Submitted:
25 August 2023
Posted:
29 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
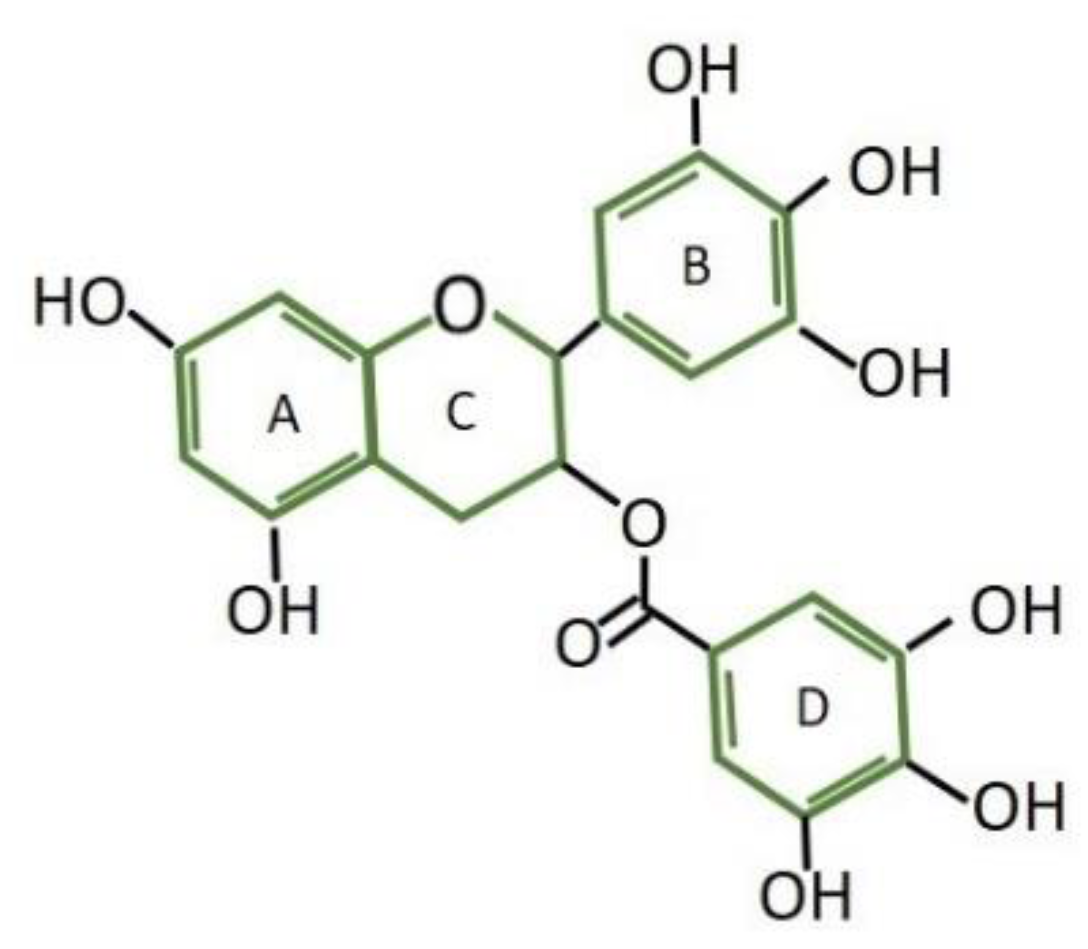
2. Results
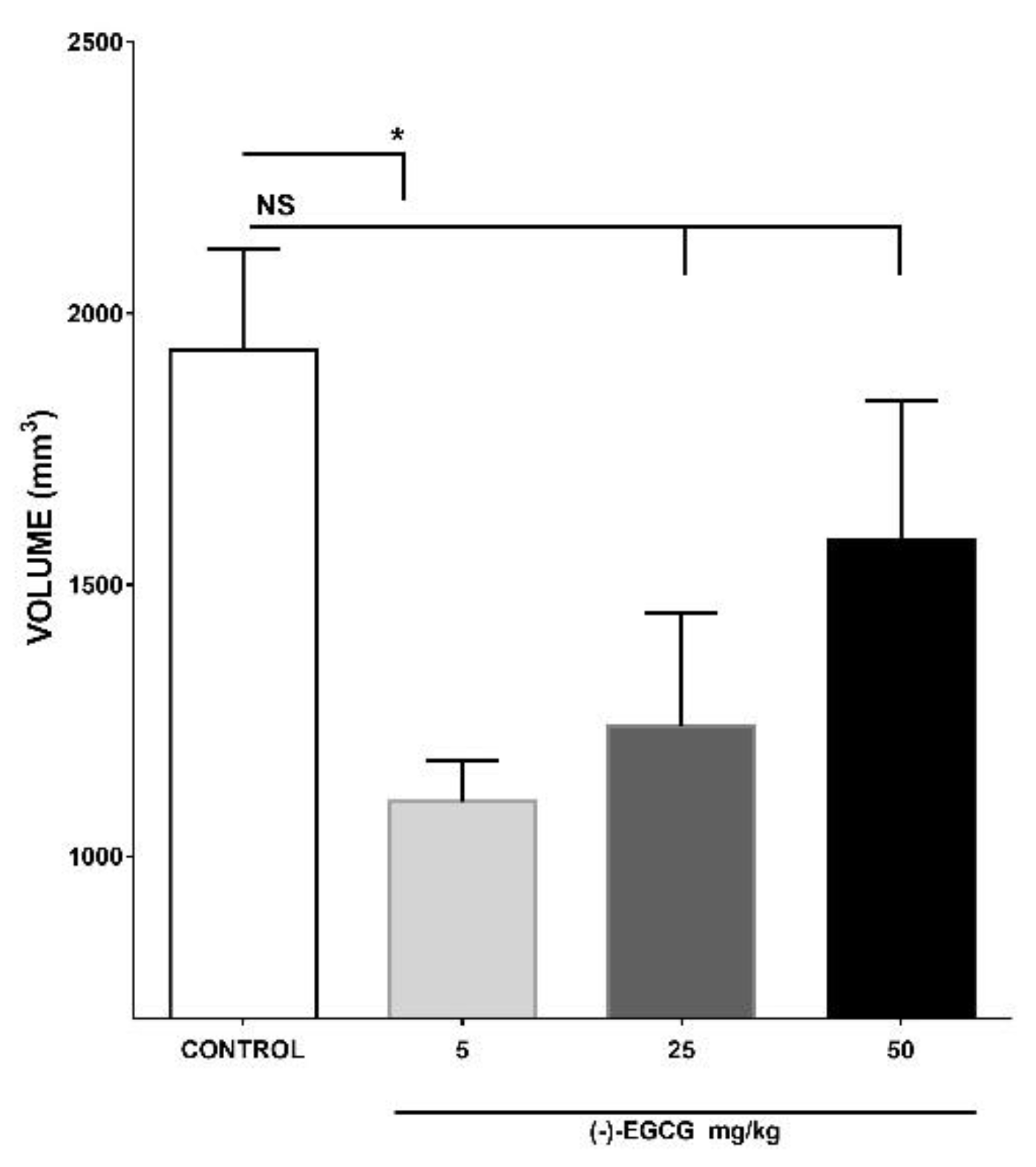
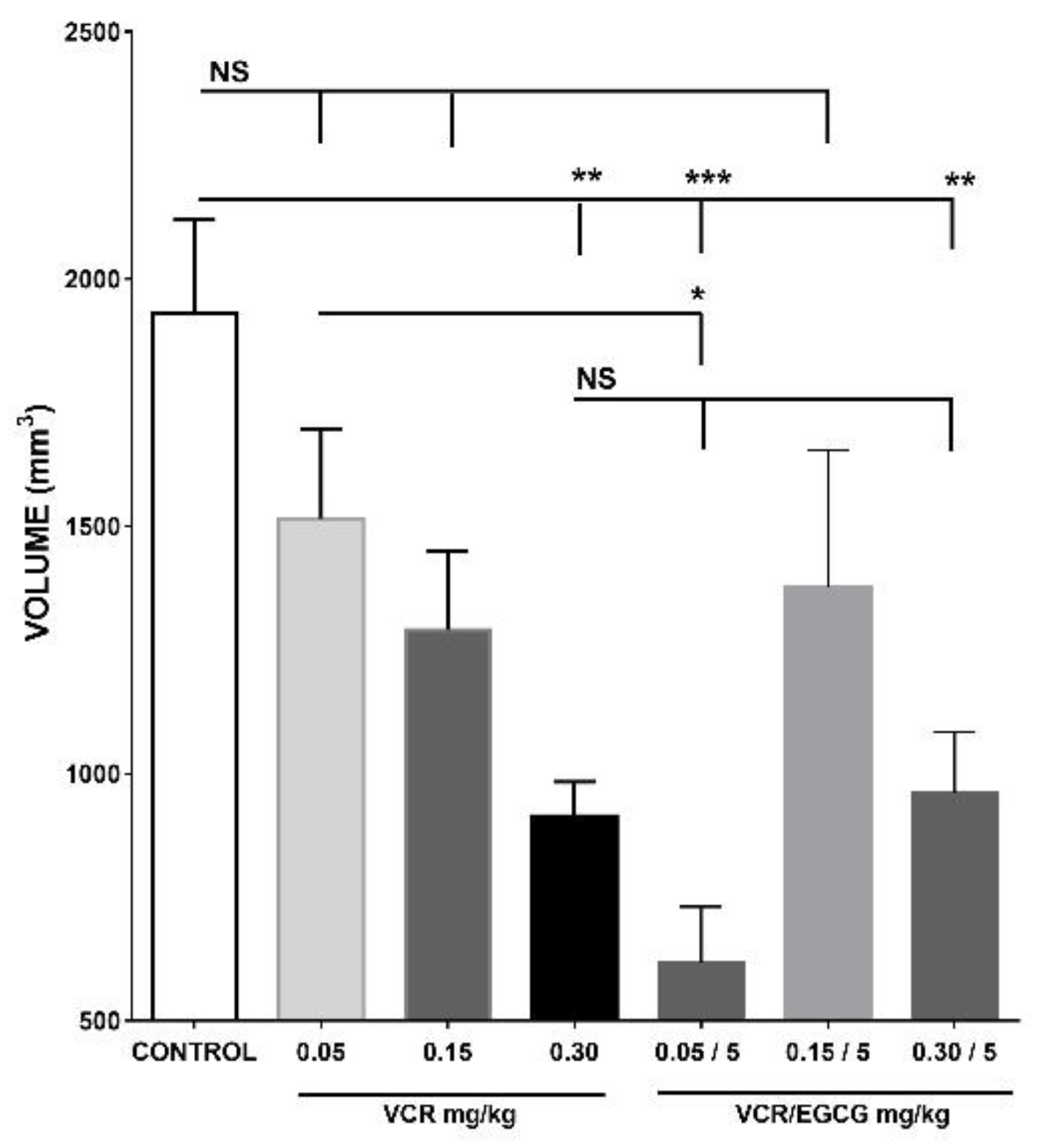
2.1. Effect of different treatments on the inhibition of tumor growth
2.1.1. Effect of (–)-EGCG on the inhibition of tumor growth
2.1.2. Effect of vincristine and its combination with EGCG in the inhibition of tumor growth
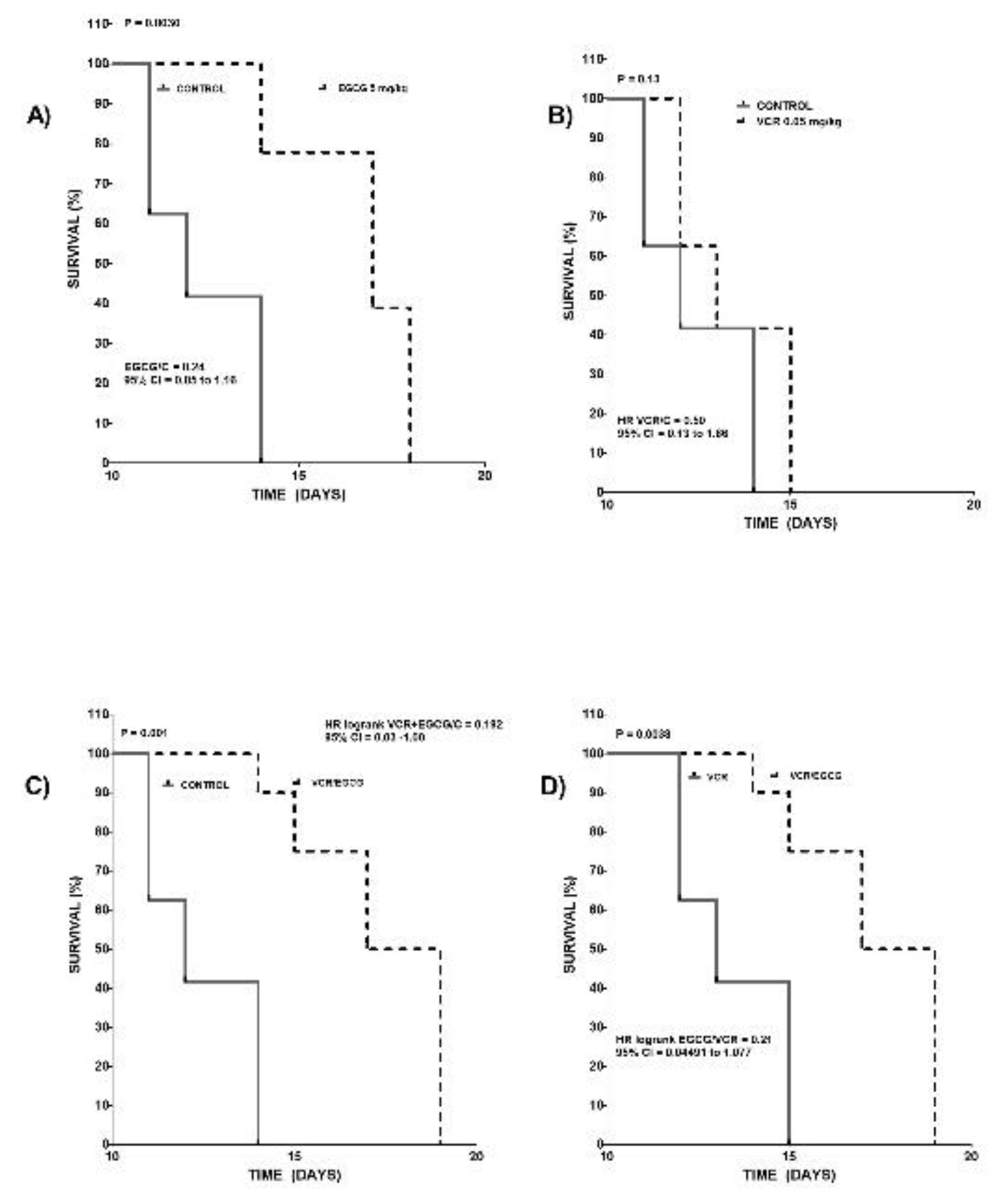
2.2. Effect of Effect of EGCG, VCR and their concomitant administration on survival
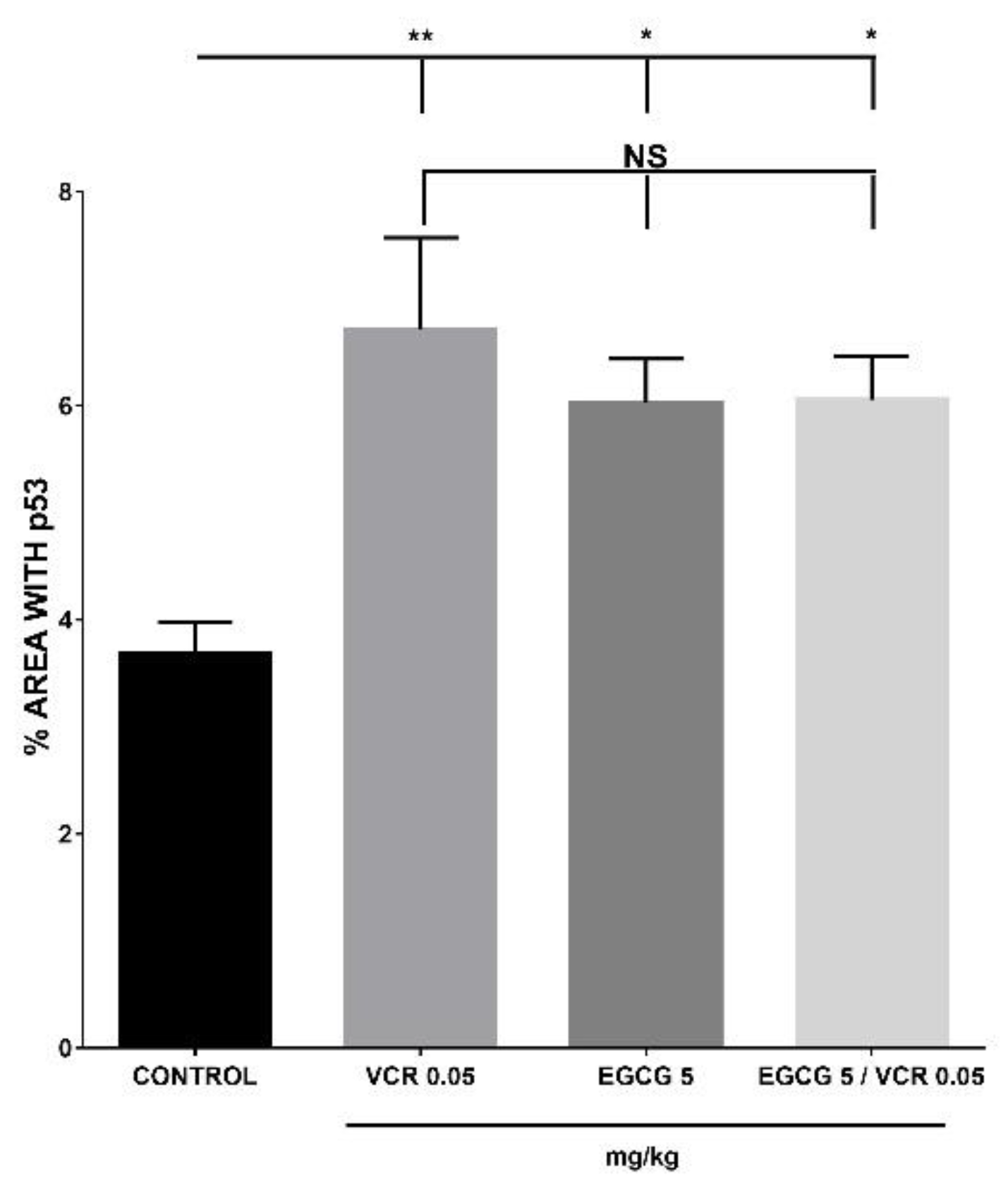
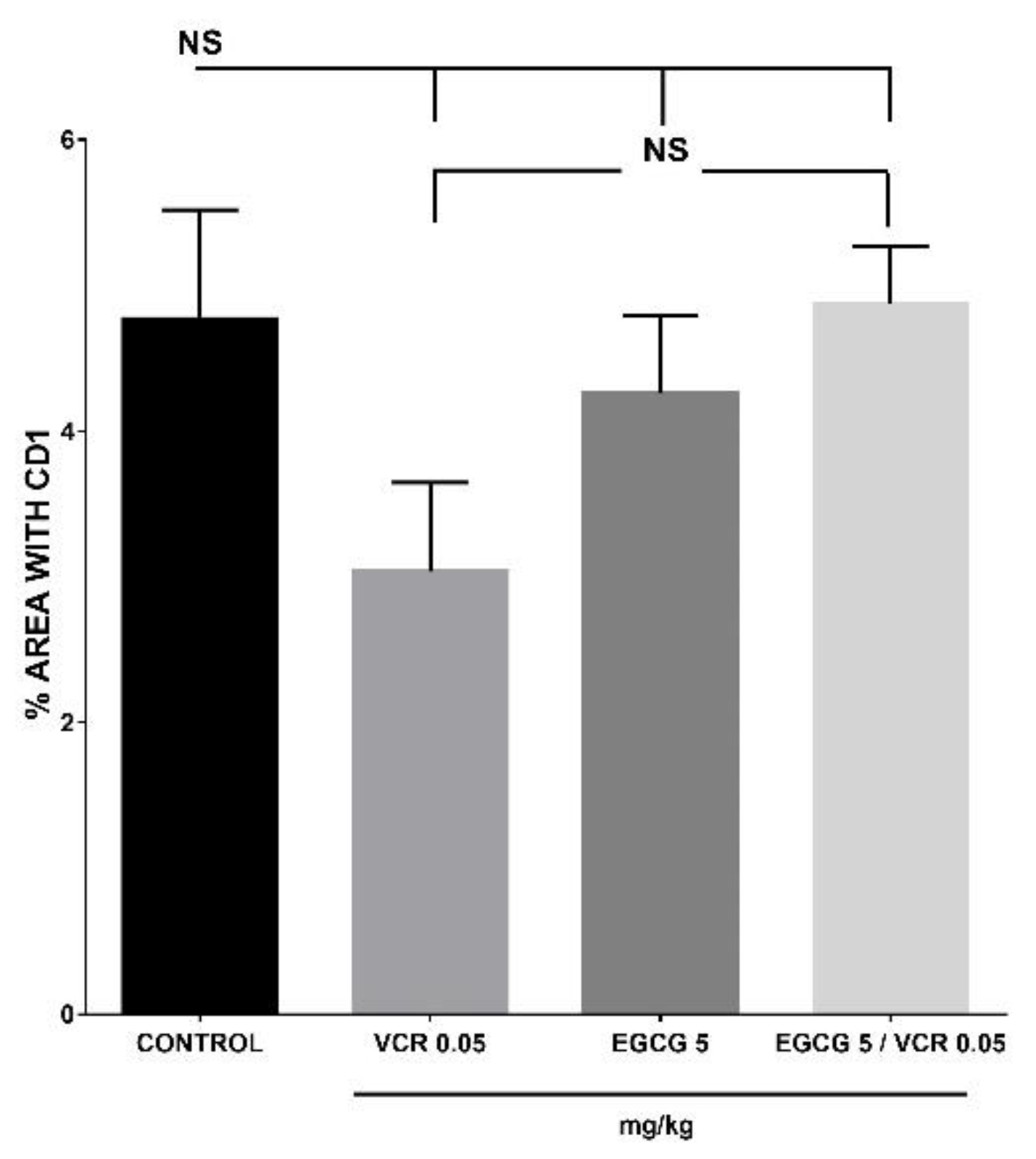
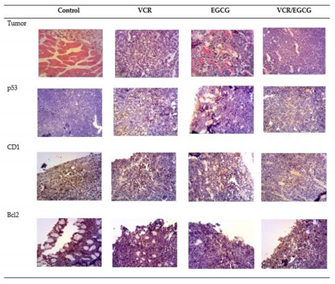
2.3. Effect of EGCG, VCR and VCR / EGCG on the determination of p53, CD1 and Bcl2 in murine lymphoma
3. Discussion
4. Materials and Methods
4.1. Determination of tumor volume and survival with (−)- EGCG, VCR and their combination.
4.2. Determination of proteins by Immunohistochemistry in L5178Y tumor with (−)- EGCG, VCR and their combination.
4.3. Statistical analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Garraway, LA.; Lander, ES. ; Lessons from the cancer genome. Cell. 2013, 28, 153, 17–37. [Google Scholar] [CrossRef]
- FAO Intergovernmental Goup on tea World tea production and trade Currente and future develoment Kaison Chang- Secretary. A subsidiary body of the Organization of the United Nations (FAO). Committee on commodity problems (CCP) Rome 2015. https://www.fao.org/3/i4480e/i4480e.pdf Accessed on 02 august 2023.
- Du, Guang-Jian. ; Zhiyu Zhang.; Xiao-Dong Wen.; Chunhao Yu.; Tyler Calway.; Chun,-Su Yuan.; Chong,-Zhi Wang. Epigallocatechin Gallate (EGCG) Is the Most Effective Cancer Chemopreventive Polyphenol in Green Tea. Nutrients 4. 2012, 11, 1679–1691. [CrossRef]
- Chen, Y.; Wang, X. Q.; Zhang, Q.; Zhu, J. Y.; Li, Y.; Xie, C. F.; Li, X. T.; Wu, J. S.; Geng, S. S.; Zhong, C. Y.; Han, H. Y. Epigallocatechin-3-Gallate Inhibits Colorectal Cancer Stem Cells by Suppressing Wnt/β-Catenin Pathway. Nutrients. 2017, 9, 6, 572 doiorg/103390/nu9060572. [Google Scholar] [CrossRef]
- Wei; Pens; Hackman; Wang; & Mackenzie. Epigallocatechin-3-Gallate (EGCG) Suppresses Pancreatic Cancer Cell Growth, Invasion and Migration partly through the Inhibition of Akt Pathway and Epithelial–Mesenchymal Transition: Enhanced Efficacy when Combined with Gemcitabine. Nutrients. 2019, 11, 8, 1856 MDPI AG. [CrossRef]
- Gu j, J.; Qiao, KS.; Sun, P.; Chen, P.; Li, Q. ; Study of EGCG induced apoptosis in lung cancer cells by inhibiting PI3K/Akt signaling pathway. Europan Rev Med Pharmaco Sci. 2018, 22, 4557–4563. [Google Scholar]
- Thangapazham, R L.; Singh, AK.; Sharma, A.; Warren, J.; Gaddipati, JP.; Maheshwari, RK. Green tea polyphenols and its constituent epigallocatechin gallate inhibits proliferation of human breast cancer cells in vitro and in vivo. Cancer letters. 245, 1-2, 232–241. [CrossRef]
- Braicu, C.; Gherman, CD.; Irimie, A. ; Berindan,NI. Epigallocatechin-3-Gallate (EGCG) inhibits cell proliferation and migratory behaviour of triple negative breast cancer cells. Journal of nanoscience and nanotechnology. 2013, 13, 1, 632–637. [Google Scholar] [CrossRef]
- Luo, K W. ; Lung, WY.; Chun-Xie, L XL.; Huang, WR. EGCG inhibited bladder cancer T24 and 5637 cell proliferation and migration via PI3K/AKT pathway. Oncotarget. 2018, 9, 15, 12261–12272. [CrossRef]
- Qin, J.; Fu, M.; Wang, J.; Huang, F.; Liu, H.; Huangfu, M.; Chen, X. PTEN/AKT/mTOR signaling mediates anticancer effects of epigallocatechin 3 gallate in ovarian cancer. Oncology Reports. 2020, 43, 1885–1896. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, SA.; Almatroudi, A. ; Khan, AAV. ; Alhumaydhi, FA.; Alsahli, M A.; Rahmani, A H. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules (Basel, Switzerland). 2020, 25, 14, 3146. [Google Scholar] [CrossRef]
- Chen, BH.; Hsieh, CH.; Tsai, SY. et al. Anticancer effects of epigallocatechin-3-gallate nanoemulsion in lung cancer cells through activation of the AMP-activated protein kinase signaling pathway. Sci Rep. 2020, 10, 5163. [Google Scholar] [CrossRef] [PubMed]
- Bimonte, S.; Cascella, M.; Barbieri, A.; Arra, C. , Cuomo, A. Current shreds of evidence on the anticancer role of EGCG in triple negative breast cancer: an update of the current state of knowledge. Infectious agents and cancer. 2020, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Arribalzaga. ; Gramatges.; Bland JM.; Altman, DG. The logrank test. BMJ. 2004, 1, 328, 7447, 1073. [CrossRef]
- Molina, A M.; Ortega, P E.; Ochoa S C. Estudios de supervivencia. Método de Kaplan-Meier. Evid Pediatr. 2022, 18:20.
- Almaguer, VG.; Resendiz, EE. ; Galnares NML. ; Montejano, ABV.; Becerril FMA.; Imbert, PH.; Molina TEM.; Balderas DC.; Bautista AM.; Montejano RJR.; Antitumor activity in lymphoma 5178Y in mice, acute toxicity and phytochemical of Decatropis bicolor Zucc. Radlk. Pharmacologyonline. 2019, 3, 224–235. [Google Scholar]
- Jackson, DV Jr. ; Bender, RA. Cytotoxic thresholds of vincristine in a murine and a human leukemia cell line in vitro. Cancer Res. 1979, 39, 11, 4346, 9 PMID: 291476. [PubMed]
- Bender, RA. ; Nichols. AP.; Norton L.; Simon RM. Lack of therapeutic synergism between vincristine and 504 methotrexate in L1210 murine leukemia in vivo. Cancer Treat Rep. 1978. 62, 7 997–1003.
- Brunton LL, Chabner BA, Knollmann BC. Brunton L.L., & Chabner B.A., & Knollmann B.C. Las Bases Farmacológicas de la terapéutica; Fármacos citotóxicos Sección VIII Tratamiento farmacológico de las enfermedades neoplásicas III, Productos naturales Vincristina; Goodman & Gilman., 12nd ed.; Chabner BA.; Bertino J.; Cleary J.; Ortiz Tv.; Lane A.; Supko J G. Ryan D.2012, 61.
- Silva, A.; Wang, Q.; Wang, M.; Ravula, SK.; Glass, JD. Evidence of direct axonal toxicity in vincristine neuropathy. J Peripheral Nerve Syst. 2006, 11, 3, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Mao, L.; Xu, P.; Zheng, X.; Hackman, R. M.; Mackenzie, G. G.; Wang, Y. Suppressing glucose metabolism with epigallocatechin-3-gallate (EGCG) reduces breast cancer cell growth in preclinical models. Food & function. 2018, 9, 11, 5682–5696. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, W.; Tu, G.; Chen, X.; Lu, Y.; Wu, L.; Zheng, D. Enhanced Chemotherapeutic Efficacy of PLGA-Encapsulated Epigallocatechin Gallate (EGCG) Against Human Lung Cancer. International journal of nanomedicine. 2020, 15, 4417–4429. [Google Scholar] [CrossRef] [PubMed]
- Tiffany, J.; Somers, E.; Marissa, J. ; Scandlyn. ; Emma, C.; Stuart.; Martin, J.; Le, N.; Sophie, P. Valentine. The combination of epigallocatechin gallate and curcumin suppresses ERa-breast cancer cell growth in vitro and in vivo Int. J. Cancer. 2008, 122, 1966–1971. [Google Scholar]
- Della, VFI. ; Shiraishi, R.N.; Santos, I. et al. Epigallocatechin-3-gallate induces apoptosis and differentiation in leukaemia by targeting reactive oxygen species and PIN1. Sci Rep. 2021, 11, 9103. [CrossRef]
- Zhu, K.; Wang, W. Green tea polyphenol EGCG suppresses osteosarcoma cell growth through upregulating miR-1. Tumor Biol. 2016, 37, 4373–4382. [Google Scholar] [CrossRef]
- Lee, WJ.; Cheng, TC.; Yen, Y.; Fang, CL.; Liao, YC.; Kuo, CC. ; Tu, SH; Lin, LC. ; Chang, HW.; Chen, LC.; Ho, YS. "Tea Polyphenol Epigallocatechin-3-Gallate Inhibits Cell Proliferation In a Patient-Derived Triple-Negative Breast Cancer Xenograft Mouse Model Via Inhibition of Proline-Dehydrogenase-Induced Effects. Journal of Food and Drug Analysis. 2021, 29, 1. [Google Scholar] [CrossRef]
- Stearns, ME. ; Amatangelo, M D. ; Varma D.; Sell, C.; Goodyear, S M. Combination therapy with epigallocatechin-3-gallate and doxorubicin in human prostate tumor modeling studies: inhibition of metastatic tumor growth in severe combined immunodeficiency mice. The American journal of pathology. 2010, 177, 6, 3169–3179. [Google Scholar] [CrossRef]
- Luo, K.; Lung, W. ; C.; Luo X.; Huang W. EGCG inhibited bladder cancer T24 and 5637 cell proliferation and migration PI3K/AKT pathway. Oncotarget. 2018; 9: 12261-12272. Retrieved from https://www.oncotarget. 2430. [Google Scholar]
- Chen, L.; Guo, X.; Hu, Y.; Li, L.; Liang, G.; Zhang, G. Epigallocatechin-3-gallate sensitises multidrug resistant oral carcinoma xenografts to vincristine sulfate. FEBS Open Bio. 2020, 107, 1403–1413. [Google Scholar] [CrossRef]
- 30. Zhou, DH.; Wang, X.; Yang, M.; Shi, X.; Huang, W.; Feng, Q. Combination of Low Concentration of Epigallocatechin Gallate (EGCG) and Curcumin Strongly Suppresses the Growth of Non-Small Cell Lung Cancer in vitro and in vivo through Causing Cell Cycle Arrest. International Journal of Molecular Sciences. 2013, 14, 6, 12023–12036. [CrossRef]
- D'Archivio, M.; Santangelo, C.; Scazzocchio, B.; Varì, R.; Filesi, C.; Masella, R.; Giovannini, C. Modulatory Effects of Polyphenols on Apoptosis Induction: Relevance for Cancer Prevention Int J Mol Sci. PMID: 19325744; PMCID: PMC2635670. 2008, 9, 3, 213–228 MDPI AG. [Google Scholar] [CrossRef]
- Ramachandran, B.; Jayavelu, S.; Murhekar, K.; Rajkumar, T. Repeated dose studies with pure Epigallocatechin-3-gallate demonstrated dose and route dependant hepatotoxicity with associated dyslipidemia. Toxicology reports. 2016, 3, 336–345. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Y.; Zhang, Y.; Wan, X.; Li, J.; Liu, K.; Wang, F.; Liu, K.; Liu, Q.; Yang, C.; Yu, P.; Huang, Y.; Wang, S.; Jiang, P.; Qu, Z.; Luan, J.; Duan, H.; Zhang, L.; Hou, A.; Jin, S.; Hsieh, TC.; Wu, E. Anti-cancer activities of tea epigallocatechin-3-gallate in breast cancer patients under radiotherapy. Curr Mol Med. 2012, 12, 2:163–76. [Google Scholar] [CrossRef]
- Mohan, L. Plant-Based Drugs as an Adjuvant to Cancer Chemotherapy. IntechOpen.; 2021. [CrossRef]
- Wang, X.; Jiang, P.; Wang, P.; Yang, CS.; Wang, X.; Feng, Q. EGCG Enhances Cisplatin Sensitivity by Regulating Expression of the Copper and Cisplatin Influx Transporter CTR1 in Ovary Cancer. PLoS ONE. 2015, 10, 4, e0125402. [Google Scholar] [CrossRef]
- Mark E., S.; Min, W. Synergistic Effects of the Green Tea Extract Epigallocatechin-3-gallate and Taxane in Eradication of Malignant Human Prostate Tumors. Translational Oncology, 2011, 4, 3, 147–156, ISSN 1936. [Google Scholar] [CrossRef]
- Wu, H.; Xin, Y.; Xu, C.; Xiao, Y. Capecitabine combined with epigallocatechin-3-gallate inhibits angiogenesis and tumor growth in nude mice with gastric cancer xenografts. Exp Ther Med. 2012, 3, 4, 650–654. [Google Scholar] [CrossRef]
- Wang, L.; Li, P.; Feng, K. EGCG adjuvant chemotherapy: Current status and future perspectives. Eur J Med Chem. 2023, 15; 250:115197. [CrossRef] [PubMed]
- Vayssade, LF.; Laurens, JB.; Jean, CA. Expression of p53-family members and associated target molecules in breast cancer cell lines in response to vincristine treatment, Biochemical Pharmacology. 2002., 63, 9, 1609-1617, ISSN 0006-2952. [CrossRef]
- Zhou, C.; Zhu, Y.; Lu, B.; Zhao, W.; & Zhao, X. Survivin expression modulates the sensitivity of A549 lung cancer cells resistance to vincristine. Oncology Letters. 2018, 16, 5466–5472. [CrossRef]
- Jin, L.; Li, C.; Xu, Y.; Wang, L.; Liu, J.; Wang, D.; Hong, C.; Jiang, Z.; Ma, Y.; Chen, Q.; Yu, F. Epigallocatechin gallate promotes p53 accumulation and activity via the inhibition of MDM2-mediated p53 ubiquitination in human lung cancer cells. Oncol Rep. 2013, 29, 5, 1983–90. [Google Scholar] [CrossRef]
- Zhao, J.; Blayney, A.; Liu, XV.; Gandy, LV.; Jin, WV.; Yan, L.; Ha, JH.; Canning, AJ.; Connelly, M.; Yang, C.; Liu, X.; Xiao, Y.; Cosgrove, MS.; Solmaz, SR.; Zhang, Y.; Ban, D.; Chen, J.; Loh, SN.; Wang, C. EGCG binds intrinsically disordered N-terminal domain of p53 and disrupts p53-MDM2 interaction. Nat Commun. 2021, 12; 12, 1,986. [CrossRef]
- Wang, H.; Guo, M.; Wei, H. et al. Targeting p53 pathways: mechanisms, structures, and advances in therapy. Sig Transduct Target Ther. 2023, 8, 92. [Google Scholar] [CrossRef]
- Alao, JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007, 6, 24. [Google Scholar] [CrossRef]
- Tu, Y.; Cheng, S.; Zhang, S.; Sun, H.; Xu, Z. Vincristine induces cell cycle arrest and apoptosis in SH-SY5Y human neuroblastoma cells. International Journal of Molecular Medicine. 2013, 31, 113–119. [Google Scholar] [CrossRef]
- Zhang, X.; Min, KW.; Wimalasena, J.; Baek, SJ. Cyclin D1 degradation and p21 induction contribute to growth inhibition of colorectal cancer cells induced by epigallocatechin-3-gallate. J Cancer Res Clin Oncol. 2012, 138, 12, 2051–60. [Google Scholar] [CrossRef]
- Srivastava, RK.; Srivastava, AR.; Korsmeyer, SJ.; Nesterova, M. ; Cho, C YS. ; Longo DL. Involvement of microtubules in the regulation of Bcl2 phosphorylation and apoptosis through cyclic AMP-dependent protein kinase. Mol Cell Biol. 1998, 18, 6, 3509–17. [Google Scholar] [CrossRef]
- Wang, J.; Xie, Y.; Feng, Y.; Zhang, L.; Huang, X.; Shen, X.; Luo, X. Epigallocatechingallate induces apoptosis in B lymphoma cells via caspase-dependent pathway and Bcl-2 family protein modulation. Int J Oncol. 2015, 46, 4, 1507–15. [Google Scholar] [CrossRef]
- Olotu, FA.; Agoni, C.; Adeniji, E.; Abdullahi, M.; Soliman, ME. Probing Gallate-Mediated Selectivity and High-Affinity Binding of Epigallocatechin Gallate: a Way-Forward in the Design of Selective Inhibitors for Anti-apoptotic Bcl-2 Proteins. Appl Biochem Biotechnol. 2019, 187, 3,1061–1080. [Google Scholar] [CrossRef]
- Chen, J. The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. Cold Spring Harb Perspect Med. 2016,1; 6, 3, a026104. [CrossRef]
- Orozco,B, A.;Peregrina SJ.; Velázquez MS.; Modelo de linfoma murino L5178Y en fase sólida. Revista de ciencias de la salud. 2017, 4-10: 23-53.
- Miranda, RN. , Briggs, RC., Kinney, MC., Veno, PA., Hammer, RD., Cousar, JB. Immunohistochemical detection of cyclin D1 using optimized conditions is highly specific for mantle cell lymphoma and hairy cell leukemia. Mod Pathol. 2000 13(12):1308-14.
- Rodriguez, CJD.; Fuchs E.; Perez, CC. Evidence of Tau Hyperphosphorylation and Dystrophic Microglia in the Common Marmoset.Frontiers in Aging Neuroscience. 2016, 8 https://www.frontiersin.org/articles/10.3389/fnagi.2016.00315. [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





