Submitted:
28 August 2023
Posted:
29 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
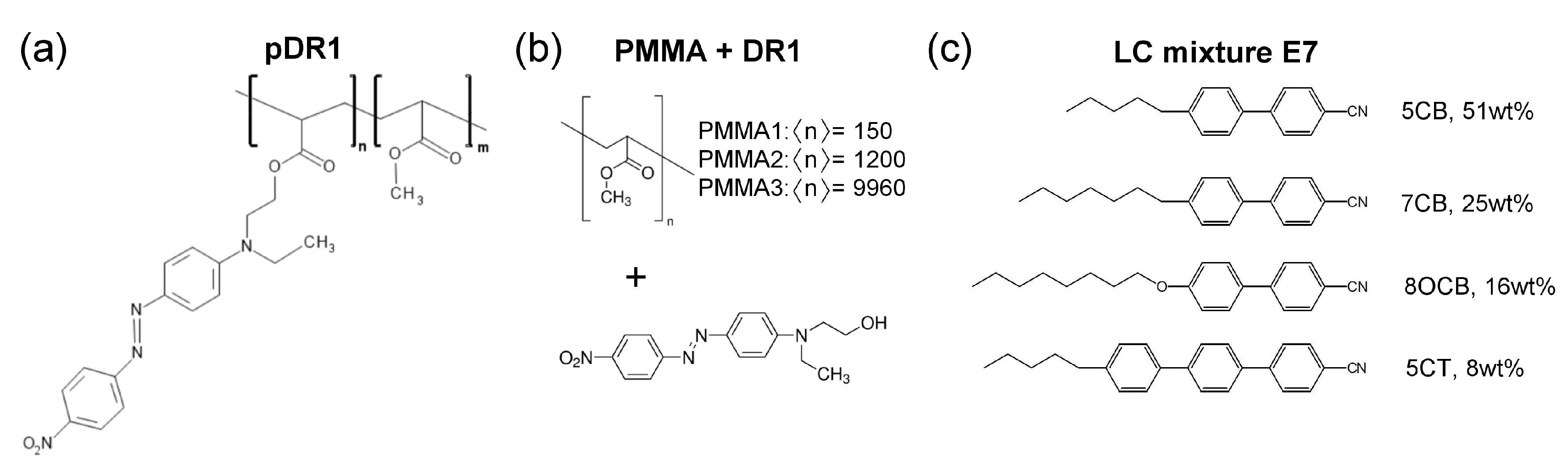
2. Materials and Methods
3. Results
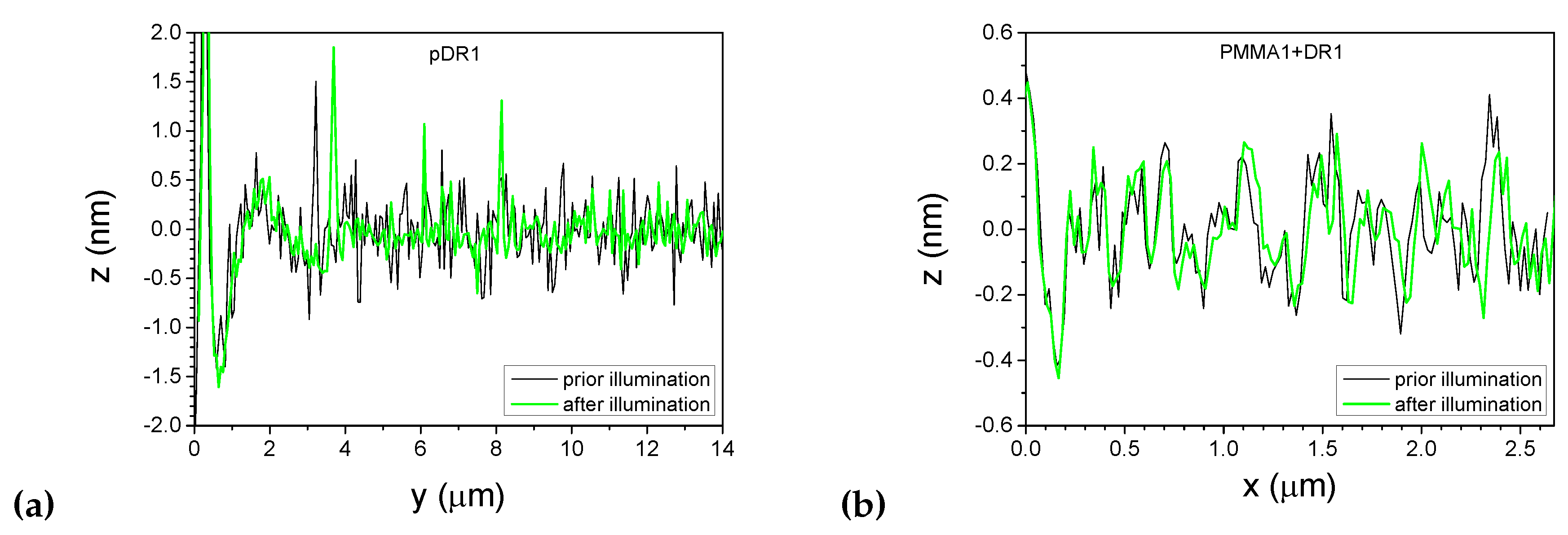
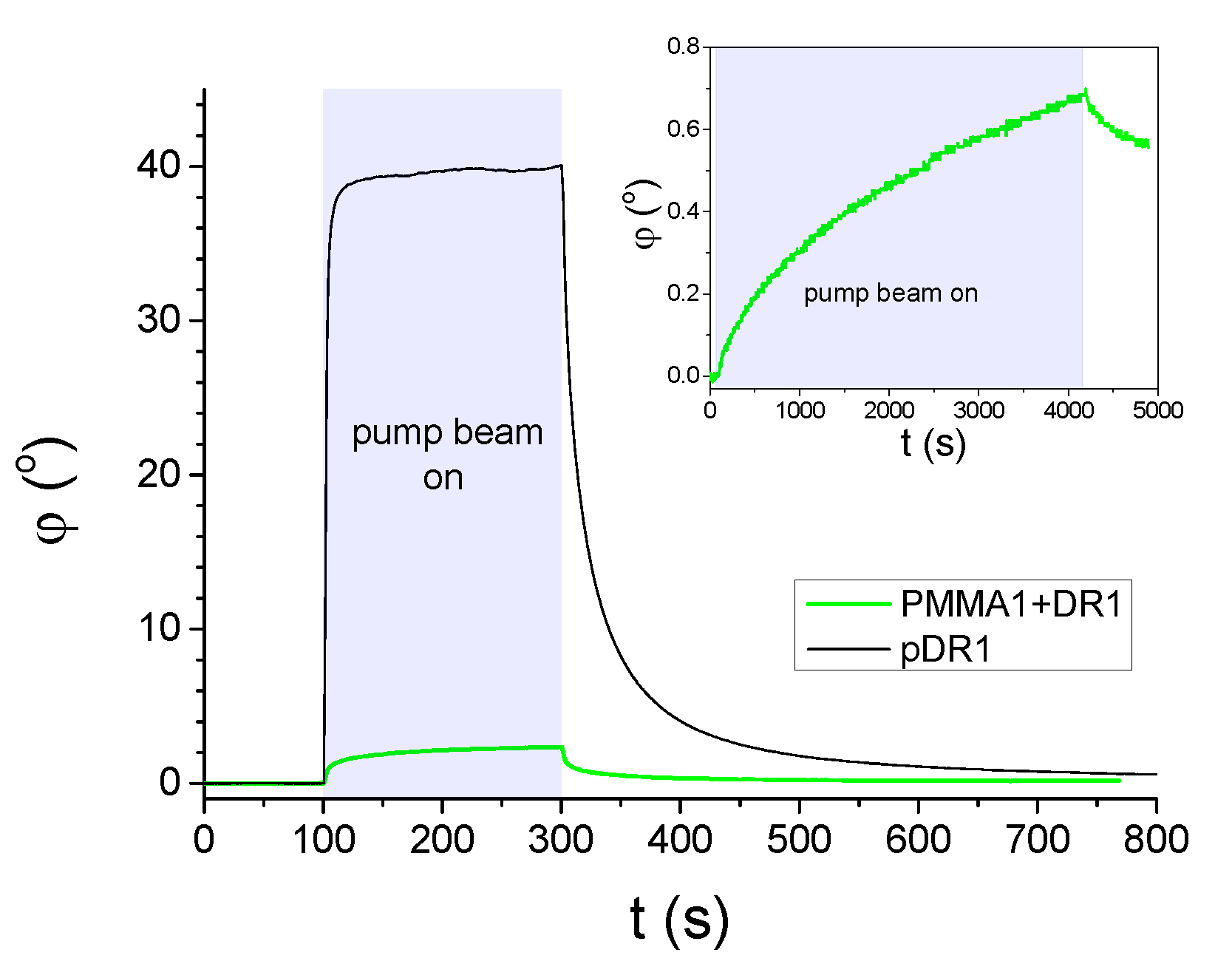
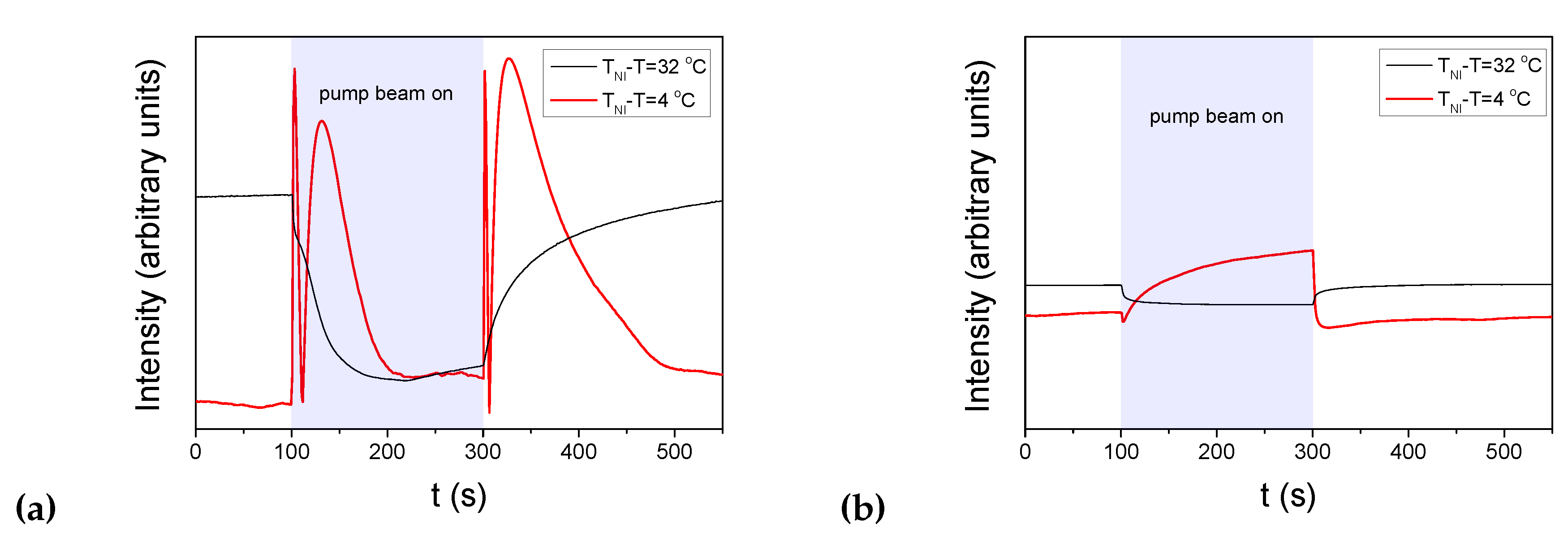
3.1. Photoalignment measurements
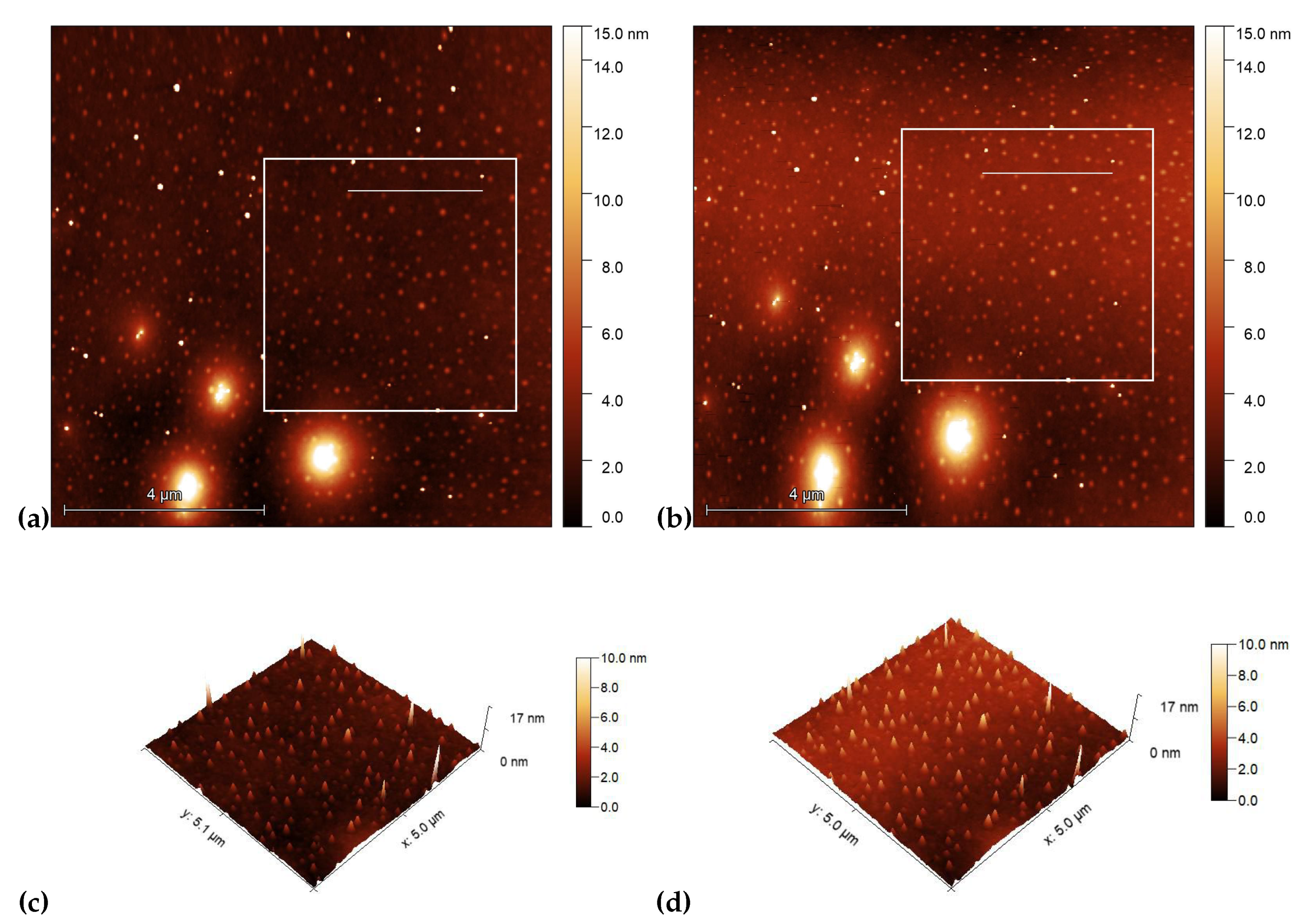
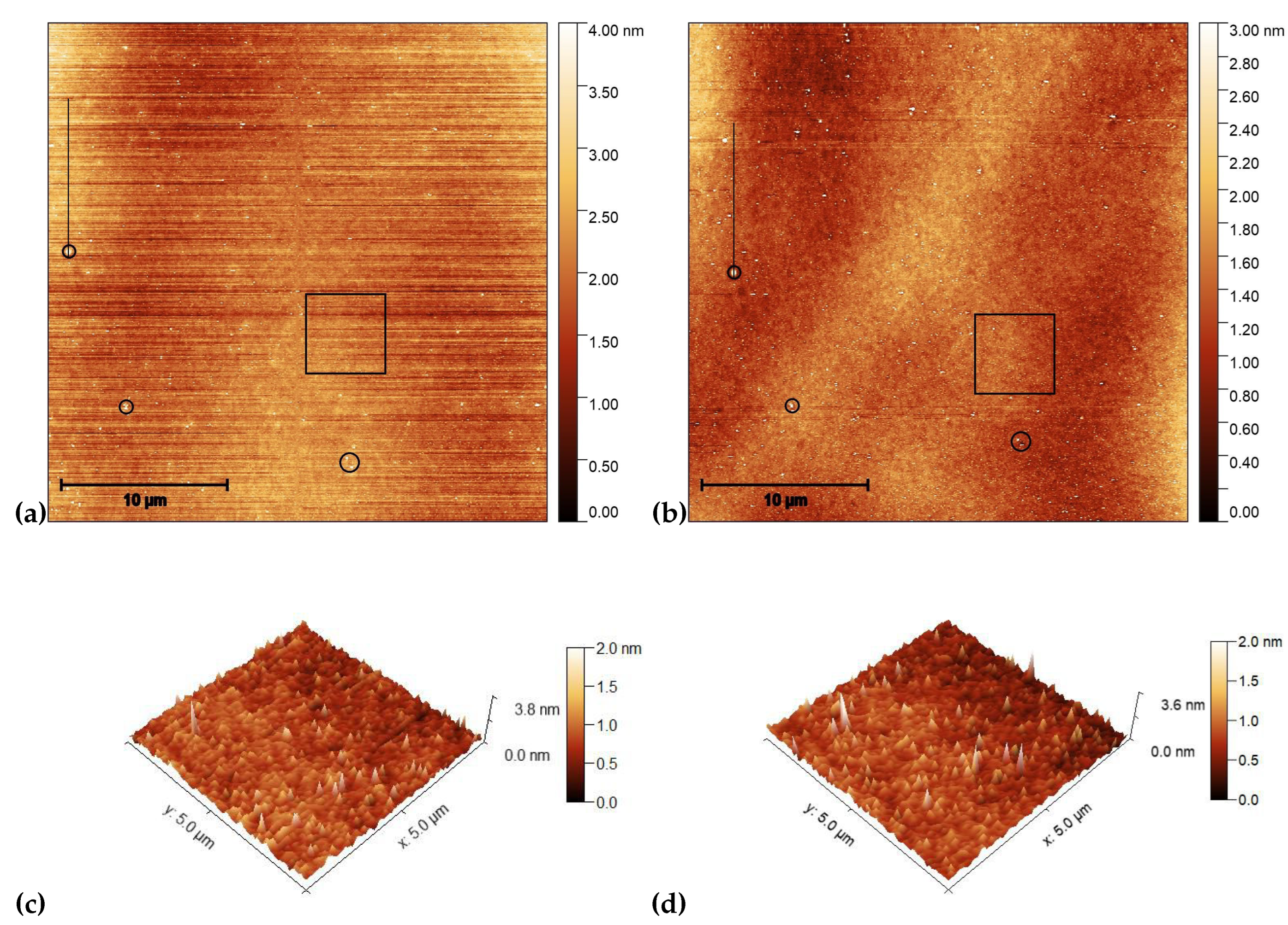
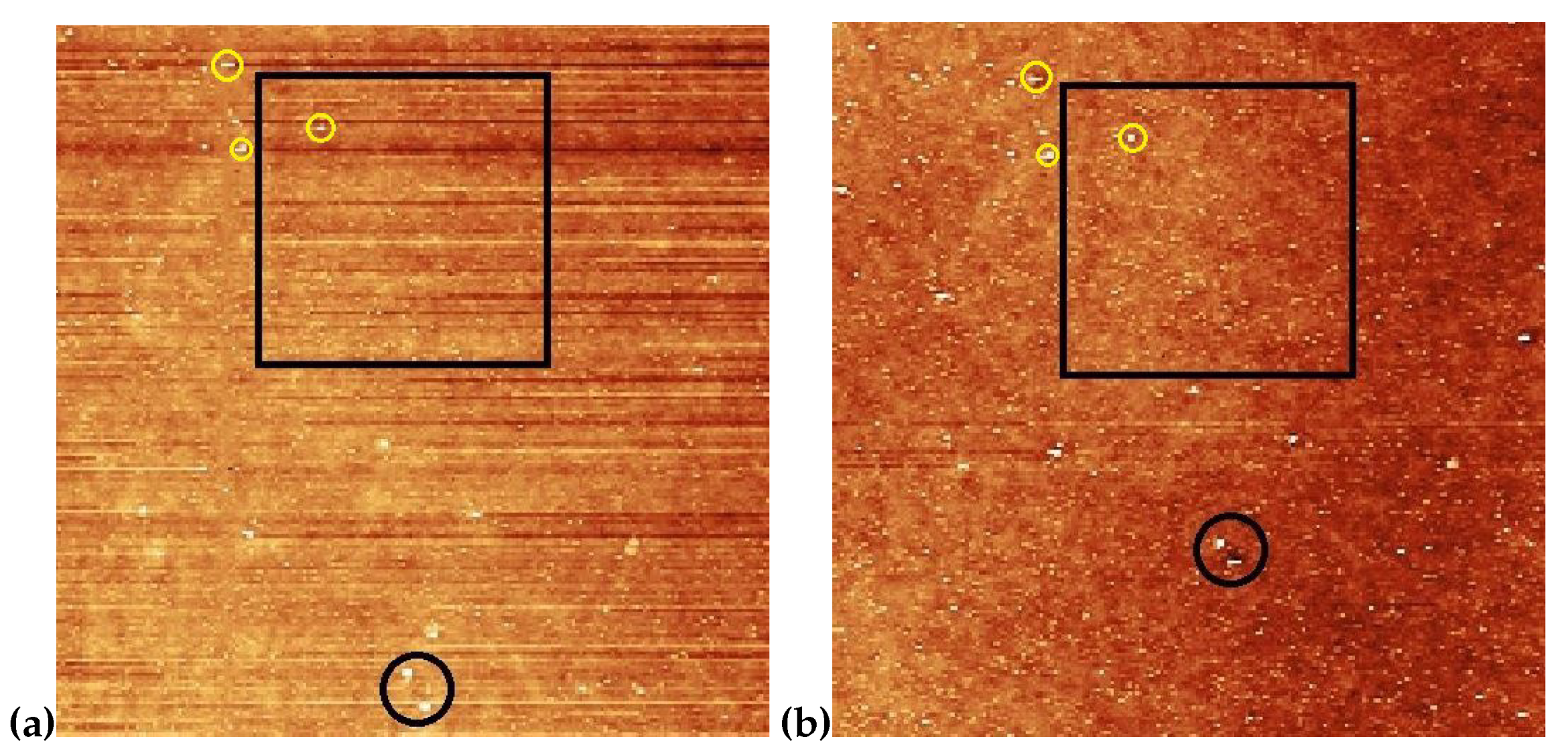
3.2. Atomic force microscopy (AFM) on photosensitive substrates
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ichimura, K. Photoalignment in liquid-crystal systems. Chem. Rev. 2000, 100, 1847–1873. [Google Scholar] [CrossRef] [PubMed]
- Seki, T. New strategies and implications for the photoalignment of liquid crystalline polymers. Polym. J. 2014, 46, 751–768. [Google Scholar] [CrossRef]
- Bisoyi, H.K.; Li, Q. Light-driven liquid crystalline materials: From photo-induced phase transitions and property modulations to applications. Chem. Rev. 2016, 116, 15089–15166. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, K.; Suzuki, J.; Seki, T.; Hosoki, A.; Aoki, K. Reversible change in alignment mode of nematic liquid crystals regulated photochemically by “command surfaces” modified with an azobenzene monolayer. Langmuir 1988, 4, 1214–1216. [Google Scholar] [CrossRef]
- Gibbons, W.M.; Shannon, P.J.; Sun, S.T.; Swetlin, B.J. Surface-mediated alignment of nematic liquid crystals with polarized laser light. Nature 1991, 351, 49–50. [Google Scholar] [CrossRef]
- Dyadyusha, A.G.; Marusii, T.; Reznikov, Y.; Khiznyak, A.; Reshetnyak, V. Orientational effect due to a change in the anisotropy of the interaction between a liquid crystal and a bounding surface. JETP Lett. 1992, 56, 17–21. [Google Scholar]
- Gibbons, W.M.; Kósa, T.; Palffy-Muhoray, P.; Shannon, P.J.; Sun, S.T. Continuous grey-scale image storage using optically aligned nematic liquid crystals. Nature 1995, 377, 43–46. [Google Scholar] [CrossRef]
- Aoki, K.; Seki, T.; Suzuki, Y.; Tamaki, T.; Hosoki, A.; Ichimura, K. Factors affecting photoinduced alignment regulation of cyclohexanecarboxylate-type nematic liquid crystals by azobenzene molecular films. Langmuir 1992, 8, 1007–1013. [Google Scholar] [CrossRef]
- Seki, T.; Sakuragi, M.; Kawanishi, A; Tamaki, T. ; Fukuda, R.; Ichimura, K. “Command surfaces” of Langmuir-Blodgett films. Photoregulations of liquid crystal alignment by molecularly tailored surface azobenzene layers. Langmuir 1993, 9, 211–218. [Google Scholar] [CrossRef]
- Ichimura, K.; Hayashi, Y.; Akiyama, H.; Ishizuki, N. Photoregulation of in-plane reorientation of liquid crystals by azobenzenes laterally attached to substrate surfaces. Langmuir 1993, 9, 3298–3304. [Google Scholar] [CrossRef]
- Yi, Y.; Farrow, M.J.; Korblova, E.; Walba, D.M.; Furtak, T.E. High-sensitivity aminoazobenzene chemisorbed monolayers for photoalignment of liquid crystals. Langmuir 2009, 25, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Jánossy, I.; Fodor-Csorba, K.; Vajda, A.; Palomares, L.O. Light-induced spontaneous pattern formation in nematic liquid crystal cells. Appl. Phys. Lett. 2011, 99, 111103. [Google Scholar] [CrossRef]
- Iimura, Y.; Kusano, J.; Kobayashi, S.; Aoyagi, T.; Sugano, T. Alignment control of a liquid crystal on a photosensitive polyvinylalcohol film. Jpn. J. Appl. Phys., Part 2 1993, 32, L93–L96. [Google Scholar] [CrossRef]
- Shannon, P.J.; Gibbons, W.M.; Sun, S.T.; Swetlin, B.J. Patterned optical properties in photopolymerized surface-aligned liquid-crystal film. Nature 1994, 368, 532–533. [Google Scholar] [CrossRef]
- Jánossy, I.; Jákli, A.; Nair, G.G.; Raina, K.K.; Kósa, T. Optical control of the alignment of a liquid crystal in the smectic A phase. Mol. Cryst. Liq. Cryst. 1999, 329, 507–516. [Google Scholar] [CrossRef]
- Jánossy, I.; Vajda, A.; Paksi, T.; Kósa, T. Photoinduced surface alignment: The role of the liquid crystalline order. Mol. Cryst. Liq. Cryst. 2001, 359, 157–166. [Google Scholar] [CrossRef]
- Ichimura, K.; Suzuki, Y.; Seki, T.; Kawanishi, Y.; Aoki, K. Reversible alignment change of a nematic liquid crystal induced by pendent azobenzene groups-containing polymer thin films. Makromol. Chem. Rapid Commun. 1989, 10, 5–8. [Google Scholar] [CrossRef]
- Kawanishi, Y.; Seki, T.; Tamaki, T.; Ichimura, K.; Ikeda, M.; Aoki, K. Reversible alignment change of nematic liquid crystals by photochromic polymer films. Polym. Adv. Technol. 1990, 1, 311–318. [Google Scholar] [CrossRef]
- Kawanishi, Y.; Tamaki, T.; Seki, T.; Sakuragi, M.; Suzuki, Y.; Ichimura, K.; Aoki, K. Multifarious liquid crystalline textures formed on a photochromic azobenzene polymer film. Langmuir 1991, 7, 1314–1315. [Google Scholar] [CrossRef]
- Ichimura, K.; Akiyama, H.; Ishizuki, N.; Kawanishi, Y. Azimuthal orientation of liquid crystals photo-controlled by an azobenzene pendent polymer. Makromol. Chem., Rapid Commun. 1993, 14, 813–817. [Google Scholar] [CrossRef]
- Akiyama, H.; Kudo, K.; Ichimura, K. Novel polymethacrylates with laterally attached azobenzene groups displaying photoinduced optical anisotropy. Macromol. Rapid Commun. 1995, 16, 35–41. [Google Scholar] [CrossRef]
- Akiyama, H.; Momose, M.; Ichimura, K.; Yamamura, S. Surface-selective modification of poly(vinyl alcohol) films with azobenzenes for in-plane alignment photocontrol of nematic liquid crystals. Macromolecules 1995, 28, 288–293. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, D.; Xie, P.; Zhang, R. Photo-driven liquid crystal cell using azobenzene-grafted ladderlike polysiloxane as command layer. Macromol. Rapid Commun. 1996, 17, 759–766. [Google Scholar] [CrossRef]
- Tang, Y.; Xie, P.; Liu, D.; Zhang, R. Performance-improved photo-driven liquid crystal cell using azobenzene-grafted ladderlike polysiloxane as command layer. Macromol. Chem. Phys. 1997, 198, 1855–1863. [Google Scholar] [CrossRef]
- Ichimura, K.; Morino, S.; Akiyama, H. Three-dimensional orientational control of molecules by slantwise photoirradiation. Appl. Phys. Lett. 1998, 73, 921–923. [Google Scholar] [CrossRef]
- Ruslim, C.; Ichimura, K. Comparative studies on isomerization behavior and photocontrol of nematic liquid crystals using polymethacrylates with 3,3’- and 4,4’-dihexyloxyazobenzenes in side chains. Macromolecules 1999, 32, 4254–4263. [Google Scholar] [CrossRef]
- Ruslim, C.; Ichimura, K. Photocontrolled alignment of chiral nematic liquid crystals. Adv. Mater. 2001, 13, 641–644. [Google Scholar] [CrossRef]
- Palffy-Muhoray, P.; Kosa, T.; Weinan, E. Dynamics of a light driven molecular motor. Mol. Cryst. Liq. Cryst. 2002, 375, 577–591. [Google Scholar] [CrossRef]
- Ryabchun, A.V.; Bobrovsky, A.Yu.; Shibaev, V.P. Photoinduced reorientation processes in thin films of photochromic LC polymers on substrates with a photocontrollable command surface. Polym. Sci. - A 2010, 52, 812–823. [Google Scholar] [CrossRef]
- Bobrovsky, A.; Ryabchun, A.; Shibaev, V. Liquid crystals photoalignment by films of side-chain azobenzene-containing polymers with different molecular structure. J. Photochem. Photobiol. A 2011, 218, 137–142. [Google Scholar] [CrossRef]
- Ryabchun, A.; Bobrovsky, A.; Chun, S.-H.; Shibaev, V. A novel generation of photoactive comb-shaped polyamides for the photoalignment of liquid crystals. J. Polym. Sci., Part A: Polym. Chem. 2013, 51, 4031–4041. [Google Scholar] [CrossRef]
- Petrov, S.; Chau, N.H.M.; Marinova, V.; Sun, C.-C.; Hsu, K.-Y.; Lin, S.-H. Controllable LC anchoring on poly1-[4-(3-carboxy-4-hydroxyphenylazo) benzenesulfonamido]-1,2-ethanediyl, sodium salt command surface. Polymer 2023, 272, 125841. [Google Scholar] [CrossRef]
- Bandara, H.M.D.; Burdette, S.C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. [Google Scholar] [CrossRef]
- Seki, T.; Nagano, S.; Hara, M. Versatility of photoalignment techniques: From nematics to a wide range of functional materials. Polymer 2013, 54, 6053–6072. [Google Scholar] [CrossRef]
- Tóth-Katona, T.; Jánossy, I. Photoalignment at the nematic liquid crystal-polymer interface: Experimental evidence of three-dimensional reorientation. J. Mol. Liq. 2019, 285, 323–329. [Google Scholar] [CrossRef]
- Nassrah, A.K.R.; Jánossy, I.; Tóth-Katona, T. Photoalignment at the nematic liquid crystal–polymer interface: The importance of the liquid crystalline molecular structure. J. Mol. Liq. 2020, 312, 113309. [Google Scholar] [CrossRef]
- Nassrah, A.K.R.; Jánossy, I.; Kenderesi, V.; Tóth-Katona, T. Polymer–nematic liquid crystal interface: On the role of the liquid crystalline molecular structure and the phase sequence in photoalignment. Polymers 2021, 13, 193. [Google Scholar] [CrossRef] [PubMed]
- Jánossy, I.; Tóth-Katona, T. Photo-orientation of liquid crystals on azo dye-containing polymers. Polymers 2022, 14, 159. [Google Scholar] [CrossRef] [PubMed]
- Shibaev, V.P.; Bobrovsky, A.Yu. Liquid crystalline polymers: Development trends and photocontrollable materials. Russ. Chem. Rev. 2017, 86, 1024–1072. [Google Scholar] [CrossRef]
- Oscurato, S.L.; Salvatore, M.; Maddalena, P.; Ambrosio, A. From nanoscopic to macroscopic photo-driven motion in azobenzene-containing materials. Nanophotonics 2018, 7, 1387–1422. [Google Scholar] [CrossRef]
- Rochon, P.; Batalla, E.; Natansohn, A. Optically induced surface gratings on azoaromatic polymer films. Appl. Phys. Lett. 1995, 66, 136–138. [Google Scholar] [CrossRef]
- Kim, D.Y. .; Tripathy, S.K.; Li, L.; Kumar, J. Laser-induced holographic surface relief gratings on nonlinear optical polymer films. Appl. Phys. Lett. 1995, 66, 1166–1168. [Google Scholar] [CrossRef]
- Kim, D.Y.; Li, L.; Jiang, X.L.; Shivshankar, V.; Kumar, J.; Tripathy, S.K. Polarized laser induced holographic surface relief gratings on polymer films. Macromolecules 1995, 28, 8835–8839. [Google Scholar] [CrossRef]
- Bian, S.; Li, L.; Kumar, J.; Kim, D.Y.; Williams, J.; Tripathy, S.K. Single laser beam-induced surface deformation on azobenzene polymer films. Appl. Phys. Lett. 1998, 73, 1817–1819. [Google Scholar] [CrossRef]
- Bian, S.; Liu, W.; Williams, J.; Samuelson, L.; Kumar, J.; Tripathy, S. Photoinduced surface relief grating on amorphous poly(4-phenylazophenol) films. Chem. Mater. 2000, 12, 1585–1590. [Google Scholar] [CrossRef]
- Lagugné Labarthet, F.; Buffeteau, C.; Sourisseau, C. Analyses of the diffraction efficiencies, birefringence, and surface relief gratings on azobenzene-containing polymer films. J. Phys. Chem. B 1998, 102, 2654–2662. [Google Scholar] [CrossRef]
- Walsh, C.B.; Franses, E.I. Ultrathin PMMA films spin-coated from toluene solutions. Thin Solid Films 2003, 429, 71–76. [Google Scholar] [CrossRef]
- Thompson, E.V. Dependence of the glass transition temperature of poly(methyl methacrylate) on tacticity and molecular weight. J. Polym. Sci. A2 1966, 4, 199–208. [Google Scholar] [CrossRef]
- Ute, K.; Miyatake, N.; Hatada, K. Glass transition temperature and melting temperature of uniform isotactic and syndiotactic poly(methyl methacrylate)s from 13mer to 50mer. Polymer 1995, 36, 1415–1419. [Google Scholar] [CrossRef]
- Haque, H.A.; Hara, M.; Nagano, S.; Seki, T. Photoinduced in-plane motions of azobenzene mesogens affected by the flexibility of underlying amorphous chains. Macromolecules 2013, 46, 8275–8283. [Google Scholar] [CrossRef]
- DSC measurement has been performed at Alphamicron Inc., Kent OH, USA.
- Jánossy, I.; Tóth-Katona, T.; Kósa, T.; Sukhomlinova, L. Super-twist generation and instabilities in photosensitive liquid crystal cells. J. Mol. Liq. 2018, 267, 177–181. [Google Scholar] [CrossRef]
- Available online:. Available online: http://gwyddion.net (accessed on 22 August 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).