Submitted:
29 August 2023
Posted:
29 August 2023
You are already at the latest version
Abstract
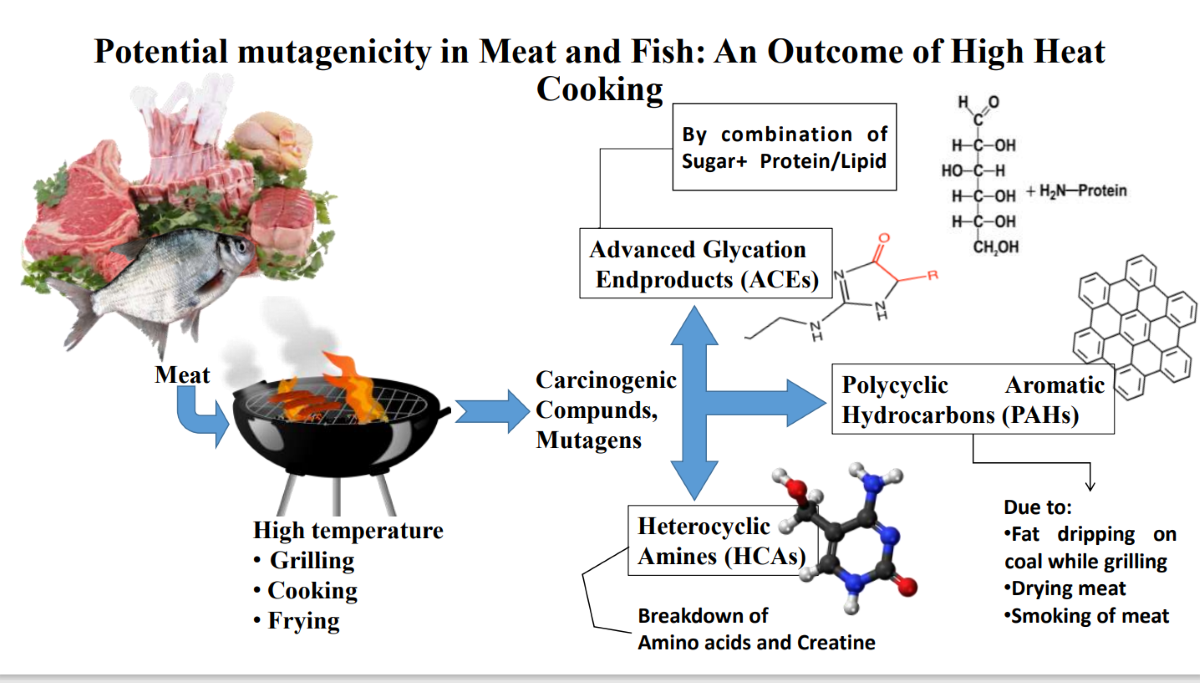
Keywords:
1. Introduction
- PhIP: a potent HCA found in cooked meat products, has been linked to increased cancer risk in breast, prostate, and colon cancer.[52].
- MeIQ: a potent HCA, has been linked to cancer in animal studies, including breast, colon, and prostate cancer [53].
- 2-Amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline (DiMeIQx): Although less effective than PhIP or MeIQ, it has been proven in animal tests to raise the risk of cancer [54].
- 2-Amino-9H-pyrido[2,3-b]indole (AαC): This HCA is produced during the cooking of meat, poultry, and fish. It has been demonstrated in animal experiments to be carcinogenic, although its potential impact on human health is currently being investigated [55].
- 2-Amino-1,6-dimethylfuro[3,2-e]imidazo[4,5-b]pyridine (IFP): This HCA is generated during the cooking of beef and has been proven in animal experiments to be mutagenic and potentially carcinogenic [56].
| Polycyclic Aromatic Hydrocarbon | Chemical Name | Carcinogenicity | References |
|---|---|---|---|
| Benzo[a]pyrene | Benzo[a]pyrene | Carcinogenic | [2] |
| Dibenzo[a,h]anthracene | Dibenzo[a,h]anthracene | Carcinogenic | [4] |
| Benzo[b]fluoranthene | Benzo[b]fluoranthene | Possibly carcinogenic | [6] |
| Benzo[k]fluoranthene | Benzo[k]fluoranthene | Possibly carcinogenic | [80] |
| Benzo[j]fluoranthene | Benzo[j]fluoranthene | Possibly carcinogenic | [21] |
| Benzo[e]pyrene | Benzo[e]pyrene | Possibly carcinogenic | [25] |
| Indeno[1,2,3-cd]pyrene | Indeno[1,2,3-cd]pyrene | Possibly carcinogenic | [28] |
| Type of AGEs | Detail | Potential Health Risk | Refrence |
| Nε-carboxymethyllysine (CML) | Highly reactive and toxic AGE that is formed through the reaction of lysine with reducing sugars or carbonyls | Causes oxidative stress, inflammation, DNA damage, and cancer development in animal models, highlighting its potential health risks | [93] |
| Methylglyoxal (MGO) | A reactive dicarbonyl compound formed during the Maillard reaction. It is capable of cross-linking proteins and nucleic acids, resulting in the creation of complex lipoxidation end-products (ALEs). | Linked to the development of several cancers, including breast cancer and stomach cancer | [34] |
| Glyoxal (GO) | Di-carbonyl compound formed during the Millard reaction and may also cross-link nucleic acids and proteins and has been found in tests to cause oxidative stress including DNA damage | In animal studies, GO has been related to the formation of cancer | [94] |
V. Mitigation strategies
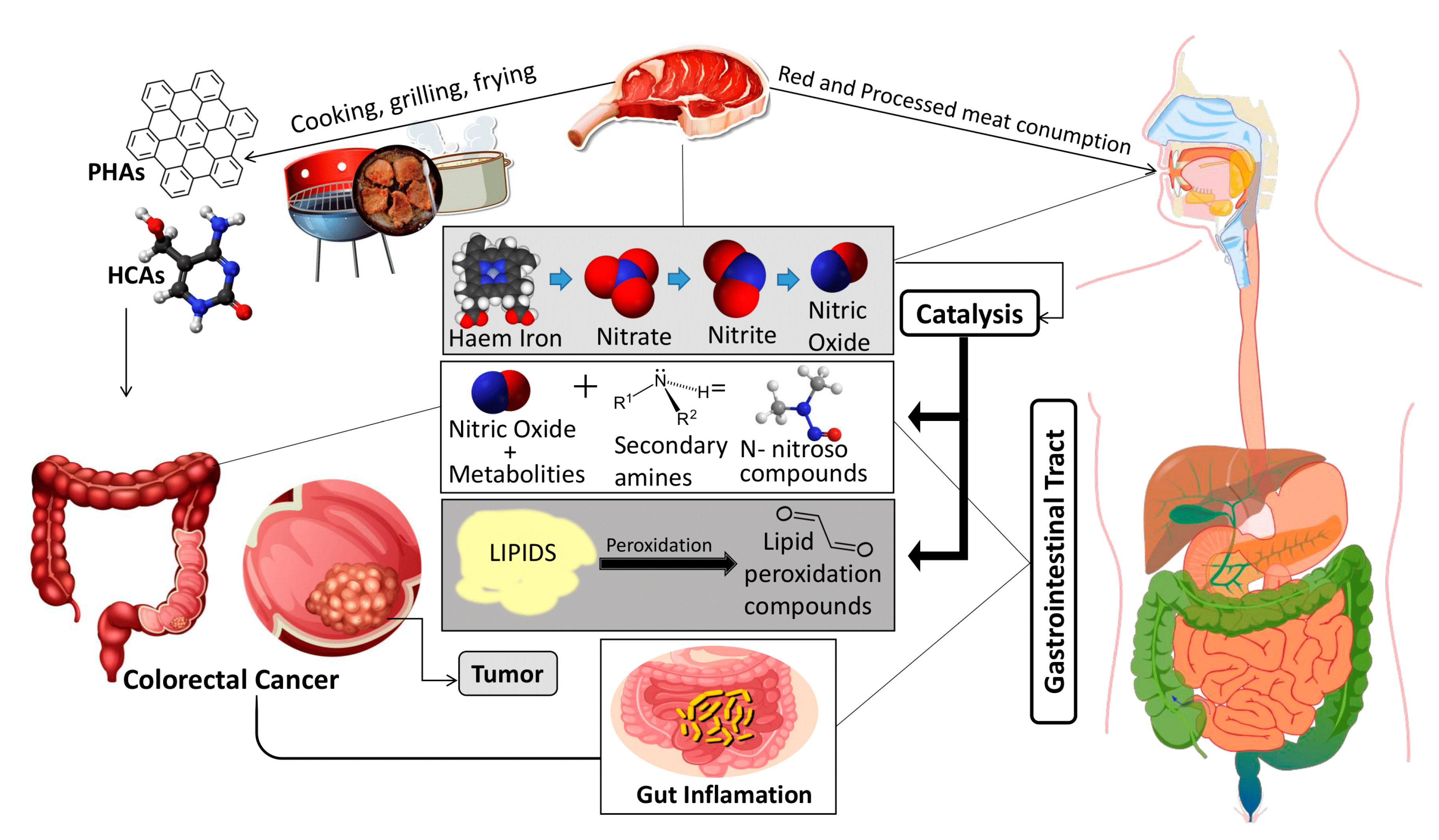
| Type of Meat |
Type of Compounds |
Subclass | Cooking Method | Mitigation Strategy | Amount found before mitigation | Amount found after mitigation Strategy | Active Ingredient | Reference |
|---|---|---|---|---|---|---|---|---|
| Pork Belly | HCA | IQx, IQ, MeIQx, 7,8-DiMeIQx, DiMeIQx, PhIP | BBQ | Natural Spice | 50.33 (ng/g) | 21.45 (ng/g) | Antioxidant Particularly Vitamin C | [120] |
| Black Current | 12.03 (ng/g) | |||||||
| Beef Patties | HCA | IQ, PhIP | Oven | Red Chilli (0.5%, 1% and 1.5%) |
10.08 (ng/g) | 5.49, 6.53, 7.62 (ng/g) | Capsaicin | [119] |
| Capsaicin (2mg, 4mg, 6 mg) | 2.03, 3.26, 5.99 (ng/g) | |||||||
| Pork Patties | HCA | IQx, IQ, MeIQx, 7,8-DiMeIQx, DiMeIQx, PhIP | High Temperature (180 to 220˚C) | Fat Replacement with Olive oil, sunflower oil and grapeseed oil (40%) | 67.56±17.29 (180˚C) 140.57±22.03 (220˚C) (ng/g) |
93.90 and 85.75% reduction at 180˚C and 220 ˚C with 40% olive oil. 91.15 and 83.01% reduction at 180˚C and 220 ˚C with 40% sunflower oil. 100 and 98.64% reduction at 180˚C and 220 ˚C with 40% grapeseed oil. |
Antioxidant in oil (vitamin E, ß-carotenes and phenolic compounds) | [131] |
| beef samples | HAAs | IQx, IQ, MeIQx, 7,8-DiMeIQx, DiMeIQx, PhIP | Oven Roasting | Artichoke | 48.00 ± 1.44 ng/g at 250˚C | 35.65 ± 0.55 ng/g (25.73% Inhibition) with 0.5% and 0.98 ± 0.08 ng/g (97.96% Inhibition) with 1% artichoke level | cafinulin and feoylquinic acid derivatives | [132] |
| Chicken breast | HAAs | IQx, IQ, MeIQx, 7,8-DiMeIQx, DiMeIQx, PhIP | Grilling | Brown sugar and Honey | 16.4±0.85 (MeIQ) 29.2±0.68 (PhIP) 46.4±0.62 (DiMeIQx) 18.9±.56 (MeIQx) 18.6±0.61 (IQ) 10.1±0.78 (IQx) ng/g |
Brown sugar reduced level of MeIQ, PIP, DiMeIQx, MeIQx, IQ and IQx to 6.14±0.52, 6.74±0.88, 25.4±2.0, 6.24±0.97, 8.85±0.60 and 6.99 ± 0.61 ng/g, respectively. Honey reduced level of MeIQ, PIP, DiMeIQx, MeIQx, IQ and IQx to 4.98±0.51, 9.87±0.35, 10.2±0.50, 8.05±0.59, 4.09±0.96 and 5.48 ± 0.81 ng/g, respectively. |
- | [133] |
| Chicken Breast | HAAs | IQx, IQ, MeIQx, 7,8-DiMeIQx, DiMeIQx, PhIP | Grilling | Tamarind, Lemon and Lime | 6.99±0.61, 6.24±0.97, 8.85±0.60, 25.4±2.0, 4.14±0.52 and 6.74±0.88 ng/g of IQx, MeIQx, IQ, DiMeIQx, MeIQ and PhIP, respectively. | Tamarind reduced level of IQx, MeIQx, IQ, DiMeIQx, MeIQ and PhIP to 2.27±0.89, 8.17±2.73, 29.3±2.31, 35.3±3.78, 2.79±1.21, 1.20±0.80 ng/g, respectively. Lemon reduced level of IQx, MeIQx, IQ, DiMeIQx, MeIQ and PhIP to 3.41±1.05, 2.38±1.09, 3.81±0.98, 16.2±1.59, 1.26±0.98, 3.63±1.60 ng/g, respectively. Lime reduced level of IQx, MeIQx, IQ, DiMeIQx, MeIQ and PhIP to 2.36±1.02, 2.39±0.97, 4.50±1.09, 29.6±3.84, 2.37±0.98, 3.36±0.97 ng/g, respectively. |
[134] | |
| Chicken wings | HCAs | Deep Frying | Sugarcane Molasses | 4.51-8.04 ng/g after 2.5 mint frying. 26.2-37.4 ng/g after 5 mint frying. |
32.5-43.9% inhibition after 2.5 minutes. 18.5-29.9% after 5 minutes frying. | Phenolic compounds | [135] |
VI. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S.Y.; Yim, D.G.; Lee, D.Y.; Kim, O.Y.; Kang, H.J.; Kim, H.S.; Jang, A.; Park, T.S.; Jin, S.K.; Hur, S.J. Overview of the effect of natural products on reduction of potential carcinogenic substances in meat products. Trends Food Sci. Technol. 2020, 99, 568–579. [Google Scholar] [CrossRef]
- Dong, H.; Xian, Y.; Li, H.; Bai, W.; Zeng, X. Potential carcinogenic heterocyclic aromatic amines (HAAs) in foodstuffs: Formation, extraction, analytical methods, and mitigation strategies. Compr. Rev. Food Sci. Food Saf. 2020, 19, 365–404. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, W.; Li, T.; Liu, Y.; Simon, T.G.; Sui, J.; Wu, K.; Giovannucci, E.L.; Chan, A.T.; Zhang, X. Meat intake and risk of hepatocellular carcinoma in two large US prospective cohorts of women and men. Leuk. Res. 2019, 48, 1863–1871. [Google Scholar] [CrossRef] [PubMed]
- Dutta, K.; Shityakov, S.; Zhu, W.; Khalifa, I. High-risk meat and fish cooking methods of polycyclic aromatic hydrocarbons formation and its avoidance strategies. Food Control. 2022, 142. [Google Scholar] [CrossRef]
- González, N.; Marquès, M.; Nadal, M.; Domingo, J.L. Meat consumption: Which are the current global risks? A review of recent (2010–2020) evidences. Food Res. Int. 2020, 137, 109341. [Google Scholar] [CrossRef]
- Aendo, P.; Thongyuan, S.; Songserm, T.; Tulayakul, P. Carcinogenic and non-carcinogenic risk assessment of heavy metals contamination in duck eggs and meat as a warning scenario in Thailand. Sci. Total. Environ. 2019, 689, 215–222. [Google Scholar] [CrossRef]
- Racovita, R.C.; Secuianu, C.; Israel-Roming, F. Quantification and risk assessment of carcinogenic polycyclic aromatic hydrocarbons in retail smoked fish and smoked cheeses. Food Control. 2020, 121, 107586. [Google Scholar] [CrossRef]
- Libera, J.; Iłowiecka, K.; Stasiak, D. Consumption of processed red meat and its impact on human health: A review. Int. J. Food Sci. Technol. 2021, 56, 6115–6123. [Google Scholar] [CrossRef]
- Dianatinasab, M.; Wesselius, A.; de Loeij, T.; Salehi-Abargouei, A.; Yu, E.Y.W.; Fararouei, M.; Brinkman, M.; Brandt, P.v.D.; White, E.; Weiderpass, E.; et al. The association between meat and fish consumption and bladder cancer risk: a pooled analysis of 11 cohort studies. Eur. J. Epidemiology 2021, 36, 781–792. [Google Scholar] [CrossRef]
- Nadeem, H.R.; Akhtar, S.; Ismail, T.; Sestili, P.; Lorenzo, J.M.; Ranjha, M.M.A.N.; Jooste, L.; Hano, C.; Aadil, R.M. Heterocyclic Aromatic Amines in Meat: Formation, Isolation, Risk Assessment, and Inhibitory Effect of Plant Extracts. Foods 2021, 10, 1466. [Google Scholar] [CrossRef]
- Negahdari, S.; Sabaghan, M.; Pirhadi, M.; Alikord, M.; Sadighara, P.; Darvishi, M.; Nazer, M. Potential Harmful Effects of Heavy Metals as a toxic and carcinogenic agent in Marine Food-An Overview. Egypt. J. Veter- Sci. 2021, 52, 379–385. [Google Scholar] [CrossRef]
- Khan, M.R.; Busquets, R.; Azam, M. Blueberry, raspberry, and strawberry extracts reduce the formation of carcinogenic heterocyclic amines in fried camel, beef and chicken meats. Food Control. 2020, 123, 107852. [Google Scholar] [CrossRef]
- Asamoah, E.K.; Nunoo, F.K.E.; Addo, S.; Nyarko, J.O.; Hyldig, G. Polycyclic aromatic hydrocarbons (PAHs) in fish smoked using traditional and improved kilns: Levels and human health risk implications through dietary exposure in Ghana. Food Control. 2020, 121, 107576. [Google Scholar] [CrossRef]
- Adeyeye, S.A.O.; Ashaolu, T.J. Heterocyclic Amine Formation and Mitigation in Processed Meat and Meat Products: A Mini-Review. J. Food Prot. 2021, 84, 1868–1877. [Google Scholar] [CrossRef]
- Sampaio, G.R.; Guizellini, G.M.; da Silva, S.A.; de Almeida, A.P.; Pinaffi-Langley, A.C.C.; Rogero, M.M.; et al. Polycyclic aromatic hydrocarbons in foods: Biological effects, legislation, occurrence, analytical methods, and strategies to reduce their formation. International Journal of Molecular Sciences 2021, 22, 6010. [Google Scholar] [CrossRef]
- Deeduah, F.; Iwuoha, G. Genotoxicity and carcinogenicity of traditionally roasted meat using indicator polycyclic aromatic hydrocarbons (PAHs), Port Harcourt, Nigeria. DF Mene and GN Iwuoha Genotoxicity and Carcinogenicity of Traditionally Roasted Meat Using Indicator Polycyclic Aromatic Hydrocarbons (PAHs), Port Harcourt. Nigeria Chemistry International. 2021, 7, 217–23. [Google Scholar]
- Iwasaki, M.; Tsugane, S. Dietary heterocyclic aromatic amine intake and cancer risk: epidemiological evidence from Japanese studies. Genes Environ. 2021, 43, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Khanverdiluo, S.; Talebi-Ghane, E.; Heshmati, A.; Mehri, F. The concentration of polycyclic aromatic hydrocarbons (PAHs) in mother milk: A global systematic review, meta-analysis and health risk assessment of infants. Saudi J. Biol. Sci. 2021, 28, 6869–6875. [Google Scholar] [CrossRef] [PubMed]
- Kossenas, K.; Constantinou, C. Epidemiology, Molecular Mechanisms, and Clinical Trials: an Update on Research on the Association Between Red Meat Consumption and Colorectal Cancer. Curr. Nutr. Rep. 2021, 10, 435–467. [Google Scholar] [CrossRef]
- Oz, E. Author response for "The impact of fat content and charcoal types on quality and the development of carcinogenic polycyclic aromatic hydrocarbons and heterocyclic aromatic amines formation of barbecued fish". 2020. [Google Scholar] [CrossRef]
- Fakhri, Y.; Nematollahi, A.; Abdi-Moghadam, Z.; Daraei, H.; Ghasemi, S.M.; Thai, V.N. Concentration of Potentially Harmful Elements (PHEs) in Trout Fillet (Rainbow and Brown) Fish: a Global Systematic Review and Meta-analysis and Health Risk Assessment. Biol. Trace Element Res. 2020, 199, 3089–3101. [Google Scholar] [CrossRef]
- Wang, X.; Gao, M.; Wang, B.; Tan, Y.; Guo, Y.; Li, Q.; Ge, S.; Lan, C.; Chen, J.; Jiangtulu, B.; et al. Risk of dietary intake of organochlorine pesticides among the childbearing-age women: A multiple follow-up study in North China. Ecotoxicol. Environ. Saf. 2021, 224, 112607. [Google Scholar] [CrossRef] [PubMed]
- Fathabad, A.E.; Tajik, H.; Najafi, M.L.; Jafari, K.; Khaneghah, A.M.; Fakhri, Y.; Thai, V.N.; Conti, G.O.; Miri, M. The concentration of the potentially toxic elements (PTEs) in the muscle of fishes collected from Caspian Sea: A health risk assessment study. Food Chem. Toxicol. 2021, 154, 112349. [Google Scholar] [CrossRef] [PubMed]
- Vernia, F.; Longo, S.; Stefanelli, G.; Viscido, A.; Latella, G. Dietary Factors Modulating Colorectal Carcinogenesis. Nutrients 2021, 13, 143. [Google Scholar] [CrossRef]
- Li, Y.; Guo, N.; Zou, X.; Li, P.; Zou, S.; Luo, J.; Yang, Y. Pollution level and health risk assessment of polycyclic aromatic hydrocarbons in marine fish from two coastal regions, the South China Sea. Mar. Pollut. Bull. 2021, 168, 112376. [Google Scholar] [CrossRef]
- Maruf MA-h Punom, N.J.; Saha, B.; Moniruzzaman, M.; Suchi, P.D.; Eshik, M.M.E.; et al. Assessment of human health risks associated with heavy metals accumulation in the freshwater fish Pangasianodon hypophthalmus in Bangladesh. Exposure and Health. 2021, 13, 337–59. [Google Scholar] [CrossRef]
- Shimomura, Y.; Sobue, T.; Zha, L.; Kitamura, T.; Iwasaki, M.; Inoue, M.; Yamaji, T.; Tsugane, S.; Sawada, N. Association between meat, fish, and fatty acid intake and incidence of acute myeloid leukemia and myelodysplastic syndrome: the Japan Public Health Center-based Prospective Study. Environ. Heal. Prev. Med. 2023, 28, 19–19. [Google Scholar] [CrossRef] [PubMed]
- Fong, F.L.Y.; El-Nezami, H.; Sze, E.T.P. Biogenic amines – Precursors of carcinogens in traditional Chinese fermented food. NFS J. 2021, 23, 52–57. [Google Scholar] [CrossRef]
- Jalandra, R.; Dalal, N.; Yadav, A.K.; Verma, D.; Sharma, M.; Singh, R.; Khosla, A.; Kumar, A.; Solanki, P.R. Emerging role of trimethylamine-N-oxide (TMAO) in colorectal cancer. Appl. Microbiol. Biotechnol. 2021, 105, 7651–7660. [Google Scholar] [CrossRef]
- Malik, S.; Prasad, S.; Kishore, S.; Kumar, A.; Upadhyay, V. A perspective review on impact and molecular mechanism of environmental carcinogens on human health. Biotechnol. Genet. Eng. Rev. 2021, 37, 178–207. [Google Scholar] [CrossRef]
- Bulanda, S.; Janoszka, B. Consumption of Thermally Processed Meat Containing Carcinogenic Compounds (Polycyclic Aromatic Hydrocarbons and Heterocyclic Aromatic Amines) versus a Risk of Some Cancers in Humans and the Possibility of Reducing Their Formation by Natural Food Additives—A Literature Review. Int. J. Environ. Res. Public Heal. 2022, 19, 4781. [Google Scholar] [CrossRef]
- Huang, S.; Fu, W.; Fang, Q.; Ni, L.; Zheng, R.; Yong, L.; Huang, Z.; Pang, J.; Lin, Z.; Lin, H.; et al. Occurrence and carcinogenic risk assessment of N-nitrosamines in some dried aquatic products in China. Food Control. 2023, 152. [Google Scholar] [CrossRef]
- Moradi, S.; Shariatifar, N.; Akbari-Adergani, B.; Aghaee, E.M.; Arbameri, M. Analysis and health risk assessment of nitrosamines in meat products collected from markets, Iran: with the approach of chemometric. J. Environ. Heal. Sci. Eng. 2021, 19, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, W. Biogenic amines and volatile N-nitrosamines in Chinese smoked-cured bacon (Larou) from industrial and artisanal origins. Food Addit. Contam. Part B 2023, 16, 143–160. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Huang, X.; Xiao, Z.; Yang, Y.; Yu, Q.; Chen, S.; He, L.; Liu, A.; Liu, S.; et al. A Review on Mechanistic Overview on the Formation of Toxic Substances during the Traditional Fermented Food Processing. Food Rev. Int. 2021, 39, 1275–1292. [Google Scholar] [CrossRef]
- Afé, O.H.I.; Kpoclou, Y.E.; Douny, C.; Anihouvi, V.B.; Igout, A.; Mahillon, J.; Hounhouigan, D.J.; Scippo, M. Chemical hazards in smoked meat and fish. Food Sci. Nutr. 2021, 9, 6903–6922. [Google Scholar] [CrossRef]
- Seo Je Park Je Lee, Y.; Do, B.; Lee Jy Kwon, H. Effect of cooking method on the concentrations of volatile N-nitrosamines in various food products. Journal of Food Processing and Preservation. 2022, 46, 16590. [Google Scholar]
- Jung, J.-W.; Kim, U.-J.; Yu, W.-J.; Park, J.-W.; Jeong, E.J. Probabilistic cancer risk assessment for dietary intake of seven nitrosamine chemicals in Korea. Hum. Ecol. Risk Assessment: Int. J. 2020, 27, 626–637. [Google Scholar] [CrossRef]
- Niklas, A.A.; Pedersen, M.; Christensen, T.; Duedahl-Olesen, L. Simultaneous determination of heterocyclic aromatic amines and N-nitrosamines in fried bacon cubes and slices using LC-(ESI/APCI)-MS/MS. Food Addit. Contam. Part A 2023, 40, 493–507. [Google Scholar] [CrossRef]
- Özbay, S.; Şireli, U.T. The effect of ascorbic acid, storage period and packaging material on the formation of volatile N-nitrosamine in sausages. J. Food Sci. Technol. 2021, 59, 1823–1830. [Google Scholar] [CrossRef]
- Wu, Y.; Qin, L.; Chen, J.; Wang, H.; Liao, E. Nitrite, biogenic amines and volatile N-nitrosamines in commercial Chinese traditional fermented fish products. Food Addit. Contam. Part B 2021, 15, 10–19. [Google Scholar] [CrossRef]
- Afé, O.H.I.; Kpoclou, Y.E.; Douny, C.; Anihouvi, V.B.; Igout, A.; Mahillon, J.; Hounhouigan, D.J.; Scippo, M. Chemical hazards in smoked meat and fish. Food Sci. Nutr. 2021, 9, 6903–6922. [Google Scholar] [CrossRef] [PubMed]
- Rudneva, I.I.; Omel’chenko, S.O. Nitrosamines in Aquatic Ecosystems: Sources, Formation, Toxicity, Environmental Risk (Review). 2. Content In Aquatic Biota, Biological Effects and Risk Assessment. Water Resour. 2021, 48, 291–299. [Google Scholar] [CrossRef]
- Omer, A.K.; Mohammed, R.R.; Ameen, P.S.M.; Abas, Z.A.; Ekici, K. Presence of Biogenic Amines in Food and Their Public Health Implications: A Review. J. Food Prot. 2021, 84, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Sallan, S.; Oral, Z.F.Y.; Kaya, M. A Review on the Role of Lactic Acid Bacteria in the Formation and Reduction of Volatile Nitrosamines in Fermented Sausages. Foods 2023, 12, 702. [Google Scholar] [CrossRef]
- Paglialunga, S.; van Haarst, A. The impact of N-nitrosamine impurities on clinical drug development. Journal of Pharmaceutical Sciences. 2023, 112, 1183–1191. [Google Scholar] [CrossRef]
- Zahra, N.; Saeed, M.K.; Raza, M.H. Nitrosamines: Incredibly unsafe contaminants in different food commodities. Chemistry International. 2023, 1, 27–36. [Google Scholar]
- Bercu, J.P.; Masuda-Herrera, M.; Johnson, G.; Czich, A.; Glowienke, S.; Kenyon, M.; Thomas, R.; Ponting, D.J.; White, A.; Cross, K.; et al. Use of less-than-lifetime (LTL) durational limits for nitrosamines: Case study of N-Nitrosodiethylamine (NDEA). Regul. Toxicol. Pharmacol. 2021, 123, 104926. [Google Scholar] [CrossRef]
- Ejike, U.D.I.; Liman, M.L. Role of Dietary Antioxidants in Chemoprevention of Nitrosamines-Induced Carcinogenesis. 2021; 23. [Google Scholar] [CrossRef]
- James, M.; Edge, T. Low-Level Determination of Mutagenic Nitrosamine Impurities in Drug Substances by LC–MS/MS. 34. [CrossRef]
- Chen, L.; Liu, R.; Wu, M.; Yu, H.; Ge, Q.; Zhang, W. Nitrosamines and Polycyclic Aromatic Hydrocarbons in Smoke-Cured Bacon (Larou) of Artisanal and Industrial Origin. Foods 2021, 10, 2830. [Google Scholar] [CrossRef]
- Nguyen, L.C.; Nguyen, B.T.; Le, N.T. A Prospective Pooled Analysis of Meat Mutagens and Colorectal Adenoma and Cancer in the US and EPIC Studies: Findings with an Emphasis on Improving Exposure Measurements. Asian Pac. J. Cancer Prev. 2022, 23, 2215–2224. [Google Scholar] [CrossRef]
- Erdoğan, B.; Özdestan-Ocak. Inhibitory effects of carob and propolis extracts on the formation of heterocyclic aromatic amines in beef meatballs cooked with different methods. J. Food Process. Preserv. 2022, 46. [Google Scholar] [CrossRef]
- Khan, I.A.; Khan, A.; Zou, Y.; Zongshuai, Z.; Xu, W.; Wang, D.; Huang, M. Heterocyclic amines in cooked meat products, shortcomings during evaluation, factors influencing formation, risk assessment and mitigation strategies. Meat Sci. 2021, 184, 108693. [Google Scholar] [CrossRef] [PubMed]
- Onjia, A.; Huang, X.; González, J.M.T.; Egbueri, J.C. Editorial: Chemometric approach to distribution, source apportionment, ecological and health risk of trace pollutants. Front. Environ. Sci. 2022, 10. [Google Scholar] [CrossRef]
- Arisekar, U.; Shakila, R.J.; Shalini, R.; Jeyasekaran, G.; Padmavathy, P. Effect of household culinary processes on organochlorine pesticide residues (OCPs) in the seafood (Penaeus vannamei) and its associated human health risk assessment: Our vision and future scope. Chemosphere 2022, 297, 134075. [Google Scholar] [CrossRef] [PubMed]
- Macit, A.; Kizil, M. The effect of olive leaf extract containing natural antioxidant on the formation of heterocyclic aromatic amines in oil free pan-cooked salmon. Clin. Nutr. ESPEN 2021, 46, S635–S636. [Google Scholar] [CrossRef]
- Yeh, G.; Ebeler, J.D.; Ebeler, S.E. Analysis of nitrosamines in foods and beverages. In Chromatographic Analysis of Environmental and Food Toxicants; CRC Press, 2021; pp. 77–91. [Google Scholar]
- Saleem, A.; Sahar, A.; Pasha, I.; Shahid, M. Determination of Adulteration of Chicken Meat into Minced Beef Mixtures using Front Face Fluorescence Spectroscopy Coupled with Chemometric. Korean J. Food Sci. Anim. Resour. 2022, 42, 672–688. [Google Scholar] [CrossRef]
- Nie, W.; Chen, Y.; Zhang, H.; Liu, J.; Peng, Z.; Li, Y. A novel colorimetric sensor array for real-time and on-site monitoring of meat freshness. Anal. Bioanal. Chem. 2022, 414, 6017–6027. [Google Scholar] [CrossRef]
- Windarsih, A.; Rohman, A.; Riswanto, F.D.O.; Dachriyanus; Yuliana, N. D.; Abu Bakar, N.K. The Metabolomics Approaches Based on LC-MS/MS for Analysis of Non-Halal Meats in Food Products: A Review. Agriculture 2022, 12, 984. [Google Scholar] [CrossRef]
- Marques, C.; Toazza, C.E.B.; Lise, C.C.; de Lima, V.A.; Mitterer-Daltoé, M.L. Prediction of food quality parameters in fish burgers by partial least square models using RGB pattern of digital images. J. Food Sci. Technol. 2022, 59, 3312–3317. [Google Scholar] [CrossRef]
- Principato, L.; Secondi, L.; Cicatiello, C.; Mattia, G. Caring more about food: The unexpected positive effect of the Covid-19 lockdown on household food management and waste. Socio-Economic Plan. Sci. 2022, 82, 100953–100953. [Google Scholar] [CrossRef]
- Omofuma, O.O.; Steck, S.E.; Olshan, A.F.; Troester, M.A. The association between meat and fish intake by preparation methods and breast cancer in the Carolina Breast Cancer Study (CBCS). Breast Cancer Res. Treat. 2022, 193, 187–201. [Google Scholar] [CrossRef]
- Saeed, R.; Feng, H.; Wang, X.; Zhang, X.; Fu, Z. Fish quality evaluation by sensor and machine learning: A mechanistic review. Food Control. 2022, 137. [Google Scholar] [CrossRef]
- Cirne, F.; Kappel, C.; Zhou, S.; Mukherjee, S.D.; Dehghan, M.; Petropoulos, J.-A.; Leong, D.P. Modifiable risk factors for prostate cancer in low- and lower-middle-income countries: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2022, 25, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Zaukuu, J.-L.Z.; Benes, E.; Bázár, G.; Kovács, Z.; Fodor, M. Agricultural Potentials of Molecular Spectroscopy and Advances for Food Authentication: An Overview. Processes 2022, 10, 214. [Google Scholar] [CrossRef]
- Reščič, N.; Mayora, O.; Eccher, C.; Luštrek, M. Food Frequency Questionnaire Personalisation Using Multi-Target Regression. Nutrients 2022, 14, 3943. [Google Scholar] [CrossRef]
- Fomena Temgoua, N.S.; Sun, Z.; Okoye, C.O.; Pan, H. Fatty acid profile, physicochemical composition, and sensory properties of atlantic salmon fish (salmo salar) during different culinary treatments. Journal of Food Quality. 2022, 2022, 1–16. [Google Scholar] [CrossRef]
- Kalla, A.; Loucif, L.; Yahia, M. Miscarriage Risk Factors for Pregnant Women: A Cohort Study in Eastern Algeria’s Population. J. Obstet. Gynecol. India 2021, 72, 109–120. [Google Scholar] [CrossRef]
- Mannino, G.; Cirlincione, F.; Gaglio, R.; Franciosi, E.; Francesca, N.; Moschetti, G.; Asteggiano, A.; Medana, C.; Gentile, C.; Settanni, L. Preliminary Investigation of Biogenic Amines in Type I Sourdoughs Produced at Home and Bakery Level. Toxins 2022, 14, 293. [Google Scholar] [CrossRef]
- Ben Akacha, B.; Švarc-Gajić, J.; Elhadef, K.; Ben Saad, R.; Brini, F.; Mnif, W.; Smaoui, S.; Ben Hsouna, A. The Essential Oil of Tunisian Halophyte Lobularia maritima: A Natural Food Preservative Agent of Ground Beef Meat. Life 2022, 12, 1571. [Google Scholar] [CrossRef]
- Hossain, M.B.; Miazie, R.; Nur, A.-A.U.; Paul, S.K.; Abu Bakar, M.; Paray, B.A.; Arai, T. Assessment of Metal Contamination in Water of Freshwater Aquaculture Farms from a South Asian Tropical Coastal Area. Toxics 2022, 10, 536. [Google Scholar] [CrossRef]
- Deng, H.; He, Y.; Cao, H.; Chen, L.; Teng, H. New insight into the effect of hydroxyl substituted flavonoids on the cytotoxicity of 2-amino-3-methylimidazo [4, 5-f] quinoline. Food Frontiers. 2023, 4, 289–296. [Google Scholar] [CrossRef]
- Ye, H.; Yang, J.; Xiao, G.; Zhao, Y.; Li, Z.; Bai, W.; Zeng, X.; Dong, H. A comprehensive overview of emerging techniques and chemometrics for authenticity and traceability of animal-derived food. Food Chem. 2023, 402, 134216. [Google Scholar] [CrossRef] [PubMed]
- Mutz, Y.S.; Rosario, D.K.A.; Bernardo, Y.A.d.A.; Vieira, C.P.; Moreira, R.V.P.; Bernardes, P.C.; Conte-Junior, C.A. Unravelling the relation between natural microbiota and biogenic amines in Brazilian dry-cured loin: a chemometric approach. Int. J. Food Sci. Technol. 2021, 57, 1621–1629. [Google Scholar] [CrossRef]
- Mercado-Molares, C. Seasonal differences in mercury accumulation in Acanthopleura granulata (Gmelin, 1791) (Polyplacophora: Mollusca) in relation to length and weight in Cartagena Bay (Bolívar-Colombia). Abstracts/Toxicology Letters 368S1. 2022, 284, S310. [Google Scholar] [CrossRef]
- Prado-Silva, L.D.; Brancini, G.T.; Braga, G. .; Liao, X.; Ding, T.; Sant’ana, A.S. Antimicrobial photodynamic treatment (aPDT) as an innovative technology to control spoilage and pathogenic microorganisms in agri-food products: An updated review. Food Control. 2021, 132, 108527. [Google Scholar] [CrossRef]
- Bangar, S.P.; Siroha, A.K. Biopolymer-based Films and Coatings: Trends and Challenges; CRC Press: Boca Raton, 2023; pp. 116–139. [Google Scholar]
- Libera, J.; Iłowiecka, K.; Stasiak, D. Consumption of processed red meat and its impact on human health: A review. Int. J. Food Sci. Technol. 2021, 56, 6115–6123. [Google Scholar] [CrossRef]
- Afé, O.H.I.; Douny, C.; Kpoclou, Y.E.; Igout, A.; Mahillon, J.; Anihouvi, V.; Hounhouigan, D.J.; Scippo, M.-L. Insight about methods used for polycyclic aromatic hydrocarbons reduction in smoked or grilled fishery and meat products for future re-engineering: A systematic review. Food Chem. Toxicol. 2020, 141, 111372. [Google Scholar] [CrossRef]
- Rascón, A.J.; Azzouz, A.; Ballesteros, E. Trace level determination of polycyclic aromatic hydrocarbons in raw and processed meat and fish products from European markets by GC-MS. Food Control. 2019, 101, 198–208. [Google Scholar] [CrossRef]
- Khalili, F.; Shariatifar, N.; Dehghani, M.H.; Yaghmaeian, K.; Nodehi, R.N.; Yaseri, M.; Moazzen, M. Polycyclic aromatic hydrocarbons (PAHs) in meat, poultry, fish and related product samples of Iran: a risk assessment study. J. Environ. Heal. Sci. Eng. 2023, 21, 215–224. [Google Scholar] [CrossRef]
- Sahin, S.; Ulusoy, H.I.; Alemdar, S.; Erdogan, S.; Agaoglu, S. The Presence of Polycyclic Aromatic Hydrocarbons (PAHs) in Grilled Beef, Chicken and Fish by Considering Dietary Exposure and Risk Assessment. Korean J. Food Sci. Anim. Resour. 2020, 40, 675–688. [Google Scholar] [CrossRef]
- Onopiuk, A.; Kołodziejczak, K.; Marcinkowska-Lesiak, M.; Poltorak, A. Determination of polycyclic aromatic hydrocarbons using different extraction methods and HPLC-FLD detection in smoked and grilled meat products. Food Chem. 2021, 373, 131506. [Google Scholar] [CrossRef]
- Aveta, A.; Cacciapuoti, C.; Barone, B.; Di Zazzo, E.; Del Giudice, F.; Maggi, M.; Ferro, M.; Terracciano, D.; Busetto, G.M.; Lucarelli, G.; et al. The Impact of Meat Intake on Bladder Cancer Incidence: Is It Really a Relevant Risk? Cancers 2022, 14, 4775. [Google Scholar] [CrossRef] [PubMed]
- Zachara, A.; Gałkowska, D.; Juszczak, L. Contamination of smoked meat and fish products from Polish market with polycyclic aromatic hydrocarbons. Food Control. 2017, 80, 45–51. [Google Scholar] [CrossRef]
- Kafouris, D.; Koukkidou, A.; Christou, E.; Hadjigeorgiou, M.; Yiannopoulos, S. Determination of polycyclic aromatic hydrocarbons in traditionally smoked meat products and charcoal grilled meat in Cyprus. Meat Sci. 2020, 164, 108088. [Google Scholar] [CrossRef] [PubMed]
- Fadnes, L.T.; Økland, J.-M.; Haaland, Ø.A.; Johansson, K.A. Estimating impact of food choices on life expectancy: A modeling study. PLoS Medicine. 2022, 19, e1003889. [Google Scholar]
- Kassis, A.; Chokor, F.A.Z.; Nasreddine, L.; Hwalla, N.; O’neill, L. Food Sources of Fiber and Micronutrients of Concern in Infants and Children in the United Arab Emirates: Findings from the Feeding Infants and Toddlers Study (FITS) and the Kids Nutrition and Health Survey (KNHS) 2020. Nutrients 2022, 14, 2819. [Google Scholar] [CrossRef]
- Liu, T.; Broverman, S.; Puffer, E.S.; Zaltz, D.A.; Thorne-Lyman, A.L.; Benjamin-Neelon, S.E. Dietary Diversity and Dietary Patterns in School-Aged Children in Western Kenya: A Latent Class Analysis. Int. J. Environ. Res. Public Heal. 2022, 19, 9130. [Google Scholar] [CrossRef]
- Shen, Q.; Song, G.; Zhao, Q.; Wang, P.; Yang, H.; Xue, J.; Wang, H.; Cui, Y.; Wang, H. Detection of lipidomics characterization of tuna meat during different wet-aging stages using iKnife rapid evaporative ionization mass spectrometry. Food Res. Int. 2022, 156, 111307. [Google Scholar] [CrossRef]
- Ylilauri, M.P.T.; Hantunen, S.; Lönnroos, E.; Salonen, J.T.; Tuomainen, T.-P.; Virtanen, J.K. Associations of dairy, meat, and fish intakes with risk of incident dementia and with cognitive performance: the Kuopio Ischaemic Heart Disease Risk Factor Study (KIHD). Eur. J. Nutr. 2022, 61, 2531–2542. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Patra, J.-K.; Shin, H.-S. Evaluation of analytical method and risk assessment of polycyclic aromatic hydrocarbons for fishery products in Korea. Food Control. 2021, 131, 108421. [Google Scholar] [CrossRef]
- Hwang, J.; Shin, D.; Kim, H.; Kwon, O. Association of maternal dietary patterns during pregnancy with small-for-gestational-age infants: Korean Mothers and Children’s Environmental Health (MOCEH) study. Am. J. Clin. Nutr. 2021, 115, 471–481. [Google Scholar] [CrossRef]
- Han, B.; Sun, Z.; Chong, J.; Lyu, N.; Rao, H.; Yang, Y. Lipid residue analysis of ceramic vessels from the Liujiawa site of the Rui State (early Iron Age, north China). J. Quat. Sci. 2021, 37, 114–122. [Google Scholar] [CrossRef]
- Pincinato, R.B.M.; Oglend, A.; Bertolini, R.M.B.; Muñoz, A.E.P. The São Paulo wholesale seafood market: A study of fish prices in Brazil. Aquac. Econ. Manag. 2022, 26, 259–282. [Google Scholar] [CrossRef]
- Louis, L.M.; Quirós-Alcalá, L.; Kuiper, J.R.; Diette, G.; Hansel, N.N.; McCormack, M.C.; et al. Variability and predictors of urinary organophosphate ester concentrations among school-aged children. Environmental research. 2022, 212, 113–192. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.A.; Wesselink, A.K.; Schildroth, S.; Calafat, A.M.; Bethea, T.N.; Geller, R.J.; Coleman, C.M.; Fruh, V.; Henn, B.C.; Botelho, J.C.; et al. Correlates of plasma concentrations of per- and poly-fluoroalkyl substances among reproductive-aged Black women. Environ. Res. 2022, 203, 111860. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.A.; Cardoso, R.; Vieira, R.; Almeida, J.C.; Gomes, M.J.; Venâncio, C.; Patarata, L. The Effect of Weaning and Slaughter Age on the Physicochemical and Sensory Characteristics of Arouquesa Beef—A PDO Portuguese Meat. Foods 2022, 11, 2505. [Google Scholar] [CrossRef]
- Xu, X.; Xue, T.; Jiang, Q.; Fan, D.; Wang, M.; Zhao, Y. Inhibitory effects of some hydrocolloids on the formation of NƐ-(carboxymethyl) lysine and NƐ-(carboxyethyl) lysine in chemical models and fish patties. LWT 2022, 162, 113431. [Google Scholar] [CrossRef]
- Johnson, J.J.; A Shaw, P.; Wooller, M.J.; A Venti, C.; Krakoff, J.; Votruba, S.B.; O’brien, D.M. Amino Acid Nitrogen Isotope Ratios Respond to Fish and Meat Intake in a 12-Week Inpatient Feeding Study of Men. J. Nutr. 2022, 152, 2031–2038. [Google Scholar] [CrossRef]
- Kumar, E.; Koponen, J.; Rantakokko, P.; Airaksinen, R.; Ruokojärvi, P.; Kiviranta, H.; Vuorinen, P.J.; Myllylä, T.; Keinänen, M.; Raitaniemi, J.; et al. Distribution of perfluoroalkyl acids in fish species from the Baltic Sea and freshwaters in Finland. Chemosphere 2021, 291, 132688. [Google Scholar] [CrossRef]
- Munsch, S.H.; Greene, C.M.; Mantua, N.J.; Satterthwaite, W.H. One hundred-seventy years of stressors erode salmon fishery climate resilience in California’s warming landscape. Glob. Chang. Biol. 2022, 28, 2183–2201. [Google Scholar] [CrossRef]
- Lewtas, J. Air pollution combustion emissions: Characterization of causative agents and mechanisms associated with cancer, reproductive, and cardiovascular effects. Mutat. Res. Mol. Mech. Mutagen. 2007, 636, 95–133. [Google Scholar] [CrossRef]
- Muroya, S.; Zhang, Y.; Otomaru, K.; Oshima, K.; Oshima, I.; Sano, M.; Roh, S.; Ojima, K.; Gotoh, T. Maternal Nutrient Restriction Disrupts Gene Expression and Metabolites Associated with Urea Cycle, Steroid Synthesis, Glucose Homeostasis, and Glucuronidation in Fetal Calf Liver. Metabolites 2022, 12, 203. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Shilan, L.; Yan, Y.; Xinran, L.; Xinli, L. Daytime restricted feeding promotes circadian desynchrony and metabolic disruption with changes in bile acids profiles and gut microbiota in C57BL/6 Male Mice. The Journal of Nutritional Biochemistry. 2022, 109, 109121. [Google Scholar]
- Fox, S.B.; Zahariadis, E.N.; McDevitt, R.; Grigorova, Y.; Wei, W.; Zernetkina, V.; dos Santos, C.R.; Cezayirli, D.; Juhasz, O.; Shearon, J.; et al. Bioactive Steroid Marinobufagenin in a Mouse Model of Early-Stage Alzheimer's Disease. FASEB J. 2022, 36. [Google Scholar] [CrossRef]
- Hanley, K.L.; Liang, Y.; Wang, G.; Lin, X.; Yang, M.; Karin, M.; et al. Concurrent disruption of the Ras/MAPK and NF-κB pathways induces circadian deregulation and hepatocarcinogenesis. Molecular Cancer Research. 2022, 20, 337–49. [Google Scholar] [CrossRef] [PubMed]
- Caini, S.; Chioccioli, S.; Pastore, E.; Fontana, M.; Tortora, K.; Caderni, G.; Masala, G. Fish Consumption and Colorectal Cancer Risk: Meta-Analysis of Prospective Epidemiological Studies and Review of Evidence from Animal Studies. Cancers 2022, 14, 640. [Google Scholar] [CrossRef]
- Tsuruwaka, Y.; Shimada, E. Reprocessing seafood waste: challenge to develop aquatic clean meat from fish cells. npj Sci. Food 2022, 6, 1–7. [Google Scholar] [CrossRef]
- Reyed, R.M. Simulate the Dynamic Interactions Across Nutritional Supplements, the Polybiome, and the Biochemical Approach Dependent on Patient-Derived Gastrointestinal Knowledge. Journal of Clinical Epidemiology & Toxicology SRC/JCET-153 2023, 133, 2–15. [Google Scholar]
- Dixon, K.A.; Michelsen, M.K.; Carpenter, C.L. Modern Diets and the Health of Our Planet: An Investigation into the Environmental Impacts of Food Choices. Nutrients 2023, 15, 692. [Google Scholar] [CrossRef]
- Zhong, C.; Feng, Y.; Xu, Y. Production of Fish Analogues from Plant Proteins: Potential Strategies, Challenges, and Outlook. Foods 2023, 12, 614. [Google Scholar] [CrossRef]
- Makokha, M.P.; Muliro, P.S.; Ngoda, P.N.; Ghemoh, C.J.; Xavier, C.; Tanga, C.M. Nutritional quality of meat from hen fed diet with full-fat black soldier fly (Hermetia illucens) larvae meal as a substitute to fish meal. J. Funct. Foods 2023, 101. [Google Scholar] [CrossRef]
- Markoulli, M.; Ahmad, S.; Arcot, J.; Arita, R.; Benitez-Del-Castillo, J.; Caffery, B.; Downie, L.E.; Edwards, K.; Flanagan, J.; Labetoulle, M.; et al. TFOS Lifestyle: Impact of nutrition on the ocular surface. Ocul. Surf. 2023, 29, 226–271. [Google Scholar] [CrossRef]
- Mahgoub, S.; Alagawany, M.; Nader, M.; Omar, S.M.; El-Hack, M.E.A.; Swelum, A.; Elnesr, S.S.; Khafaga, A.F.; Taha, A.E.; Farag, M.R.; et al. Recent Development in Bioactive Peptides from Plant and Animal Products and Their Impact on the Human Health. Food Rev. Int. 2021, 1–26. [Google Scholar] [CrossRef]
- Kido, S.; Chosa, E.; Tanaka, R. The effect of six dried and UV-C-irradiated mushrooms powder on lipid oxidation and vitamin D contents of fish meat. Food Chem. 2023, 398, 133917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Jia, J.; Peng, H.; Qian, Q.; Pan, Z.; Liu, D. Nitrite and nitrate in meat processing: Functions and alternatives. Curr. Res. Food Sci. 2023, 6, 100470. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Deng, H.; Zhang, C.; Cao, H.; Huang, Q.; Chen, L. The role of flavonoids in mitigating food originated heterocyclic aromatic amines that concerns human wellness. Food Sci. Hum. Wellness 2023, 12, 975–985. [Google Scholar] [CrossRef]
- Adeyeye, S.A.O. Heterocyclic Amines and Polycyclic Aromatic Hydrocarbons in Cooked Meat Products: A Review. Polycycl. Aromat. Compd. 2018, 40, 1557–1567. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, P.; Zhao, F.-J. Dietary cadmium exposure, risks to human health and mitigation strategies. Critical Reviews in Environmental Science and Technology. 2023, 53, 939–63. [Google Scholar] [CrossRef]
- Farhadian, A.; Jinap, S.; Faridah, A.; Zaidul, I.S. Effects of marinating on the formation of polycyclic aromatic hydrocarbons (benzo [a] pyrene, benzo [b] fluoranthene and fluoranthene) in grilled beef meat. Food Control. 2012, 28, 420–5. [Google Scholar] [CrossRef]
- Xu, Y.; Cheng, Y.; Zhu, Z.; Guo, H.; Bassey, A.P.; Huang, T.; Huang, Y.; Huang, M. Inhibitory effect of mulberry leaf (Morus alba L.) extract on the formation of free and bound heterocyclic amines in pan-fried muscovy duck (Cairina moschata) patties. Food Control. 2023, 144. [Google Scholar] [CrossRef]
- Bulanda, S.; Janoszka, B. Consumption of Thermally Processed Meat Containing Carcinogenic Compounds (Polycyclic Aromatic Hydrocarbons and Heterocyclic Aromatic Amines) versus a Risk of Some Cancers in Humans and the Possibility of Reducing Their Formation by Natural Food Additives—A Literature Review. Int. J. Environ. Res. Public Heal. 2022, 19, 4781. [Google Scholar] [CrossRef]
- Buja, A.; Pierbon, M.; Lago, L.; Grotto, G.; Baldo, V. Breast cancer primary prevention and diet: an umbrella review. International journal of environmental research and public health. 2020, 17, 4731. [Google Scholar] [CrossRef] [PubMed]
- Meurillon, M.; Anderson, C.; Angénieux, M.; Mercier, F.; Kondjoyan, N.; Engel, E. Sensory acceptability of antioxidant-based formulations dedicated to mitigate heterocyclic aromatic amines in cooked meat. Meat Sci. 2023, 198, 109088. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wang, X.; Huang, C.; Liu, X.; Zhao, J.; Yu, I.T.; Christiani, D.C. Consumption of salted meat and its interactions with alcohol drinking and tobacco smoking on esophageal squamous-cell carcinoma. Int. J. Cancer 2015, 137, 582–589. [Google Scholar] [CrossRef]
- Zheng, W.; McLaughlin, J.K.; Gridley, G.; Bjelke, E.; Schuman, L.M.; Silverman, D.T.; Wacholder, S.; Co-Chien, H.T.; Blot, W.J.; Fraumeni, J.F., Jr. A cohort study of smoking, alcohol consumption, and dietary factors for pancreatic cancer (United States). Cancer Causes Control 1993, 4, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Zhang, M.; He, Z.; Qin, F.; Tao, G.; Zhang, S.; Gao, Y.; Chen, J. Inhibitory profiles of chilli pepper and capsaicin on heterocyclic amine formation in roast beef patties. Food Chem. 2017, 221, 404–411. [Google Scholar] [CrossRef]
- Lu, F.; Kuhnle, G.K.; Cheng, Q. Vegetable oil as fat replacer inhibits formation of heterocyclic amines and polycyclic aromatic hydrocarbons in reduced fat pork patties. Food Control. 2017, 81, 113–125. [Google Scholar] [CrossRef]
- Tengilimoglu-Metin, M.M.; Kizil, M. Reducing effect of artichoke extract on heterocyclic aromatic amine formation in beef and chicken breast meat. Meat Sci. 2017, 134, 68–75. [Google Scholar] [CrossRef]
- Hasnol, N.; Jinap, S.; Sanny, M. Effect of different types of sugars in a marinating formulation on the formation of heterocyclic amines in grilled chicken. Food Chem. 2014, 145, 514–521. [Google Scholar] [CrossRef]
- Jinap, S.; Hasnol, N.; Sanny, M.; Jahurul, M. Effect of organic acid ingredients in marinades containing different types of sugar on the formation of heterocyclic amines in grilled chicken. Food Control. 2018, 84, 478–484. [Google Scholar] [CrossRef]
- Cheng, Y.; Yu, Y.; Wang, C.; Zhu, Z.; Huang, M. Inhibitory effect of sugarcane (Saccharum officinarum L.) molasses extract on the formation of heterocyclic amines in deep-fried chicken wings. Food Control. 2021, 119. [Google Scholar] [CrossRef]
- Mendoza, L.C.; Nolos, R.C.; Villaflores, O.B.; Apostol, E.M.D.; Senoro, D.B. Detection of Heavy Metals, Their Distribution in Tilapia spp., and Health Risks Assessment. Toxics 2023, 11, 286. [Google Scholar] [CrossRef] [PubMed]
| HCA Type | Potential Carcinogenicity | References |
|---|---|---|
| 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) | Most abundant and potent HCA; linked to breast, prostate, and colon cancer | [57] |
| 2-Amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) | Potent HCA; linked to colon, liver, and lung cancer | [58] |
| 2-Amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline (DiMeIQx) | Potent HCA; linked to colon and liver cancer | [59] |
| 2-Amino-9H-pyrido[2,3-b]indole (AαC) | Potent HCA; linked to colon and lung cancer | [42] |
| 2-Amino-6-methyldipyrido[1,2-a:3’,2’-d]imidazole (Glu-P-1) | Potent HCA; linked to colon and liver cancer | [58] |
| 2-Amino-3-methylimidazo[4,5-f]quinoline (IQ) | Moderate HCA; linked to colon and liver cancer | [50] |
| 2-Amino-3,7,8-trimethylimidazo[4,5-f]quinoxaline (MeIQx) | Moderate HCA; linked to colon and liver cancer | [33] |
| 2-Amino-6-heterocyclic-4-alkylamino-3-methylimidazo[4,5-f]quinolines (IHLNs) | Moderate HCA; linked to colon cancer | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




