Submitted:
27 August 2023
Posted:
30 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Epidemiology of Tuberculosis
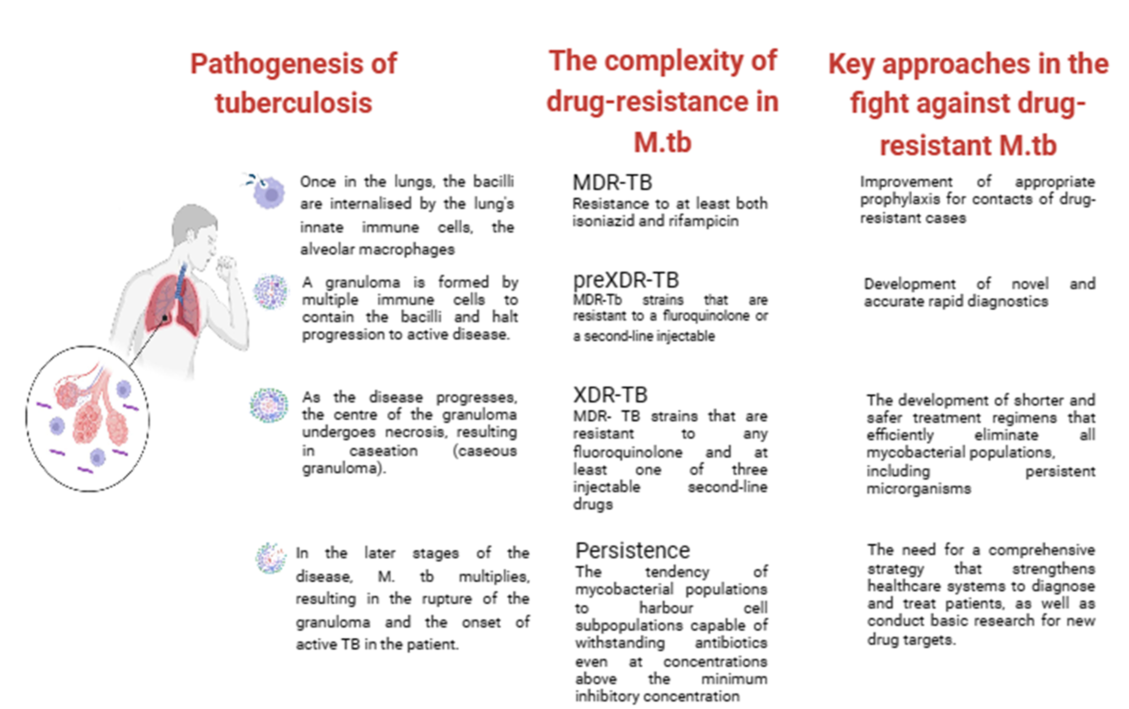
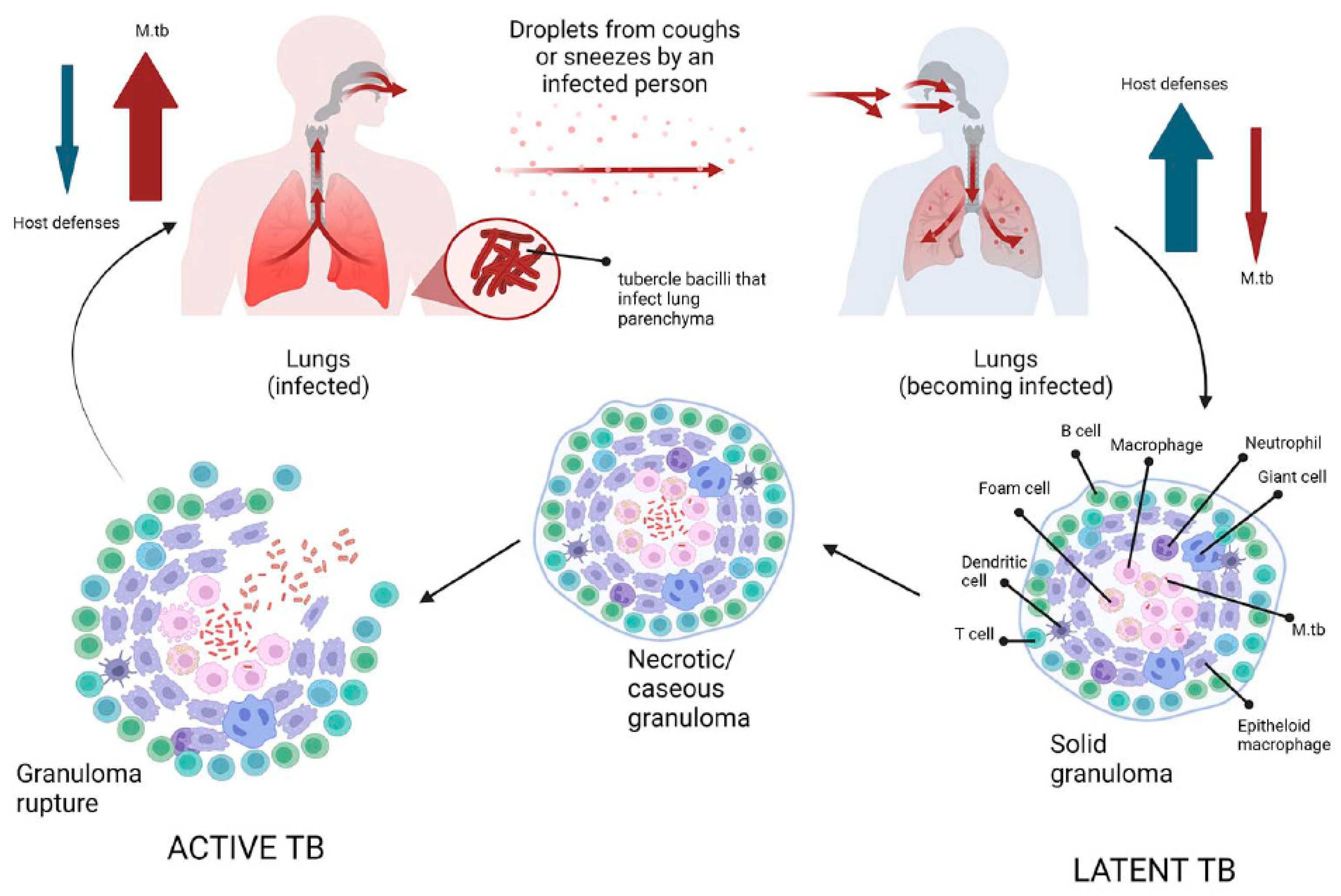
3. Insights into Pathogenesis of Tuberculosis
4. Effects of SARS-CoV-2 infection on latent TB
5. Treatment of LTBI and TB Disease
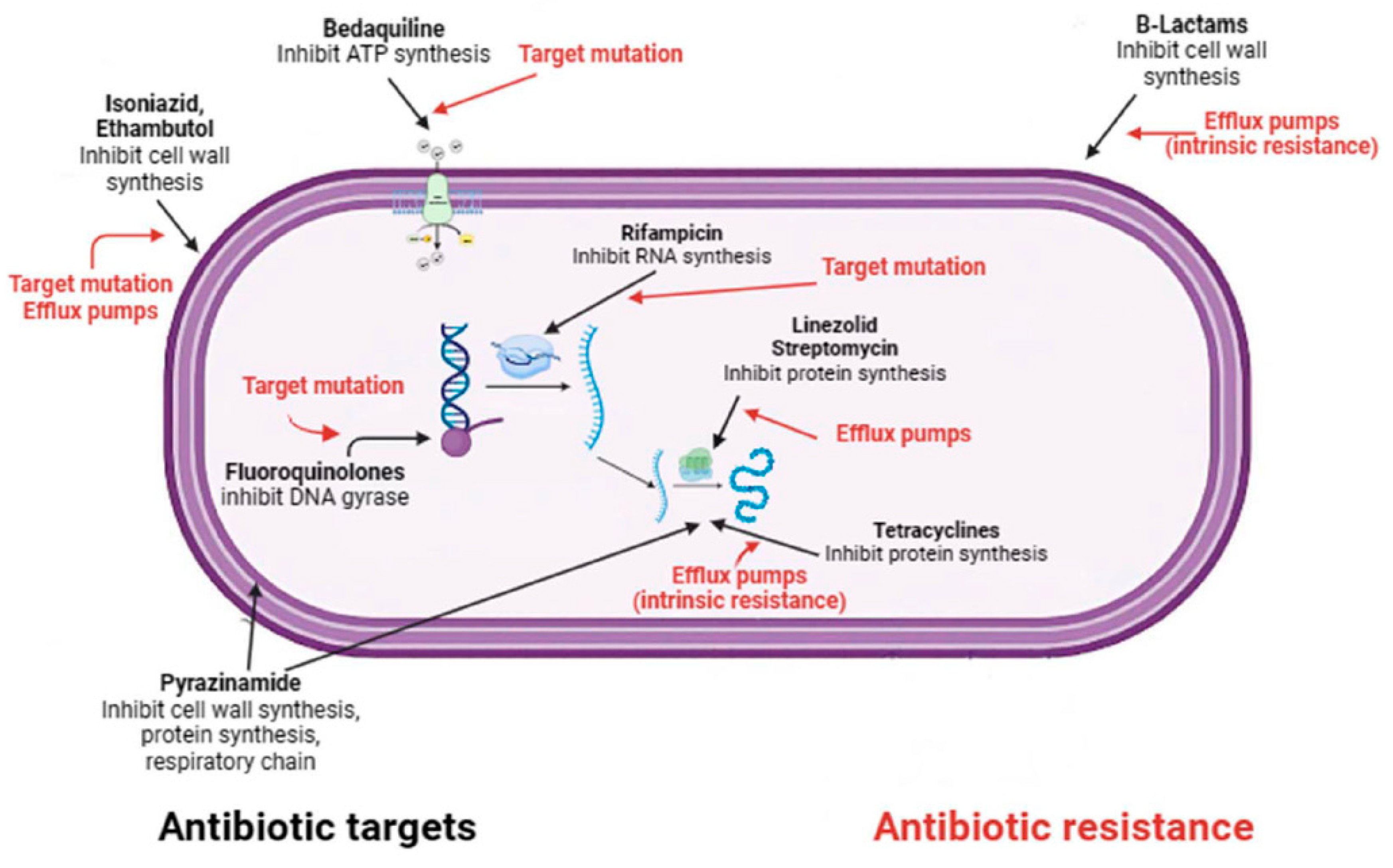
6. The complexity of drug resistance in M.tb
7. New insights into the treatment of MDR-TB
8. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shuaib, Y.A.; Utpatel, C.; Kohl, T.A.; Barilar, I.; Diricks, M.; Ashraf, N.; Wieler, L.H.; Kerubo, G.; Mesfin, E.A.; Diallo, A.B.; et al. Origin and Global Expansion of Mycobacterium tuberculosis Complex Lineage 3. Genes 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Coscolla, M.; Gagneux, S.; Menardo, F.; Loiseau, C.; Ruiz-Rodriguez, P.; Borrell, S.; Otchere, I.D.; Asante-Poku, A.; Asare, P.; Sanchez-Buso, L.; et al. Phylogenomics of Mycobacterium africanum reveals a new lineage and a complex evolutionary history. Microbial genomics 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.L.; Ca, B.; Osorio, N.S.; Rodrigues, P.N.S.; Maceiras, A.R.; Saraiva, M. tuberculosis caused by Mycobacterium africanum: Knowns and unknowns. PLoS pathogens 2022, 18, e1010490. [Google Scholar] [CrossRef] [PubMed]
- Kanabalan, R.D.; Lee, L.J.; Lee, T.Y.; Chong, P.P.; Hassan, L.; Ismail, R.; Chin, V.K. Human tuberculosis and Mycobacterium tuberculosis complex: A review on genetic diversity, pathogenesis and omics approaches in host biomarkers discovery. Microbiological research 2021, 246, 126674. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.A.; Laver, P.N.; Michel, A.L.; Williams, M.; van Helden, P.D.; Warren, R.M.; Gey van Pittius, N.C. Novel Mycobacterium tuberculosis complex pathogen, M. mungi. Emerging infectious diseases 2010, 16, 1296–1299. [Google Scholar] [CrossRef]
- van Ingen, J.; Rahim, Z.; Mulder, A.; Boeree, M.J.; Simeone, R.; Brosch, R.; van Soolingen, D. Characterization of Mycobacterium orygis as M. tuberculosis complex subspecies. Emerging infectious diseases 2012, 18, 653–655. [Google Scholar] [CrossRef]
- Ghodbane, R.; Drancourt, M. Non-human sources of Mycobacterium tuberculosis. Tuberculosis 2013, 93, 589–595. [Google Scholar] [CrossRef]
- Grange, J.M. The biology of the genus Mycobacterium. Society for Applied Bacteriology symposium series 1996, 25, 1S–9S. [Google Scholar] [CrossRef]
- Phillips, C.J.; Foster, C.R.; Morris, P.A.; Teverson, R. The transmission of Mycobacterium bovis infection to cattle. Research in veterinary science 2003, 74, 1–15. [Google Scholar] [CrossRef]
- Somoskovi, A.; Dormandy, J.; Mayrer, A.R.; Carter, M.; Hooper, N.; Salfinger, M. "Mycobacterium canettii" isolated from a human immunodeficiency virus-positive patient: first case recognized in the United States. Journal of clinical microbiology 2009, 47, 255–257. [Google Scholar] [CrossRef]
- Comas, I.; Gagneux, S. A role for systems epidemiology in tuberculosis research. Trends in microbiology 2011, 19, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Abascal, E.; Genestet, C.; Valera, A.; Herranz, M.; Martinez-Lirola, M.; Munoz, P.; Dumitrescu, O.; Garcia de Viedma, D. Assessment of closely related Mycobacterium tuberculosis variants with different transmission success and in vitro infection dynamics. Scientific reports 2021, 11, 11041. [Google Scholar] [CrossRef] [PubMed]
- Saelens, J.W.; Viswanathan, G.; Tobin, D.M. Mycobacterial Evolution Intersects With Host Tolerance. Frontiers in immunology 2019, 10, 528. [Google Scholar] [CrossRef]
- Ma, J.; Vongpradith, A.; Ledesma, J.R.; Novotney, A.; Yi, S.; Lim, K.; Hay, S.I.; Murray, C.J.L.; Kyu, H.H. Progress towards the 2020 milestones of the end TB strategy in Cambodia: estimates of age and sex specific TB incidence and mortality from the Global Burden of Disease Study 2019. BMC infectious diseases 2022, 22, 904. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, R.; Glaziou, P.; Harris, J.B.; Date, A.; Floyd, K.; Kasaeva, T. Epidemiology of Tuberculosis and Progress Toward Meeting Global Targets - Worldwide, 2019. MMWR. Morbidity and mortality weekly report 2021, 70, 427–430. [Google Scholar] [CrossRef]
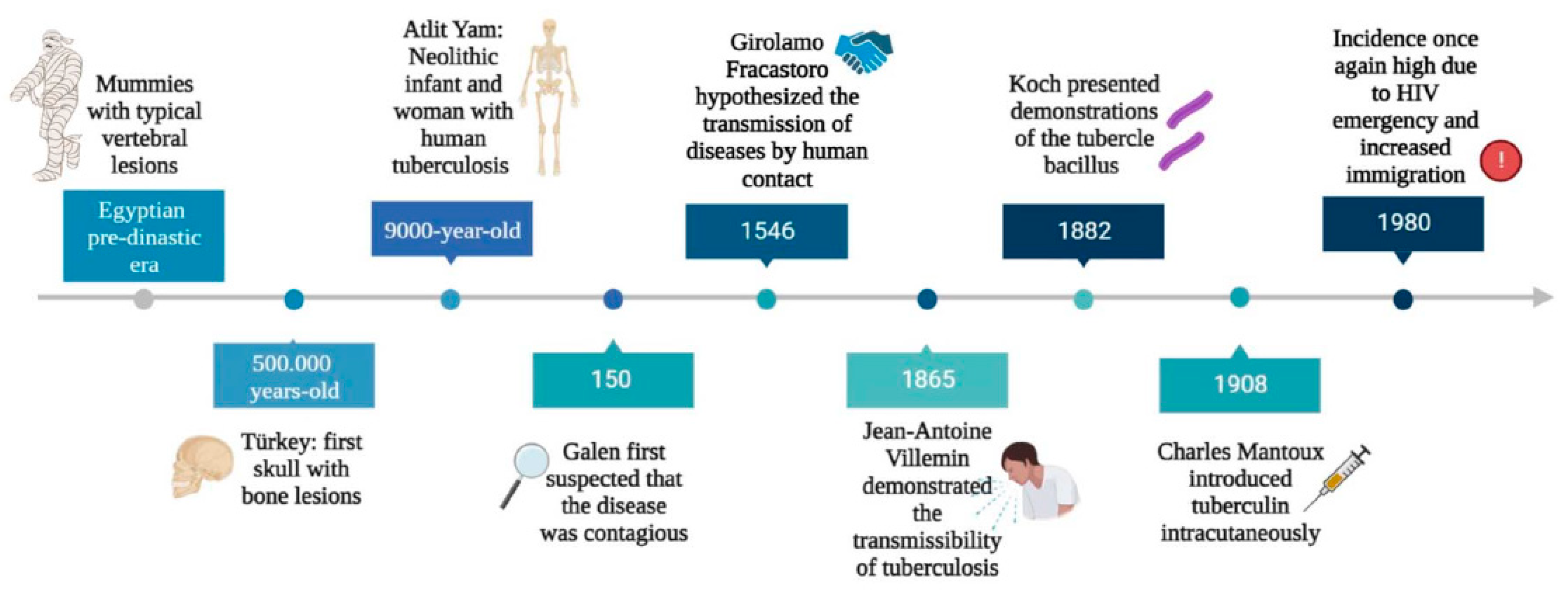
- Barberis, I.; Bragazzi, N.L.; Galluzzo, L.; Martini, M. The history of tuberculosis: from the first historical records to the isolation of Koch's bacillus. Journal of preventive medicine and hygiene 2017, 58, E9–E12. [Google Scholar]
- Buzic, I.; Giuffra, V. The paleopathological evidence on the origins of human tuberculosis: a review. Journal of preventive medicine and hygiene 2020, 61, E3–E8. [Google Scholar] [CrossRef]
- Hershkovitz, I.; Donoghue, H.D.; Minnikin, D.E.; Besra, G.S.; Lee, O.Y.; Gernaey, A.M.; Galili, E.; Eshed, V.; Greenblatt, C.L.; Lemma, E.; et al. Detection and molecular characterization of 9,000-year-old Mycobacterium tuberculosis from a Neolithic settlement in the Eastern Mediterranean. PloS one 2008, 3, e3426. [Google Scholar] [CrossRef]
- Holloway, K.L.; Link, K.; Ruhli, F.; Henneberg, M. Skeletal lesions in human tuberculosis may sometimes heal: an aid to palaeopathological diagnoses. PloS one 2013, 8, e62798. [Google Scholar] [CrossRef]
- McDonald, S.K.; Matisoo-Smith, E.A.; Buckley, H.R.; Walter, R.K.; Aung, H.L.; Collins, C.J.; Cook, G.M.; Kardailsky, O.; Krause, J.; Knapp, M. 'TB or not TB': the conundrum of pre-European contact tuberculosis in the Pacific. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 2020, 375, 20190583. [Google Scholar] [CrossRef]
- Ojo, O.O.; Nadarajah, S.; Kebe, M. Integer time series models for tuberculosis in Africa. Scientific reports 2023, 13, 11443. [Google Scholar] [CrossRef] [PubMed]
- Cambau, E.; Drancourt, M. Steps towards the discovery of Mycobacterium tuberculosis by Robert Koch, 1882. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases 2014, 20, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Daniel, V.S.; Daniel, T.M. Old Testament biblical references to tuberculosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 1999, 29, 1557–1558. [Google Scholar] [CrossRef] [PubMed]
- Pesapane, F.; Marcelli, S.; Nazzaro, G. Hieronymi Fracastorii: the Italian scientist who described the "French disease". Anais brasileiros de dermatologia 2015, 90, 684–686. [Google Scholar] [CrossRef]
- Oren, E., McDermid, J.M. . Tuberculosis. In Nutrition and Health in a Developing World de Pee, S., Taren, D., Bloem, M., Ed.; Humana Press, Cham: 2017.
- Sandhu, G.K. Tuberculosis: current situation, challenges and overview of its control programs in India. Journal of global infectious diseases 2011, 3, 143–150. [Google Scholar] [CrossRef]
- Riva, M.A. From milk to rifampicin and back again: history of failures and successes in the treatment for tuberculosis. The Journal of antibiotics 2014, 67, 661–665. [Google Scholar] [CrossRef]
- Kestler, B.; Tyler, S.K. Latent tuberculosis testing through the ages: the search for a sleeping killer. American journal of physiology. Lung cellular and molecular physiology 2022, 322, L412–L419. [Google Scholar] [CrossRef]
- Yang, H.; Kruh-Garcia, N.A.; Dobos, K.M. Purified protein derivatives of tuberculin--past, present, and future. FEMS immunology and medical microbiology 2012, 66, 273–280. [Google Scholar] [CrossRef]
- Dutour, O.; Colombo, A.; Coqueugniot, H. Was the rise of TB contemporaneous with the industrial revolution? Epidemiological evolution of TB in France (17th-20th centuries) inferred from osteoarchaeological and historical archives. International journal of paleopathology 2021, 34, 130–133. [Google Scholar] [CrossRef]
- Tripp, L.; Sawchuk, L.A. Insights into secular trends of respiratory tuberculosis: The 20th century Maltese experience. PloS one 2017, 12, e0183296. [Google Scholar] [CrossRef]
- Al-Humadi, H.W.; Al-Saigh, R.J.; Al-Humadi, A.W. Addressing the Challenges of Tuberculosis: A Brief Historical Account. Frontiers in pharmacology 2017, 8, 689. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.D. The world-wide increase in tuberculosis: how demographic changes, HIV infection and increasing numbers in poverty are increasing tuberculosis. Annals of medicine 2003, 35, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Cummings, K.J. Tuberculosis control: challenges of an ancient and ongoing epidemic. Public health reports 2007, 122, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Borgdorff, M.W.; van Soolingen, D. The re-emergence of tuberculosis: what have we learnt from molecular epidemiology? Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases 2013, 19, 889–901. [Google Scholar] [CrossRef]
- Porter, J.D.; McAdam, K.P. The re-emergence of tuberculosis. Annual review of public health 1994, 15, 303–323. [Google Scholar] [CrossRef]
- Rossi, G. [The re-emergence of tuberculosis: the endemia of multiple-antibiotic-resistant strains]. Recenti progressi in medicina 1996, 87, 487–488. [Google Scholar]
- Vall Mayans, M.; Maguire, A.; Miret, M.; Alcaide, J.; Parron, I.; Casabona, J. The spread of AIDS and the re-emergence of tuberculosis in Catalonia, Spain. Aids 1997, 11, 499–505. [Google Scholar] [CrossRef]
- Soko, R.N.; Burke, R.M.; Feasey, H.R.A.; Sibande, W.; Nliwasa, M.; Henrion, M.Y.R.; Khundi, M.; Dodd, P.J.; Ku, C.C.; Kawalazira, G.; et al. Effects of Coronavirus Disease Pandemic on Tuberculosis Notifications, Malawi. Emerging infectious diseases 2021, 27, 1831–1839. [Google Scholar] [CrossRef]
- Hargreaves, J.R.; Boccia, D.; Evans, C.A.; Adato, M.; Petticrew, M.; Porter, J.D. The social determinants of tuberculosis: from evidence to action. American journal of public health 2011, 101, 654–662. [Google Scholar] [CrossRef]
- Zwerling, A.; Hanrahan, C.; Dowdy, D.W. Ancient Disease, Modern Epidemiology: A Century of Progress in Understanding and Fighting Tuberculosis. American journal of epidemiology 2016, 183, 407–414. [Google Scholar] [CrossRef]
- Barry, C.E. Lessons from seven decades of antituberculosis drug discovery. Current topics in medicinal chemistry 2011, 11, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Cegielski, J.P. Extensively drug-resistant tuberculosis: "there must be some kind of way out of here". Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2010, 50 Suppl 3, S195-200. [CrossRef]
- Yang, Q.; Han, J.; Shen, J.; Peng, X.; Zhou, L.; Yin, X. Diagnosis and treatment of tuberculosis in adults with HIV. Medicine 2022, 101, e30405. [Google Scholar] [CrossRef] [PubMed]
- Glaziou, P.; Floyd, K.; Raviglione, M.C. Global Epidemiology of Tuberculosis. Seminars in respiratory and critical care medicine 2018, 39, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Chakaya, J.; Petersen, E.; Nantanda, R.; Mungai, B.N.; Migliori, G.B.; Amanullah, F.; Lungu, P.; Ntoumi, F.; Kumarasamy, N.; Maeurer, M.; et al. The WHO Global Tuberculosis 2021 Report - not so good news and turning the tide back to End TB. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases 2022, 124 Suppl 1, S26-S29. [CrossRef]
- Hamada, Y.; Getahun, H.; Tadesse, B.T.; Ford, N. HIV-associated tuberculosis. International journal of STD & AIDS 2021, 32, 780–790. [Google Scholar] [CrossRef]
- Cai, L.; Hu, X.; Huang, Y.; Huang, X.; Tong, Y. Editorial: Updates on tuberculosis control and management. Frontiers in public health 2022, 10, 1126429. [Google Scholar] [CrossRef]
- Bloom, B.R.; Atun, R.; Cohen, T.; Dye, C.; Fraser, H.; Gomez, G.B.; Knight, G.; Murray, M.; Nardell, E.; Rubin, E.; et al. Tuberculosis. In Major Infectious Diseases, 3rd ed.; Holmes, K.K., Bertozzi, S., Bloom, B.R., Jha, P., Eds.; Washington (DC), 2017.
- Chakaya, J.; Khan, M.; Ntoumi, F.; Aklillu, E.; Fatima, R.; Mwaba, P.; Kapata, N.; Mfinanga, S.; Hasnain, S.E.; Katoto, P.; et al. Global Tuberculosis Report 2020 - Reflections on the Global TB burden, treatment and prevention efforts. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases 2021, 113 Suppl 1, S7-S12. [CrossRef]
- Jang, J.G.; Chung, J.H. Diagnosis and treatment of multidrug-resistant tuberculosis. Yeungnam University journal of medicine 2020, 37, 277–285. [Google Scholar] [CrossRef]
- (WHO), W.H.O. Tuberculosis. 2023.
- Li, T.; Du, X.; Kang, J.; Luo, D.; Liu, X.; Zhao, Y. Patient, Diagnosis, and Treatment Delays Among Tuberculosis Patients Before and During COVID-19 Epidemic - China, 2018-2022. China CDC weekly 2023, 5, 259–265. [Google Scholar] [CrossRef]
- Dheda, K.; Perumal, T.; Moultrie, H.; Perumal, R.; Esmail, A.; Scott, A.J.; Udwadia, Z.; Chang, K.C.; Peter, J.; Pooran, A.; et al. The intersecting pandemics of tuberculosis and COVID-19: population-level and patient-level impact, clinical presentation, and corrective interventions. The Lancet. Respiratory medicine 2022, 10, 603–622. [Google Scholar] [CrossRef]
- Organization, W.H. Tuberculosis deaths and disease increase during the COVID-19 pandemic. Global Tuberculosis Report 2022 2022. [Google Scholar]
- Dean, A.S.; Tosas Auguet, O.; Glaziou, P.; Zignol, M.; Ismail, N.; Kasaeva, T.; Floyd, K. 25 years of surveillance of drug-resistant tuberculosis: achievements, challenges, and way forward. The Lancet. Infectious diseases 2022, 22, e191–e196. [Google Scholar] [CrossRef]
- Kostyukova, I.; Pasechnik, O.; Mokrousov, I. Epidemiology and Drug Resistance Patterns of Mycobacterium tuberculosis in High-Burden Area in Western Siberia, Russia. Microorganisms 2023, 11. [Google Scholar] [CrossRef]
- Khabibullina, N.F.; Kutuzova, D.M.; Burmistrova, I.A.; Lyadova, I.V. The Biological and Clinical Aspects of a Latent Tuberculosis Infection. Tropical medicine and infectious disease 2022, 7. [Google Scholar] [CrossRef] [PubMed]
- Behr, M.A.; Edelstein, P.H.; Ramakrishnan, L. Revisiting the timetable of tuberculosis. Bmj 2018, 362, k2738. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, A.; Jansson, M.; Skold, M.; Rottenberg, M.E.; Kallenius, G. Tuberculosis and HIV co-infection. PLoS pathogens 2012, 8, e1002464. [Google Scholar] [CrossRef] [PubMed]
- Patterson, B.; Wood, R. Is cough really necessary for TB transmission? Tuberculosis 2019, 117, 31–35. [Google Scholar] [CrossRef]
- Ryndak, M.B.; Laal, S. Mycobacterium tuberculosis Primary Infection and Dissemination: A Critical Role for Alveolar Epithelial Cells. Frontiers in cellular and infection microbiology 2019, 9, 299. [Google Scholar] [CrossRef]
- Nardell, E.A. Transmission and Institutional Infection Control of Tuberculosis. Cold Spring Harbor perspectives in medicine 2015, 6, a018192. [Google Scholar] [CrossRef]
- Delogu, G.; Sali, M.; Fadda, G. The biology of Mycobacterium tuberculosis infection. Mediterranean journal of hematology and infectious diseases 2013, 5, e2013070. [Google Scholar] [CrossRef]
- Chandra, P.; Grigsby, S.J.; Philips, J.A. Immune evasion and provocation by Mycobacterium tuberculosis. Nature reviews. Microbiology 2022, 20, 750–766. [Google Scholar] [CrossRef]
- Smith, I. Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clinical microbiology reviews 2003, 16, 463–496. [Google Scholar] [CrossRef]
- Ahmad, F.; Rani, A.; Alam, A.; Zarin, S.; Pandey, S.; Singh, H.; Hasnain, S.E.; Ehtesham, N.Z. Macrophage: A Cell With Many Faces and Functions in Tuberculosis. Frontiers in immunology 2022, 13, 747799. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.B.; Gern, B.H.; Delahaye, J.L.; Adams, K.N.; Plumlee, C.R.; Winkler, J.K.; Sherman, D.R.; Gerner, M.Y.; Urdahl, K.B. Alveolar Macrophages Provide an Early Mycobacterium tuberculosis Niche and Initiate Dissemination. Cell host & microbe 2018, 24, 439–446. [Google Scholar] [CrossRef]
- Khan, A.; Singh, V.K.; Hunter, R.L.; Jagannath, C. Macrophage heterogeneity and plasticity in tuberculosis. Journal of leukocyte biology 2019, 106, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Behar, S.M.; Martin, C.J.; Booty, M.G.; Nishimura, T.; Zhao, X.; Gan, H.X.; Divangahi, M.; Remold, H.G. Apoptosis is an innate defense function of macrophages against Mycobacterium tuberculosis. Mucosal immunology 2011, 4, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.J.; Booty, M.G.; Rosebrock, T.R.; Nunes-Alves, C.; Desjardins, D.M.; Keren, I.; Fortune, S.M.; Remold, H.G.; Behar, S.M. Efferocytosis is an innate antibacterial mechanism. Cell host & microbe 2012, 12, 289–300. [Google Scholar] [CrossRef]
- Garcia-Bengoa, M.; Meurer, M.; Goethe, R.; Singh, M.; Reljic, R.; von Kockritz-Blickwede, M. Role of phagocyte extracellular traps during Mycobacterium tuberculosis infections and tuberculosis disease processes. Frontiers in microbiology 2023, 14, 983299. [Google Scholar] [CrossRef]
- Ravesloot-Chavez, M.M.; Van Dis, E.; Stanley, S.A. The Innate Immune Response to Mycobacterium tuberculosis Infection. Annual review of immunology 2021, 39, 611–637. [Google Scholar] [CrossRef]
- Cooper, A.M. Cell-mediated immune responses in tuberculosis. Annual review of immunology 2009, 27, 393–422. [Google Scholar] [CrossRef]
- Esmail, H.; Barry, C.E., 3rd; Wilkinson, R.J. Understanding latent tuberculosis: the key to improved diagnostic and novel treatment strategies. Drug discovery today 2012, 17, 514–521. [Google Scholar] [CrossRef]
- Gengenbacher, M.; Kaufmann, S.H. Mycobacterium tuberculosis: success through dormancy. FEMS microbiology reviews 2012, 36, 514–532. [Google Scholar] [CrossRef]
- Petersen, H.J.; Smith, A.M. The role of the innate immune system in granulomatous disorders. Frontiers in immunology 2013, 4, 120. [Google Scholar] [CrossRef] [PubMed]
- Alsayed, S.S.R.; Gunosewoyo, H. Tuberculosis: Pathogenesis, Current Treatment Regimens and New Drug Targets. International journal of molecular sciences 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Wainwright, H.C.; Locketz, M.; Bekker, L.G.; Walther, G.B.; Dittrich, C.; Visser, A.; Wang, W.; Hsu, F.F.; Wiehart, U.; et al. Caseation of human tuberculosis granulomas correlates with elevated host lipid metabolism. EMBO molecular medicine 2010, 2, 258–274. [Google Scholar] [CrossRef] [PubMed]
- Ulrichs, T.; Kosmiadi, G.A.; Trusov, V.; Jorg, S.; Pradl, L.; Titukhina, M.; Mishenko, V.; Gushina, N.; Kaufmann, S.H. Human tuberculous granulomas induce peripheral lymphoid follicle-like structures to orchestrate local host defence in the lung. The Journal of pathology 2004, 204, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Biondo, C.; Midiri, A.; Gerace, E.; Zummo, S.; Mancuso, G. SARS-CoV-2 Infection in Patients with Cystic Fibrosis: What We Know So Far. Life 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Biondo, C.; Ponzo, E.; Midiri, A.; Ostone, G.B.; Mancuso, G. The Dark Side of Nosocomial Infections in Critically Ill COVID-19 Patients. Life 2023, 13. [Google Scholar] [CrossRef]
- Pathak, L.; Gayan, S.; Pal, B.; Talukdar, J.; Bhuyan, S.; Sandhya, S.; Yeger, H.; Baishya, D.; Das, B. Coronavirus Activates an Altruistic Stem Cell-Mediated Defense Mechanism that Reactivates Dormant Tuberculosis: Implications in Coronavirus Disease 2019 Pandemic. The American journal of pathology 2021, 191, 1255–1268. [Google Scholar] [CrossRef]
- Hildebrand, R.E.; Chandrasekar, S.S.; Riel, M.; Touray, B.J.B.; Aschenbroich, S.A.; Talaat, A.M. Superinfection with SARS-CoV-2 Has Deleterious Effects on Mycobacterium bovis BCG Immunity and Promotes Dissemination of Mycobacterium tuberculosis. Microbiology spectrum 2022, 10, e0307522. [Google Scholar] [CrossRef]
- Group, T.C.-G.S. Tuberculosis and COVID-19 co-infection: description of the global cohort. The European respiratory journal 2022, 59. [Google Scholar] [CrossRef]
- Luke, E.; Swafford, K.; Shirazi, G.; Venketaraman, V. TB and COVID-19: An Exploration of the Characteristics and Resulting Complications of Co-infection. Frontiers in bioscience 2022, 14, 6. [Google Scholar] [CrossRef]
- Oh, C.E.; Menzies, D. Four months of rifampicin monotherapy for latent tuberculosis infection in children. Clinical and experimental pediatrics 2022, 65, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Sterling, T.R.; Njie, G.; Zenner, D.; Cohn, D.L.; Reves, R.; Ahmed, A.; Menzies, D.; Horsburgh, C.R., Jr.; Crane, C.M.; Burgos, M.; et al. Guidelines for the Treatment of Latent Tuberculosis Infection: Recommendations from the National Tuberculosis Controllers Association and CDC, 2020. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports 2020, 69, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Assefa, D.G.; Bedru, A.; Zeleke, E.D.; Negash, S.E.; Debela, D.T.; Molla, W.; Mengistu, N.; Woldesenbet, T.T.; Bedane, N.F.; Kajogoo, V.D.; et al. Efficacy and safety of different regimens in the treatment of patients with latent tuberculosis infection: a systematic review and network meta-analysis of randomized controlled trials. Archives of public health = Archives belges de sante publique 2023, 81, 82. [Google Scholar] [CrossRef] [PubMed]
- In WHO consolidated guidelines on tuberculosis: Module 4: treatment - drug-resistant tuberculosis treatment, 2022 update; WHO Guidelines Approved by the Guidelines Review Committee; Geneva, 2022.
- Dartois, V.A.; Rubin, E.J. Anti-tuberculosis treatment strategies and drug development: challenges and priorities. Nature reviews. Microbiology 2022, 20, 685–701. [Google Scholar] [CrossRef]
- Khan, S.R.; Manialawy, Y.; Siraki, A.G. Isoniazid and host immune system interactions: A proposal for a novel comprehensive mode of action. British journal of pharmacology 2019, 176, 4599–4608. [Google Scholar] [CrossRef]
- Campbell, E.A.; Korzheva, N.; Mustaev, A.; Murakami, K.; Nair, S.; Goldfarb, A.; Darst, S.A. Structural mechanism for rifampicin inhibition of bacterial rna polymerase. Cell 2001, 104, 901–912. [Google Scholar] [CrossRef]
- Gopal, P.; Gruber, G.; Dartois, V.; Dick, T. Pharmacological and Molecular Mechanisms Behind the Sterilizing Activity of Pyrazinamide. Trends in pharmacological sciences 2019, 40, 930–940. [Google Scholar] [CrossRef]
- Batt, S.M.; Burke, C.E.; Moorey, A.R.; Besra, G.S. Antibiotics and resistance: the two-sided coin of the mycobacterial cell wall. Cell surface 2020, 6, 100044. [Google Scholar] [CrossRef]
- Occhineri, S.; Matucci, T.; Rindi, L.; Tiseo, G.; Falcone, M.; Riccardi, N.; Besozzi, G. Pretomanid for tuberculosis treatment: an update for clinical purposes. Current research in pharmacology and drug discovery 2022, 3, 100128. [Google Scholar] [CrossRef]
- Bagcchi, S. WHO's Global Tuberculosis Report 2022. The Lancet. Microbe 2023, 4, e20. [Google Scholar] [CrossRef]
- Chowdhury, K.; Ahmad, R.; Sinha, S.; Dutta, S.; Haque, M. Multidrug-Resistant TB (MDR-TB) and Extensively Drug-Resistant TB (XDR-TB) Among Children: Where We Stand Now. Cureus 2023, 15, e35154. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Pereiro, J.; Sanchez-Montalva, A.; Aznar, M.L.; Espiau, M. MDR Tuberculosis Treatment. Medicina 2022, 58. [Google Scholar] [CrossRef] [PubMed]
- Kherabi, Y.; Frechet-Jachym, M.; Rioux, C.; Yazdanpanah, Y.; Mechai, F.; Pourcher, V.; Robert, J.; Guglielmetti, L.; Group, M.-T.M. Revised Definitions of Tuberculosis Resistance and Treatment Outcomes, France, 2006-2019. Emerging infectious diseases 2022, 28, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.S.; Sharma, D.; Hussain, T.; Pati, S. Molecular mechanisms of underlying genetic factors and associated mutations for drug resistance in Mycobacterium tuberculosis. Emerging microbes & infections 2020, 9, 1651–1663. [Google Scholar] [CrossRef]
- Gygli, S.M.; Borrell, S.; Trauner, A.; Gagneux, S. Antimicrobial resistance in Mycobacterium tuberculosis: mechanistic and evolutionary perspectives. FEMS microbiology reviews 2017, 41, 354–373. [Google Scholar] [CrossRef]
- Allue-Guardia, A.; Garcia, J.I.; Torrelles, J.B. Evolution of Drug-Resistant Mycobacterium tuberculosis Strains and Their Adaptation to the Human Lung Environment. Frontiers in microbiology 2021, 12, 612675. [Google Scholar] [CrossRef]
- Li, H.; Guo, H.; Chen, T.; Yu, L.; Chen, Y.; Zhao, J.; Yan, H.; Chen, M.; Sun, Q.; Zhang, C.; et al. Genome-wide SNP and InDel mutations in Mycobacterium tuberculosis associated with rifampicin and isoniazid resistance. International journal of clinical and experimental pathology 2018, 11, 3903–3914. [Google Scholar]
- Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2023, 12. [Google Scholar] [CrossRef]
- Bakhtiyariniya, P.; Khosravi, A.D.; Hashemzadeh, M.; Savari, M. Detection and characterization of mutations in genes related to isoniazid resistance in Mycobacterium tuberculosis clinical isolates from Iran. Molecular biology reports 2022, 49, 6135–6143. [Google Scholar] [CrossRef]
- Jian, Z.; Zeng, L.; Xu, T.; Sun, S.; Yan, S.; Yang, L.; Huang, Y.; Jia, J.; Dou, T. Antibiotic resistance genes in bacteria: Occurrence, spread, and control. Journal of basic microbiology 2021, 61, 1049–1070. [Google Scholar] [CrossRef]
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic resistance and persistence-Implications for human health and treatment perspectives. EMBO reports 2020, 21, e51034. [Google Scholar] [CrossRef] [PubMed]
- Urbaniec, J.; Xu, Y.; Hu, Y.; Hingley-Wilson, S.; McFadden, J. Phenotypic heterogeneity in persisters: a novel 'hunker' theory of persistence. FEMS microbiology reviews 2022, 46. [Google Scholar] [CrossRef] [PubMed]
- Lanni, A.; Iacobino, A.; Fattorini, L.; Giannoni, F. Eradication of Drug-Tolerant Mycobacterium tuberculosis 2022: Where We Stand. Microorganisms 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Al-Saeedi, M.; Al-Hajoj, S. Diversity and evolution of drug resistance mechanisms in Mycobacterium tuberculosis. Infection and drug resistance 2017, 10, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.M.; Adams, K.N.; Eldesouky, H.E.; Sherman, D.R. The evolving biology of Mycobacterium tuberculosis drug resistance. Frontiers in cellular and infection microbiology 2022, 12, 1027394. [Google Scholar] [CrossRef]
- Vanino, E.; Granozzi, B.; Akkerman, O.W.; Munoz-Torrico, M.; Palmieri, F.; Seaworth, B.; Tiberi, S.; Tadolini, M. Update of drug-resistant tuberculosis treatment guidelines: A turning point. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases 2023, 130 Suppl 1, S12-S15. [CrossRef]
- Conradie, F.; Bagdasaryan, T.R.; Borisov, S.; Howell, P.; Mikiashvili, L.; Ngubane, N.; Samoilova, A.; Skornykova, S.; Tudor, E.; Variava, E.; et al. Bedaquiline-Pretomanid-Linezolid Regimens for Drug-Resistant Tuberculosis. The New England journal of medicine 2022, 387, 810–823. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




