Introduction
Fermented dairy products has a long and vibrant history in many cultures of the world. In India, it plays an important place in day to day diet. It’s been proved by several research the various health benefits of fermented products by providing the nutrition and therapeutic properties (Shiby et al., 2013). Due to health awareness among the young Indian population, the cheese and yoghurt is getting popular. Cheese production is growing at approximately 15 percent per year, in India due to increasing growing demand by India’s young demography and increasing urban middle class (Global agricultural information network, USDA, 2015). India’s Yogurt market can hit $1bn by 2021- 2022 (Dairy reporter, 2016).
The phages has been known since it is reported in the waters of Ganges and Yamuna in India by Ernest Hanbury Hankin in the 18th century that had marked antibacterial action against cholera and could pass through a very fine porcelain filter. In 19th century British bacteriologist Frederick Twort, and French –Canadian Microbiologist Felix d’Herelle discovered a small agent capable of infecting bacteria by their independent research (Tammelin et al., 1992). In the dairy environment, the presence of phages in the cheese manufacturing unit was first reported from New Zealand (Whitehead et al., 1953), still then the problem of bacteriophages in the dairy environment is still exists in the fermentation industry. The phages are present in the environment where the bacterial prevalence is more, like in food fermentation areas. The industry is been dealing with the control of phages by different means like culture rotation, phage resistant strains, improved sanitation conditions, modified factory designs, process changes, modified starter medium. But the problem of phages attacking the starter culture is a constant exists, leading to loss of failure in the fermentation process and reduced quality of the final product (Beatriz del Río et al., 2012).
The prevalence of lysogeny in the lactic acid bacteria has been reported by several workers (Canchaya et al., 2003, Ventura et al., 2007, Suárez et al., 2008). There are reports that certain environmental conditions such as heat, salt, antimicrobials, or starvation, may activate the induction prophages that will replicate, leading to the release of new virions, which can potentially infect sensitive starter cultures (Davidson et al., 1990). The presence of prophages as well as the risk of their spontaneous induction should be carefully monitored when selecting strains and designing cultures for specific industrial fermentation processes (Emond et al., 2007).
There is an increasing consumer demand for the product with high quality and safety. The requirement of quality products with sustained availability is essential to meet the growing requirement. This put the effort of using the starter microorganisms in a sustainable way to produce the high-quality product without any uncertainty. Therefore, controlling the function of specific microorganisms or the succession of microorganisms that dominate the microflora is therefore advantageous, because it can increase product quality, functionality and value of the final product (Plessas et al., 2011). Evaluation and monitoring of the production environment plays a major role in the quality enhancement of fermented food products.
The aim of this study is the monitoring of the dairy production and processing area for the presence of phages as part of the quality assurance system to improve the quality and safety of the fermented dairy product.
Materials and methods
Eight starter cultures (including Cheese and Yoghurt) maintained in the department of Dairy microbiology section of ICAR-NDRI, Bangalore was utilized for the screening purpose. cheese and yoghurt have been used. The Cheese culture is the mixed culture and the yoghurt is of streptococcus. Both these cultures were maintained as liquid culture and the weekly propagation has been carried out.
The cultures were sub cultured in de Mann Rogosa Sharpe (MRS) (Hi Media Pvt. Ltd., India). Both the starter cultures have been maintained as a liquid culture in the lab. During propagation, the cultures were streaked in the MRS agar and single colony been isolated and propagated in the MRS broth. To make the respective solid medium 15 gl-1 agar was added. The cultures were handled as per quality and safety norms and according to protocols and rules of the biosecurity and bioethical committees of the institute where work was conducted.
The cultures were sub cultured every week and incubated at 37 °C overnight for growth. The cultures were stored at 4 °C between transfers and were sub cultured before experimental use.
A total of 410 samples from Dairy processing area (260 samples) and from livestock research Centre (150 samples) of NDRI, Bangalore were collected. Minimum of 10 samples from each section has been collected for screening and for the cheese whey, dahi and paneer whey, the samples collected are more because of the positive presence of phages. The samples were centrifuged at 3000rpm for 20 min and filtered with Millipore membrane (pore diameter, 0.45um) and stored at 4 °C. according to Svenson and Christiansson (1991) stored at 4 °C.
The assay has been standardized as follows with modification from Zhang et al.,(2006). In Clearance test, overnight starter cultures grown in MRS broth were used for screening. The samples were passed through 0.45 µ syringe filter (0.45 µm, PVDF, 33 mm, gamma sterilized, PVDF, Millipore, India)- Filtrate collected and stored at 4 °C.
The procedure is as follows, in a 96 well flat bottom plates, 150 µl of overnight grown starter cultures has added to each well followed by addition of 1 drop of 1M CaCl2 to each well and followed by 150 µl of samples was added to respective wells. The plate was incubated at 37 °C for overnight incubation. After that the plates were subjected to OD measurement at 600 nm by using spectrophotometer.
The assay has been standardised as follows with slight modification from Mullan et al. 2002. Briefly, 1 ml of phage suspected material is added to 10 ml of 10X MRS broth followed by 100 ul of 1M Cacl2. It was incubated for 2 days at 37 °C followed by centrifugation at 3000 rpm for 10 minutes at 4 °C and filtered through 0.2 um syringe filter and the filtrate is stored at 4 °C. The enriched phage suspected solution (100 ul) was added to a sterile test tube along with the starter cultures (300 ul) followed by 1 drop of 1M Cacl2 and incubated at 37 °C for 15–30 minutes. The adsorbed sample is then added over to 3 ml MRS soft agar (45 °C) and then poured over the base agar (MRS agar) and incubated at 37 °C for 24 hours.
The assay has been optimized using the mrthod described by Kilic et al., (1996). one 100ul of overnight grown cheese and yoghurt culture is added to a 10 ml MRS broth along with 1 ug/ml of mitomycin and incubated overnight at 37 °C. The induced culture is centrifuged at 3000 rpm for 10 minutes at 4 °C and the supernatant is collected. About 10 ug of mitomycin is added to 100ul of supernatant. The basal MRS agar plate is seeded with 100ul of respective starter culture, allowed to dry and the 10 ul of supernatant with mitomycin is spotted over the centre and incubated at 37 °C for overnight.
Samples which shown positive in the spot test was subjected to transmission electron microscopic studies to observe the morphology of bacteriophage. Bacteriophage morphology was observed by transmission electron microscopy. Phage suspensions were concentrated by ultra- centrifugation. A drop of concentrated viral suspension was placed on 300 mesh copper grids coated with carbon film; the grids were then dipped in phosphotungstic acid (2% w/v, pH 4·5) for 30 seconds to negatively stain the viral particles. After drying, the preparation was examined on a Tecnai T12 G2 spirit electron microscope (NCBS, India, Bangalore) at different magnitudes.
Result and Discussion
The departmental cheese and yoghurt culture has been used to screen the samples from different sectors of dairy environment. The clearance test has been carried out in the 96 well bottomed plate, so that large number of samples can be screened for the phages at a single time. The samples which shown the OD value of more than 1 is taken as negative for phages and the samples which shown the OD value of less than 0.4 is taken as positive for phages according to the Muyad et al., (2002) and Khakhria et al., (1990). The
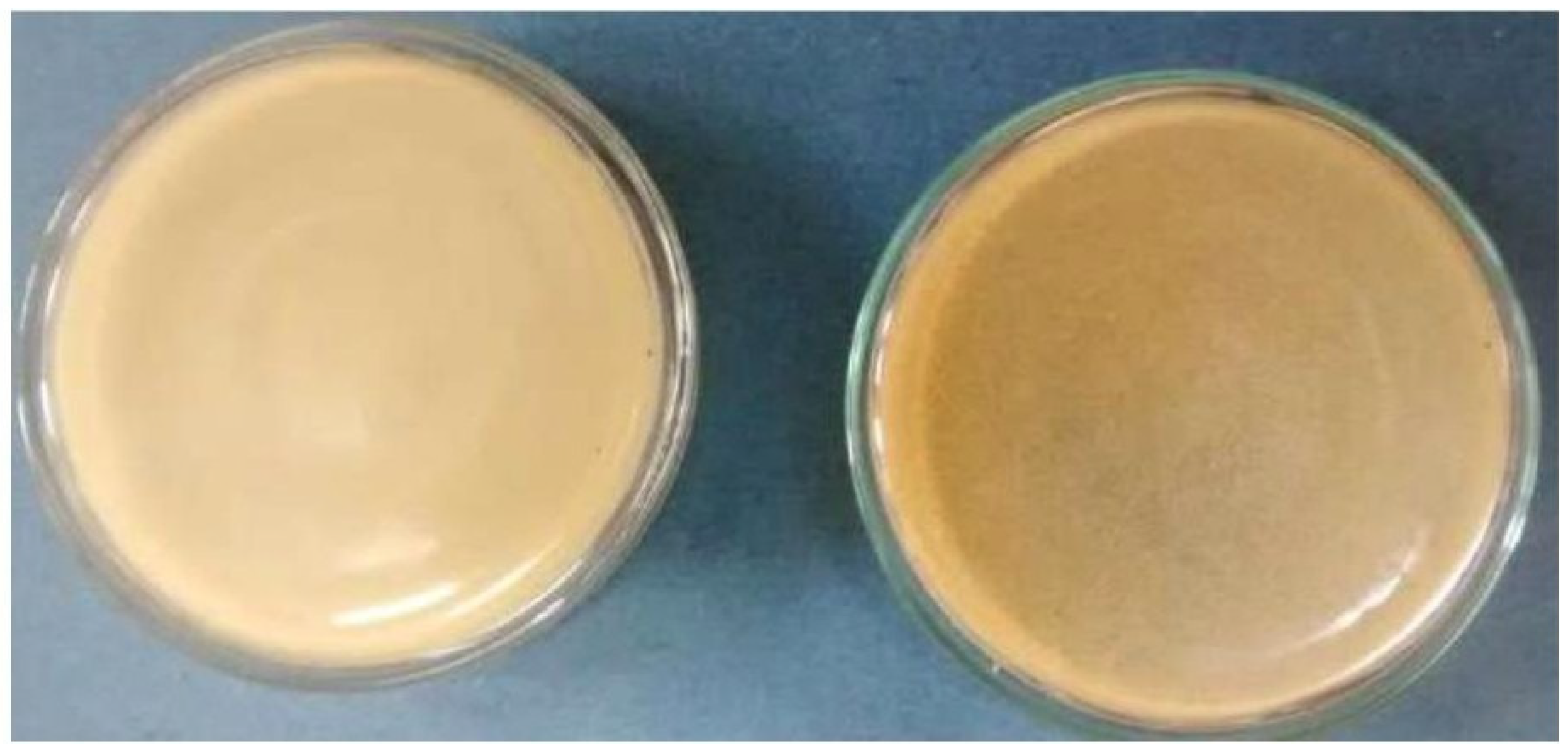
Figure 1, shows that the phage suspected sample shows the clear supernatant solution compared to the turbidity of the samples, in which starter culture have grown.
The samples of yoghurt, Paneer dip solution, cheese whey, cheese drip solution has been positive for phages by forming the clear supernatant.
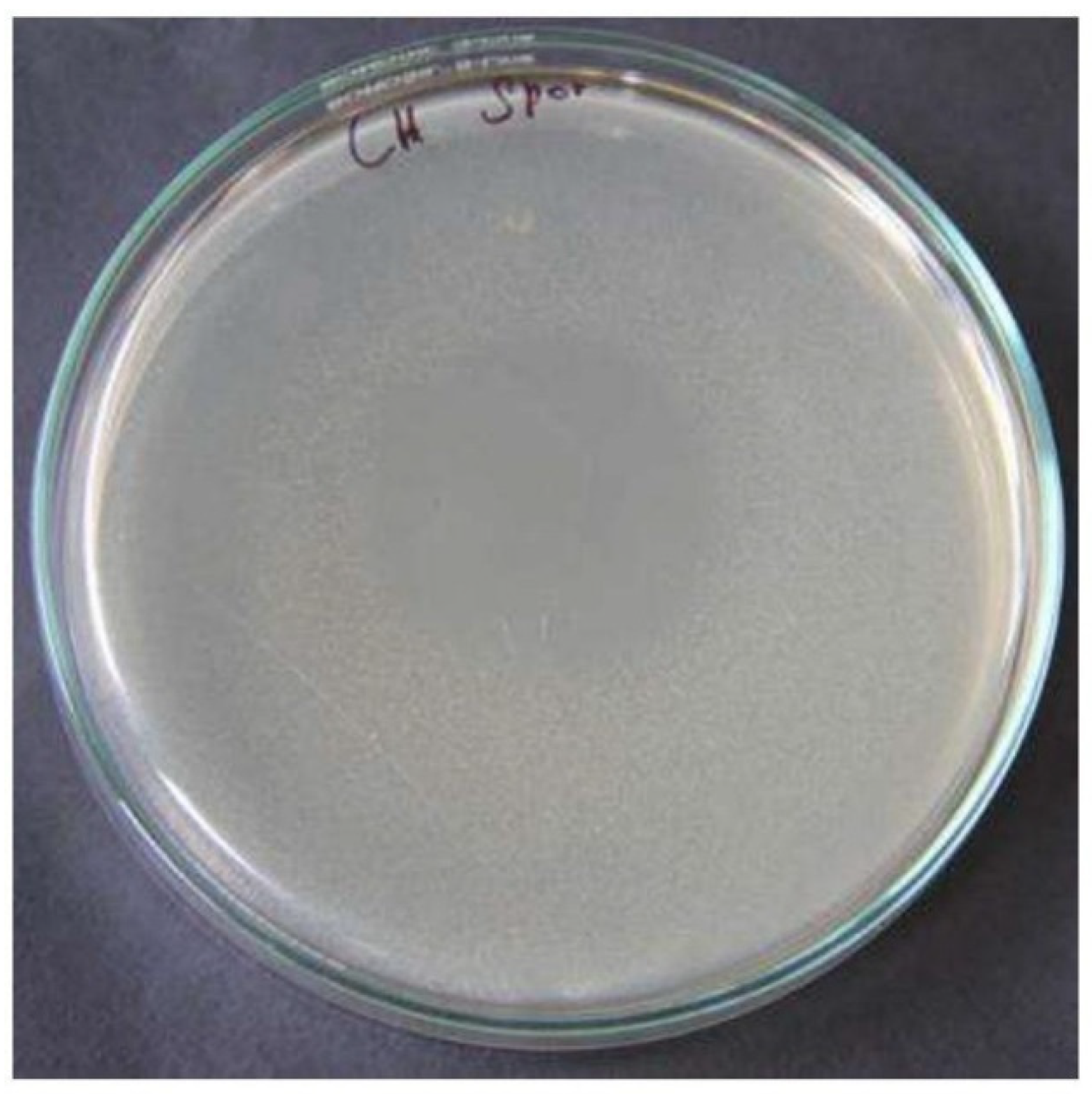
Figure 3.
The turbid appearance of the phage suspected sample when compared to the uniform control plate.
Figure 3.
The turbid appearance of the phage suspected sample when compared to the uniform control plate.
The
Figure 1 shows the OD of less than 0.4 in B7, B9, F3, F6, F7 and F9, (the clear supernatant) Where B 7 and 9 columns are cheese whey and cheese drip solution respectively and F 3, 6, 7 and 9 columns are yoghurt vessel, Paneer dip solution and Cheese whey and cheese drip solution respectively. Earlier research by Zhang et al. 2006 also used the turbidity clearance test as a confirming test for the presence of phages, where there is comparison of turbidity between phage suspected samples with the control. The screening of large number of samples by turbidity test has been carried out in this study with 96 well bottomed plate is a time saving technique.
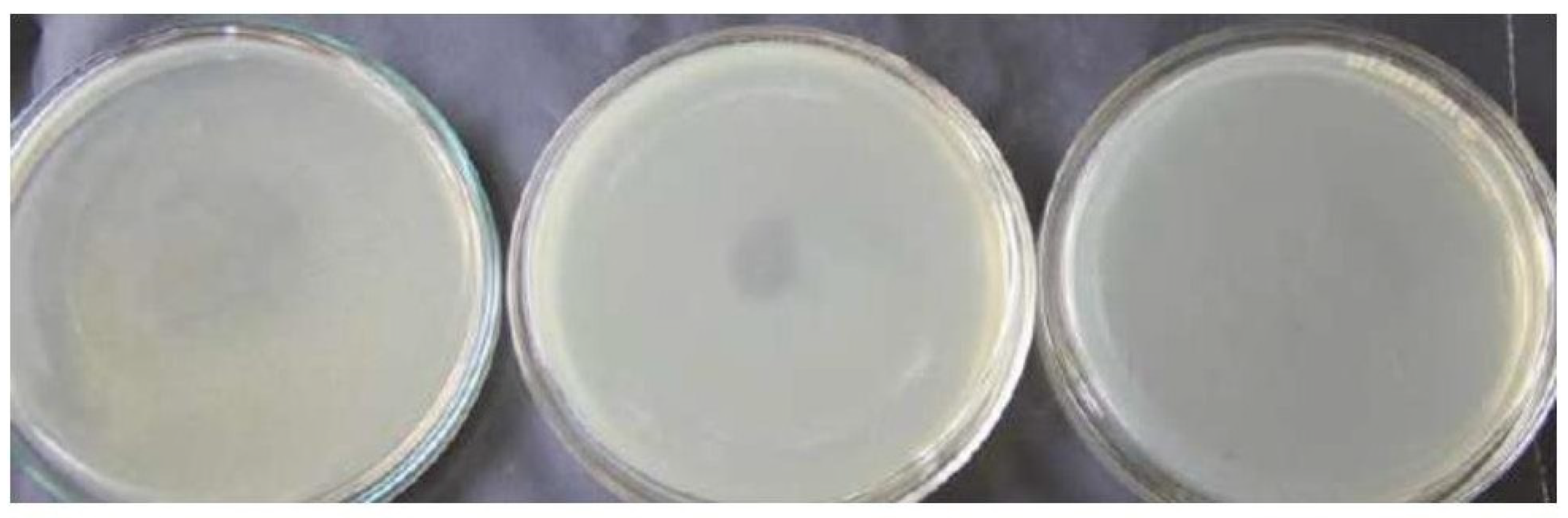
The samples which showed positive in the turbidity test has been subjected to double layer plaque assay. The results showed there was no complete lysis of the starter culture. The speckled appearance of the plates with the suspected samples when compared to control plates suggests there is a host – phage interaction. There are reports by several researchers about the formation of small and turbid plaques by some bacteriophages (Santos B Silvio et al. 2009) and pin holed size plaques by (Mullan et al. 2002). The appearance of plates when compared to control shows the presence of temperate phages. The research by Lillehaug et al. 1996 also discusses the difficulty in finding the temperate phages affecting the lactic starter cultures by double layer plaque assay. It should be noted that the inability to confirm the presence of temperate phages in the dairy environment leads to the poor evaluation of the dairy environment for the presence of phages and their prevalence is underestimated.
The enrichment of phages has been carried out prior to the double layer plaque assay to improve the phage concentration. A study carried out by Müller-Merbach et al. 2005 support the enrichment step to increase the phage concentration. It is interesting to note that the calcium ion concentration plays an important for the growth of phages affecting the starter culture as the mild variation of the concentration, the phage growth is disturbed. This is supported by the Jończyk, et al.,. 2011 review work in which several researchers has reported the stability of different phages at the different ions and pH. In this study, the addition of 1M CaCl2 at the enrichment and adsorption steps has given the optimum result. As the addition of MgCl2 also carried out, which yielded the negative result.
The samples which shown the presence of turbid plaques has been subjected to the mitomycin induction for overnight incubation. Following the exposure with mitomycin, grossly there is a pellicle formation in the test tube inoculated with suspected sample when compared to the control group (
Figure 4), which is formed by certain lactococcal strains infected with phages, which has been supported by the work of Carine Farenc et al., (2014) where he concluded that the lactococcal pellicles recognized by certain phages possess a semi conserved gene lead to phage-strain specificity. The mitomycin concentration also tested for various concentrations viz., 0.1 µg, 0.5µg, 1µg, 2 µg to 5 µg. In this the concentration of 0.5 µg provided the optimum result of forming pellicle and spot.
Figure 4.
The
Figure 4 shows lysis of bacteria (right) test tube in which the starter culture has been induced with mitomycin compared to the control; turbidity indicates the bacterial growth (Left).
Figure 4.
The
Figure 4 shows lysis of bacteria (right) test tube in which the starter culture has been induced with mitomycin compared to the control; turbidity indicates the bacterial growth (Left).
Figure 5.
The zone of inhibition of 3 cm formed by the cheese culture after induction with mitomycin.
Figure 5.
The zone of inhibition of 3 cm formed by the cheese culture after induction with mitomycin.
Figure 6.
The zone of inhibition of 1.2 cm formed by the yoghurt culture after induction with mitomycin.
Figure 6.
The zone of inhibition of 1.2 cm formed by the yoghurt culture after induction with mitomycin.
Figure 7.
The plate showing zone of inhibition when compared to the control.
Figure 7.
The plate showing zone of inhibition when compared to the control.
Figure 8.
The isometric head phages of the cheese culture by TEM at 50nm.
Figure 8.
The isometric head phages of the cheese culture by TEM at 50nm.
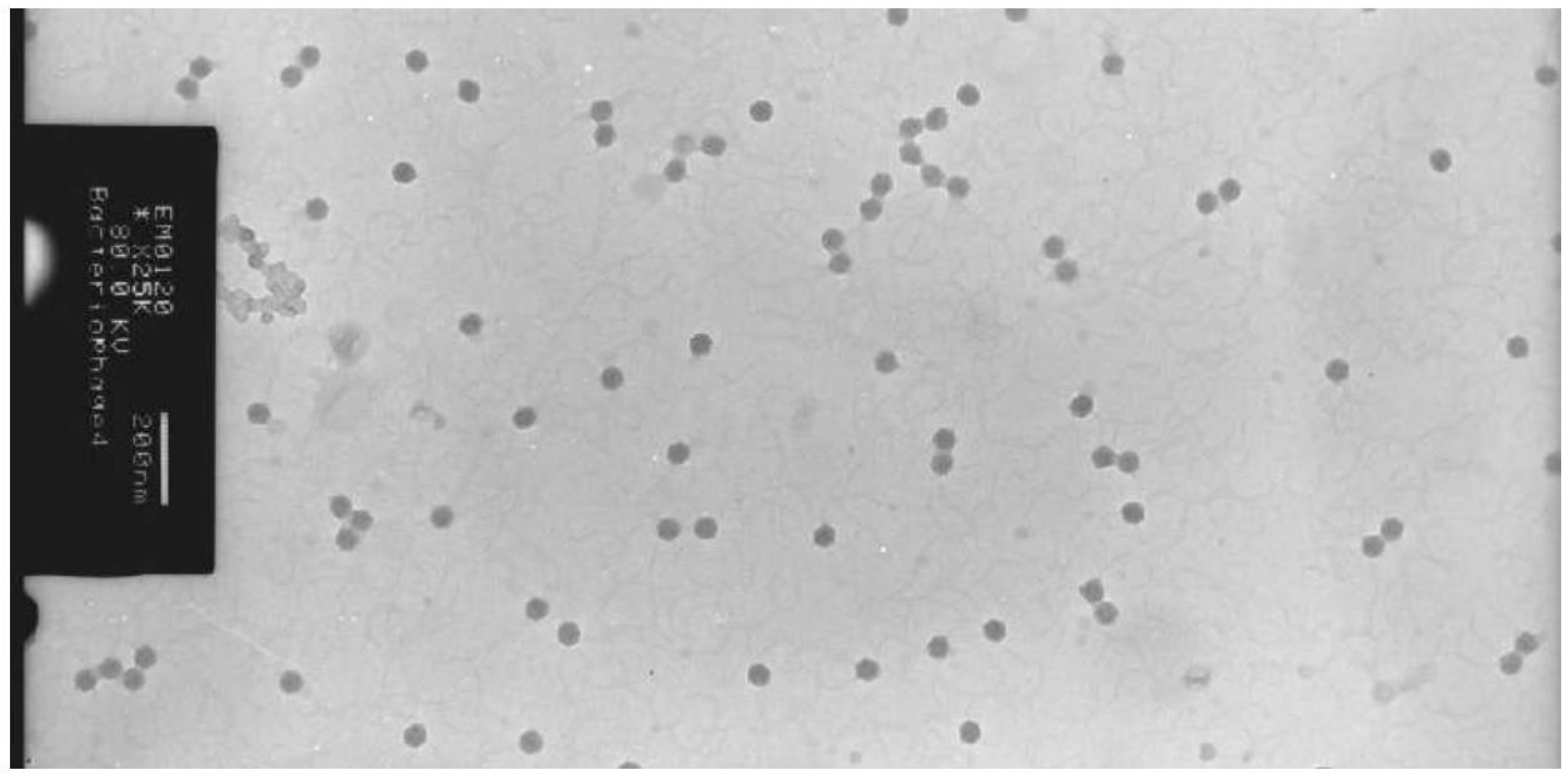
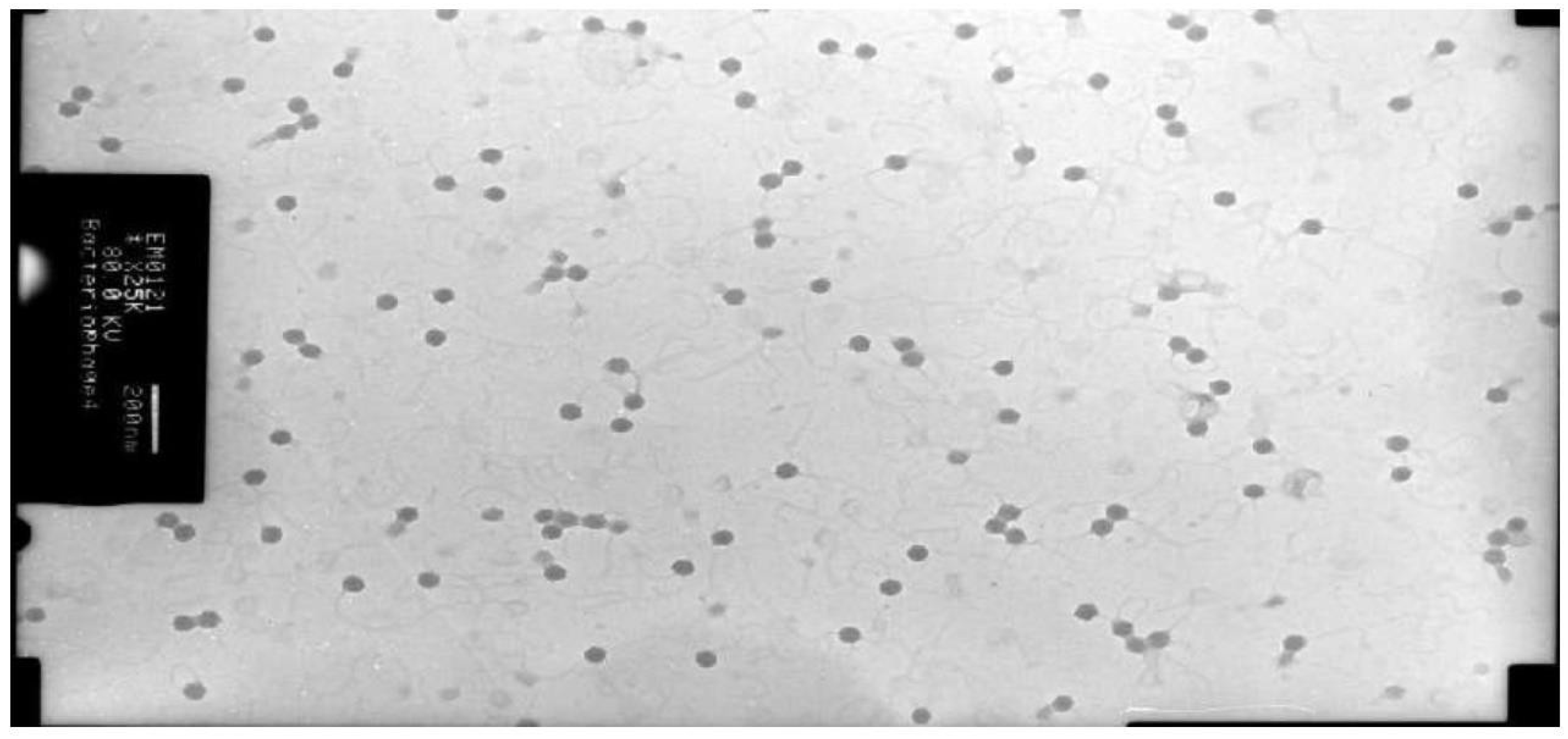
Figure 9.
The isometric head phages of the cheese culture by TEM at 200 nm.
Figure 9.
The isometric head phages of the cheese culture by TEM at 200 nm.
Figure 10.
The prolate head phages against the yoghurt culture at 200 nm.
Figure 10.
The prolate head phages against the yoghurt culture at 200 nm.
There are two different zone of inhibition with two different starter culture. The cheese culture provided a wide zone of inhibition measuring 3cm in a 8 cm petriplate. While the yoghurt culture provided the inhibition zone of 1.2 cm in a 8 cm petriplate.The formation of zone of inhibition for the mixed culture or strains has been clearly seen.
Two different morphology has been observed with the electron microscopy studies. Both the phages showed the presence of tail, which put them in the Siphoviridae family of classification. The cheese culture showed the isometric heads of 50 nm diameter and long flexible tail with non- contractile sheath, which belong to Bradley’s group B (Bradley et al. 1967) or the Siphoviridae family (Morphotype B1) according to International committee on Taxonomy of viruses. The other phage morphology of yoghurt culture exhibited the prolate head with characteristic collar structure that was no the common feature in phages of dairy starter culture. (Jarvis et al. 1989).
The S.thermophilus phages showed isometric heads and long flexible tails with noncontractile sheaths. Phages belonged to Bradley’s group B or the Siphoviridae family according to the international committee on taxonomy of viruses. The morphology of two phages was comparable with the morphology of the Streptococci phages
The investigation of phages in the dairy environment showed the presence of temperate phages. The use of these strains in fermentation process is a high risk as the fermentation can be halted at any stressful or uncertain conditions leads to the quality instability of the fermented dairy products.
Molecular studies to investigate the diversity of phages are underway at our laboratory. Periodic monitoring of phage presence should be performed in order to design effective phage defense systems, but always as a complement of effective sanitation programs, since a controlled phage level is needed to improve the starter activities during cheese and yoghurt manufacturing process.
Conclusion
This study provides the status of phage prevalence in the different sectors of dairy environment. The screening of the several areas in the dairy environment using starter culture allowed us to isolate the phages for the starter cultures. Many small dairy factories, use their own starter cultures rather than DVS culture because of their novelty and specific sensory characteristic it provides to their products. But the main disadvantage of using this novel strains are that the quality instability of the fermented dairy products. Starter culture rotation is not always possible to retain the novel strains and these novel starters are easily suffered from the bacteriophage attack in the long run.
The presence of phages in the paneer section, where there is no usage of starter culture indicates the probable air borne transmission of the phages, where the finely divided particles of whey emitted from the whey separator appeared to be the main vehicle for the air-borne phage although whey- contaminated dust probably also played a part. The question of the origin of bacteriophage is discussed in the light of the recognition of the prevalence of air-borne phage. The occurrence of air- borne infection could account for most of the instances of starter failures which were previously thought to be due to spontaneous phage development within cultures. So, the preventive measures should aim at the separate whey handling systems, whey storage tanks, aerosol fumigation, control airflow etc. The knowledge of the phage presence in the dairy plants is important for the efficient use of preventive measures and solutions to prevent the future attack / failure of starter cultures.
References
- Beatriz del Río, María Cruz Martín, Víctor Ladero, Noelia Martínez, Daniel M. Linares, María Fernández and Miguel A. Alvarez (2012). Bacteriophages in Dairy Industry: PCR Methods as Valuable Tools, Bacteriophages, Dr. Ipek Kurtboke (Ed.), InTech. [CrossRef]
- Canchaya C, Proux C, Fournous G, Bruttin A, Brüssow H.(2003). Prophage genomics. Microbiol Mol Biol Rev. 67:238–76. [CrossRef]
- Carine Farenc, Silvia Spinelli, Evgeny Vinogradov, Denise Tremblay, Stéphanie Blangy, Irina Sadovskaya, Sylvain Moineau, and Christian Cambillau (2014). Molecular Insights on the Recognition of a Lactococcus lactis Cell Wall Pellicle by the Phage 1358 Receptor Binding Protein. J Virol. 2014 Jun; 88(12): 7005–7015. [CrossRef]
- D. Lillehaug (1997). An improved plaque assay for poor plaque-producing temperate lactococcal bacteriophages. Journal of Applied Microbiology 1997, 83, 85–90. [CrossRef]
- Dairy reporter, (2016). Yogurt market can hit $1bn by 2021 in India: Report.
- Davidson BE, Powell IB, Hillier AJ. Temperate bacteriophages and lysogeny in lactic acid bacteria. (1990). FEMS Microbiol Rev.7:79–90.
- E. Jończyk, M. Kłak, R. Międzybrodzki, and A. Górski (2011) The influence of external factors on bacteriophages—review Folia Microbiol (Praha). May; 56(3): 191–200. [CrossRef]
- Emond E, Moineau S. Bacteriophages and food fermentations. In: McGrath S, van Sinderen D, eds. (2007). Bacteriophage: Genetics and Molecular Biology. Horizon Scientific Press/Caister Academic Press 2007; 93- 124.
- Global agricultural information network, (2015). USDA Foreign agricultural service. GAIN report number: IN5125 dated 30.09.2015.
- Jarvis, A.W. (1989). Bacteriophages of lactic acid bacteria. J.Dairy Sci. 72:3406-3428. [CrossRef]
- Khakhria R, Duck D, Lior H (1990). Extended phage-typing scheme for Escherichia coli O157:H7.. Dec;105(3):511-20. [CrossRef]
- Mullan, W.M.A. (2002). Plaque formation. [On-line]. Available from:https://www.dairyscience.info/index.php/enumeration-of-lactococcal-bacteriophages/plaque- formation.html. Accessed: 14 June, 2016. Updated January 2015.
- Muyad M. M. (2009). Molecular identification of Pseudomonas auroginosa and its phage effect on those bacterial strain.
- S. Plessas, A. Alexopoulos, C. Voidarou, E. Stavropoulou, E. Bezirtzoglou. (2011). Microbial ecology and quality assurance in food fermentation systems. The case. [CrossRef]
- Silvio B Santos, Carla M Carvalho, Sanna Sillankorva, Ana Nicolau, Eugenio C Ferreira and Joana Azeredo (2009). The use of antibiotics to improve phage detection and enumeration by the double –layer agar technique. BMC Microbiology, 2009, 9: 148. [CrossRef]
- Whitehead and Huntera1 Starter cultures for cheese manufacture: Further attempts to eliminate failures due to bacteriophage (2009). Journal of Dairy Research volume 12 / Issue 01 / January 1941, pp 63-70. [CrossRef]
- Suárez V, Zago M, Quiberoni A, Carminati D, Giraffa G, Reinheimer J.(2008). Lysogeny in Lactobacillus delbrueckii strains and characterization of two new temperate prolate-headed bacteriophages. J Appl Microbiol. 2008;105:1402–11. [CrossRef]
- Suarez, V.B, Quiberoni. A, Binetti, A.G and Reinheimer, J.A (2002). Thermophilic lactic acid bacteria phages isolated from Argentinian Dairy Industries. Journal of Food protection, Vol 65, No.10, 2002, pages 1597-1604. [CrossRef]
- Svenson, U., and A. Christiansson. (1991). Methods for phage monitoring. Bull. FIL-IDF 263(4): 29-39.
- Tammelin A. (1992). Staphylococus aureus surgical wound infection; possibility of preventing wound infection by use of bacteriophages. Nature. 22:26–31.
- V. K. Shiby & H. N. Mishra (2013) Fermented Milks and Milk Products as Functional Foods—A Review, Critical Reviews in Food Science and Nutrition, 53:5, 482-496. [CrossRef]
- Ventura M, Zomer A, Canchaya C, O’Connell-Motherway M, Kuipers O, Turroni F, et al. (2007). Comparative analyses of prophage-like elements present in two Lactococcus lactis strains. Appl Environ Microbiol. 73:7771–80. [CrossRef]
- Whitehead, H.R. (1953). Bacteriophage in cheese manufacture. Bacteriol.Rev.17:109-123.
- X. Zhang,J. Kong,Y. Qu. (2006). Isolation and characterization of a Lactobacillus fermentum temperate bacteriophage from Chinese yogurt. Journal of Applied Microbiology. Volume 102, Issue 2, 607. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).