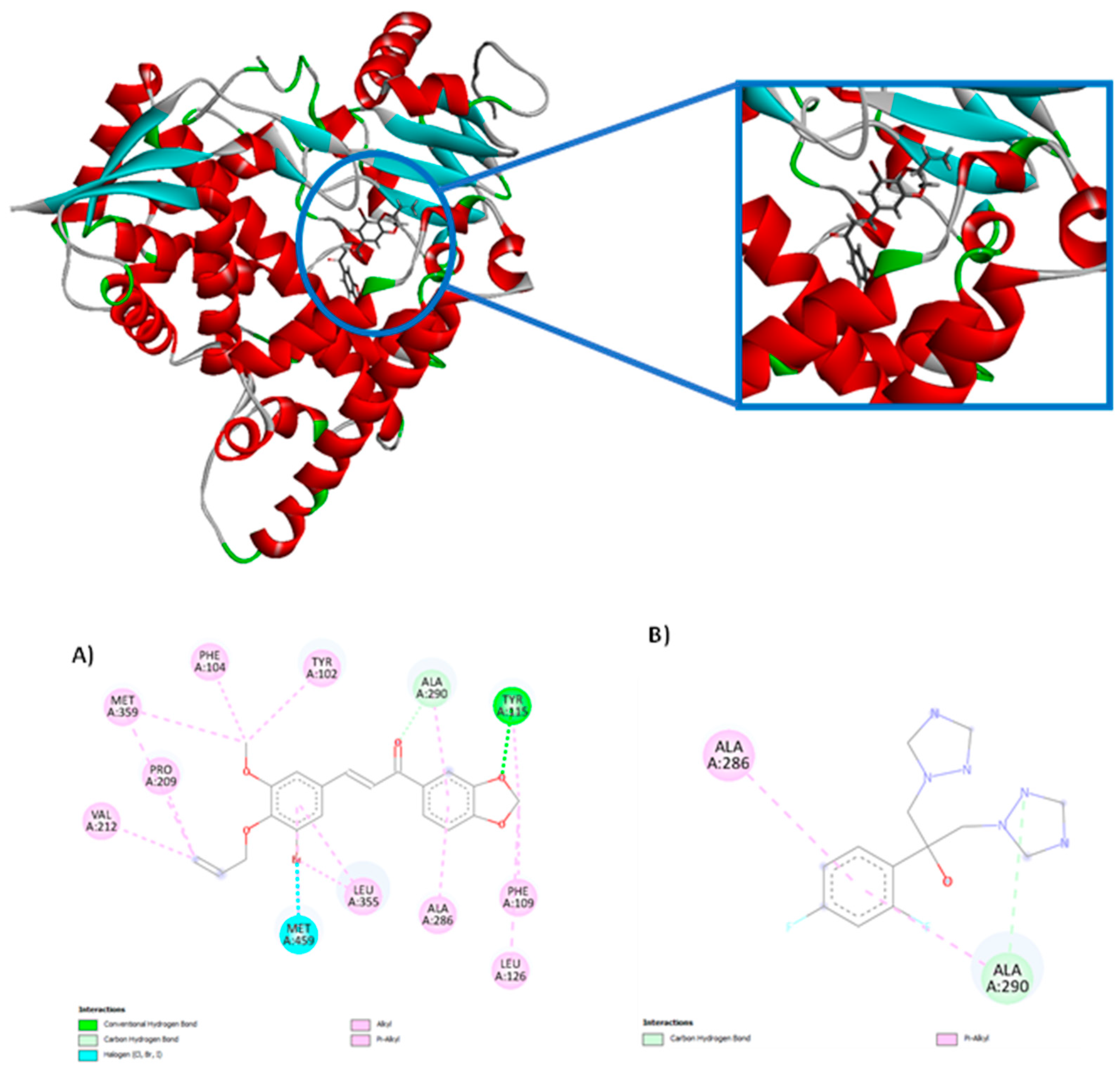
2.4.1. Molecular docking
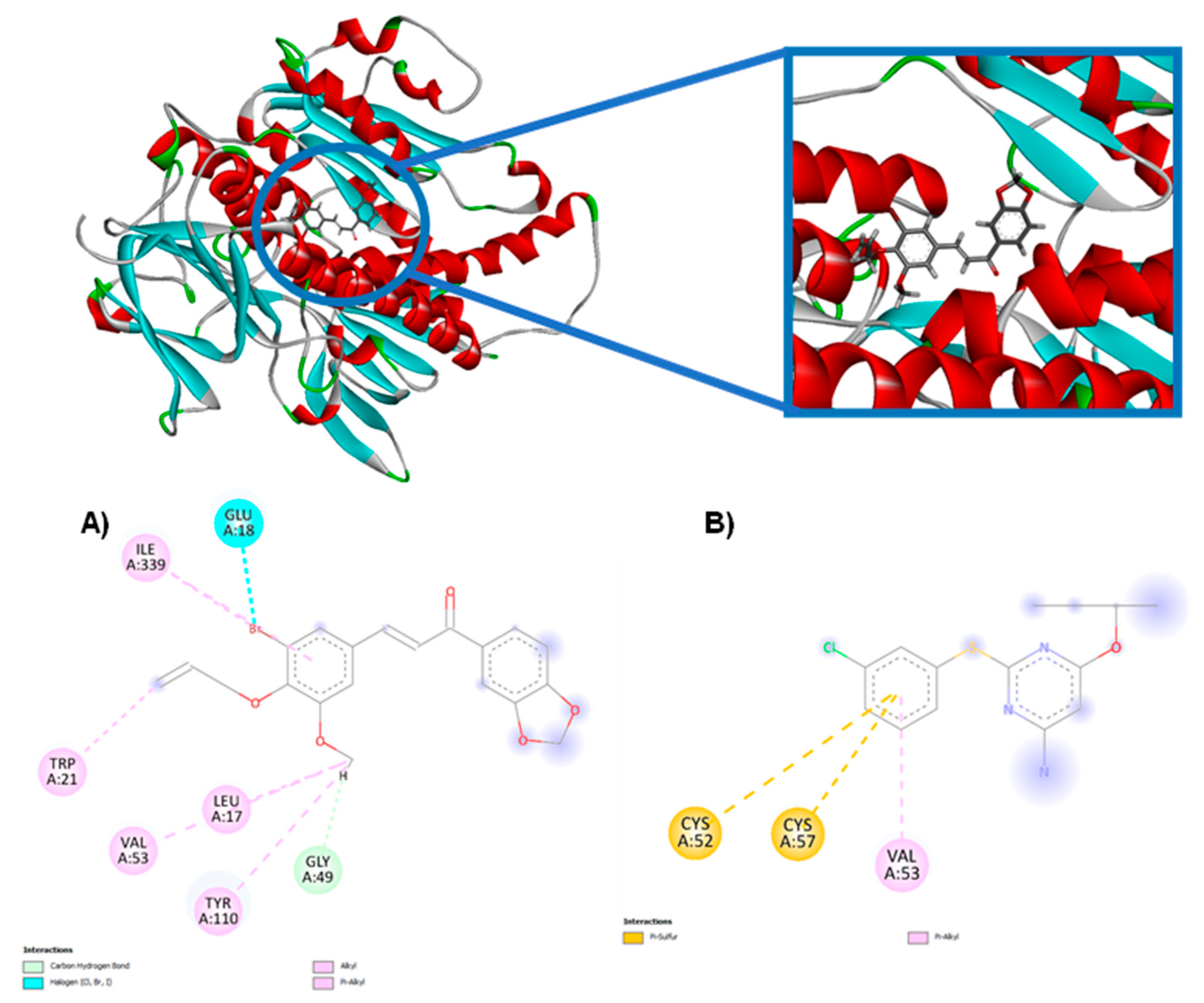
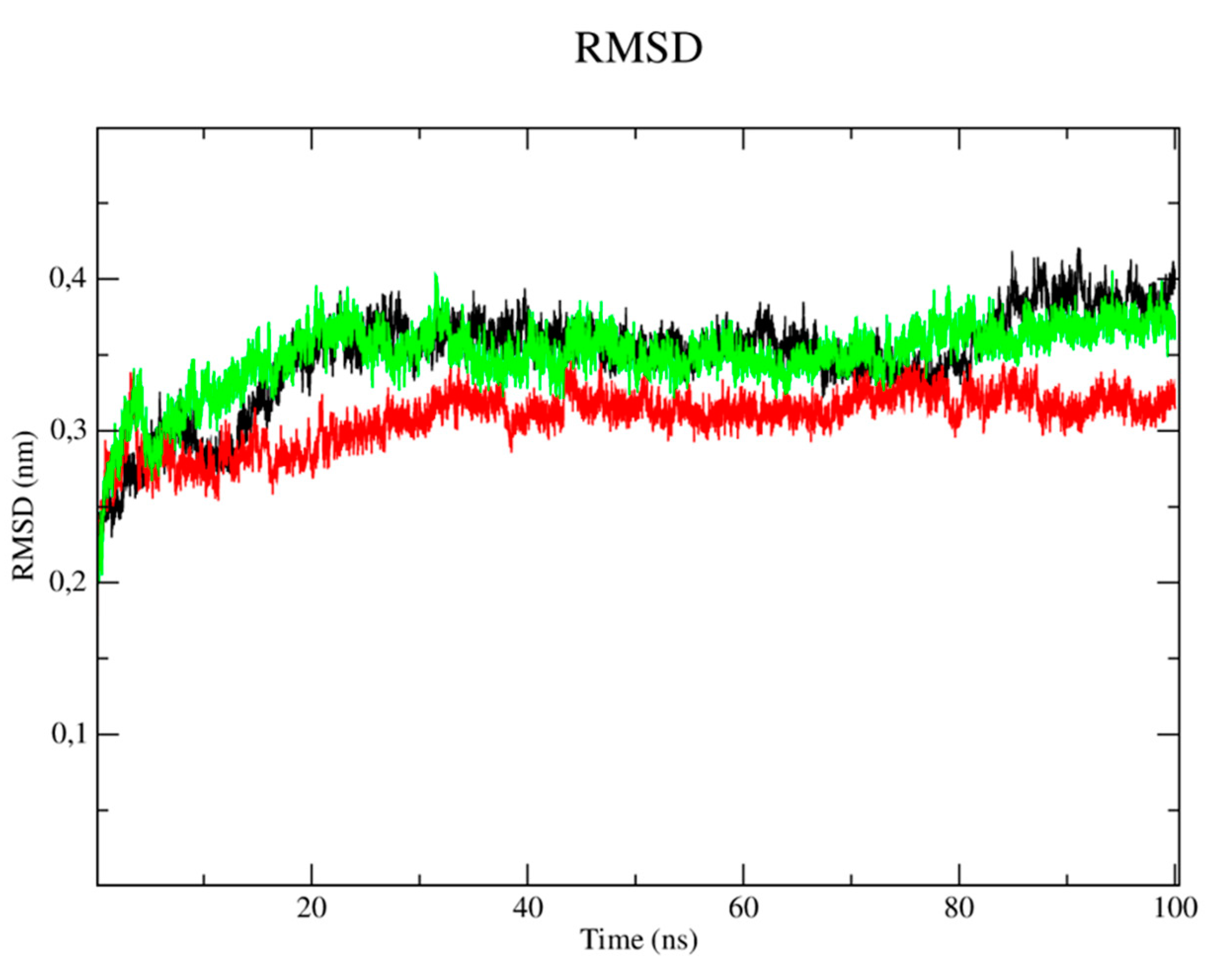
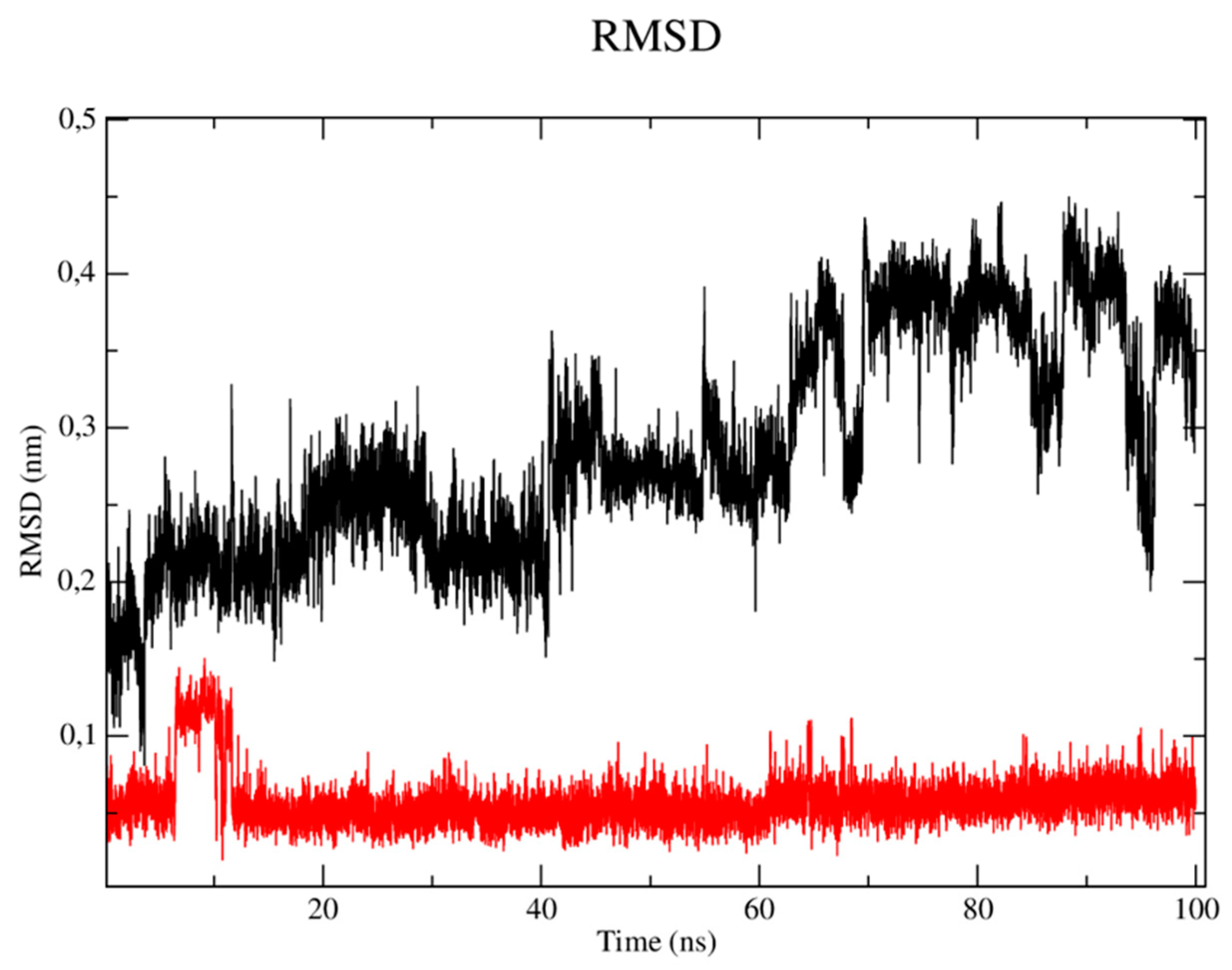
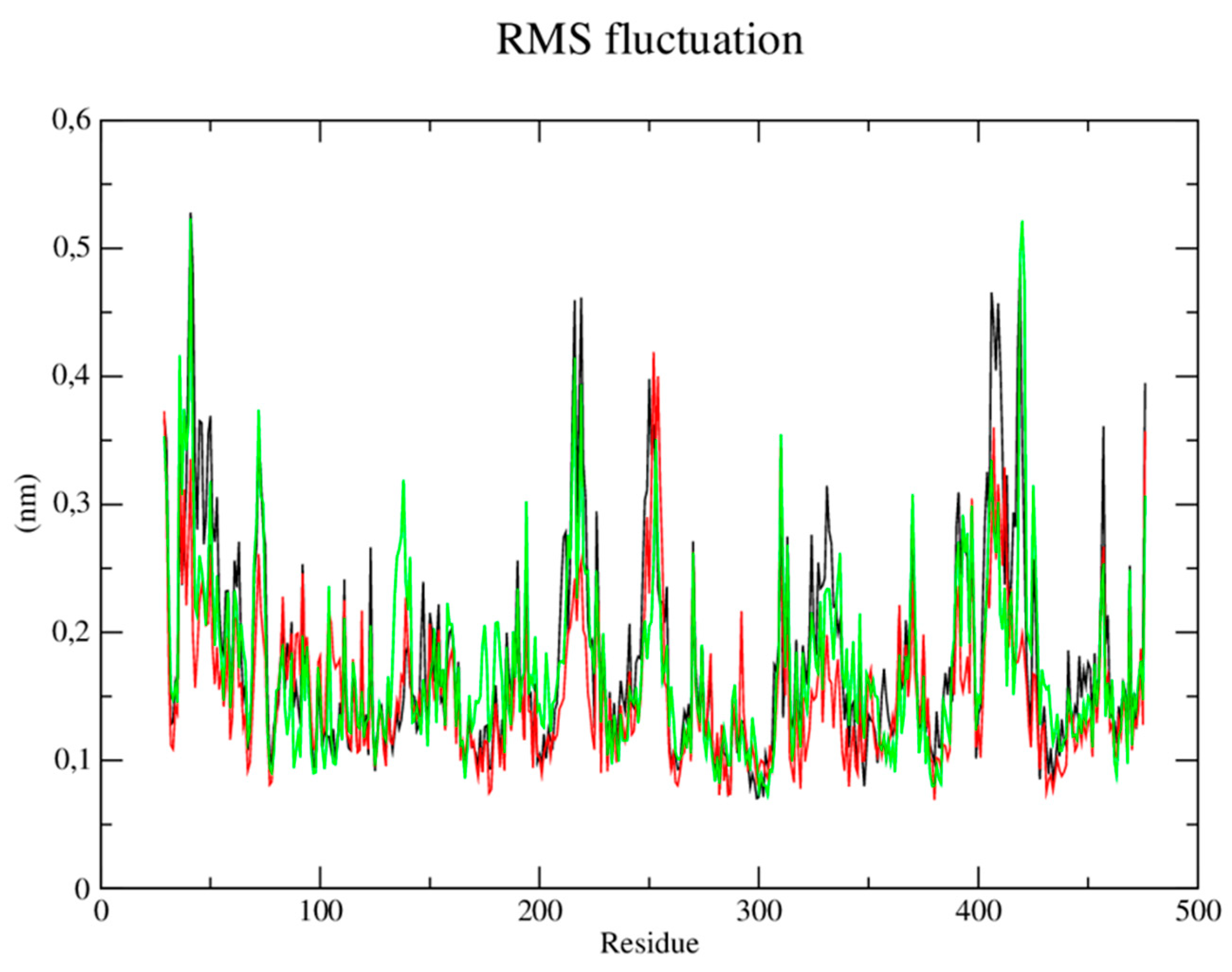
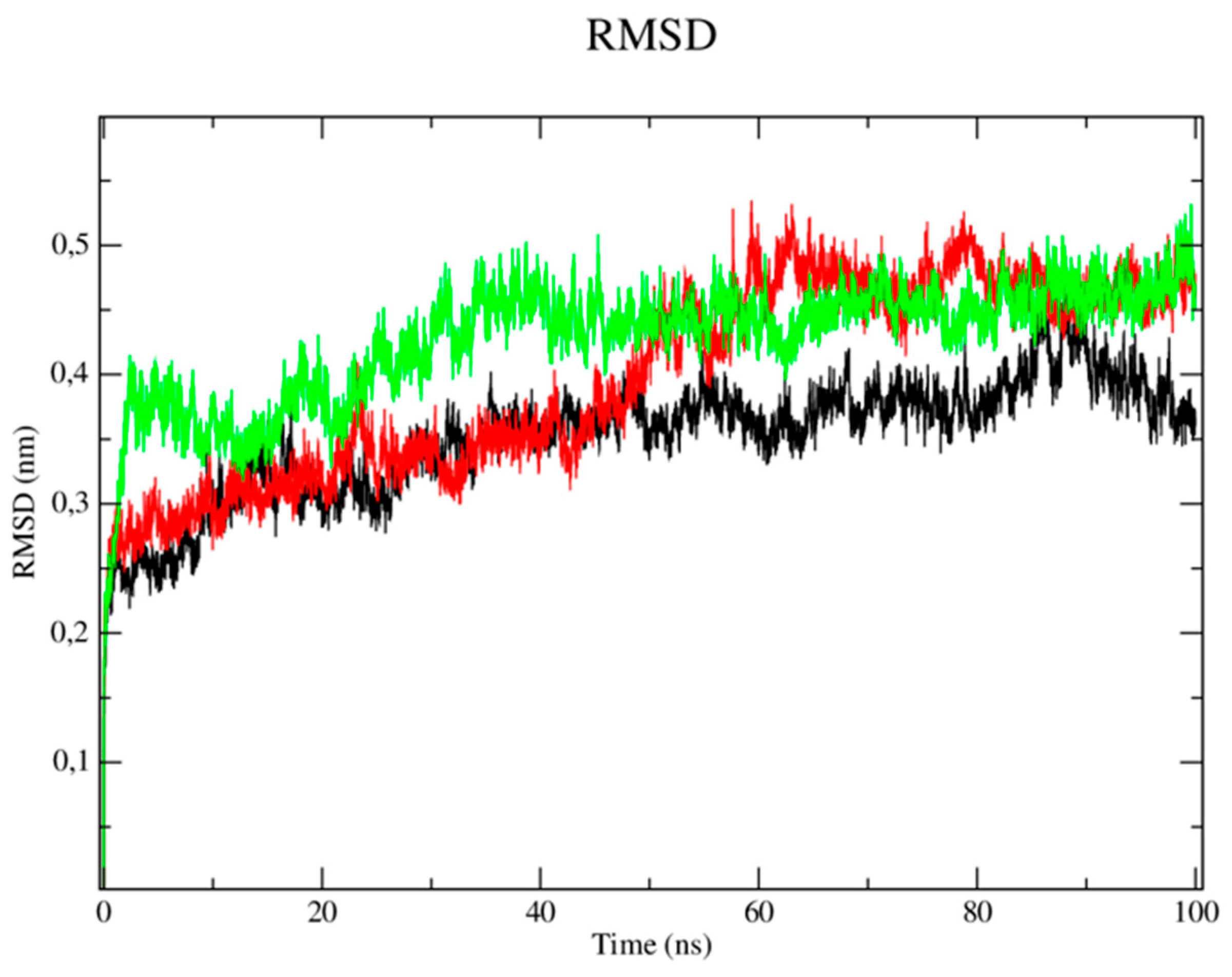
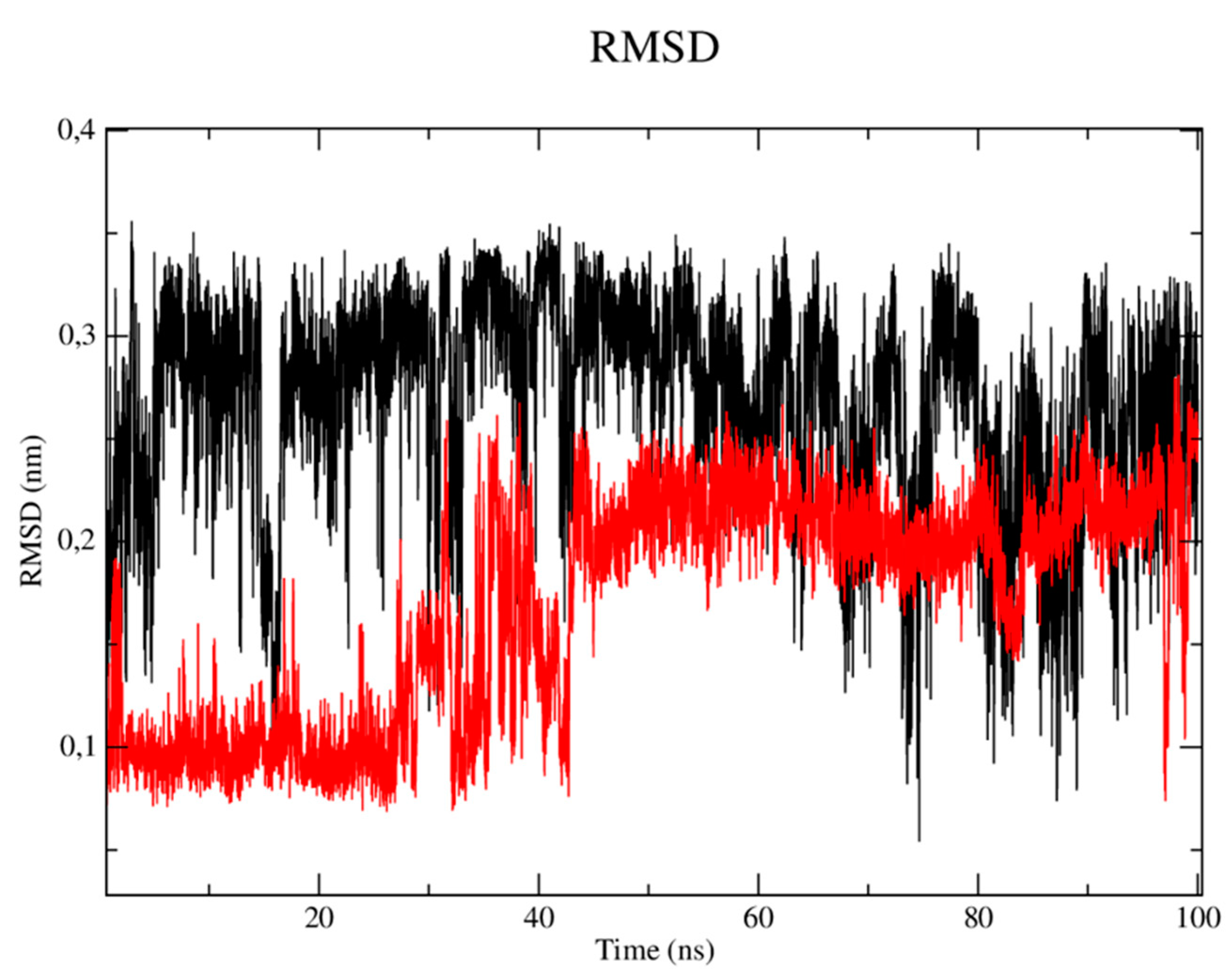
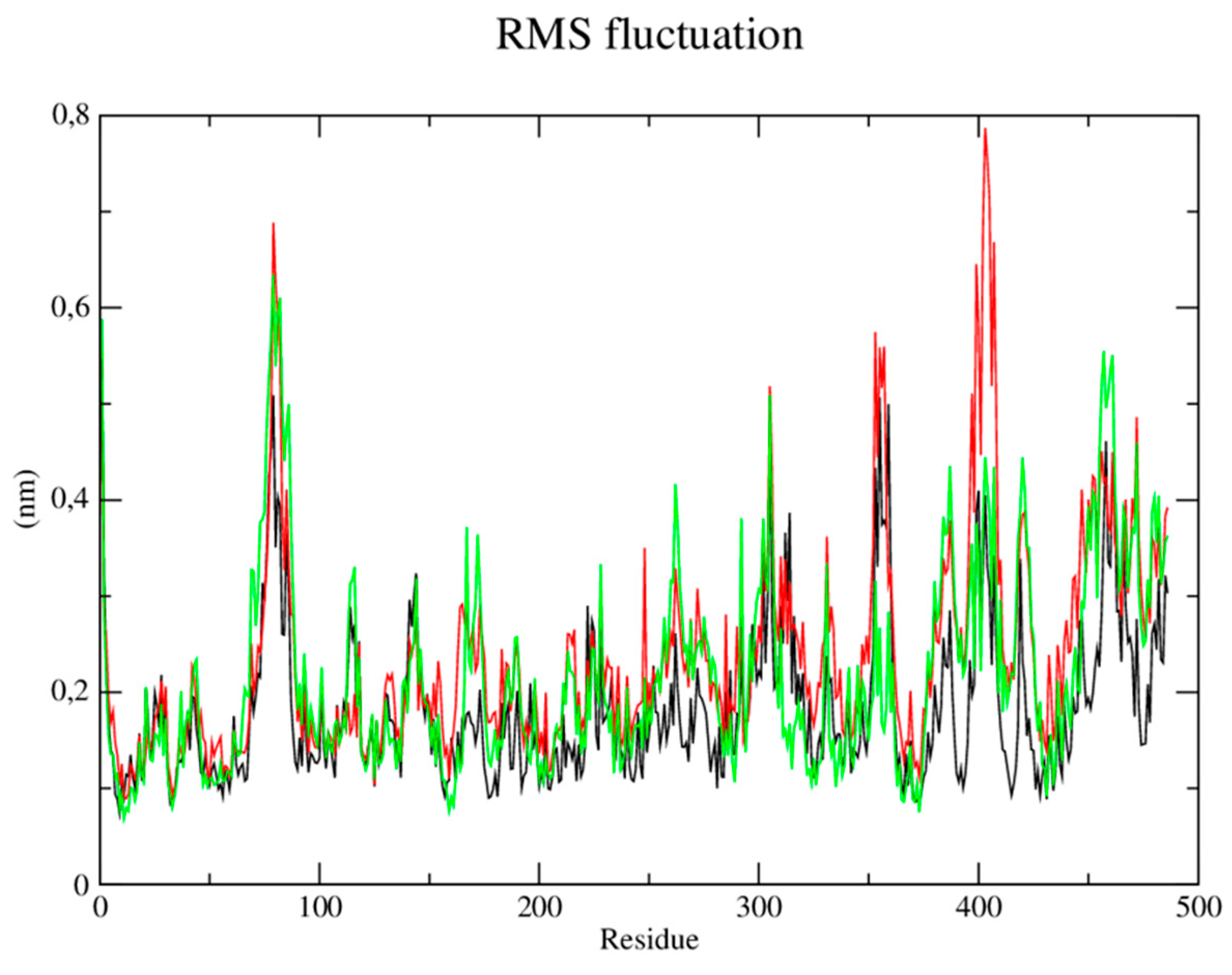
2.4.2. Molecular dynamics
- -
- CYP-51 (PDB: 3L4D):
- -
- Trypanothione Reductase (PDB: 5EBK):
2.4.3. Analysis of ADME parameters (Absorption, Distribution, Metabolism and Excretion)
3. Discussion
4. Materials and Methods
4.1. Chemical
4.1.1. Vanillin bromination
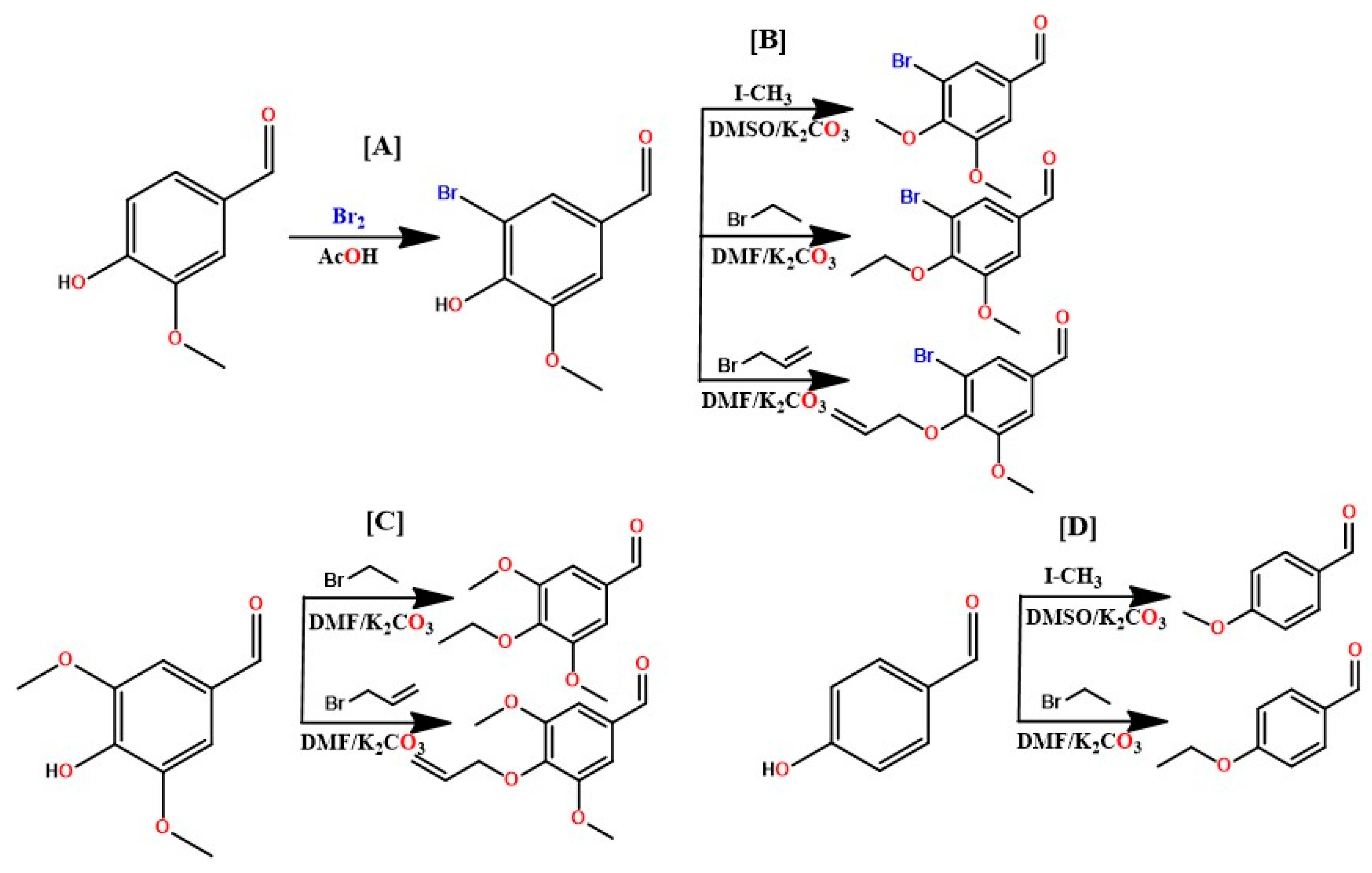
4.1.2. Etherification of bromine vanillin, 4-hydroxybenzaldehyde and syringealdehyde
4.1.3. Chalcone synthesis
4.1.4. Spectral data and physicochemical characteristics of the synthesized compounds
4.2. Evaluation of anti-Leishmania activity
4.2.1. Evaluation of hemolytic activity in human red blood cells
4.2.2. Statistical analysis
5. Computational chemistry
5.1. Molecular docking simulations
5.2. Molecular dynamics
5.3. Evaluation of ADME parameters
6. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization, Leishmaniasis. 2018. Available online: http://www.who.int/mediacentre/factsheets/fs375/en/ (accessed on 7 August 2023).
- Diotallevi, A.; Buffi, G.; Ceccarelli, M.; Neitzke-Abreu, H.C. Real-time PCR to differentiate among Leishmania (Viannia) subgenus, Leishmania (Leishmania) infantum and Leishmania (Leishmania) amazonensis: Application on Brazilian clinical samples. Acta Trop. 2020, 201, 105178. [Google Scholar] [CrossRef] [PubMed]
- Maurício, I.L.; Bruschi, F. Leishmania Taxonomy. In The Leishmaniases: Old Neglected Tropical Diseases; Bruschi, F., Gradoni, L., Eds.; Springer International Publishing: New York City, USA, 2018; pp. 15–30. [Google Scholar]
- Kmetiuk, L.B.; Camopos, M.P.; Bach, R.V.W. Serosurvey of anti-Leishmania (Leishmania) infantum antibodies in hunting dogs and hunters in Brazil. Vet. World. 2021, 14, 2735–2738. [Google Scholar] [CrossRef]
- Rougeron, V.; Meeûs, T.; Bañuls, A.L. Reproduction in Leishmania: a focus on genetic exchange. Infect. Genet. Evol. 2017, 50, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.L.; Zhang, Z.W.; Lekkala, R.; Alsulami, H. Chalcone hybrids as privileged scaffolds in antimalarial drug discovery: A key review. Eur. J. Med. Chem. 2020, 193, 112215. [Google Scholar] [CrossRef] [PubMed]
- Akbari, M.; Oryan, A.; Hatam, G. Application of nanotechnology in treatment of leshmaniasis: A Review. Acta Trop. 2017, 172, 86–90. [Google Scholar] [CrossRef]
- Barbosa, J.F.; Figueiredo, S.M. New approaches on Leishmaniasis treatment and prevention: A review of recent patents. Recent Pat. Endocr. Metab. Immune Drug Discov. 2015, 9, 90–102. [Google Scholar] [CrossRef]
- Ortalli, M.; Llari, A.; Colotti, G.; Lonna, I.; Battista, T. Identification of chalcone-based antileishmanial agents targeting trypanothione reductase. Eur. J. Med. Chem. 2018, 152, 527–541. [Google Scholar] [CrossRef]
- Moreno, M.A.; Alonso, A.; Alcolea, P.J.; Abramov, A. Tyrosine aminotransferase from Leishmania infantum: A new drug target candidate. Int. J. Parasitol.-DRUG. 2014, 4, 347–354. [Google Scholar] [CrossRef]
- Hermoso, A.; Jimérez, I.A.; Mamani, Z.A. Antileishmanial activities of dihydrochalcones from Piper elongatum and synthetic related compounds. Structural requirements for activity. Bioorg. Med. Chem. 2003, 11, 3975–3980. [Google Scholar] [CrossRef]
- Rodrigues, I.A.; Mazzoto, A.M.; Cardoso, V. Natural products: Insights into Leishmaniasis inflammatory response. Mediators Inflamm. 2015, 2015, 835910. [Google Scholar] [CrossRef]
- Souza, J.M.; Carvalho, É.A.A.; Candido, A.C.B.B. Licochalcone a exhibits leishmanicidal activity in vitro and in experimental model of Leishmania (Leishmania) infantum. Front. Vet. Sci. 2020, 7, 527. [Google Scholar] [CrossRef] [PubMed]
- Rosa, G.P.; Seca, A.M.L.; Barreto, M.C.; Silva, A.M.S. Chalcones and flavanones bearing hydroxyl and/or methoxyl groups: Synthesis and biological assessments. Appl. Sci. 2019, 9, 2846. [Google Scholar] [CrossRef]
- Passalacqua, T.G.; Torres, F.A.E.; Nogueira, C.T. The 2’,4’-dihydroxychalcone could be explored to develop new inhibitors against the glycerol-3-phosphate dehydrogenase from Leishmania species. Bioorg. Med. Chem. Lett. 2015, 25, 3564–3568. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.E.; Rittle, K.E.; Bock, M.G. Methods for drug discovery: development of potent, selective, orally effective cholecystokinin antagonists. J. Med. Chem. 1988, 31, 2235–2246. [Google Scholar] [CrossRef]
- Romagnoli, R.; Baraldi, P.G.; Carrion, M.D. Hybrid α-bromoacryloylamido chalcones. Design, synthesis and biological evaluation. Bioorg. Med. Chem. Lett. 2009, 19, 2022–2028. [Google Scholar] [CrossRef]
- Pozzetti, L.; Ibba, S.; Rossi, S.; Taglialatela-Scafati, O. Total synthesis of the natural chalcone lophirone E, synthetic studies toward benzofuran and indole-based analogues, and investigation of anti-leishmanial activity. Molecules. 2022, 27, 463. [Google Scholar] [CrossRef]
- Garcia, A.R.; Oliveira, D.M.P.; Jesus, J.B.; Souza, A.M.T. Identification of chalcone derivatives as inhibitors of Leishmania infantum arginase and promising antileishmanial agents. Front. Chem. 2021, 8, 624678. [Google Scholar] [CrossRef]
- Mello, M.V.P.; Abrahim-Vieira, B.A. A comprehensive review of chalcone derivatives as antileishmanial agents. Eur. J. Med. Chem. 2018, 150, 920–929. [Google Scholar] [CrossRef]
- Escrivani, D.O.; Charlton, R.L.; Caruso, M.B. Chalcones identify cTXNPx as a potential antileishmanial drug target. PLoS Negl. Trop. Dis, 2021, 15, 9951. [Google Scholar] [CrossRef]
- Sundar, S.; Singh, B. Emerging therapeutic targets for treatment of leishmaniasis. Expert Opin. Ther. Targets. 2018, 22, 467–486. [Google Scholar] [CrossRef]
- Hargrove, T.Y.; Wawrzak, Z.; Liu, J.; Nes, W.D. Substrate Preferences and Catalytic Parameters Determined by Structural Characteristics of Sterol 14α-Demethylase (CYP51) from Leishmania Infantum. J. Biol. Chem. 2011, 286, 26838–26848. [Google Scholar] [CrossRef] [PubMed]
- Maiorov, V.N.; Crippen, G.M. Significance of root-mean-square deviation in comparing three-dimensional structures of globular proteins. J. Mol. Biol. 1994, 235, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Guilbert, C.; James, T.L. Docking to RNA via root-mean-square-deviation-driven energy minimization with flexible ligands and flexible targets. J. Chem. Inf. Model. 2008, 48, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Ciccotti, G.; Ryckaert, J.P. Molecular Dynamics Simulation of Rigid Molecules. Comput. Phys. Reports. 1986, 4, 346–392. [Google Scholar] [CrossRef]
- Giraud, F.; Loge, C.; Pagniez, F.; Crepin, D. Design, synthesis and evaluation of 3-(imidazol- 1-ylmethyl)indoles as antileishmanial agents. Part II. J. Enzyme Inhib. Med. Chem. 2009, 24, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Cuellar, J.E.; Quiñones, W.; Robledo, S.; Gil, J.; Duramgo, D. Coumaro-chalcones synthesized under solvent-free conditions as potential agents against malaria, leishmania and trypanosomiasis. Heliyon. 2022, 8, e08939. [Google Scholar] [CrossRef]
- Santiago-Silva, K.M.; Bortoleti, B.T.S.; Oliveira, L.N. Antileishmanial Activity of 4,8-Dimethoxynaphthalenyl Chalcones on Leishmania amazonensis. Antibiotics. 2022, 11, 1402. [Google Scholar] [CrossRef]
- Insuasty, B.; Ramírez, J.; Becerra, D.; Echeverry, C. An efficient synthesis of new caffeine-based chalcones, pyrazolines and pyrazolo[3,4-b][1,4]diazepines as potential antimalarial, antitrypanosomal and antileishmanial agents. Eur. J. Med. Chem. 2015, 93, 401–413. [Google Scholar] [CrossRef]
- Brajtburg, J.; Elberg, S.; Schwartz, D.R. Involvement of oxidative damage in erythrocyte lysis induced by amphotericin B. Antimicrob. Agents Chemother. 1985, 27, 172–176. [Google Scholar] [CrossRef]
- Sousa-Batista, A.J.; Pacienza-Lima, W. Depot Subcutaneous Injection with Chalcone CH8-Loaded Poly(Lactic-Co-Glycolic Acid) Microspheres as a Single-Dose Treatment of Cutaneous Leishmaniasis. Antimicrob. Agents Chemother, 2018, 62, e01822–17. [Google Scholar] [CrossRef]
- Debrabant, A.; Joshi, M.B.; Pimenta, P.F.P. Generation of Leishmania Donovani Axenic Amastigotes: Their Growth and Biological Characteristics. Int. J. Parasitol. 2004, 34, 205–217. [Google Scholar] [CrossRef]
- Almeida, F.S.; Moreira, V.P.; Silva, E.S.; Cardoso, L.L. Leishmanicidal Activity of Guanidine Derivatives against Leishmania infantum. Trop. Med. Infect. Dis. 2003, 8, 141. [Google Scholar] [CrossRef]
- Jain, K.; Verma, A.K.; Mishra, P.R. Surface-Engineered Dendrimeric Nanoconjugates for Macrophage-Targeted Delivery of Amphotericin B: Formulation Development and in Vitro and in Vivo Evaluation. Antimicrob. Agents Chemother. 2015, 59, 2479–2487. [Google Scholar] [CrossRef] [PubMed]
- Saccoliti, F.; Angiulli, G.; Pupo, G.; Pescatori, L. Inhibition of Leishmania Infantum Trypanothione Reductase by Diaryl Sulfide Derivatives. J. Enzyme Inhib. Med. Chem. 2017, 32, 304–310. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C. GROMACS: High Performance Molecular Simulations through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX. 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Van der Spoel, D. A Message-Passing Parallel Molecular Dynamics Implementation. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- Bondi, A. Van Der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure Visualization for Researchers, Educators, and Developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef]
- Nachbagauer, R.; Feser, J.; Naficy, A.; Bernstein, D.I.; Guptill, J.; Walter, E.B.; Berlanda-Scorza, F.; Stadlbauer, D.; Wilson, P.C.; Aydillo, T.; et al. A Chimeric Hemagglutinin-Based Universal Influenza Virus Vaccine Approach Induces Broad and Long-Lasting Immunity in a Randomized, Placebo-Controlled Phase I Trial. Nat. Med. 2021, 27, 106–114. [Google Scholar] [CrossRef]
- Rodrigues, G.S.; Avelino, J.A.; Siqueira, A.L.N.; Ramos, L.F.P. O uso de softwares livres em aula praítca sobre filtros moleculares de biodisponibilidade oral de fármacos. Quím. Nova. 2021, 44, 1036–1044. [Google Scholar]
- Rodrigues, J.S.M.; Costa, E.D. Previsão in silico ADME/T de novos inibidores potenciais contra o vírus da dengue. Res. Soc. Develop. 2021, 10, e53010414459. [Google Scholar] [CrossRef]
- Daina, A.; Michelin, O.; Zoete, V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Mchelin, O.; Zoete, V. iLOGP: a simple, robust, and efficient description of n-octanol/water partition coefficient for drug design using the GB/SA approach. J.Chem. Inf. Model. 2014, 54, 3284–3301. [Google Scholar] [CrossRef] [PubMed]
- Tavares, G.G.; Alves, S.F.; Borges, L.L. Investigação in silico de compostos bioativos de Croton linearifolius Müll. Arg com atividade antidepressiva. Rev.Bras. Mil. Cien. 2020, 6, 8–14. [Google Scholar]
- Daina, A.; Zoete, V. A boiled-egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem. 2016, 11, 1117–1121. [Google Scholar] [CrossRef]
| Compound | IC50 (uM) | EC50 (uM) |
|---|---|---|
| CP01 | Inactive | |
| CP02 | Inactive | |
| CP03 | 10.96 ± 0.27 | 62.52 ± 0.05 |
| CP04 | 13.64 ± 0.25 | 18.92 ± 0.05 |
| CP05 | 39.04 ± 0.19 | |
| CP06 | 11.19 ± 0.22 | 22.42 ± 0.05 |
| CP07 | Inactive | |
| CP08 | Inactive | |
| CP09 | 60.48 ± 0.27 | |
| CP10 | 42.21 ± 0.25 | |
| AmpB | 0.13 ± 0.02 | 0.15 ± 0.02 |
| Compound | IC50 (μM) | RBC50 (μM) | SI |
|---|---|---|---|
| CP03 | 10.96 ± 0.27 | >1012 | >92.33 |
| CP04 | 13.64 ± 0.25 | >1012 | >74.19 |
| CP06 | 11.19 ± 0.22 | >1012 | >90.43 |
| AmpB | 0.13 ± 0.02 | 58.9 ± 0.14 | 245.41 |
| Protein | PDB ligand ID | RMSD |
|---|---|---|
| CYP-51 (PDB: 3L4D) | Fluconazole (TPF) | 0.2122 |
| Trypanothione Reductase (PDB: 5EBK) | 6-sec-Butoxy-2-[(3-chlorophenyl)sulfanyl]-4-pyrimidinamine (RDS) | 1.5733 |
| Compound | CYP-51 (PDB: 3L4D) |
Trypanothione Reductase (PDB: 5EBK) |
|---|---|---|
| CP01 | -82.2604 | -28.1816 |
| CP02 | -92.3169 | -20.2976 |
| CP03 | -85.9169 | -45.2721 |
| CP04 | -94.0758 | -50.5692 |
| CP05 | -89.5499 | -17.9649 |
| CP06 | -91.2791 | -28.3599 |
| CP07 | -75.0916 | -29.6601 |
| CP08 | -84.5501 | -27.6151 |
| CP09 | -71.7698 | -36.0213 |
| CP10 | -75.2075 | -29.9171 |
| PDB ligand (fluconazole) | -72.482 | -16.29 |
| Compound | MW (g/mol) | LogP | nHBA | nHBD | TPSA (Ų) | LogKP (cm/s) | GIA | VLR |
|---|---|---|---|---|---|---|---|---|
| CP01 | 296.32 | 3.15 | 4 | 0 | 44.76 | -5.56 | High | 0 |
| CP02 | 296.32 | 3.48 | 4 | 0 | 83.61 | -5.38 | High | 0 |
| CP03 | 405.24 | 4.08 | 5 | 0 | 53.99 | -5.58 | High | 0 |
| CP04 | 417.25 | 4.29 | 5 | 0 | 53.99 | -5.45 | High | 0 |
| CP05 | 356.37 | 3.44 | 6 | 0 | 63.22 | -5.79 | High | 0 |
| CP06 | 368.38 | 3.68 | 6 | 0 | 63.22 | -5.66 | High | 0 |
| CP07 | 286.71 | 3.70 | 3 | 0 | 35.53 | -5.12 | High | 0 |
| CP08 | 295.33 | 3.16 | 3 | 0 | 38.77 | -5.52 | High | 0 |
| CP09 | 258.29 | 3.16 | 3 | 0 | 63.77 | -5.59 | High | 0 |
| CP10 | 270.26 | 3.46 | 4 | 0 | 35.53 | -5.39 | High | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





