Submitted:
28 August 2023
Posted:
30 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calderón Díaz, J.A.; Fitzgerald, R.M.; Shalloo, L.; Rodrigues da Costa, M.; Niemi, J.; Leonard, F.C.; Kyriazakis, I.; García Manzanilla, E. Financial Analysis of Herd Status and Vaccination Practices for Porcine Reproductive and Respiratory Syndrome Virus, Swine Influenza Virus, and Mycoplasma Hyopneumoniae in Farrow-to-Finish Pig Farms Using a Bio-Economic Simulation Model. Front. Vet. Sci. 2020, 7, 1–14. [Google Scholar] [CrossRef]
- Renken, C.; Nathues, C.; Swam, H.; Fiebig, K.; Weiss, C.; Eddicks, M.; Ritzmann, M.; Nathues, H. Application of an Economic Calculator to Determine the Cost of Porcine Reproductive and Respiratory Syndrome at Farm-Level in 21 Pig Herds in Germany. Porc. Heal. Manag. 2021, 7, 1–12. [Google Scholar] [CrossRef]
- Itch, M. Aujeszky ’ s Disease Auj Eszky ’ s Disease. 2017, 1–7. [Google Scholar]
- Garcia-Morante, B.; Noguera, M.; Klocke, S.; Sommer, K.; Bridger, P. Duration of Immunity against Heterologous Porcine Parvovirus 1 Challenge in Gilts Immunized with a Novel Subunit Vaccine Based on the Viral Protein 2. BMC Vet. Res. 2020, 16, 1–10. [Google Scholar] [CrossRef]
- Glišić, D.; Milićević, V.; Veljović, L.; Milovanović, B.; Kureljušić, B.; Đorđević, I.; Anđelković, K.; Petković, J.; Dačić, M. Patterns of ASFV Transmission in Domestic Pigs in Serbia. Pathogens 2023, 12, 1–10. [Google Scholar] [CrossRef]
- Martínez-López, B.; Alexandrov, T.; Mur, L.; Sánchez-Vizcaíno, F.; Sánchez-Vizcaíno, J.M. Evaluation of the Spatial Patterns and Risk Factors, Including Backyard Pigs, for Classical Swine Fever Occurrence in Bulgaria Using a Bayesian Model. Geospat. Health 2014, 8, 489–501. [Google Scholar] [CrossRef]
- Извештај o Стању у Пoљoпривреди у Републици Србији у 2018. Гoдини. 2018, 81, 1.
- Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Herskin, M.; Miranda Chueca, M.Á.; et al. African Swine Fever and Outdoor Farming of Pigs. EFSA J. 2021, 19. [Google Scholar] [CrossRef]
- Prodanov-Radulović, J.; Đurđević, B.; Petrović, J.; Mirčeta, J.; Polaček, V. African Swine Fever: A Biosecurity Challenge for Domestic Pig Production in Serbia. Arch. Vet. Med. 2022, 15, 21–38. [Google Scholar] [CrossRef]
- Nešković, M.; Ristić, B.; Došenović, R.; Grubač, S.; Petrović, T.; Prodanov-Radulović, J.; Polaček, V. African Swine Fever Outbreak Investigation on Large Commercial Pig Farm in Serbia. Acta Vet. Brno. 2021, 71, 219–229. [Google Scholar] [CrossRef]
- Milićević, V. Prevalence of Enzootic Viral Diseases in Wild Boar (Sus Scrofa) and Risk Analysis for Central Serbia Region, University in Belgrade, 2016.
- Milicevic, V.; Radojicic, S.; Valcic, M.; Ivovic, V.; Radosavljevic, V. Evidence of Aujeszky’s Disease in Wild Boar in Serbia. BMC Vet. Res. 2016, 12. [Google Scholar] [CrossRef]
- Maes, D.G.D.; Dewulf, J.; Piñeiro, C.; Edwards, S.; Kyriazakis, I. A Critical Reflection on Intensive Pork Production with an Emphasis on Animal Health and Welfare. J. Anim. Sci. 2020, 98, S15–S26. [Google Scholar] [CrossRef]
- María, E.; Antonio, M. De; Dauphin, G.; Fusaro, A.A.; Cavicchio, L.; Campalto, M.; Tassoni, L.; Pastori, A.; Belfanti, I.; Carrino, M.; et al. ESFLU Abstract Book. 2023, 25–27.
- Maksimović Zorić, J.; Milićević, V.; Stevančević, O.; Chiapponi, C.; Potkonjak, A.; Stojanac, N.; Kureljušić, B.; Veljović, L.; Radosavljević, V.; Savić, B. Phylogenetic Analysis of HA and Na Genes of Swine Influenza Viruses in Serbia in 2016-2018. Acta Vet. Brno. 2020, 70, 110–125. [Google Scholar] [CrossRef]
- Djurdjević, B.; Polaček, V.; Pajić, M.; Petrović, T.; Vučićević, I.; Vidanović, D.; Aleksić-Kovačević, S. Highly Pathogenic Avian Influenza H5N8 Outbreak in Backyard Chickens in Serbia. Animals 2023, 13. [Google Scholar] [CrossRef]
- Chauhan, R.P.; Gordon, M.L. A Systematic Review of Influenza A Virus Prevalence and Transmission Dynamics in Backyard Swine Populations Globally. Porc. Heal. Manag. 2022, 8, 1–18. [Google Scholar] [CrossRef]
- Novosel, D.; Petrovic, T.; Acinger-Rogic, Ž.; Štukelj, M. Epidemiology and Status of Porcine Reproductive and Respiratory Syndrome in the Western Balkan Region: Challenges and Prospects. Slov. Vet. Res. 2016, 53, 185–193. [Google Scholar]
- Nemes, I.; Molnár, T.; Abonyi, T.; Terjék, Z.; Bálint, Á.; Szabó, I. Eradication of PRRS from Backyard Swine Herds in Hungary between 2012 and 2018. Acta Vet. Hung. 2019, 67, 543–552. [Google Scholar] [CrossRef]
- Touloudi, A.; Valiakos, G.; Athanasiou, L. V.; Birtsas, P.; Giannakopoulos, A.; Papaspyropoulos, K.; Kalaitzis, C.; Sokos, C.; Tsokana, C.N.; Spyrou, V.; et al. A Serosurvey for Selected Pathogens in Greek European Wild Boar. Vet. Rec. Open 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Rodríguez-Prieto, V.; Kukielka, D.; Martínez-López, B.; de las Heras, A.I.; Barasona, J.Á.; Gortázar, C.; Sánchez-Vizcaíno, J.M.; Vicente, J. Porcine Reproductive and Respiratory Syndrome (PRRS) Virus in Wild Boar and Iberian Pigs in South-Central Spain. Eur. J. Wildl. Res. 2013, 59, 859–867. [Google Scholar] [CrossRef]
- Streck, A.F.; Truyen, U. Porcine Parvovirus. Curr. Issues Mol. Biol. 2020, 37, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Pacini, M.I.; Forzan, M.; Cilia, G.; Bertelloni, F.; Fratini, F.; Mazzei, M. Detection and Characterization of Viral Pathogens Associated with Reproductive Failure in Wild Boars in Central Italy. Animals 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Sookhoo, J.R.V.; Brown-Jordan, A.; Blake, L.; Holder, R.B.; Brookes, S.M.; Essen, S.; Carrington, C.V.F.; Brown, I.H.; Oura, C.A.L. Seroprevalence of Economically Important Viral Pathogens in Swine Populations of Trinidad and Tobago, West Indies. Trop. Anim. Health Prod. 2017, 49, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Deka, D.; Barman, N.N.; Deka, N.; Batth, B.K.; Singh, G.; Singh, S.; Agrawal, R.K.; Mukhopadhyay, C.S. ; Ramneek Sero-Epidemiology of Porcine Parvovirus, Circovirus, and Classical Swine Fever Virus Infections in India. Trop. Anim. Health Prod. 2021, 53. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, G.; Longobardi, C.; D’ambrosi, F.; Amoroso, M.G.; D’alessio, N.; Damiano, S.; Ciarcia, R.; Iovane, V.; Iovane, G.; Pagnini, U.; et al. Aujeszky’s Disease in South-Italian Wild Boars (Sus Scrofa): A Serological Survey. Animals 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Freuling, C.M.; Hlinak, A.; Schulze, C.; Sehl-Ewert, J.; Wysocki, P.; Szentiks, C.A.; Schmitt, K.; Wohlsein, P.; Kluth, G.; Reinhardt, I.; et al. Suid Alphaherpesvirus 1 of Wild Boar Origin as a Recent Source of Aujeszky’s Disease in Carnivores in Germany. Virol. J. 2023, 20, 1–11. [Google Scholar] [CrossRef]
- Konjević, D.; Sučec, I.; Turk, N.; Barbić, L.; Prpić, J.; Krapinec, K.; Bujanić, M.; Jemeršić, L.; Keros, T. Epidemiology of Aujeszky Disease in Wild Boars (Sus Scrofa L.) in Croatia. Vet. Res. Commun. 2023, 47, 631–639. [Google Scholar] [CrossRef] [PubMed]
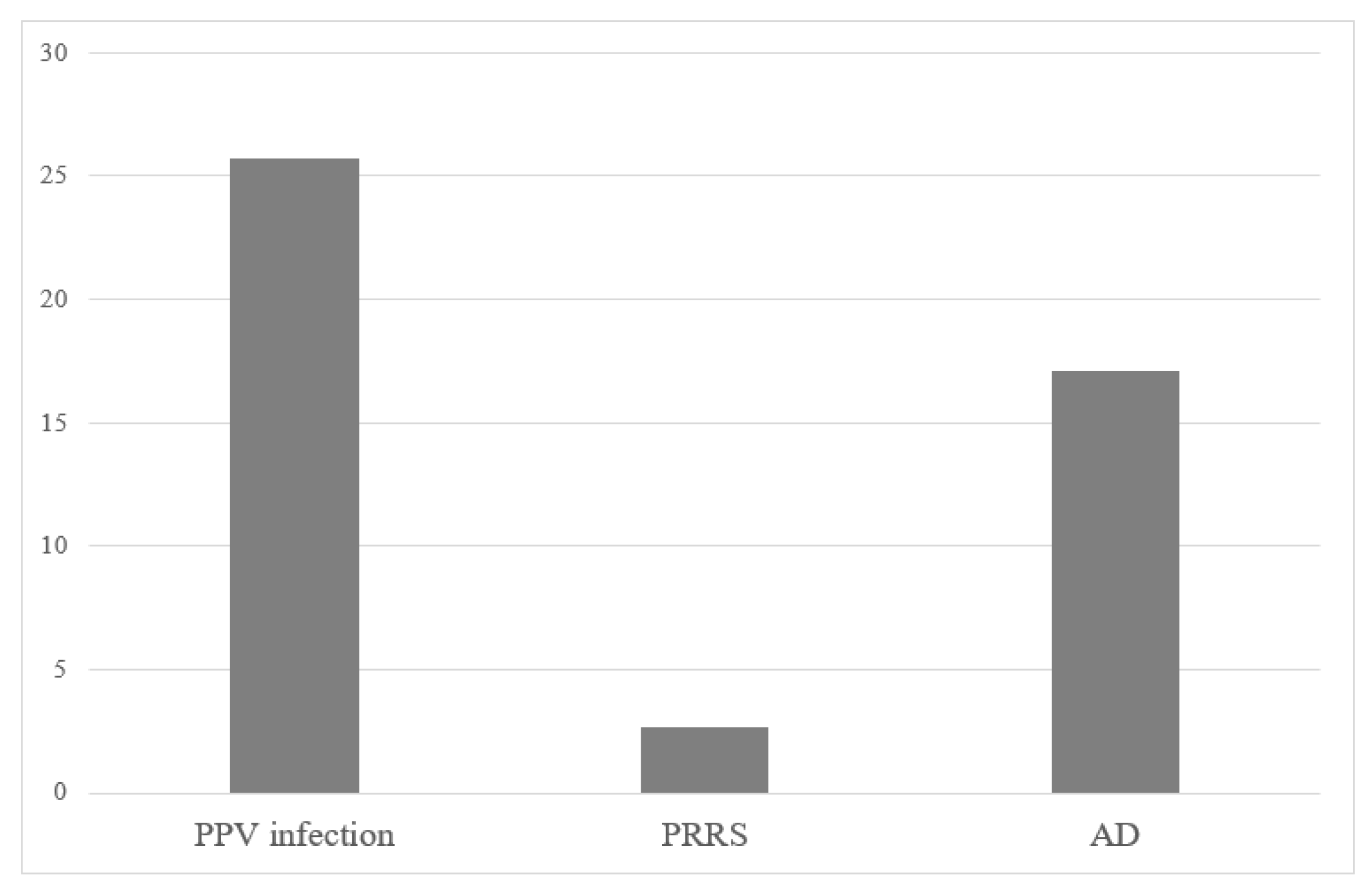
| Farm category | No (%) | PRRS | PPV infection | AD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| neg | pos | % | neg | pos | % | neg | pos | % | ||
| No sows | 3 (4.4) | 3 | 0 | 0.00 | 2 | 1 | 33.33 | 2 | 1 | 33.33 |
| 1-2 sows | 46 (66.7) | 45 | 1 | 2.17 | 29 | 17 | 36.96 | 35 | 11 | 23.91 |
| 3-10 sows | 20 (29) | 19 | 1 | 5.00 | 12 | 8 | 40.00 | 13 | 7 | 35.00 |
| SUM | 67 | 2 | 2.9 | 43 | 26 | 37.7 | 50 | 19 | 27.5 | |
| No sows | 1-2 sows | 3-10 sows | SUM % | ||||
|---|---|---|---|---|---|---|---|
| No | % | No | % | No | % | ||
| Negative | 2 | 66.7 | 24 | 52.2 | 8 | 40.0 | 49.3 |
| Mono PRRS | 0 | 0.0 | 1 | 2.2 | 1 | 5.0 | 2.9 |
| Mono PPV | 0 | 0.0 | 10 | 21.7 | 4 | 20.0 | 20.3 |
| Mono AD | 0 | 0.0 | 4 | 8.7 | 3 | 15.0 | 10.1 |
| PPV+AD | 1 | 33.3 | 7 | 15.2 | 4 | 20.0 | 17.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





