Submitted:
29 August 2023
Posted:
30 August 2023
You are already at the latest version
Abstract
Keywords:
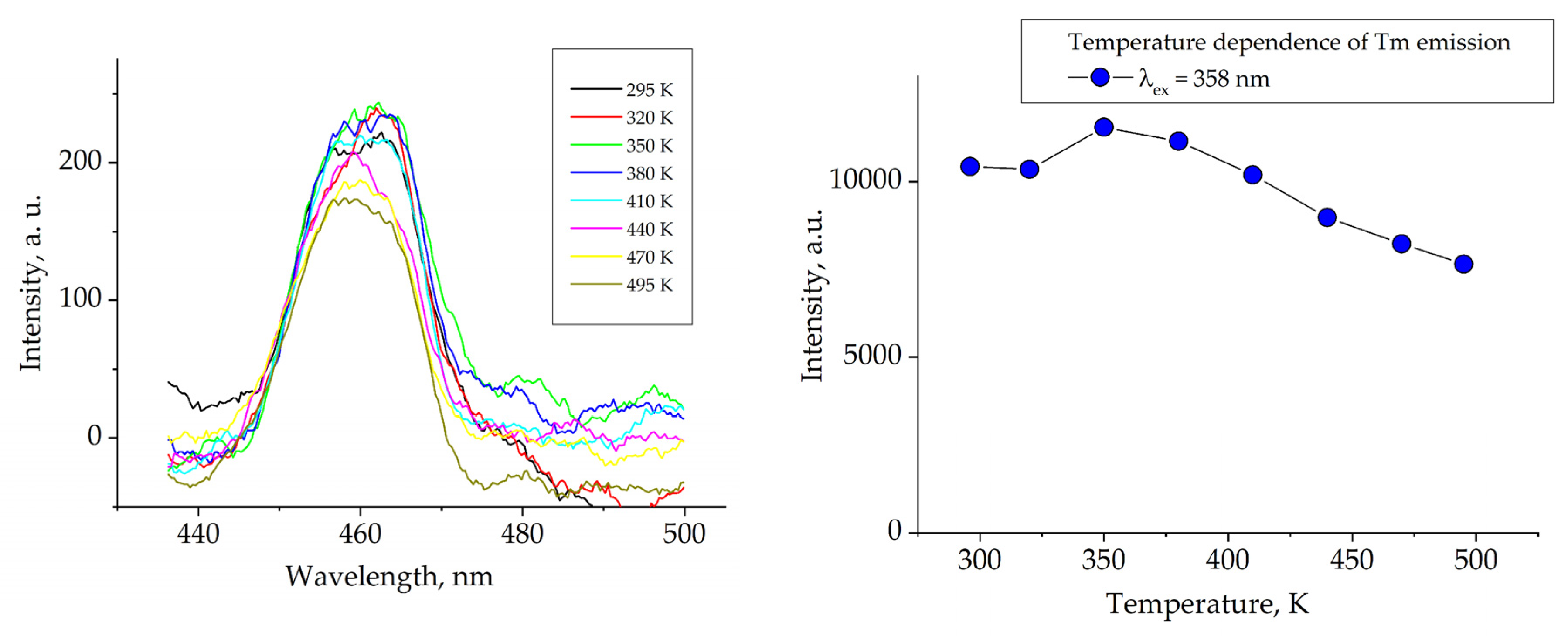
1. Introduction
2. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
References
- Leonyuk, N.I.; Maltsev, V.V.; Volkova, E.A. Crystal chemistry of high-temperature borates. Molecules 2020, 25(10), 2450. [Google Scholar] [CrossRef] [PubMed]
- Hölsä, J.; Leskelä, M. Fluorescence spectrum, energy level scheme and crystal field analysis of europium(+III) doped lanthanum magnesium borate LaMgB5O10:Eu3+. Molecular Physics 1985, 54(3), 657–667. [Google Scholar] [CrossRef]
- Mitina, D.D.; Maltsev, V.V.; Deineko, D.V.; Volkova, E.A.; Koporulina, E.V.; Kuzmin, N.N.; Kosorukov, V.L.; Jiliaeva, A.I. Luminescent properties of lanthanum-magnesium pentaborate crystals doped by Tb3+ and Eu3+ ions. J. Inorganic chemistry 2023, 9. (in press). [Google Scholar]
- Saubat, B.; Vlasse, M.; Fouassier, C. Synthesis and structural study of the new rare earth magnesium borates LnMgB5O10 (Ln = La, …, Er). J. Solid State Chem. 1980, 34(3). 271–277. [CrossRef]
- Fouassier, C.; Saubat, B.; Hagenmuller, P. Self-quenching of Eu3+ and Tb3+ luminescencein LaMgB5O10: a host structure allowing essentially one-dimensional interactions. J. Lumin. 1981, 23, 405–412. [Google Scholar] [CrossRef]
- Saubat, B.; Fouassier, C.; Hagenmuller, P. Luminescent efficiency of Eu3+ and Tb3+ in LaMgB5O10 - type borates under excitation from 100 to 400 nm. Mater. Res. Bull. 1981, 16, 193–198. [Google Scholar] [CrossRef]
- Knitel, M.J.; Dorenbos, P.; van Eijk, C.W.E.; Plasteig, B.; Viana, B.; Kahn-Harari, A.; Vivien, D. Photoluminescence, and scintillation/thermoluminescence yields of several Ce3+ and Eu2+ activated borates. Nuclear Instruments and Methods in Physics Research A 2000, 443(2-3), 364–374. [CrossRef]
- Lin, C.K.; Yu, M.; Pang, M.L.; Lin, J. Photoluminescent properties of sol–gel derived (La,Gd)MgB5O10:Ce3+/Tb3+ nanocrystalline thin films. Opt. Mater. 2006, 28(8-9), 913–918. [CrossRef]
- El Jouhari, N.; Parent, C.; Le Flem, G. Photoluminescence of Ce3+, Tb3+, and Mn2+ in Glasses of Base Composition LaMgB5O10. J. Solid State Chem. 1996, 123(2), 398–407. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, H.; Sun, S.; Yuan, F.; Zhang, L.; Lin, Z.; Zhang, G.; Wang, G. Growth, thermal, spectral and laser properties of Nd:LaMgB5O10 crystal – A new promising laser material. J. Alloys Compd. 2015, 646, 1083–1088. [Google Scholar] [CrossRef]
- Sun, S.; Wei, Q.; Li, B.; Shi, X.; Yuan, F.; Lou, F.; Zhang, L.; Lin, Z.; Zhong, D.; Huang, Y.; Teng, B. The YMgB5O10 crystal preparation and attractive multi-wavelength emission characteristics of doping Nd3+ ions. J. Mater. Chem. C, 2021, 9, 1945. [Google Scholar] [CrossRef]
- Huang, Y.; Lou, F.; Sun, S.; Yuan, F.; Zhang, L.; Lin, Z.; You, Z. Spectroscopy and laser performance of Yb3+:GdMgB5O10 crystal. J. Lumin. 2017, 188, 7–11. [Google Scholar] [CrossRef]
- Mitina, D.D.; Maltsev, V.V.; Leonyuk, N.I.; Gorbachenya, K.N.; Deineka, R.V.; Kisel, V.E.; Yasukevich, A.S.; Kuleshov, N.V. Growth and Characterization of RMgB5O10 (R = Y, La, Gd) Crystals. Inorg. Mater. 2020, 56(2), 211–222. [Google Scholar] [CrossRef]
- Maltsev, V.V.; Mitina, D.D.; Belokoneva, E.L. , Volkova, E.A.; Koporulina E.V.; Jiliaeva, A.I. Synthesis and flux-growth of rare-earth magnesium pentaborate crystals RMgB5O10 (R=Y, Gd, La, Tm and Yb). J. Cryst. Growth 2022, 587, 126628. [Google Scholar] [CrossRef]
- Leonyuk, N.I.; Leonyuk, L.I. Growth and characterization of RM3(BO3)4 crystals. Progr. Cryst. Growth Charact. 1995, 31, 179–278. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction, CrysAlis Pro Software System, Version 1.171.64.119a, Rigaku Corporation, Oxford, UK, 2018.
- Giannozzi, P.; Baroni, S.; Bonini, N. et al. QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke,K. ; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77(18), 3865–3868. [Google Scholar] [CrossRef]
- Lin, J.S.; Qteish, A.; Payne, M. C.; Heine, V. Optimized and transferable nonlocal separableab initiopseudopotentials. Phys. Review B 1993, 47(8), 4174–4180. [Google Scholar] [CrossRef]
- Petricek, V.; Dusek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features, Z. Kristallogr. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Zhang, J.; Tao, X.; Cai, G.; Jin, Z. Phase relation, structure, and properties of borate MgYB5O10 in MgO–Y2O3–B2O3 system. Powder Diffraction 2017, 32(02), 97–106. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, W.; Sun, S.; Yuan, F.; Zhang, L.; Zhao, W.; Wang, G.; Lin, Z. Growth, structure, spectral and laser properties of Yb3+:LaMgB5O10– a new laser material. CrystEngComm 2015, 17(38), 7392–7397. [Google Scholar] [CrossRef]
- Palatinus, L. Ab initio determination of incommensurately modulated structures by charge-flipping in super space. Acta Cryst. A 2004, 60, 604–610. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Gorbachenya, K.N.; Yasukevich, A.S.; Lazarchuk, A.I.; Kisel, V.E.; Kuleshov, N.V.; Volkova, E.A.; Maltsev, V.V.; Koporulina, E.V.; Yapaskurt, V.O.; Kuzmin, N.N.; et al. Growth and Spectroscopy of Yb:YMgB5O10 Crystal. Crystals 2022, 12, 986. [Google Scholar] [CrossRef]
- Yao, L.; Chen, G.; Yang, T.; Yuan, C.; Zhou, C. Energy transfer, optical and luminescent properties in Tm3+/Tb3+/Sm3+ tri-doped borate glasses. J. Mater. Sci. Mater. Electron. 2016, 28(1), 553–558. [Google Scholar] [CrossRef]
- Kuwik, M.; Kowalska, K.; Pisarska, J.; Pisarski, W.A. Spectroscopic Properties of Pr3+, Tm3+, and Ho3+ in Germanate-Based Glass Systems Modified by TiO2. Materials 2023, 16, 61. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Pérez, J.A.; González, F.; Meza-Avendaño, C.A.; De Los Santos, I.M.; López-Juárez, R.; Hernández, I.; Alonso-Guzman, E.M.; Martinez-Molina, W.; Chavez-Garcia, H.L. Structural, optical and photoluminescence properties of TiO2 and TiO2: Tm3+ nanopowders. Optik 2020, 166083. [Google Scholar] [CrossRef]
- Guerbous, L.; Derbal, M.; Chaminade, J.P. Photoluminescence and energy transfer of Tm3+ doped LiIn (WO4)2 blue phosphors. J. Lumin. 2010, 130(12), 2469–2475. [Google Scholar] [CrossRef]
- Kozhan, T.M.; Kuznetsova, V.V.; Pershukevich, P.P.; Sergeev, I.I.; Khomenko, V.S.; Chernyavskii, V.A. Concentration dependence of the quantum yield of luminescence of Tm3+ ions in gadolinium oxychloride. J. Appl. Spectrosc. 2004, 71(6), 829–836. [Google Scholar] [CrossRef]
- Makhov, V.N.; Uvarova, T.V.; Kirm, M.; Vielhauer, S. Thermal quenching of luminescence of BaY2F8 crystals activated with Er3+ and Tm3+ ions. Bulletin of the Lebedev Physics Institute 2016, 43(12), 348–351. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Xu, S.; Yu, H.; Zhang, J.; Zhang, X.; Cao, Y.; Cheng, L.; Sun, J.; Chen, B. Temperature-dependent luminescence properties of Dy3+, Tm3+ single-/co-doped YNbO4 phosphors. Optik 2021, 238, 166524. [Google Scholar] [CrossRef]

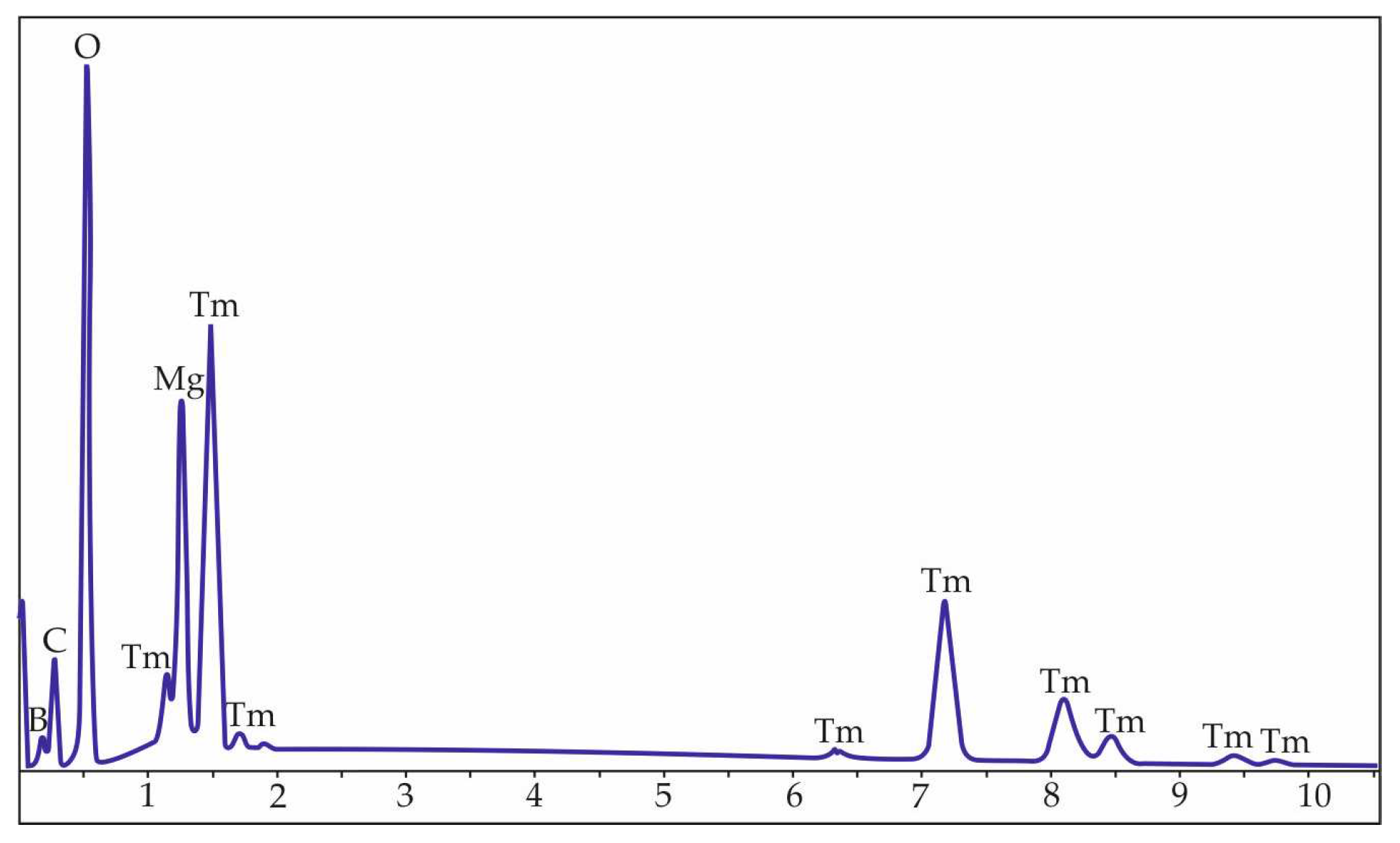

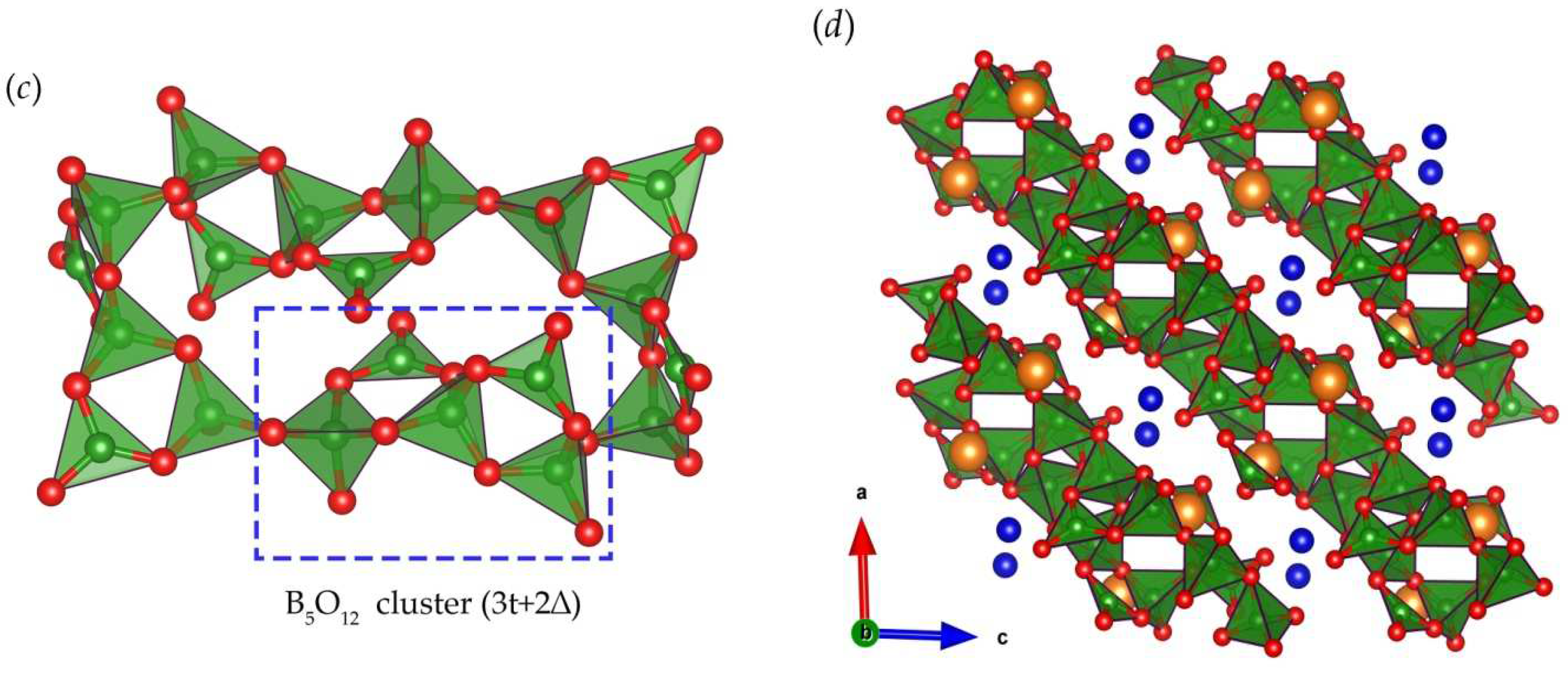
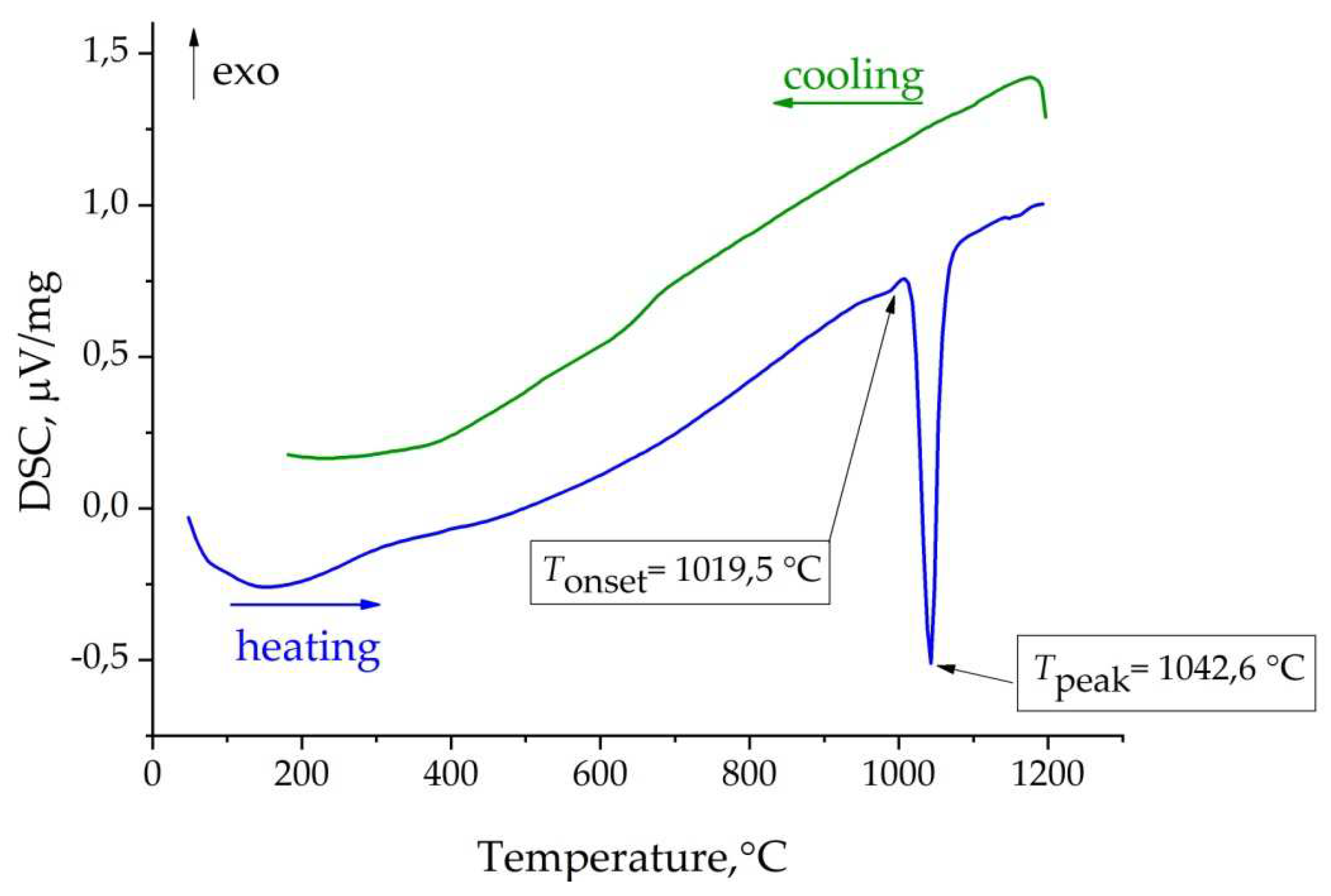
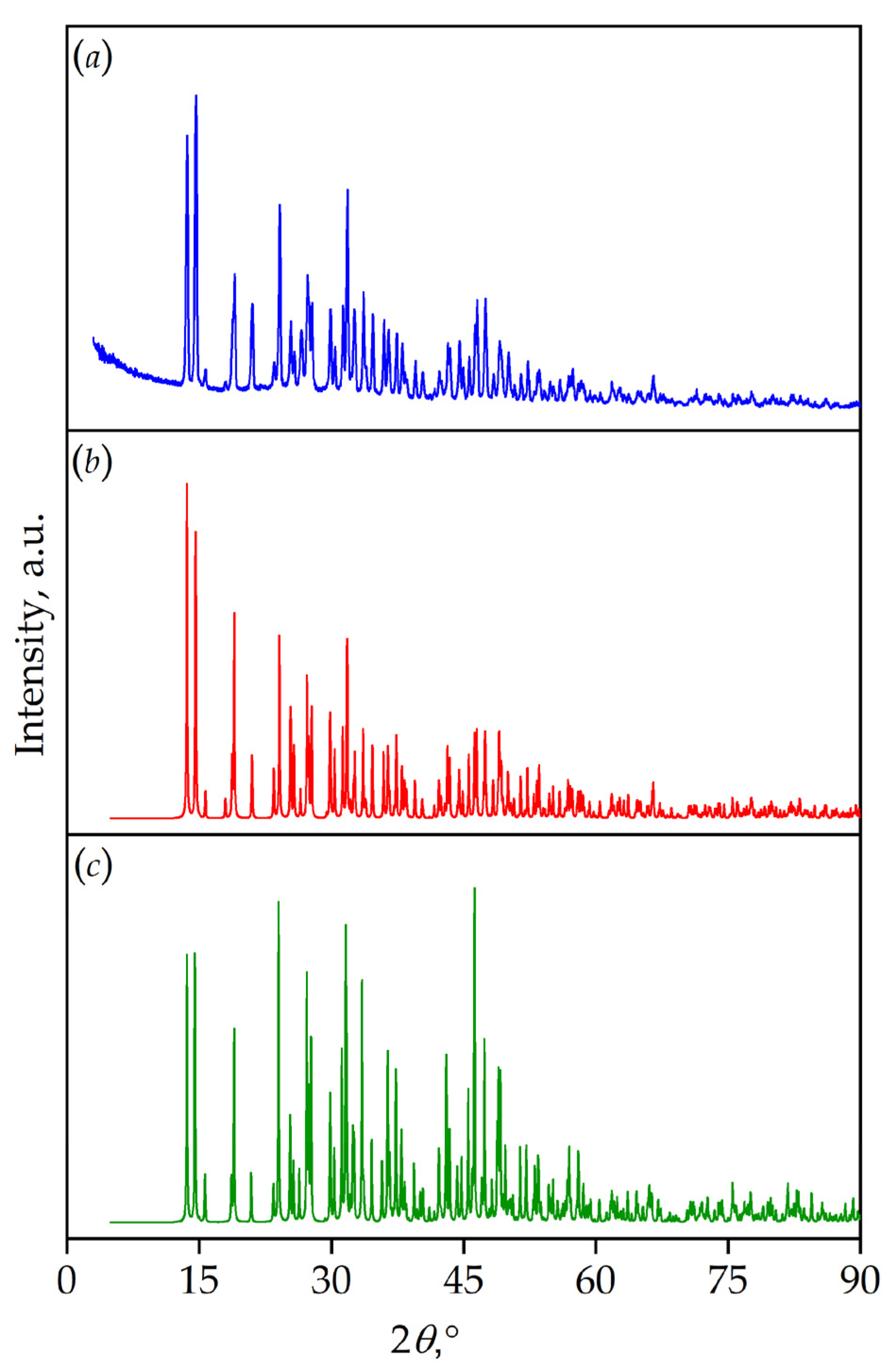
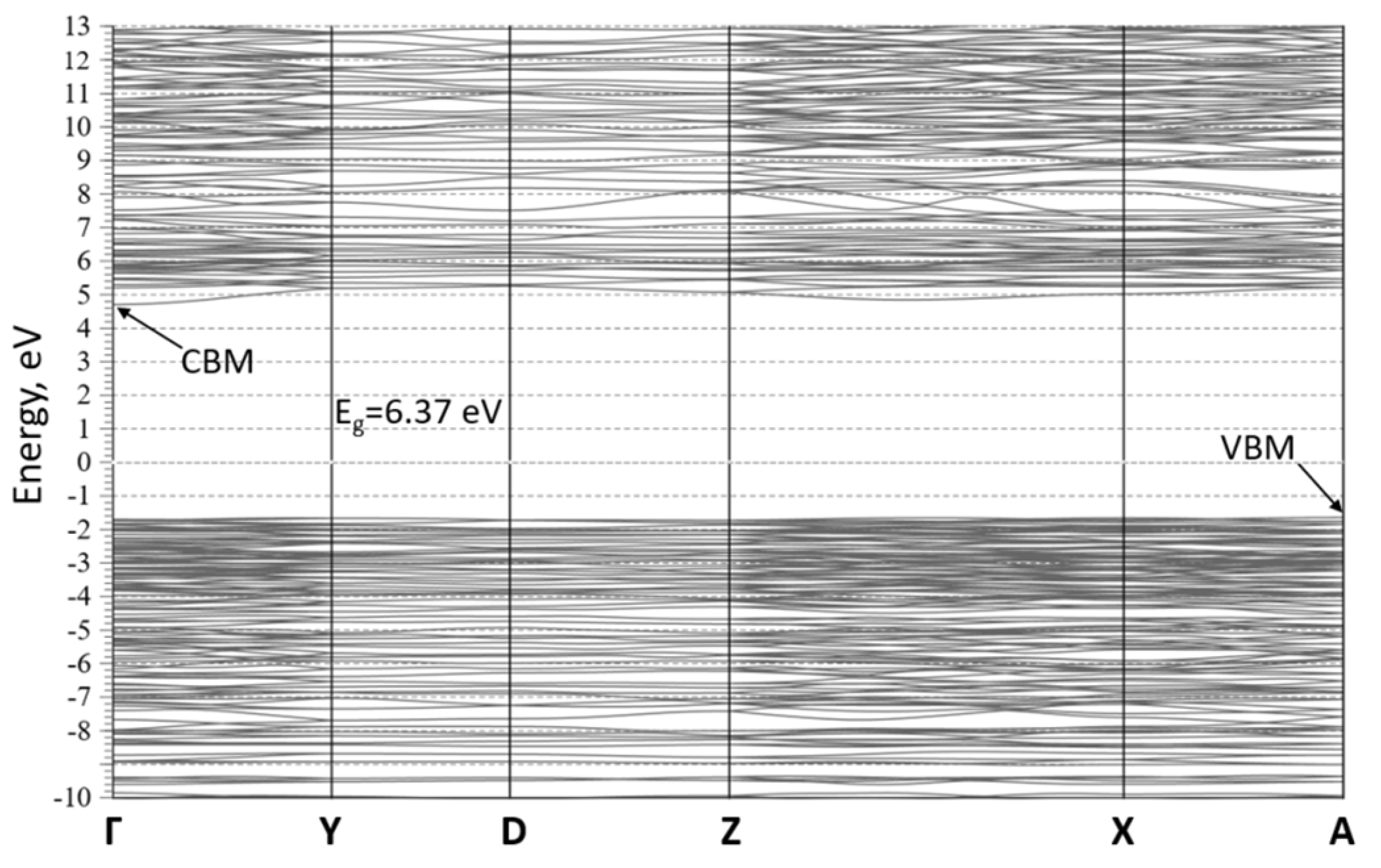
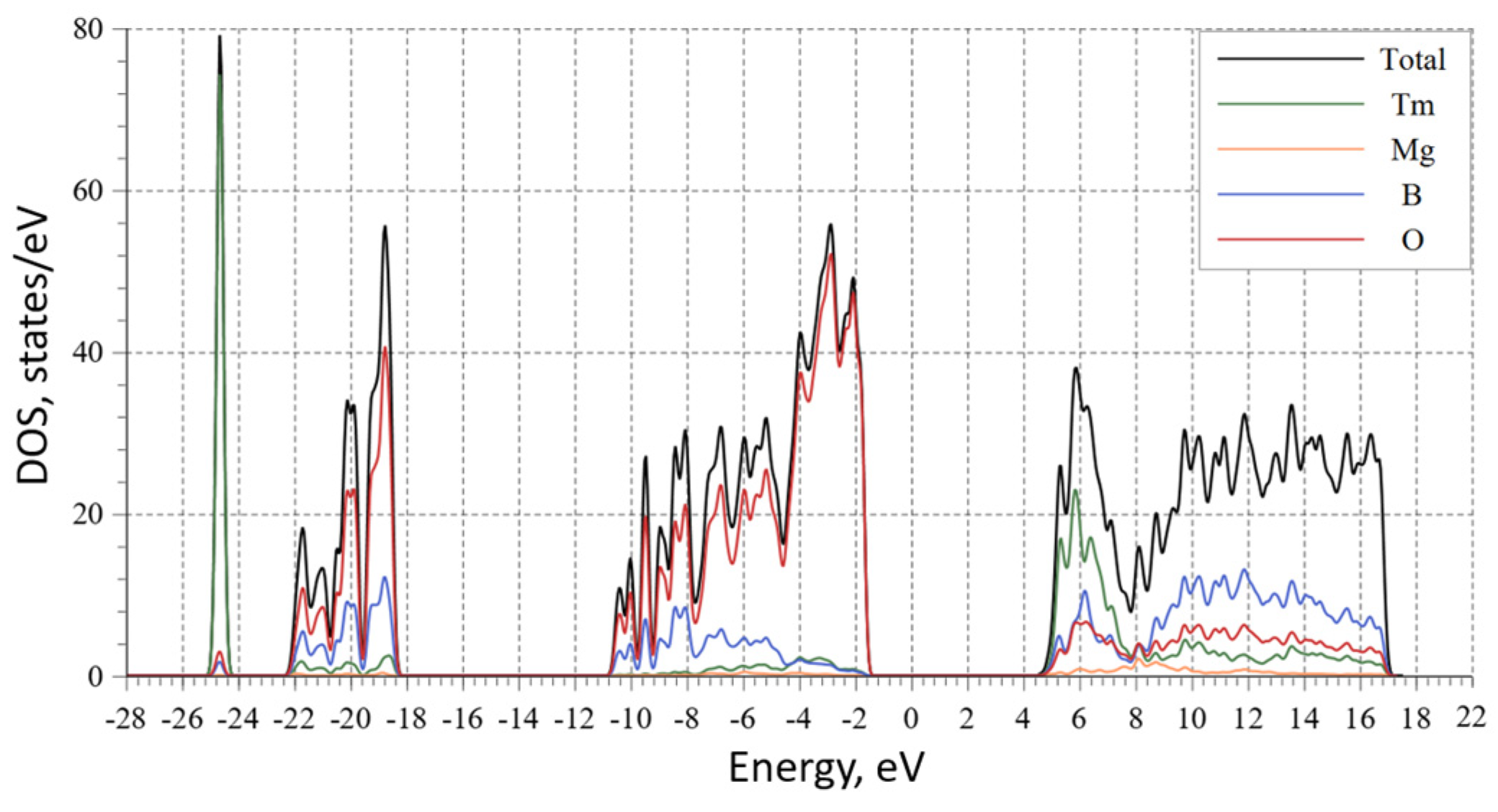
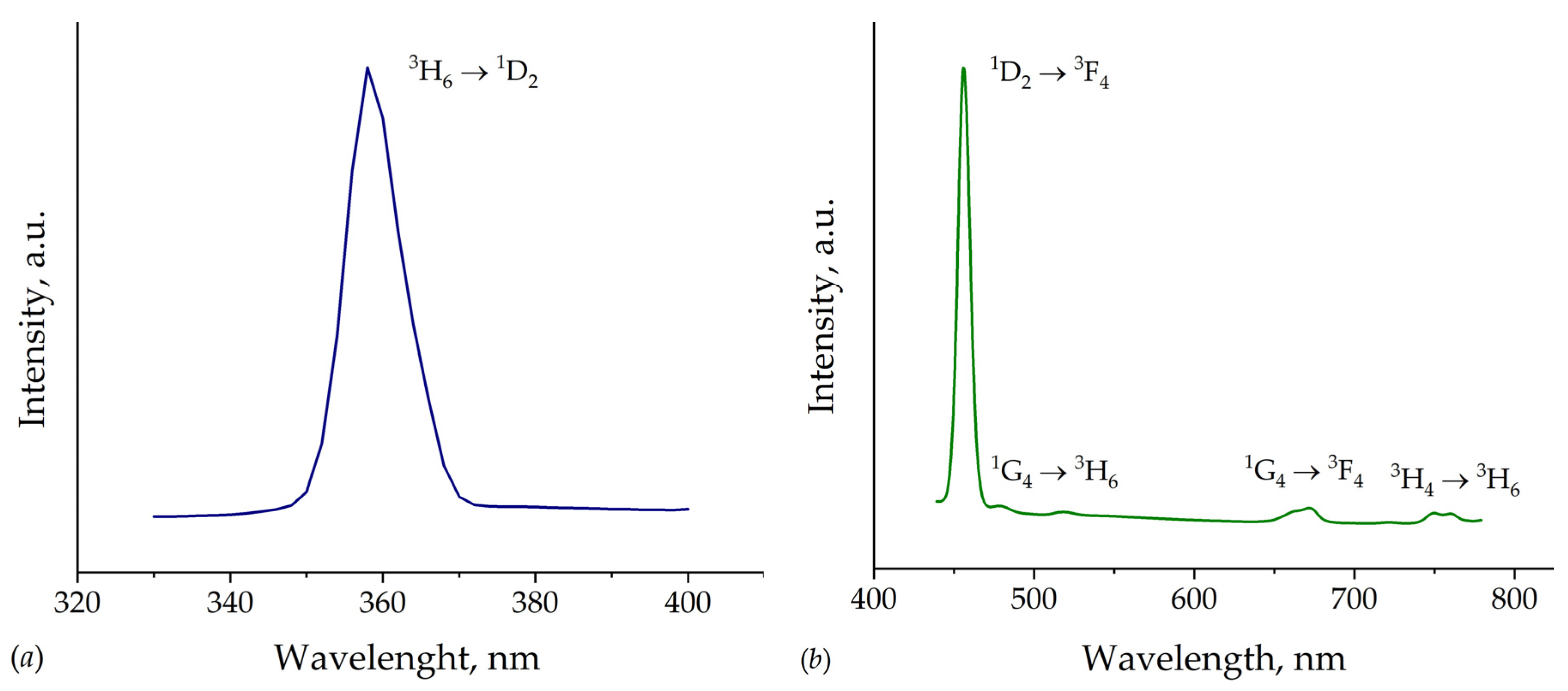
| Chemical formula | TmMgB5O10 |
|---|---|
| Sp.gr., Z | P21/n, 4 |
| а, b, c, Å | 8.476(1) 7.577(1) 9.368(1) |
| β, ° | 94.035(3) |
| V, Å | 600.1(1) |
| D, g/cm3 | 4.508 |
| Radiation; λ, Å | MoKα; 0.71069 |
| μ, mm–1 | 14.946 |
| Т, K | 295 |
| Diffractometer | Xcalibur |
| Scan type | ω |
| Absorption coefficient; Тmin/Tmax | By cut; 0.125/1.000 |
| θmax, ° | 30.61 |
| Limitation of h, k, l | –11 ≤ h ≤ 12; –10 ≤ k ≤ 10; –12 ≤ l ≤ 13 |
| The number of reflections: measured/ independent (N1) / I > 3σ(I) (N2) |
8445 / 1601 / 1303 |
| Rint, % | 19.2 |
| The method of refinement | OLS by F2 |
| The number of refined parameters | 71 |
| Consideration of extinction, k | Type 2 |
| R1/Rw2 on N1 | 0.0543/0.1045 |
| R1/Rw2 on N2 | 0.0638/0.1056 |
| S | 2.42 |
| Δρmin/Δρmax | –5.54 / 5.50 |
| Atom | x/a | y/b | z/c | Ueq, Å2 |
|---|---|---|---|---|
| Tm1 | 0.3152(1) | 0.6865(1) | 0.2582(1) | 0.0065(1) |
| Mg1 | 0.596(1) | 0.406(1) | 0.130(1) | 0.005(1) |
| B1 | 0.335(1) | 0.898(1) | 0.491(1) | 0.006(1) |
| B2 | 0.436(1) | 0.186(1) | -0.089(1) | 0.006(1) |
| B3 | -0.084(1) | 0.573(1) | 0.254(1) | 0.008(1) |
| B4 | 0.517(1) | 0.676(1) | 0.602(1) | 0.003(1) |
| B5 | 0.224(1) | -0.034(1) | -0.056(1) | 0.006(1) |
| O1 | 0.250(1) | 0.945(1) | 0.370(1) | 0.006(1) |
| O2 | 0.320(1) | 0.124(1) | -0.011(1) | 0.007(1) |
| O3 | 0.812(1) | 0.532(1) | 0.121(1) | 0.006(1) |
| O4 | 0.682(1) | 0.150(1) | 0.140(1) | 0.006(1) |
| O5 | 0.451(1) | 0.770(1) | 0.475(1) | 0.007(1) |
| O6 | 0.508(1) | 0.350(1) | -0.073(1) | 0.006(1) |
| O7 | 0.490(1) | 0.093(1) | -0.201(1) | 0.008(1) |
| O8 | 0.583(1) | 0.472(1) | 0.351(1) | 0.007(1) |
| O9 | 0.038(1) | 0.705(1) | 0.228(1) | 0.002(1) |
| O10 | 0.677(1) | 0.611(1) | 0.580(1) | 0.007(1) |
| Atoms | Distance, Å | Atoms | Distance, Å |
|---|---|---|---|
| Tm1 – O1 | 2.304(7) | B2 – O2 | 1.347(13) |
| Tm1 – O1 | 2.236(7) | B2 – O6 | 1.387(12) |
| Tm1 – O2 | 2.747(7) | B2 – O7 | 1.363(13) |
| Tm1 – O5 | 2.354(7) | <B2 – O> | 1.365 |
| Tm1 – O6 | 2.389(7) | B3 – O3 | 1.512(12) |
| Tm1 – O7 | 2.441(7) | B3 – O4 | 1.456(13) |
| Tm1 – O8 | 2.875(6) | B3 – O7 | 1.452(13) |
| Tm1 – O9 | 2.355(6) | B3 – O9 | 1.467(12) |
| Tm1 – O10 | 2.713(7) | <B3 – O> | 1.472 |
| Tm1 – O10 | 2.497(1) | B4 – O5 | 1.462(12) |
| <Tm1 – O> | 2.4911 | B4 – O8 | 1.492(12) |
| Mg1 – O3 | 2.067(7) | B4 – O9 | 1.485(12) |
| Mg1 – O4 | 2.071(8) | B4 – O10 | 1.472(12) |
| Mg1 – O6 | 2.040(7) | <B4 – O> | 1.478 |
| Mg1 – O6 | 2.104(7) | B5 – O2 | 1.490(12) |
| Mg1 – O8 | 2.137(7) | B5 – O4 | 1.455(13 |
| Mg1 – O9 | 2.371(7) | B5 – O8 | 1.504(11) |
| <Mg1 – O> | 2.131 | B5 – O10 | 1.483(13) |
| B1 – O1 | 1.347(12) | <B5 – O> | 1.483 |
| B1 – O3 | 1.353(13) | ||
| B1 – O5 | 1.398(12) | ||
| <B1 – O> | 1.366 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





