Submitted:
28 August 2023
Posted:
30 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. The target hosts
2.2. Diagnostic procedures
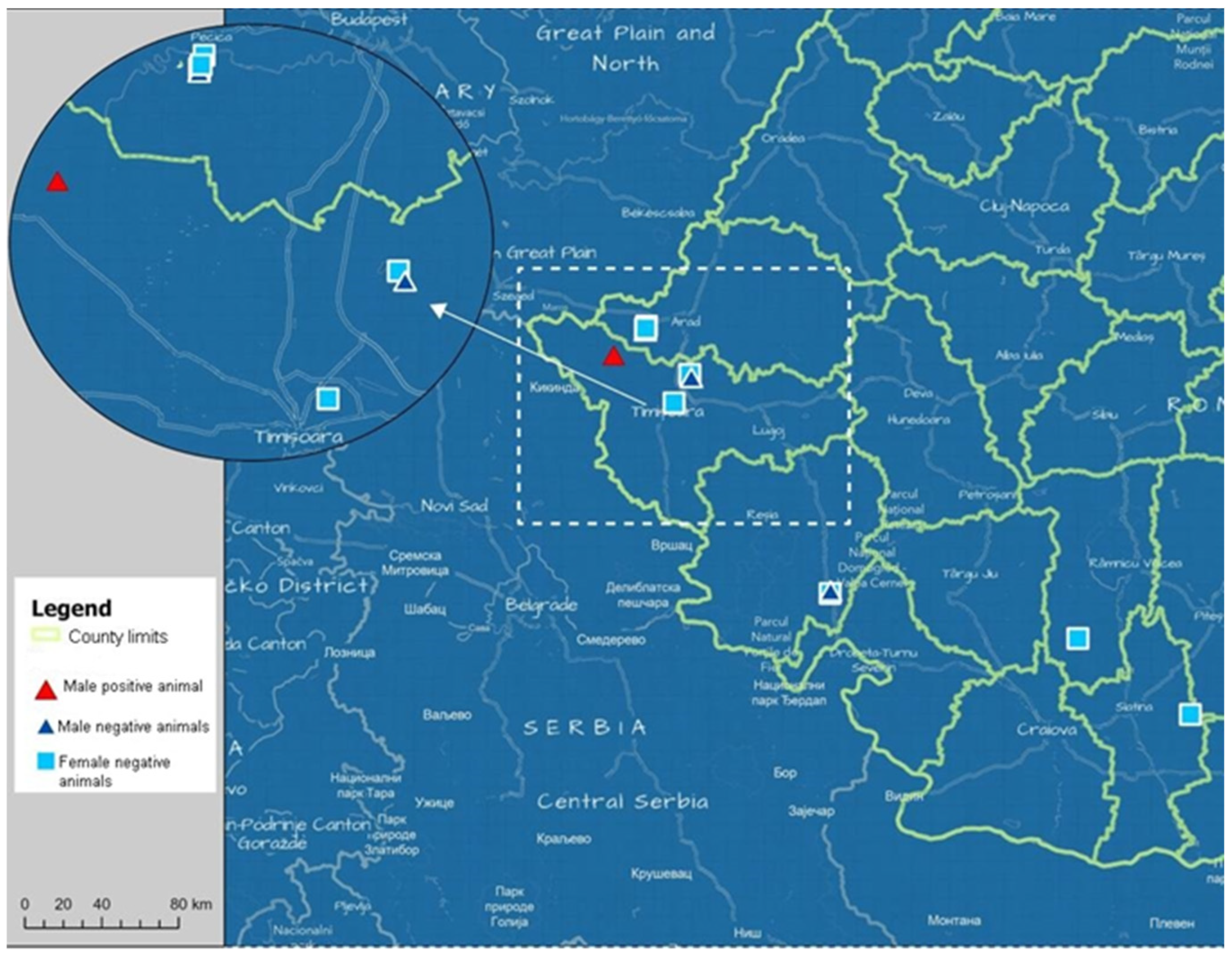
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Johnson, D.D.P.; Macdonald, D.W.; Dickman, A.J. Wildlife Conservation Research Unit, Department of Zoology, University of Oxford, South Parks Road, Oxford OX1 3PS, UK Mammal Rev. 2000, 30, 171–196.
- Cotta, V.; Bodea, M.; Micu, I. Vânatul și vânătoarea în România. Ed. Ceres. Romania. 2001, 786.
- Koepfli, K.P.; Deere, K.A.; Slater, G.J.; Begg, C.; Begg, K.; Grassman, L.; Lucherini, M.; Veron G.; Wayne, R.K. Multigene phylogeny of the Mustelidae: Resolving relationships, tempo and biogeographic history of a mammalian adaptive radiation. BMC Biol. 2008, 6, 10. [CrossRef]
- Pozio, E.; Zarlenga, D.S. New pieces of the Trichinella puzzle. Int. J. Parasitol. 2013, 43, 983-97. [CrossRef]
- Pozio, E. Trichinella pseudospiralis an elusive nematode. Vet. Parasitol. 2016, 231, 97–101. [CrossRef]
- Gherman, C.M.; Boros, Z.; Băieș, M.H.; Cozma-Petruț, A.; Cozma, V. A review of Trichinella species infection in wild animals in Romania, Food Waterborne Parasitol. 2022, 27, e00178. [CrossRef]
- Marin, A.M.; Oprescu, I.; Dărăbuş, G.; Imre, M.; Hădărugă, N.; Moraru, M.M.F.; Ghilean, B.M.; Mederle, N. Updates on Trichinella species infection in wild boar (Sus scrofa) from Southern Romania, J. Agroaliment. Processes Technol. 2022, 28, 388-393.
- Pozio, E. Trichinellosis in the European union: epidemiology, ecology and economic impact. Parasitol. Today. 1998, 14, 35-38. [CrossRef]
- Pozio, E.; Murrell, K.D. Systematics and epidemiology of Trichinella. Adv. Parasitol. 2006, 63, 367-439. [CrossRef]
- Moraru, M.M.F.; Herman, V.; Oprescu, I.; Fodor, J.T.; Morariu, S.; Ilie, M.; Marin, A.M.; Mederle, N. Wild boar - The source of human contamination with Trichinella spp. in Romania. Rev. Rom. Med. Vet. 2022, 32, 38-42.
- Deak, G.; Ionică, A.M.; Gherman, C.M.; Mihalca, A.D. Diversity of Crenosoma species in mustelids with the first molecular characterization of C. melesi and C. petrowi. Front. Vet. Sci. 2023, 10, 1094554. [CrossRef]
- Commission Implementing Regulation (EU) 2015/1375 of 10 August 2015 laying down specific rules on official controls for Trichinella in meat (codification). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32015R1375 (accessed on 27.08.2023).
- Pozio E, Zarlenga D. International Commission on Trichinellosis: Recommendations for genotyping Trichinella muscle stage larvae. Food and Waterborne Parasitol. 2019, 12, e00033. [CrossRef]
- Pozio, E.; Rinaldi, L.; Marucci, G.; Musella, V.; Galati, F.; Cringoli, G.; Boireau, P.; La Rosa, G. Hosts and habitats of Trichinella spiralis and Trichinella britovi in Europe. Int. J. Parasitol. 2009, 39, 71-79. [CrossRef]
- Varlamova, A.I.; Romashov, B.; Romashova, E.; Odoevskaya, I.M. Modern aspects of the epizootology of trichinellosis in the central black earth region of Russia. 26TH International Conference of the World Association for the Advancement of Veterinary Parasitology. Kuala Lumpur, Malaysia, (04–08.09.2017).
- Akkoc, N.; Kuruuzum, Z.; Akar, S.; Yuce, A.; Onen, F.; Yapar, N.; Ozgenc, O.; Turk, M.; Ozdemir, D.; Avci, M.; Guruz, Y.; Oral, A.M.; Pozio, E. A large scale outbreak of trichinellosis due to Trichinella britovi in Turkey, Zoonoses Public Health. 2009, 56, 65-70. [CrossRef]
- Pozio, E. Epidemiology and control prospects of foodborne parasitic zoonoses in the European Union. Parassitologia. 2008, 50, 17-24.
- Marin, A.M.; Dărăbuș, G.; Herman, V.; Morariu, S.; Olariu, T.R.; Dărăbuș, R.G.; Sîrbu, B.; Mederle, N. Study on the prevalence and larval burden of the nematode Trichinella spp. in red foxes from hunting grounds in Timis County. Rev. Rom. Med. Vet. 2021, 31, 23-26.
- Pozio, E.; Kapel, C.M.O.; Gajadhar, A.A.; Boireau, P.; Dupouy-Camet, J.; Gamble, H.R. Trichinella in pork: current knowledge on the suitability of freezing as a public health measure, Euro Surveill. 2006, 11, E061116. [CrossRef]
- van der Giessen J.W.; Rombout, Y.; Franchimont, H.J.; La Rosa, G.; Pozio, E. Trichinella britovi in foxes in The Netherlands. J. Parasitol. 1998, 84, 1065-1068. [CrossRef]
- Frey, C.F.; Basso, W.U.; Zürcher-Giovannini, S.; Marti, I.; Borel, S.; Guthruf, S.; Gliga, D.; Lundström-Stadelmann, B.; Origgi, F.C.; Ryser-Degiorgis, M.P. The golden jackal (Canis aureus): A new host for Echinococcus multilocularis and Trichinella britovi in Switzerland. Schweiz. Arch. Tierheilkd. 2022, 164, 71-78. [CrossRef]
- Kärssin, A.; Häkkinen, L.; Niin, E.; Peik, K.; Vilem, A.; Jokelainen, P.; Lassen, B. Trichinella spp. biomass has increased in raccoon dogs (Nyctereutes procyonoides) and red foxes (Vulpes vulpes) in Estonia. Parasites Vectors. 2017, 10, 609. [CrossRef]
- Cvetkovic, J.; Teodorovic, V.; Marucci, G.; Vasilev, D.; Vasilev, S.; Cirovic, D.; Sofronic-Milosavljevic, L. First report of Trichinella britovi in Serbia, Acta Parasitol. 2011, 56, 232–235. [CrossRef]
- Fonseca-Salamanca, F.; Nogal-Ruiz, J.J.; García-Sánchez, R.N.; Bolas-Fernandez, F.; Jiménez, S.; Alamo, R.; Gárate, T.; Martínez-Fernandez, A.R. Prevalence of Trichinella spp. in North Spain wild fauna and new variety of Trichinella britovi identification. Vet. Parasitol. 2009, 159, 222-224. [CrossRef]
- Kołodziej-Sobocińska, M.; Yakovlev, Y.; Schmidt, K.; Hurníková, Z.; Ruczyńska, I.; Bednarski, M.; Tokarska, M. Update of the helminth fauna in Eurasian lynx (Lynx lynx) in Poland. Parasitol. Res. 2018, 117, 2613-2621. [CrossRef]
- Moskwa, B.; Goździk, K.; Bień, J.; Bogdaszewski, M.; Cabaj, W. Molecular identification of Trichinella britovi in martens (Martes martes) and badgers (Meles meles); new host records in Poland. Acta Parasitol. 2012, 57, 402–405. [CrossRef]
- Badagliacca, P.; Di Sabatino, D.; Salucci, S.; Romeo, G.; Cipriani, M.; Sulli, N.; Dall'Acqua, F.; Ruggieri, M.; Calistri, P.; Morelli, D. The role of the wolf in endemic sylvatic Trichinella britovi infection in the Abruzzi region of Central Italy. Vet. Parasitol. 2016, 231, 124-127. [CrossRef]
- Segliņa, Z.; Bakasejevs, E.; Deksne, G.; Spuņģis, V.; Kurjušina, M. New finding of Trichinella britovi in a European beaver (Castor fiber) in Latvia. Parasitol. Res. 2015, 114, 3171-3173. [CrossRef]
- Orusa, R.; Banch, C.; Peracino, V.; Domenis, L. First report of Trichinella britovi infection in the wild boar of Aosta. Valley. Z. Jagdwiss. 2002, 48, 247-255. [CrossRef]
- Schynts, F.; van der Giessen, J.; Tixhon, S.; Pozio, E.; Dorny, P.; de Borchgrave, J. First isolation of Trichinella britovi from a wild boar (Sus scrofa) in Belgium. Vet. Parasitol. 2006, 135, 191-194. [CrossRef]
- Kärssin, A.; Häkkinen, L.; Vilem, A.; Jokelainen, P.; Lassen, B. Trichinella spp. in Wild Boars (Sus scrofa), Brown Bears (Ursus arctos), Eurasian Lynxes (Lynx lynx) and Badgers (Meles meles) in Estonia, 2007-2014. Animals (Basel). 2021, 11, 183. [CrossRef]
- Klun, I.; Ćosić, N.; Ćirović, D.; Vasilev, D.; Teodorović, V.; Djurković-Djaković, O. Trichinella spp. in wild mesocarnivores in an endemic setting. Acta Vet. Hung. 2019, 67, 34-39. [CrossRef]
- Senutaitė, J.; Grikienienė, J. Prevalence of Trichinell in Muscles of Some Domestic and Wild Mammals in Lithuania and their Impact on the Organism. Acta Zool. Litu. 2001, 11, 395-404. [CrossRef]
- Zelyazkov, P.; Todev, I.; Ivanov, L.; Mirchev, R.; Lalkovski, N. Epizootological stuides on trichinelosis (Trichinella sp.) among wild carnivores in Bulgaria. Sbornik dokladi ot nauchnata konferentsiya: Traditsii i s'vrenmennost v'v veterinarnata meditsina. 2009, 2009, 335-340.
- Hurníková, Z.; Chovancová, B.; Bartková, D.; Dubinský, P. The role of wild carnivores in the maintenance of trichinellosis in the Tatras National Park, Slovakia. Helminthol. 2007, 44, 18-20. [CrossRef]
- Hurníková, Z.; Miterpáková, M.; Chovancová, B. The important zoonoses in the protected areas of the Tatra National Park (TANAP). Wiad. Parazytol. 2009, 55, 395-398.
- Bakasejevs, E.; Daukšte, A.; Zolovs, M.; Zdanovska, A. Investigation of Trichinella in wildlife in Latgale region (Latvia). Acta Biol. Univ. Daugavp. 2012, 12, 1-5.
- Deksne, G.; Segliņa, Z.; Jahundoviča, I.; Esīte, Z.; Bakasejevs, E.; Bagrade, G.; Keidāne, D.; Interisano, M.; Marucci, G.; Tonanzi, D.; Pozio, E.; Kirjušina, M. High prevalence of Trichinella spp. in sylvatic carnivore mammals of Latvia. Vet. Parasitol. 2016, 231, 118-123. [CrossRef]
- Kirjušina, M.; Bakasejevs, E.; Pezzotti, P.; Pozio, E. Trichinella britovi biomass in naturally infected pine martens (Martes martes) of Latvia. Vet Parasitol. 2016, 231, 110-114. [CrossRef]
- Berzina, Z.; Stankeviciute, J.; Sidlauskas, G.; Bakasejevs, E.; Zdankovska, A.; Gackis, M. Trichinella sp Infection in Martens (Martes martes, Martes foina) in Latvia and Lithuania (Kaunas region). Rural Development. 2013, 6, 34-37.
- Berzina, Z.; Jahundovica, I.; Kirjusina, M. Trichinella species variety in pine marten (Martes martes) and stone marten (Martes foina) in Latvia and Lithuania (Kaunas region). Proceedings of Conference "Research and Practice in Veterinary Medicine - 2014" Jelgava, Latvia, (02.02.2014).
- Piekarska, J.; Gorczykowski, M.; Kicia, M.; Pacoń, J.; Sołtysiak, Z.; Merta, D. Trichinella spp. (Nematoda) in free-living carnivores (Mammalia: Carnivora) from the Lower Silesia (Poland). Ann. Parasitol. 2016, 62, 64.
- Cybulska, A.; Kornacka, A.; Skopek, R.; Moskwa, B. Trichinella britovi infection and muscle distribution in free-living martens (Martes spp.) from the Głęboki Bród Forest District, Poland. Int. J. Parasitol. Parasites Wildl. 2020, 12, 176-180. [CrossRef]
- Hirvelä-Koski, V.; Aho, M.; Asplund, K.; Hatakka, M.; Hirn, J. Trichinella spiralis in wild animals, cats, mice, rats and farmed fur animals in Finland. Nord Vet. Med. 1985, 37, 234-42.
- Pozio, E.; Christensson, D.; Stéen, M.; Marucci, G.; La Rosa, G.; Bröjer, C.; Mörner, T.; Uhlhorn, H.; Agren, E.; Hall M. Trichinella pseudospiralis foci in Sweden. Vet. Parasitol. 2004, 125, 335-342. [CrossRef]
- Bourque, M. A Survey of Trichinella spiralis in Wild Carnivores in Southwestern Quebec. Can. Vet. J. 1985, 26, 203-204.
- Blaga, R.; Gherman, C.; Cozma, V.; Zocevic, A.; Pozio, E.; Boireau, P. Trichinella species circulating among wild and domestic animals in Romania. Vet. Parasitol. 2009, 159, 218-221. [CrossRef]
- Blaga, R.; Gherman, C.; Seucom, D.; Cozma, V.; Boireau, P. First identification of Trichinella spp. in golden jackal (Canis aureus) in Romania. J. Wildl. Dis. 2008, 44, 457-459. [CrossRef]
- Boros, Z.; Ionica, A.M.; Deak, G.; Mihalca, A.D.; Chisamera, G.B.; Gyorke, A.; Gherman, C.M.; Cozma, V. The European badger, Meles, as a new host for Trichinella britovi in Romania. Vet. Parasitol. 2021, 297, 109545. [CrossRef]
- Boros, Z.; Ionica, A.M.; Deak, G.; Mihalca, A.D.; Chișamera, G.B.; Constantinescu, I.C.; Adam, C.; Gherman, C.; Cozma, V. Trichinella spp. infection in European polecats (Linnaeus, 1758) from Romania. Helminthol. 2021, 58, 323–327. [CrossRef]
- Iacob, O. Epidemiological surveillance of Trichinella spp. infection in animals in the eastern part of Romania (Moldova) and the potential risk of human infection. In: Book of Abstracts of the Symposium “Actual Problems of Zoology and Parasitology: Achievements and Prospects”, 13 October 2017, Chișinau, Moldavia, 2017, 161.
- Imre, K.; Pozio, E.; Tonanzi, D.; Sala, C.; Ilie, M.S.; Imre, M.; Morar, A. The red fox (Vulpes vulpes) plays a minor role in the epidemiology of the domestic cycle of Trichinella in Romania. Vet. Parasitol. 2015, 212, 448–450. [CrossRef]
- Nicorescu, I.M.D.; Ionița, M.; Ciupescu, L.; Buzatu, C.V.; Tanasuica, R.; Mitrea, I.L. New insights into the molecular epidemiology of Trichinella infection in domestic pigs, wild boars, and bears in Romania. Vet. Parasitol. 2015, 212, 257–261. [CrossRef]
- Oltean, M.; Kalmar, Z.; Kiss, B.J.; Marinov, M.; Vasile, A.; Sandor, A.D.; Rosenthal, B.M. European mustelids occupying pristine wetlands in the Danube delta are infected with Trichinella likely derived from domesticated swine. J. Wildl. Dis. 2014, 50, 972–975. [CrossRef]
- Dmitric, M.; Debeljak, Z.; Vidanovic, D.; Sekler, M.; Vaskovic, N.; Matovic, K.; Karabasil, N. Trichinella britovi in Game Meat Linked to Human Trichinellosis Outbreak in Serbia. J. Parasitol. 2018, 104, 557-559. [CrossRef]
- Pavic, S.; Andric, A.; Sofronic-Milosavljevic, L.J.; Gnjatovic, M.; Mitic, I.; Vasilev, S.; Sparic, R.; Pavic, A. Trichinella britovi outbreak: Epidemiological, clinical, and biological features, Med. Mal. Infect. 2020, 50, 520-524. [CrossRef]
- Vasilev, S.; Mitic, I.; Mirilovic, M.; Plavsa, D.; Milakara, E.; Plavsic, B.; Sofronic-Milosavljevic, L. Trichinella infection in Serbia from 2011 to 2020: a success story in the field of One Health. Epidemiol. Infect. 2023, 151, e20. [CrossRef]
- Vieira-Pinto, M.; Fernandes, A.R.G.; Santos, M.H.; Marucci, G. Trichinella britovi infection in wild boar in Portugal, Zoonoses Public Health, 2021, 68, 103-109. [CrossRef]
- Fichi, G.; Stefanelli, S.; Pagani, A.; Luchi, S.; De Gennaro, M.; Gómez-Morales, M.A.; Selmi, M.; Rovai, D.; Mari, M.; Fischetti, R.; Pozio, E. Trichinellosis outbreak caused by meat from a wild boar hunted in an Italian region considered to be at negligible risk for Trichinella. Zoonoses Public Health. 2015, 62, 285-291. [CrossRef]
- Romano, F.; Motta, A.; Melino, M.; Negro, M.; Gavotto, G.; Decasteli, L.; Careddu, E.; Bianchi, C.; Bianchi, D.M.; Pozio, E. Investigation on a focus of human trichinellosis revealed by an atypical clinical case: after wild-boar (Sus scrofa) pork consumption in northern Italy. Parasite. 2011, 18, 85-87. [CrossRef]
- Stroffolini, G.; Rossi, L.; Lupia, T.; Faraoni, S.; Paltrinieri, G.; Lipani, F.; Calcagno, A.; Bonora, S.; Di Perri, G.; Calleri, G. Trichinella britovi outbreak in Piedmont, North-West Italy, 2019-2020: Clinical and epidemiological insights in the one health perspective. Travel Med. Infect. Dis. 2022, 47, 102308. [CrossRef]
- Troiano, G.; Nante, N. Human Trichinellosis in Italy: an epidemiological review since 1989. J. Prev. Med. Hyg. 2019, 60, 71-75. [CrossRef]
- Turiac, I.A.; Cappelli, M.G.; Olivieri, R.; Angelillis, R.; Martinelli, D.; Prato, R.; Fortunato, F. Trichinellosis outbreak due to wild boar meat consumption in southern Italy. Parasit. Vectors. 2017, 10, 107. [CrossRef]
- Gallardo, M.T.; Mateos, L.; Artieda, J.; Wesslen, L.; Ruiz, C.; García, M.A.; Galmés-Truyols, A.; Martin, A.; Hernández-Pezzi, G.; Andersson, Y.; Gárate, T.; Christensson, D. Outbreak of trichinellosis in Spain and Sweden due to consumption of wild boar meat contaminated with Trichinella britovi. Euro Surveill. 2007, 12, E070315.1. [CrossRef]
- Perez-Perez, A.; Bescos, J.G.; Gracia, A.D.C.; Aznar, C.M.; Cuenca, S.M.; Brieba, A.A.; Belanche, M.A.L.; Lacambra, I.S.; Dea, C.C. Trichinellosis outbreaks in Aragón (1998-2017). Rev. Esp. Salud. Publica. 2019, 93, e201902005.
- Dimzas, D.; Diakou, A.; Koutras, C.; Morales, M.A.G.; Psalla, D.; Keryttopoulos, P.; Deligianni, D.; Kontotasios, K.; Pozio, E. Human trichinellosis caused by Trichinella britovi in Greece, and literature review. J. Helminthol., 2019, 94, e33. [CrossRef]
- Borhani, M.; Fathi, S.; Harandi, M.F.; Simsek, S.; Ahmed, H.; Wu, X.; Liu, M. Trichinella infections in animals and humans of Iran and Turkey. Front. Med. (Lausanne). 2023, 10, 1088507. [CrossRef]
- Gaillard, T.; Martinaud,C.; Kérébel, S.; Cellarier, G.; Muzellec, Y.; Brisou, P. About two cases of trichinellosis caused by Trichinella britovi. Ann. Biol. Clin. (Paris). 2007, 65, 308-312.
- Gari-Toussaint, M.; Tieulié, N.; Baldin, J.; Dupouy-Camet, J.; Delaunay, P.; Fuzibet, J.G.; Le Fichoux, Y.; Pozio, E.; Marty P. Human trichinellosis due to Trichinella britovi in southern France after consumption of frozen wild boar meat. Euro Surveill. 2005, 10, 117-118. [CrossRef]
- Peju, M.; Granier, B.; Garnaud, C.; Brenier-Pinchart, M.P.; Vallee, I.; Chevillot, A.; Merel, C.; Chereau, F.; Deher, M.; Rogeaux, O.; Yera, H. A Trichinella britovi outbreak in the Northern Alps of France: investigation by a local survey network. Parasite. 2023, 30, 14. [CrossRef]
- Antolová, D.; Fecková, M.; Valentová, D.; Hurníková, Z.; Miklisová, D.; Avdičová, M.; Halánová, M. Trichinellosis in Slovakia - epidemiological situation in humans and animals (2009-2018). Ann. Agric. Environ. Med. 2020, 27, 361-367. [CrossRef]
- Dubinský, P.; Stefancíková, A.; Kinceková, J.; Ondriska, F.; Reiterová, K.; Medvedová, M. Trichinellosis in the Slovak Republic. Parasite. 2001, 8, 100-102. [CrossRef]
- Nezri, M.; Ruer, J.; De Bruyne, A.; Cohen-Valensi, R.; Pozio, E.; Dupouy-Camet, J. First report of a human case of trichinellosis due to Trichinella britovi after jackal (Canis aureus) meat consumption in Algeria. Bull. Soc. Pathol. Exot. 2006, 99, 94-95.
- Dubinský, P.; Antolová, D.; Reiterová, K. Human Trichinella infection outbreaks in Slovakia, 1980-2008. Acta Parasitol. 2016, 61, 205-211. [CrossRef]
- Gomez-Garcia, V.; Hernandez-Quero, J.; Rodriguez-Osorio, M. Short report: Human infection with Trichinella britovi in Granada, Spain. Am. J. Trop. Med. Hyg. 2003, 68, 463-464. [CrossRef]
- López Hernández, B.; Velázquez de Castro, M.T.; Galicia García, M.D.; Sabonet, J.C. Outbreak of Trichinella britovi infection in Granada in the spring of 2000. Rev. Esp. Salud. Publica. 2001, 75, 467-473.
- Krivokapich, S.J.; Gatti, G.M.; Prous, C.L.G.; Degese, M.F.; Arbusti, P.A.; Ayesa, G.E.; Bello, G.V.; Salomon, M.C. Detection of Trichinella britovi in pork sausage suspected to be implicated in a human outbreak in Mendoza, Argentina. Parasitol. Int. 2019, 71, 53-55. [CrossRef]
- Vutova, K.; Velev, V.; Chipeva, R.; Yancheva, N.; Petkova, S.; Tomov, T.; Pozio, E.; Robertson, LJ. Clinical and epidemiological descriptions from trichinellosis outbreaks in Bulgaria. Exp. Parasitol. 2020, 212, 107874. [CrossRef]
- Pozio, E.; Mesina, P.; Sechi, F.; Pira, M.; Liciardi, M.; Cossu, P.; Marucci, G.; Garippa, G.; Firinu, A.; Human outbreak of trichinellosis in the Mediterranean island of Sardinia, Italy. Vet. Parasitol. 2006, 140, 177-180. [CrossRef]
- Ruetsch, C.; Delaunay, P.; Armengaud, A.; Peloux-Petiot, F.; Dupouy-Camet, J.; Vallee, I.; Polack, B.; Boireau, P.; Marty, P. Inadequate labeling of pork sausages prepared in Corsica causing a trichinellosis outbreak in France. Parasite. 2016, 23, 27. [CrossRef]
- Neghina, R.; Neghina, A.M.; Marincu, I.; Moldovan, R.; Iacobiciu, I. Evidence of Trichinella spiralis in Timis County, Romania: a report of a winter trichinellosis outbreak in 2008 due to consumption of contaminated pork. Vector Borne Zoonotic Dis. 2010, 10, 931-933. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




