1. Introduction
More than 17 million people worldwide experience cardiovascular diseases (CVDs) each year (World Health Organisation [
1,
2]. CVDs have various forms such as ischemic heart disease, cerebrovascular disease, and congestive heart failure [
3]. The top cause of death is ischemic heart disease (IHD) resulting in acute myocardial infarction (AMI or heart attack) [
4]. In Singapore, IHD is the third leading cause of hospitalization and mortality in 2019 [
4].
AMI reflects irreversible damages to the heart muscle resulting from diminished blood supply to the heart [
5]. People experiencing AMI reported severe and diffuse chest pain, which may radiate to arms, jaws and back [
6]. Evidence suggests that stressful life, diabetes, hypertension, hypercholesterolemia, smoking, and alcohol consumption increase the risk of developing AMI [
7]. Patients post-AMI reported lowest quality of life (QoL) after an AMI episode [
8]. Low QoL is linked with the feeling of shock after receiving the AMI diagnosis, fatigue, loss of job, loss of income, and changes in social and family life [
9,
10].
Stress refers to nonspecific physiological or psychological responses of the body to noxious stimuli from environment [
11]. A vicious cycle between stress and AMI is observed. On the one hand, stress may contribute to the development of AMI, medical complications, harmful life style and psychological symptoms, higher mortality risk and hospital admission [
12,
13,
14]. On the other hand, AMI may traumatize the patients, and thus increases their stress. Women reported significantly higher stress post-AMI than male [
15] and post-AMI stress is linked with psychological symptoms including depression and anxiety [
16].
Depression is characterized by persistent depressed mood, anhedonia, changes in body weight, sleep problems, fatigue, poor concentration, feeling of guilt and suicidal ideation [
17]. The risk of suicidal thought was increased during the first month of AMI conditions [
18]. Anxiety is manifested by persistent and excessive worry about various things, restlessness, fatigue, poor concentration, muscle aches and sleep problems [
17]. The prevalence of anxiety is in the range of 24% - 31% among patients post AMI [
16] and anxiety may trigger the next episode of AMI. There is a need to help patients post-AMI manage their stress, depression and anxiety more effectively.
Symptom self-management refers to an individual’s ability to use their cognitive and behavioural efforts to regulate symptoms resulting from AMI (19). To our knowledge, there are limited studies examining symptom self-management interventions for people post-AMI. However, a systematic review suggested that face-to-face psychosocial interventions improved stress and anxiety among people with CVDs [
20]. A randomized controlled trial (RCT) suggested that a face-to-face psychosocial intervention improved anxiety, depression and perceived relaxation on inpatients with IHD in Singapore (21).
Virtual reality (VR) is a set of technologies comprising graphical presentations, sounds, and synthesized forces/sensations that generate a convincing interface where an individual is immersed in an interactive virtual world [
22]. VR has two major components: immersion and presence. The former signifies how the technical aspects (such as visual, audio and tactile sensations) are offered to generate illusions of reality [
22]. The latter reflects a subjective psychological state and indicates how the users are absorbed in the virtual world and respond physically and emotionally to it like being in the actual place [
22].
The first immersive head mounted display for virtual reality (HMD-VR) was invented in 1968 [
23]. The device was later modified and it has become more portable, affordable and desirable [
23]. A scoping review suggested that HMD-VR can be used to deliver interventions for patients with stroke, spinal cord injuries, and congenital limb deficiency [
24]. Another systematic review indicated that HMD-VR were effective in delivering educations for healthcare providers and students [
25]. The HMD-VR appeared to be salient, motivating, and engaging; and non-inferior to a face-to-face traditional method of teaching [
24]. Nevertheless, it is unclear if the non-immersive HMD will produce similar positive effects.
Thus far, there is limited evidence on the effectiveness of face-to-face symptom self-management programs for people post-AMI. Furthermore, it is unknown if non-immersive HMD can be used to deliver self-management programs for people post-AMI. Consequently, we developed a symptom self-management program that can be delivered via non-immersive HMD (IManage-HSet) and traditional, face-to-face method (IManage-FF). Contents of the interventions were adapted from the previous systematic review concerning other psychosocial interventions (19) and pilot RCT conducted in Singapore (21). Both IManage-HSet and IManage-FF had the same education contents. This study aimed to evaluate the two programs on people post-AMI. Research questions are:
In comparison with a control group, will the symptom self-management program delivered via non-immersive HMD (IManage-HSet) enhance participants’ knowledge, perceived relaxation and satisfaction; while reducing objective stress, subjective stress, anxiety and depression?
In relation to the control group, will the symptom self-management program delivered face-to-face (IManage-FF) increase participants’ knowledge, perceived relaxation and satisfaction; while decreasing objective stress, subjective stress, anxiety and depression?
2. Materials and Methods
2.1. Research Design
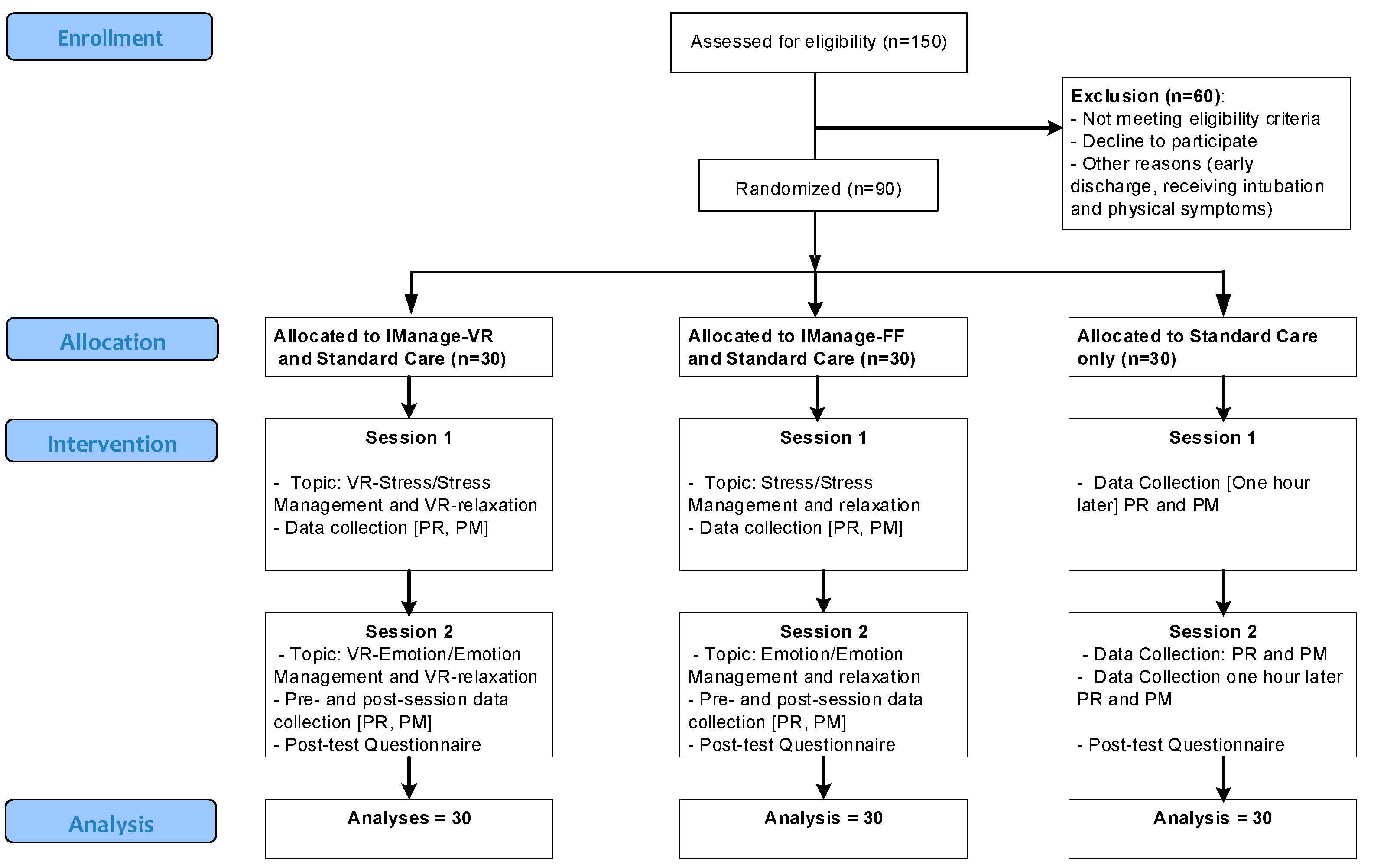
This study was a single-center RCT using three arms in 1:1:1 ratio. Participants were randomly allocated to one of these groups: a) IManage-HSet, b) IManage-FF, or c) control group (
Figure 1). A random allocation list was generated by the principal investigator using a computer generation software. The allocation list was concealed from other researchers until recruited participants completed baseline measurements [
26]. All participants were assigned a unique identification number (ID) sequentially according to order of entry to the study. After the baseline assessment, they were allocated to the group that contained the number that match with their ID. Participants were aware of the group allocation. However, we asked them not to reveal any research information with other patients. All healthcare providers in hospital were blinded to the group allocation. However, research staff were not blinded as they had to coordinated with hospital staff and delivered the interventions.
2.2. Participants
A target population included adults post-AMI who were hospitalized at a cardiac care unit (CCU) in a tertiary hospital in Singapore. A convenience sample was used to recruit potential participants. Eligibility criteria encompassed adult inpatients who: a) aged 21-65 years, b) had a diagnosis of AMI by their physician, and c) were able to communicate in English. Participants would be excluded if they had severe complications such as congestive heart failure, unstable angina, uncontrolled arrythmia, and severe hypertension with blood pressure more than 200/120 mmHg. Those with a confirmed diagnosis of mental disorders would also be excluded as they might affect study outcomes or add more stress to the participants.
Power analysis for repeated measure of variance (RMANOVA) was used to estimate a sample size (27). The following elements were required: correlations among study variables ranging from 0.50 to 0.75, type-I error (α) = 0.05, type II error (β) = 0.2, the number of pre-measurement measures = 7, and the number of post-measurement measures = 7. Taken all together, an adequate sample size for each group would be at least 20 (27). However, to account for possible attrition, 30 participants would be recruited for each group, making a total sample size of 90.
1.3. Intervention
Participants in the intervention groups received standard care plus IManage-HSet or IManage-FF programs. The control group received only standard care.
1.3.1. The IManage-Hset Program
The IManage-HSet program entailed two one-hour individual-based symptom self-management sessions delivered on two consecutive days (
Table 1). This program, delivered via a headset device, comprised an educational component and practice of relaxation techniques. Participants sat or lied down in a comfortable position while wearing a headset SONY HMZ-T2 (28).
The SONY HMZ-T2 is a light device with an adjustable headband with attaching earbuds. With this headset, a user can watch 3D movies with a big-screen cinematic experience, play 3D games and hear sound effects produced by the virtual-phone technology (28). Although a non-interactive mode was used in our study, this headset offered a high definition screen (1280 x 720) and allowed vivid video experiences with virtual surround sound (26). It was also expected that participants would engage in pleasant learning experiences without environmental distractions.
During the Stress and Stress Management session (session 1), participants viewed an education video, Stress Demolishing Room, via the headset. To make the education more interesting, the video was presented as a story, where a student nurse spoke to a viewer and described information regarding AMI, its treatments, stress and available strategies to manage stress. Case scenarios, stories, images and video clips were also presented. Subsequently, the participants viewed two relaxation videos (A fantastic journey to relaxation Track a and b) to guide their practice on abdominal breathing and muscle relaxation. The abdominal breathing track contained beach-theme images while the muscle relaxation track showed meadow, mountain and rain forest images. A soothing female narrator was also presented with calming music.
During the Emotion and Emotion Management education (session 2), participants watched an education video,
Emotional Wellness Room. This video was presented in a scenario where a nurse and a patient with post-AMI having a conversation about emotions, emotional problems (anxiety and depression) and possible strategies to manage the emotional problems. Case scenarios, images and video clips were also used to maximize the learning process. Subsequently, participant watched three short videos (via the headset) to guide relaxation practice. The videos encompassed abdominal breathing, a fantastic trip to Japan (images concerning vibrant flowers and colourful gardens), and a relaxation trip to Bhutan: A land of happiness (images about nature, mountain landscape, and river valley). (Supplementary file:
Appendix A, B).
2.3.2. The IManage-FF Program
The IManage-FF Program is a two one-hour individual-based symptom self-management intervention delivered on two consecutive days. It contained two major components; education and the practice of relaxation techniques (
Table 2). However, this program was delivered using a conventional face-to-face method. The first session delivered knowledge concerning stress and stress management. Then, participants practiced abdominal breathing and muscle relaxation guided by audiotape instruction (relaxation CD). The second session covered emotion and emotion management. Similar to session 1, participants practiced abdominal breathing and muscle relaxations using relaxation CD.
Both IManage-HSet and IManage-FF interventions were delivered at a private room in CCU by two researchers (a student nurse and registered nurse). Before delivering the interventions, both researchers received two training sessions conducted by the first author to ensure the standardizations of the intervention procedures. Participants in both groups received an education booklet (containing knowledge imparted during the programs). This booklet might help maximize outcomes as participants would be able to review materials at their convenience.
2.3.3. Standard Care
Standard care included usual services provided by the study hospital. All patients in CCU received a single-session, individual-based, one-hour patient education about AMI and management of AMI. Clinical staff at CCU delivered the patient education and they were blinded to participants’ group allocation.
2.4. Measures
2.4.1. Objective Stress
Objective stress was measured by bio-physiological instruments. Heart rate was assessed in beats/min and blood pressure was measured in mmHg. Skin temperature was measured by Stress Thermometer TM #SC911 [
29], in which lower skin temperature reflects a higher level of objective stress. An electrode of the stress thermometer was placed at participant’s fingertip for one minute. Validity of the stress thermometer was established among inpatients in Singapore [
30].
2.4.2. Subjective Stress, Anxiety, and Depression
Subjective stress, anxiety, and depression were measured with corresponding subscales of the Depression, Anxiety, and Stress scale [
31]. Each subscale has seven items with four response categories ranging from 0 (Did not apply to me at all) to 3 (Applied to me most of the time). Possible scores of each subscale are in the range of 0 - 21 with higher scores indicate higher stress (anxiety or depression) levels. Cronbach’s alphas of the stress (0.86 – 0.90), anxiety subscale (0.86-0.90), and depression (0.82 - 0.90) are in acceptable ranges [
32].
2.4.3. Perceived Relaxation
Perceived relaxation was measured by the perceived relaxation scale (PRC) [
33]. Participants rated their relaxation level on a single-item Numeric Rating Scale: a 10-centrimeter continuum line starting from “0” (very tensed) to “10” (very relaxed). Validity of the PRC was established in previous research on people with CVD in Singapore [
33].
2.4.4. Knowledge
Knowledge was measured by the 6-item IManage knowledge scale (IKS) developed by the researchers. Two set of items (three items each) were determined by contents thoughts in the IManage programs. The first set tested knowledge concerning stress and stress management strategies and it was administered before and after the intervention session one. Another set, addressing knowledge about emotion and emotion management, was administered before and after the intervention session two. Respondents answered 1 (True) or 0 (False) and a total score would add to 3 for each set of items.
2.4.5. Patient Satisfaction
Patient satisfaction was measured by the 4-item satisfaction with the IManage program scale (SAT-IM) developed by the researchers. The scale captures how respondents were satisfied with the overall programs, contents, education materials and relaxation practice. They responded on five categories from 1 (Very dissatisfied) to 5 (Very satisfied) and they also provided qualitative feedback for the programs.
2.5. Statistical Analysis
We entered all research data into IBM SPSS Statistics 26.0 and then verified the accuracy of data entry by two researchers. The intent-to-treat principles were used to guide the data analyses. Descriptive statistics were used to analyse patient satisfaction and content analyses were used to summarize qualitative data. Analysis of variance (ANOVA) and RMANOVA were used to compare study variables. Partial eta square (η
p2) was used to determine an effect size of the interventions. The value of η
p2 0.01, 0.06, and 0.14 would be interpreted as small, medium and large effect size respectively [
34].
2.6. Ethical Considerations
This research received ethical approval from the Domain Specific Review Board of the study hospital (Ref: 00801, RCB No: 200002150H). The researchers approached eligible patients at their bedside, provided details of this research, distributed patient information sheet (PIS), answered questions that they might have and requested their participation. The researchers also emphasized that participation in this study would be voluntary and their information would be kept strictly confidential. All interested participants were asked to sign a written consent form before partaking in this study.
3. Results
3.1. Participant Characteristics
In total, 150 inpatients were assessed for eligibility, 90 of which fulfilled the inclusion criteria and were randomly assigned to the three groups, 30 each (
Figure 1). Participants had an average age of 51 years (SD=8.68). They were predominantly male, Singaporean citizen, and married (
Table 3). A significant difference in marital status was observed (χ
2= 12.77, p < 0.01). Nevertheless, there were no significant differences in other characteristics across the three groups.
3.2. Efficacy of the IManage Interventions
3.2.1. Knowledge
Knowledge was measured twice at the end of intervention session 1 and 2 (
Table 4). Knowledge scores concerning stress and stress management (session 1) for the IManage-HSet (Mean=3.00, SD=0.00) and IManage-FF (Mean=2.90, SD=0.40) were significantly higher than those in the control group (Mean=1.90, SD=0.55) with a large effect size (η
p2=0.62). Furthermore, knowledge scores regarding emotion and emotion management (session 2) for the IManage-HSet (Mean=3.00, SD=0.00) and IManage-FF (Mean=3.00, SD=0.00) were significantly higher than those in the control group (Mean=0.67, SD=1.12) with a large effect size (η
p2=0.75).
3.2.2. Subjective Stress, Depression and Anxiety
Subjective stress, depression and anxiety were measured twice at baseline and the end of intervention session 2. The IManage-HSet group had higher changed scores on all three variables than the IManage-FF and control groups (
Table 4). Nonetheless, the changes did not achieve statistical significance. Effect sizes were small for subjective stress (η
p2=0.04), depression (Partial η
2=0.01), and anxiety (η
p2=0.03).
3.2.3. Objective Stress
To capture immediate changes, all measures of objective stress were measured before and after each intervention session, making a total of 4 assessment points (
Table 5). At session 1, changed scores in skin temperature for the IManage-HSet (changed scores=0.43, SD=1.48, p=.01) and IManage-FF group (changed scores=0.57, SD=1.59, p=.00) were significantly higher than that of the control group (changed scores=-0.99, SD=2.18) with a strong effect size (η
p2= 0.14). These finding suggested that both intervention groups had lower objective stress than the control.
At session 2, changed scores for the IManage-HSet (changed scores=0.55, SD=1.39, p=.76) and IManage-FF groups (changed scores=1.29, SD=2.14, p=.07) were higher than that of the control group (changed scores=0.21, SD=1.69) with a medium effect size (ηp2=0.06). However, there were no significant differences in other measures of objective stress including heart rate, systolic BP, and diastolic BP across the three groups.
3.2.4. Perceived Relaxation
To capture immediate changes, perceived relaxation was assessed before and after each intervention session, adding to 4 assessment points (
Table 5). At session 1, only a changed score of the IManage-HSet (changed scores=6.10, SD=3.64, p=.01) had significantly higher than that of the control group (changed scores=3.00, SD=3.85) with a medium effect size (η
p2=0.11). At session 2; however, changed scores of the IManage-HSet (changed scores=5.65, SD=3.78, p=.02) and IManage-FF group (changed scores=5.52, SD=4.20 p=.02) had significantly higher than that of the control group (changed scores=2.63, SD=3.76) with a medium effect size (η
p2=0.12).
3.2.5. Patient Satisfaction
All respondents (n=30) were either satisfied or very satisfied with the overall IManage-HSet program, its contents, educational materials and relaxation practice. Respondents provided positive comments on the use of headset and videos for patient education, which was more effective than a face-to-face approach (
Table 6). The program contents were perceived to be very good, clear, effective, understandable, straightforward, helpful, and sufficient. Furthermore, the relaxation instructions were clear, easy, and helpful; and the practice induced focus, relaxation, happiness, and good feelings.
Similarly, those who attended the IManage-FF program (n=30) were either satisfied or very satisfied with the overall program, contents, educational materials and relaxation practice (using audio-guided instructions) (
Table 7). Respondents commented that the program was clear, useful, helpful, understandable, and informative. The relaxation audios helped patients to relax, feel calm and think positive.
Table 5.
Comparisons of changed scores of study variables.
Table 5.
Comparisons of changed scores of study variables.
| Variables |
Session 1
Pre-test |
Session 1
Post-test |
Changed
score |
Partial eta2
|
Session 2
Pre-test |
Session 2
Post-test |
Changed
score |
Partial eta2
|
| Mean |
SD |
Mean |
SD |
Mean |
SD |
|
Mean |
SD |
Mean |
SD |
Mean |
SD |
|
| Perceived Relaxation |
|
|
|
|
|
|
0.11d |
|
|
|
|
|
|
0.12d |
| - Control |
3.53 |
3.94 |
6.53 |
3.47 |
3.00 |
3.85 |
- |
3.70 |
4.13 |
6.33 |
3.93 |
2.63 |
3.76 |
- |
| - IManage-FF |
3.77 |
4.19 |
9.10 |
1.27 |
5.33 |
4.23 |
.08 |
4.07 |
4.25 |
9.58 |
0.67 |
5.52 |
4.20 |
.02**
|
| - IManage-HSet |
3.03 |
3.62 |
9.13 |
1.07 |
6.10 |
3.64 |
.01**
|
4.00 |
3.88 |
9.65 |
0.73 |
5.65 |
3.78 |
.02**
|
| Skin Temperature |
|
|
|
|
|
|
0.14d |
|
|
|
|
|
|
0.06d |
| - Control |
28.89 |
3.75 |
27.90 |
3.22 |
-0.99 |
2.18 |
- |
28.50 |
3.18 |
28.71 |
3.46 |
0.21 |
1.69 |
- |
| - IManage-FF |
29.64 |
3.39 |
30.22 |
3.19 |
0.57 |
1.59 |
.00**
|
28.83 |
3.94 |
30.12 |
3.54 |
1.29 |
2.14 |
.07 |
| - IManage-HSet |
29.23 |
3.59 |
29.66 |
3.24 |
0.43 |
1.48 |
.01**
|
29.64 |
3.50 |
30.19 |
3.24 |
0.55 |
1.39 |
.76 |
| Heart Rate |
|
|
|
|
|
|
0.06d |
|
|
|
|
|
|
0.06d |
| - Control |
75.83 |
11.14 |
76.13 |
9.90 |
-0.30 |
4.93 |
- |
73.97 |
9.60 |
75.13 |
8.32 |
-1.17 |
3.58 |
- |
| - IManage-FF |
74.40 |
9.98 |
74.47 |
8.62 |
-0.07 |
5.83 |
.99 |
74.00 |
9.15 |
73.47 |
7.54 |
0.53 |
3.81 |
.26 |
| - IManage-HSet |
77.13 |
12.88 |
74.50 |
10.77 |
2.63 |
5.36 |
.11 |
75.77 |
11.48 |
74.43 |
10.77 |
1.33 |
4.50 |
.06 |
| Systolic BP |
|
|
|
|
|
|
0.01d |
|
|
|
|
|
|
0.03d |
| - Control |
117.83 |
20.75 |
118.53 |
18.95 |
-0.70 |
5.85 |
- |
115.77 |
14.20 |
116.20 |
13.39 |
-0.43 |
12.60 |
- |
| - IManage-FF |
115.93 |
18.45 |
114.57 |
15.66 |
1.37 |
9.83 |
.68 |
118.93 |
15.12 |
115.97 |
13.75 |
2.97 |
5.60 |
.34 |
| - IManage-HSet |
117.47 |
18.99 |
117.43 |
20.21 |
0.03 |
10.64 |
.95 |
113.17 |
14.14 |
112.83 |
13.39 |
0.33 |
6.90 |
.95 |
| Diastolic BP |
|
|
|
|
|
|
0.03d |
|
|
|
|
|
|
0.00d |
| - Control |
73.33 |
12.86 |
74.33 |
12.47 |
-1.00 |
5.48 |
- |
73.67 |
8.27 |
72.80 |
8.78 |
0.87 |
7.07 |
- |
| - IManage-FF |
72.93 |
9.36 |
73.37 |
9.46 |
-0.43 |
8.13 |
.99 |
73.40 |
8.32 |
72.50 |
7.48 |
0.90 |
6.14 |
.99 |
| - IManage-HSet |
77.40 |
19.46 |
73.70 |
13.72 |
3.70 |
20.60 |
.39 |
70.97 |
9.83 |
69.80 |
11.26 |
1.17 |
6.07 |
.98 |
Table 6.
Satisfaction with the IManage-HSet programme (n=30).
Table 6.
Satisfaction with the IManage-HSet programme (n=30).
| |
Very
Dissatisfied
n (%) |
Dissatisfied
n (%) |
Neutral
n (%) |
Satisfied
n (%) |
Very
Satisfied
n (%) |
Additional comments
|
| 1. Overall, how satisfied are you with the IManage-HSet programme? |
0 |
0 |
0 |
25 (83.3) |
5(16.7) |
“very good”
“Not bad”
“Not so lengthy”
“There must be buddy in guiding you” |
| 2. How satisfied are you with the content of the programme? |
0 |
0 |
0 |
25 (83.3) |
5(16.7) |
“Good and clear explanation” (n=4)
“Presentation & explanation are very effective to the patient”
“Straight to point and clear explanation”
“Contents of the videos and booklets are sufficient”
“Helpful, the contents are generally sufficient.” |
| 3. How satisfied are you with the educational materials? |
0 |
0 |
0 |
26(86.7) |
4(13.3) |
“Nice gadget”
“Different method (attractive)”
“Interesting mode of education”
“Interesting from the other mode of education”
“More interesting than usual face to face education”
“Understand more by watching video”
“Clear explanation with visual presentations, interesting”
“Audio very relaxing” |
| 4. How satisfied are you with the relaxation practice? |
0 |
0 |
0 |
26(86.7) |
4(13.3) |
“Easy techniques for relaxation” (n=2)
“Very relaxing: Help to relax” (n=3)
“Help the patient to focus”
“Make me so happy and have a good body”
“Clearer instructions on how to breathe and use techniques” |
Table 7.
Satisfaction with the IManage-FF programme (n=30).
Table 7.
Satisfaction with the IManage-FF programme (n=30).
| |
Very
Dissatisfied
n (%) |
Dissatisfied
n (%) |
Neutral
n (%) |
Satisfied
n (%) |
Very
Satisfied
n (%) |
Additional comments
|
| 1. Overall, how satisfied are you with the IManage-FF programme? |
0 |
0 |
0 |
27 (90.0) |
3(10.0) |
“Good approach” “Good techniques”
“Clear explanation easy to understand”
“Not so lengthy, session period just nice”
“It is useful and helpful”
“Practical interventions” |
| 2. How satisfied are you with the content of the programme? |
0 |
0 |
1(3.3) |
25 (83.3) |
4(13.3) |
“Education on stress management”
“It helps us to understand and focus on actions to take to overcome our stress/depression/anxiety”
“I am able to understand the education”
“More knowledgeable to stress, relax, and depression”
“Very good for me, study a lot on things good on my heart”
“Very informative” |
| 3. How satisfied are you with the educational materials? |
0 |
0 |
2(6.7) |
25(83.3) |
4(13.3) |
“Contents of the booklet are enough and understandable” techniques are easy to follow”
“Good explanation; relaxing audio”
“Good explanation; easy to understand. I can refer to the booklet” |
| 4. How satisfied are you with the relaxation practice? |
0 |
0 |
0 |
24(80.0) |
6(20.0) |
“Clear explanation on the relaxation techniques”
“Easy to understand the relaxion audios”
“Gives time to relax” “So relaxing”
“Help us relax and think positive”
“Made me feel calm”
“Tell people how to make themselves into a relaxed body, not having stress”
“The relaxation techniques were very useful.” |
4. Discussion
This RCT evaluated the efficacy of two symptoms self-management programs on inpatients post-AMI in Singapore. Findings suggested that respondents in the IManage-HSet group had improvement in knowledge (large effect size), perceived relaxation (large effect size) and periphery skin temperature (objective stress, medium effect size) than those of the control group. Similarly, the IManage-FF group demonstrated improvement in knowledge score (large effect size), perceive relaxation (large effect size) and skin temperature (medium effect size). Nearly all participants were satisfied or very satisfied with both programs and positive comments were provided.
Stress increases the risk of AMI, aggravates AMI-related complications, leads to poorer clinical outcomes and increases mortality risks at post discharge (12, 13). Acute stress activates sympathetic nervous responses and hypothalamus-pituitary-adrenal axis (HPA axis) by triggering the secretion of stress-related hormones such as catecholamines and glucocorticoids (35). People under stress would exhibit higher heart rate, blood pressure, respiration, and periphery vasoconstriction, leading to a rapid drop in skin temperature (36). On contrary, a relaxation state generates lower heart rate, blood pressure and respiration; but higher skin temperature (periphery vasodilation) (35, 36). In our study, it may be logical to conclude that the IManage-HSet and I-Manage-FF programs helped minimize objective stress. Similarly, a previous feasibility study revealed that the eight-week virtual world program in Second Life mitigated perceived stress among healthy Americans (38).
Relaxation response reflects a biophysiological state that counteracts the stress responses and Relaxation is found to mitigate stress-related illness [
38]. Patients in our study revealed improvement in perceived relaxation after attending both symptom self-management interventions. Relaxation audios used in the IManage-FF program might induce relaxation responses among the respondents. For the IManage-HSet program, the use of the non-immersive headset might enhance the experiences of relaxations. Respondents practiced abdominal breathing, muscle relaxation and total body relaxation. The headset is perceived to generate deeper and faster relaxation as it concurrently offers visual presentations (e.g., peaceful and pleasant sceneries) and audios (e.g., music and voice instruction). Moreover, the headset might reduce environmental distractions. Similarly, a previous RCT reported that immersive VR experiences, DVD and audio tapes produced significant improvement in relaxation and skin conductance parameters [
36,
37].
At the end of each intervention sessions, participants had significantly higher knowledge and satisfaction levels than controls with a large effect size. For the IManage-FF, the intervention facilitator delivered knowledge concerning stress and emotion management; and assisted with the relaxation practice. Such interactive individual sessions might enhance the learning process and satisfaction. Moreover, participants in the IManage-HSet viewed educational videos (stress demolishing room and emotion wellness room) through the headset. They felt that the device was an attractive gadget and more interesting mode of patient education. The videos contained useful information, case scenarios, stories, colourful images, examples of activities (such as exercise and healthy life styles) and interactive conversations among actors, which might enhance learning outcomes. Participants also viewed relaxation videos (A Fantastic Journey to Relaxation) via the SONY headset and concurrently practiced relaxation. An education booklet was distributed to all respondents so that they could reveal the learned information at their convenience. An intervention facilitator was also presented to help respondents used the headset, ensure their comfort levels, provide emotional support, answer questions regarding education videos, and assisted with relaxation practice.
In comparison to controls, both interventions had higher changed scores on subjective stress, depression, anxiety and other measures of objective stress (heart rate and blood pressure) with small effect sizes. Nevertheless, such scores did not achieve statistical significance. A possible explanation could be that the IManage-HSet and IManage-FF programs might have an immediate effect on skin temperature (objective stress) but a delayed effect on subjective stress. Hence, it might take longer durations and more sessions to induce significant changes on subjective stress. Another possible explanation could be that the contents of interventions did not directly improve subjective stress, anxiety and depression. Furthermore, scores of blood pressure and heart rate were already in a normal range for participants across three groups. Therefore, there was not much room to improve the scores. Similarly, a previous RCT found that a virtual reality-based cardiac rehabilitation program did not improve subjective stress, anxiety and depression among people with coronary artery disease [
39]. The researchers concluded that the relationships between depression and cardiovascular diseases are complex and more research is required to explore such relationships.
4.1. Limitations
Strengths of this research lied on the use of RCT to examine the effects of two interventions and the computation of sample size through power analysis. However, there are some limitations and the first one is the lack of a follow-up assessment. Secondly, the cardiac care unit used a centralized air conditioning. Temperature in the patient rooms was low and could not be manually adjusted. Such low temperature might affect the accuracy of skin temperature scores. Thirdly, due to limited manpower, we were unable to blind the outcome assessor, which might increase the risk of detection bias. However, this study also included physiological measures, which produced objective data and might help minimize the bias. Fourthly, neither researchers and participants were blinded due to the nature of interventions, which might lead to performance bias. Finally, nearly all participants were male and the generalizability of findings might be limited.
4.2. Future Directions
This study has implications to future research. More RCTs with a larger sample size and multi-site arrangements may be carried out to test both IManage-HSet and IManage-FF programs. Booster sessions of the programs may be developed and offered to patients at post-hospitalization. Several objective data (such as salivary cortisol, salivary alpha amylase, periphery skin temperature, blood pressure and heart rate) and subjective data (such as subjective stress, self-efficacy, and motivation to change) may be collected. Follow-up assessments should be utilized to measure long-term effects of the interventions. A qualitative approach may be added to elicit in-depth information concerning participants’ experiences of the program.
5. Conclusions
This is the first study to examine the symptom self-management programs on patients post-AMI at a tertiary hospital in Singapore. Apparently, it is feasible to conduct the two-session interventions at the cardiac care unit (CCU) in the study hospital. Findings suggested that the program delivered via the SONY headset (IManage-HSet) and traditional face-to-face approach (IManage-FF) had the potential to improve objective stress, perceived relaxations, knowledge and satisfaction.
Author Contributions
P.K.Y. made a substantial contribution to the concept and design of this RCT, analyse and interpret data, draft and finalised the manuscript. K.W.L., A.A. and P.C. involved in planning the RCT, recruiting participants and approving the manuscript. Y.L. planning the RCT, interpret the findings and approving the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Ministry of Health, Health Service Research New Investigator Grant, Singapore [Grant NO. HSRNIG12NOV005].
Institutional Review Board Statement
This research received ethical approval from the Domain Specific Review Board of the study hospital (Ref: 00801, RCB No: 200002150H.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article or Supplementary Materials.
Public Involvement Statement: No public involvement in any aspect of this research.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
CONSORT 2010 checklist of information to include when reporting a randomised trial*
| Section/Topic |
Item No |
Checklist item |
Reported on page No |
| Title and abstract |
| |
1a |
Identification as a randomised trial in the title |
1 |
| 1b |
Structured summary of trial design, methods, results, and conclusions (for specific guidance see CONSORT for abstracts) |
1 |
| Introduction |
| Background and objectives |
2a |
Scientific background and explanation of rationale |
2-3 |
| 2b |
Specific objectives or hypotheses |
3 |
| Methods |
| Trial design |
3a |
Description of trial design (such as parallel, factorial) including allocation ratio |
3 |
| 3b |
Important changes to methods after trial commencement (such as eligibility criteria), with reasons |
n/a |
| Participants |
4a |
Eligibility criteria for participants |
3 |
| 4b |
Settings and locations where the data were collected |
3 |
| Interventions |
5 |
The interventions for each group with sufficient details to allow replication, including how and when they were actually administered |
4-6 |
| Outcomes |
6a |
Completely defined pre-specified primary and secondary outcome measures, including how and when they were assessed |
6 |
| 6b |
Any changes to trial outcomes after the trial commenced, with reasons |
n/a |
| Sample size |
7a |
How sample size was determined |
3 |
| 7b |
When applicable, explanation of any interim analyses and stopping guidelines |
n/a |
| Randomisation: |
|
|
|
| Sequence generation |
8a |
Method used to generate the random allocation sequence |
3 |
| 8b |
Type of randomisation; details of any restriction (such as blocking and block size) |
3 |
| Allocation concealment mechanism |
9 |
Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned |
3 |
| Implementation |
10 |
Who generated the random allocation sequence, who enrolled participants, and who assigned participants to interventions |
3 |
| Blinding |
11a |
If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how |
3 |
| 11b |
If relevant, description of the similarity of interventions |
5-6 |
| Statistical methods |
12a |
Statistical methods used to compare groups for primary and secondary outcomes |
6 |
| 12b |
Methods for additional analyses, such as subgroup analyses and adjusted analyses |
n/a |
| Results |
| Participant flow (a diagram is strongly recommended) |
13a |
For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analysed for the primary outcome |
7, Figure 1
|
| 13b |
For each group, losses and exclusions after randomisation, together with reasons |
7 |
| Recruitment |
14a |
Dates defining the periods of recruitment and follow-up |
n/a |
| 14b |
Why the trial ended or was stopped |
n/a |
| Baseline data |
15 |
A table showing baseline demographic and clinical characteristics for each group |
7, Table 3
|
| Numbers analysed |
16 |
For each group, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups |
Table 4-5 |
| Outcomes and estimation |
17a |
For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% confidence interval) |
Table 4-5 |
| 17b |
For binary outcomes, presentation of both absolute and relative effect sizes is recommended |
n/a |
| Ancillary analyses |
18 |
Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing pre-specified from exploratory |
n/a |
| Harms |
19 |
All important harms or unintended effects in each group (for specific guidance see CONSORT for harms) |
n/a |
| Discussion |
| Limitations |
20 |
Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses |
16 |
| Generalisability |
21 |
Generalisability (external validity, applicability) of the trial findings |
16 |
| Interpretation |
22 |
Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence |
16-17 |
| Other information |
|
| Registration |
23 |
Registration number and name of trial registry |
2 |
| Protocol |
24 |
Where the full trial protocol can be accessed, if available |
2 |
| Funding |
25 |
Sources of funding and other support (such as supply of drugs), role of funders |
18 |
References
- World Health Organisation. Cardiovascular diseases. http://www.who. int/mediacentre/factsheets/fs317/en/index.html. (2020, accessed 1 July 2020).
- World Health Organization. Global health estimates: deaths by cause, age, sex and country, 2000–2012. http://www.who.int/healthinfo/global_burden_disease/en/ (2014, accessed 1 July 2020).
- World Health Organization. World Health Organization Heart: Technical package for cardiovascular disease management in primary health care. Switzerland: World Health Organization; 2016.
- Health Promotion Board. Singapore myocardial infarction registry annual report 2018. https://www.nrdo.gov.sg/docs/librariesprovider3/default-document-library/smir-web-report-2018.pdf?sfvrsn=ccde799a (2020, accessed 12 July 2020).
- Mechanic OJ, Grossman SA. Acute Myocardial Infarction. In: StatPearls [Internet]. Treasure Island (FL): StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK459269/ (2020, accessed 12 August 2020).
- Panday R, Gupta N, Wander G. Diagnosis of acute myocardial infarction. J Assoc Physician India 2015; 59, 8-13.
- Bahall M, Seemungal, T, Legall, G. Risk factors for first-time acute myocardial infarction patients in Trinidad. BMC public health 2018; 18(1), 161. [CrossRef]
- Bahall M, Khan, K. Quality of life of patients with first-time AMI: a descriptive study. Health Qual Life Outcomes 2018, 16(1), 32. [CrossRef]
- Alsén P, Brink E. Fatigue after myocardial infarction - a two-year follow-up study. J Cli Nurs 2013, 22(11-12), 1647-1652. [CrossRef]
- Dreyer RP, Xu X, Zhang W, Du, X, Strait KM, Bierlein M. Return to work after acute myocardial infarction: comparison between young women and men. Circ Cardiovasc Qual Outcomes 2016, 9, S45–52. [CrossRef]
- Selye H. The stress concept: Past, present, and future. In C. L. Cooper (Eds.), Stress research: Issues for the eighties. New York: John Wiley & sons; 1983.
- Arnold SV, Smolderen KG, Buchanan DM, Li Y, Spertus JA. Perceived stress in myocardial infarction: long-term mortality and health status outcomes. J Am Coll Cardiol 2012, 60(18), 1756-1763. [CrossRef]
- Shah SJ, Krumholz HM, Reid KJ, Rathore SS, Mandawat A, Spertus JA, Ross JS. Financial stress and outcomes after acute myocardial infarction. PLoS One 2012, 7(10), e47420. [CrossRef]
- Chan C, Elliott J, Troughton R, Frampton C, Smyth D, Crozier I, Bridgman P. Acute myocardial infarction and stress cardiomyopathy following the Christchurch earthquakes. PLoS One 2013, 8(7), e68504. [CrossRef]
- Xu X, Bao H, Strait KM, Edmondson DE, Davidson KW, Beltrame JF, Krumholz, HM. Perceived stress after acute myocardial infarction: A comparison between young and middle-aged women versus Men. Psychosom Med 2017, 79(1), 50-58. [CrossRef]
- Kumar M, Nayak PK. Psychological sequelae of myocardial infarction. Biomed Pharmacother 2017, 95, 487-496. [CrossRef]
- American Psychiatric Association. Anxiety disorders. In Diagnostic and statistical manual of mental disorders (5th ed.). [CrossRef]
- Klarsen KK, Agerbo E, Christensen B, Søndergaard J, Vestergaard M. Myocardial infarction and risk of suicide a population-based case-control study. Circulation 2010, 12(23), 2388–2393. [CrossRef]
- Klainin-Yobas, P., Koh, K. W. L., Ambhore, A., Chai, P., Chan, S. W. C., &. He, H.G. A study protocol of a randomised controlled trial examining the efficacy of a symptom self-management programme for people with acute myocardial infarction. Journal of Advanced Nursing 2015, 71(6), 1299-1309. [CrossRef]
- Klainin-Yobas, P., Ng S., Stephen, P. D. M., & Lau, Y. Efficacy of psychosocial interventions on psychological outcomes among people with cardiovascular diseases: A systematic review and meta-analyses. Patient Education and Counselling 2016, 99(4), 512-521. http://doi.org/10.1016/j.pec.2015.10.020.
- Ramos, P. Testing the efficacy of the psychosocial intervention with standard care on inpatients with ischemic heart disease in Singapore: A pilot randomized controlled trial. Unpublished Honours Thesis, Alice Lee Centre for Nursing Studies, Young Loo Lin School of Medicine, National University of Singapore, 2013.
- Bohil C, Owen C, Jeong E, Alicea B, Biocca, F. Virtual Reality and Presence. https://www.researchgate.net/publication/263619590. (2009, accessed 1 August 2020).
- Sherman, W. R., & Craig, A. B. Understanding virtual reality: Interface, application, and design. Burlington, MA: Morgan Kaufmann, 2002.
- Saldana D, Neureither M, Schmiesing A, et al. Applications of Head-Mounted Displays for Virtual Reality in Adult Physical Rehabilitation: A Scoping Review. Am J Occup Ther. 2020, 74(5), 1-15. [CrossRef]
- Barteit S, Lanfermann L, Bärnighausen T, Neuhann F, Beiersmann C Augmented, Mixed, and Virtual Reality-Based Head-Mounted Devices for Medical Education: Systematic Review. JMIR Serious Games 2021, 9(3), e29080 URL: https://games.jmir.org/2021/3/e29080. [CrossRef]
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, Altman DG CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c869. [CrossRef]
- Frison L, Pocock SJ. Repeated measures in clinical trials: analysis using mean summary statistics and its implications for design. Stat Med 1992, 11(13), 1685–1704. [CrossRef]
- Sony Corporation. SONY Head mounted display: Reference guide. https://www.sony.com/electronics/support/res/manuals/4439/44390203M.pdf (2012, accessed 23 September 2020).
- Stress Market. Stress Thermometer SC 911. http://www.cliving.org/SC911/SC911instr.pdf (2011, accessed 23 September 2020).
- Klainin-Yobas P, Ignacio J, Wong PHH, La Y, He HG, Ngooi B, Koh SQD. Effects of the stress management (S-Manage) program for inpatients with mental disorders: A feasibility study. Biol Res Nurs 2016, 18(2), 213-220.
- Lovibond SH, Lovibond PF. Manual for the Depression Anxiety Stress Scales (2nd ed.). Sydney: Psychology Foundation; 1995.
- Mahmoud JSR, Hall LA, Staten R. The psychometric properties of the 21-Item Depression Anxiety and Stress Scale (DASS-21) among a sample of Pritchard, M. (2009). Managing anxiety in the elective surgical patient. Br J Nurs 2010, 18(7), 416-419. [CrossRef]
- Lee, S. Y. Developing a stress management intervention for adult patients with hypertension and ischemic heart disease: A pilot study. Unpublished Honours Thesis, Alice Lee Centre for Nursing Studies, National University of Singapore; 2010.
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates, Publishers; 1988.
- Halm MA. Relaxation: a self-care healing modality reduces harmful effects of anxiety. Am J Crit Care 2009, 18(2), 169-172. [CrossRef]
- Herborn KA, Graves JL, Jerem P, Evans NP, Nager R, McCafferty DJ, McKeegan DE. Skin temperature reveals the intensity of acute stress. Physiol Behav 2015, 152(Pt A), 225-230. [CrossRef]
- Villani D, Riva G. Presence and relaxation: A preliminary controlled study. PsychNology J 2008, 6(1), 7-26.
- Hoch DB, Watson AJ, Linton DA, Bello HE, Senelly M, Milik MT, .Kvedar JC. The feasibility and impact of delivering a mind-body intervention in a virtual world. PLoS One 2012; 7(3), e33843. [CrossRef]
- Vieira A, Melo MCDA, Noites SPARS, Machado J, Gabriel MMJ. The effect of virtual reality on a home-based cardiac rehabilitation program on body composition, lipid profile and eating patterns: A randomized controlled trial. Eur J Integr Med 2017, 9, 69-78. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).