Submitted:
25 August 2023
Posted:
31 August 2023
You are already at the latest version
Abstract
Keywords:
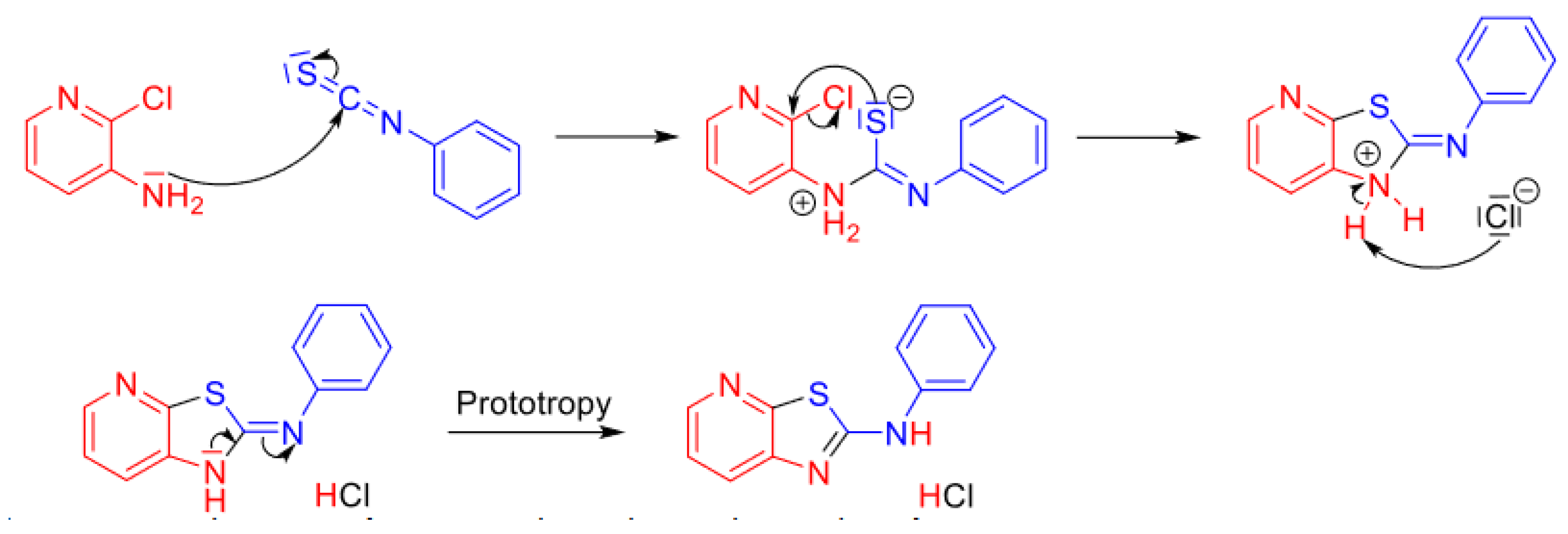
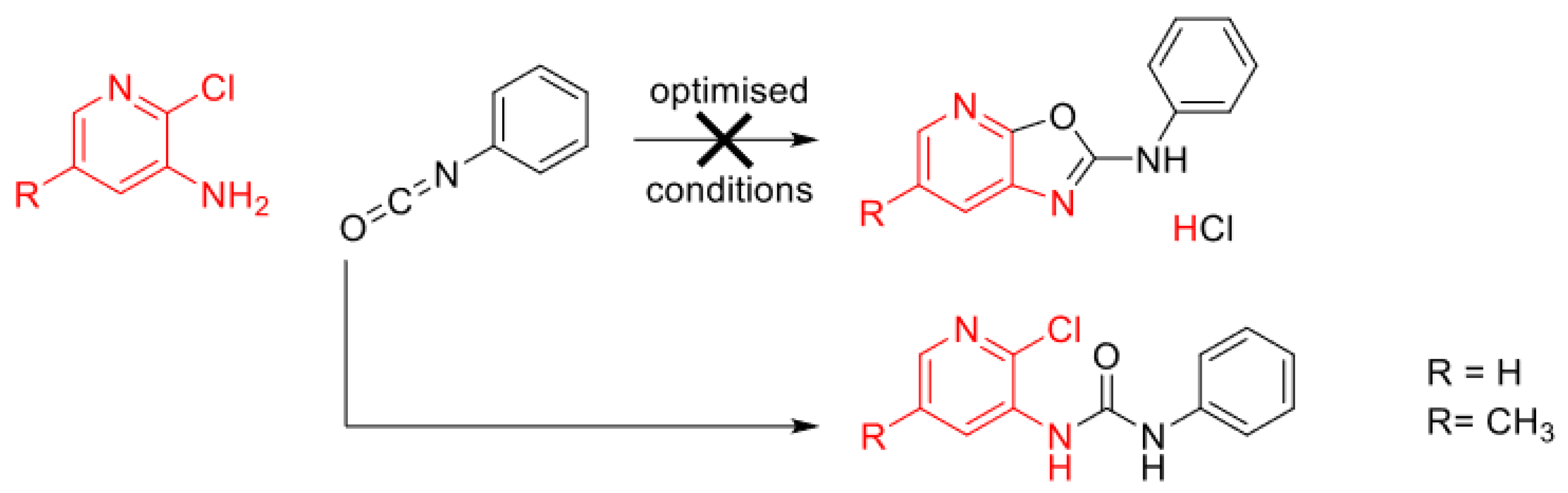
1. Introduction
2. Results and Discussion
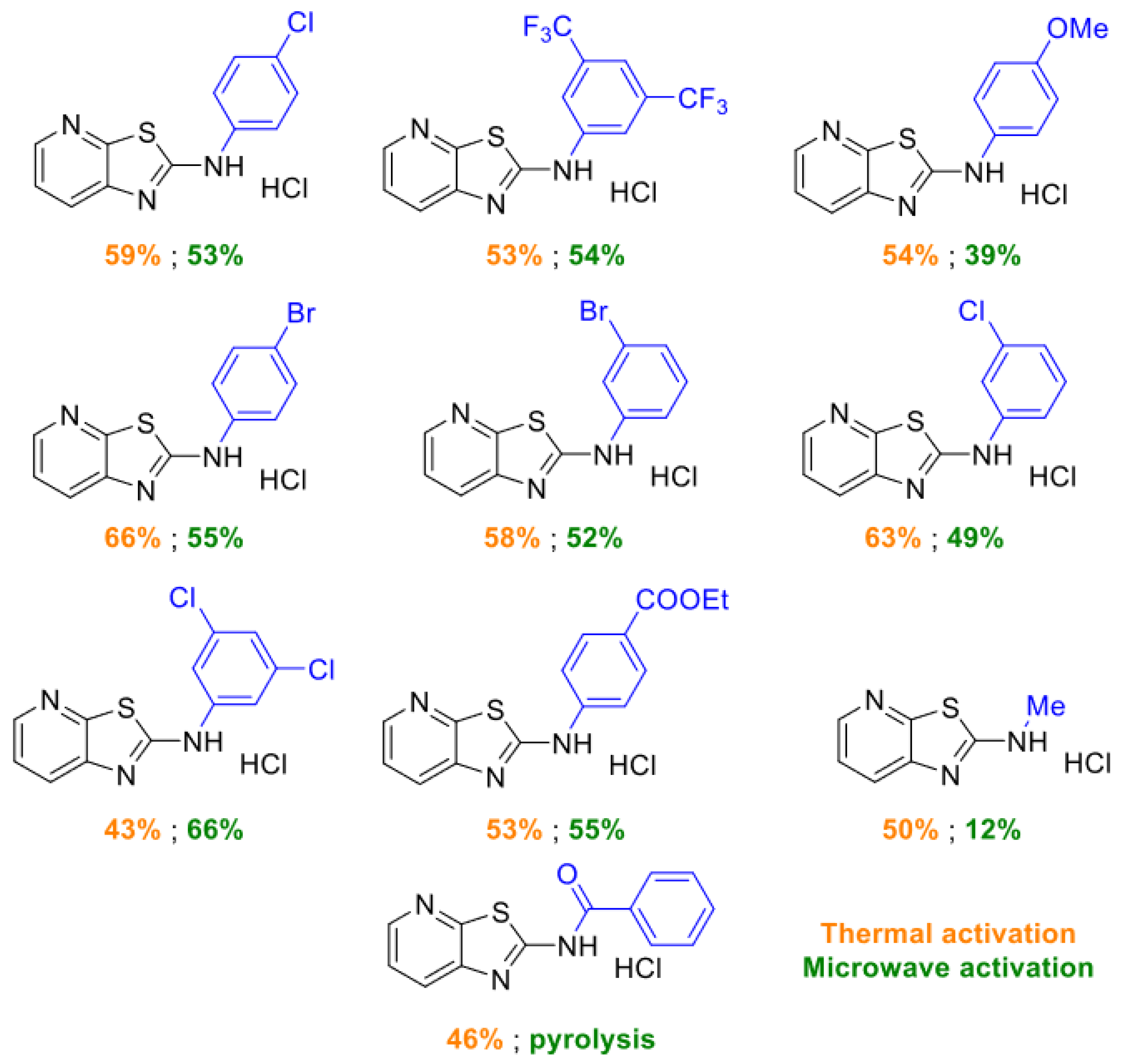
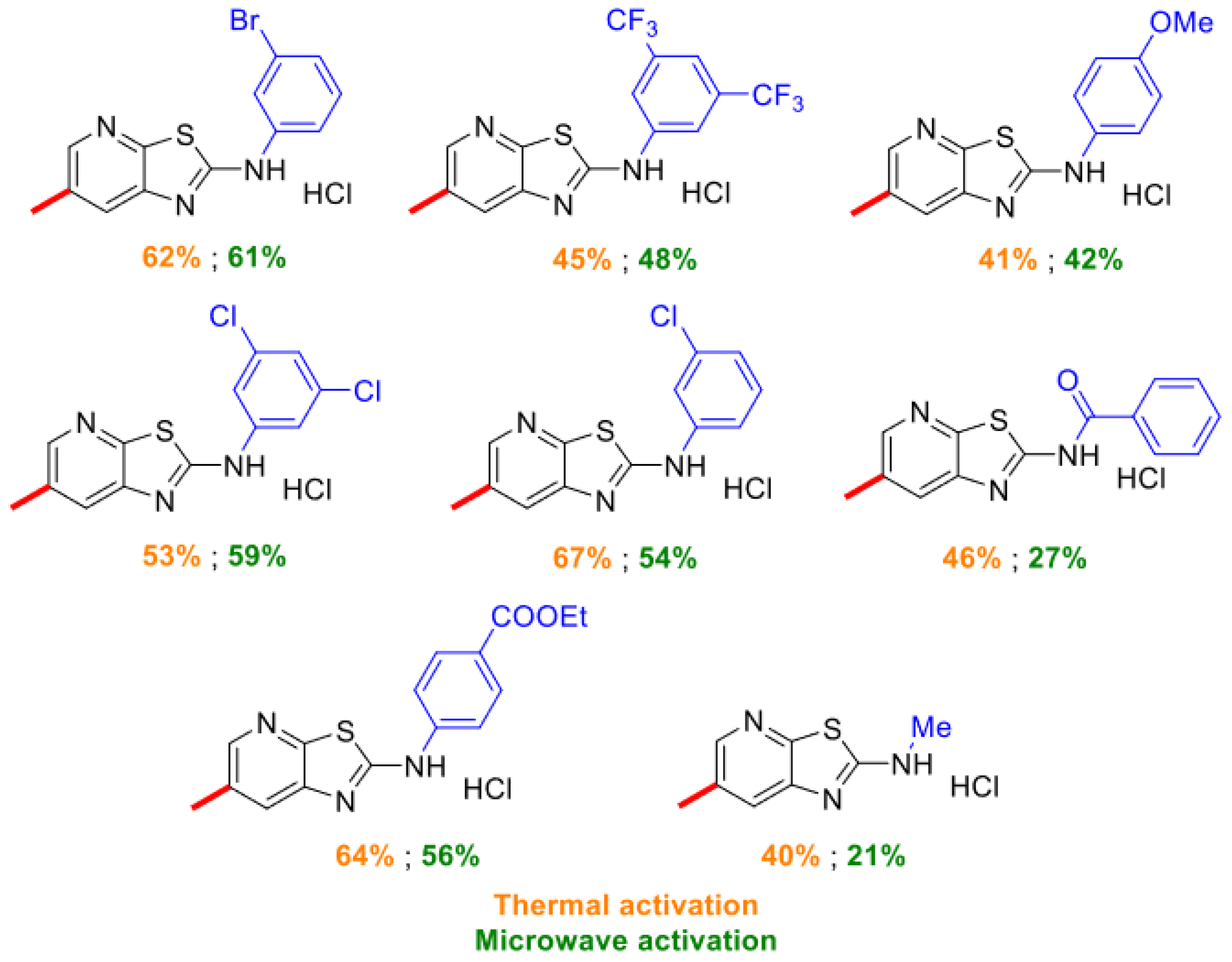
2.1. Thiazolo-pyridine synthesis in various standard and green solvents :
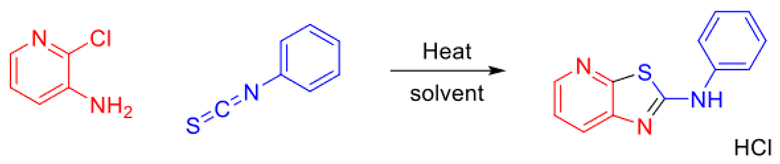
| Entry | 2-chloro-3-amino-pyridine (equiv.) | Isothiocyanate (equiv.) | Reaction time | TP (°C) | Solvent | Yields |
|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 4 | 100 | Eucalyptol | 59% |
| 2 | 1 | 1 | 16 | 100 | Eucalyptol | 75% |
| 3 | 1 | 1 | 4 | 100 | CPME | 63% |
| 4 | 1 | 1 | 16 | 100 | CPME | 71% |
| 5 | 1.1 | 1 | 16 | 100 | CPME | 79% |
| 6 | 1 | 1 | 4 | 100 | Limonene | 65% |
| 7 | 1 | 1 | 16 | 100 | Limonene | 70% |
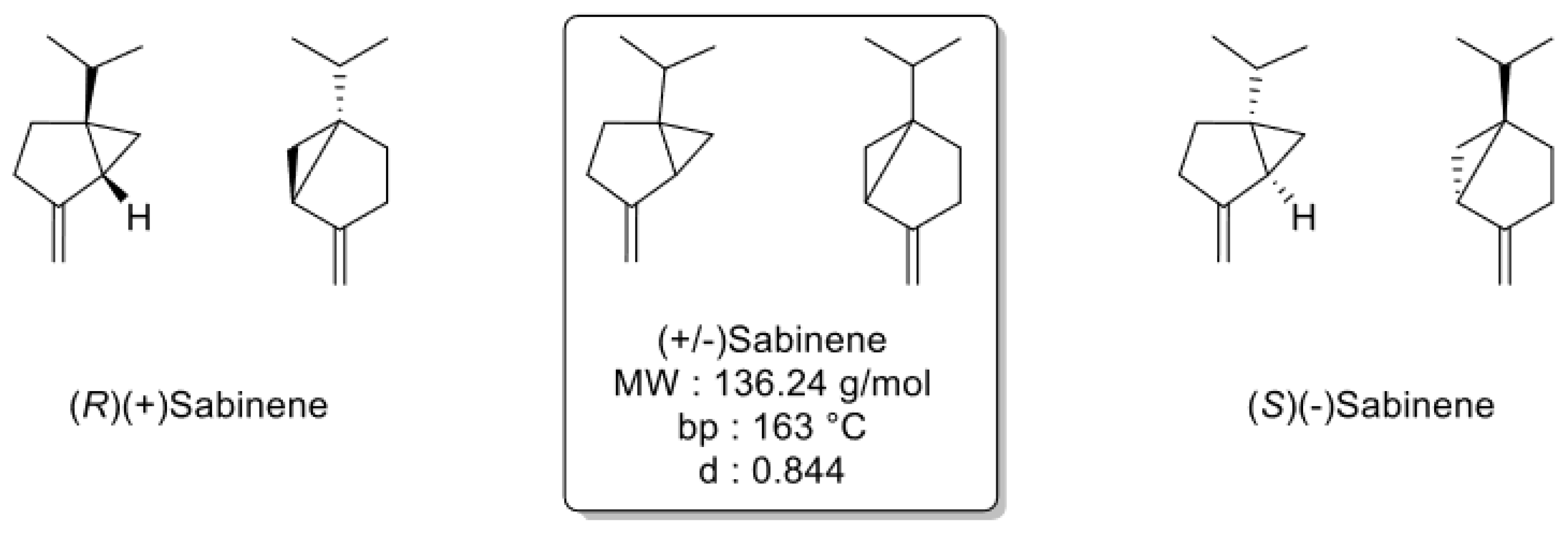
| 8 | 1 | 1 | 4 | 100 | Sabinene | 36% |
| 9 | 1.1 | 1 | 4 | 100 | Sabinene | 38% |
| 10 | 1 | 1.1 | 4 | 100 | Sabinene | 33% |
| 11 | 1 | 1 | 16 | 100 | Sabinene | 68% |
| 12 | 1.1 | 1 | 16 | 100 | Sabinene | 76% |
| 13 | 1.1 | 1 | 16 | 100 | Sabinene | 61% |
| 14 | 1.1 | 1 | 16 | 100 | Distilled Sabinene | 62% |
| 15 | 1 | 1 | 16 | 100 | Distilled Sabinene | 58% |
2.2. Optimization of thiazolo-pyridine synthesis in sabinene under microwaves irradiation
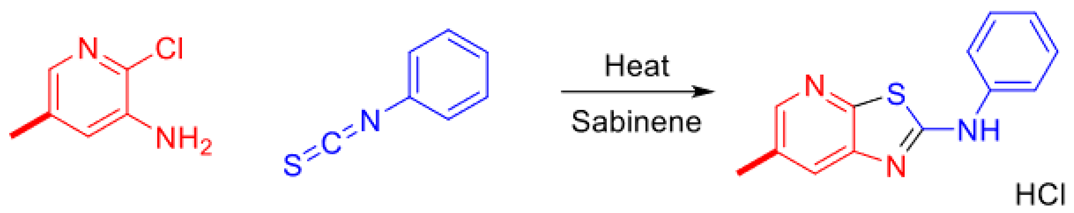
| Entry | Reaction time | Pyridine (equiv.) | Isothiocyante (equiv.) | Rinsing solvent | Yield | NMR observation |
|---|---|---|---|---|---|---|
| 1 | 5 h | 1 | 1 | Diethyl ether | 46 % | Clear |
| 2 | 5 h | 1.1 | 1 | Diethyl ether | 55 % | Parasite peak |
| 3 | 5 h | 1 | 1.1 | Diethyl ether | 53 % | Parasite peak |
| 4 | 16 h | 1.1 | 1 | Diethyl ether | 75 % | Parasite peak |
| 5 | 16 h | 1.1 | 1 | Ethyl acetate | 66 % | Clear |
3. Materials and Methods
3.1. General Information
3.2. General procedure (1)
3.3. General procedure (2)
3.4. General procedure (3)
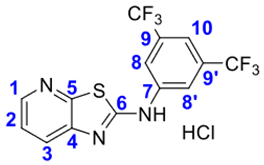
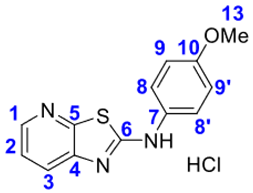
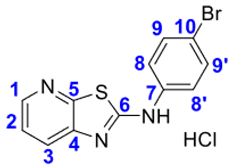
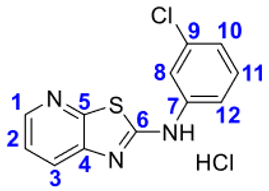
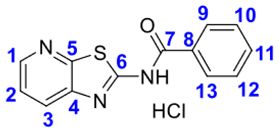
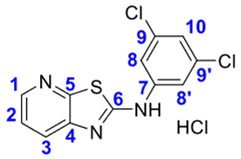
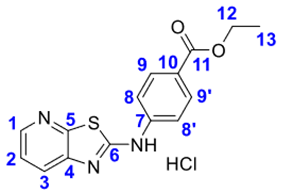
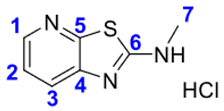
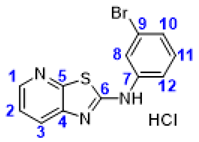
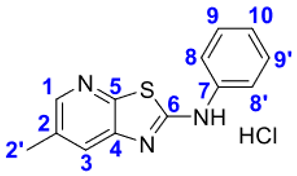
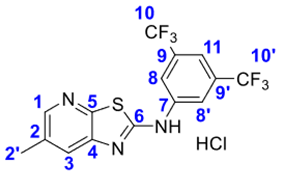
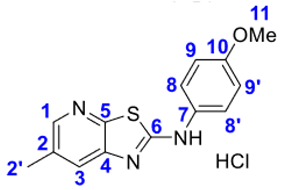
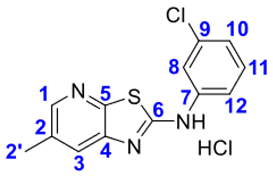
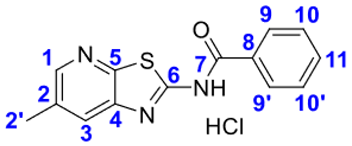
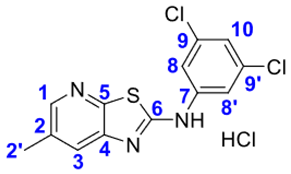
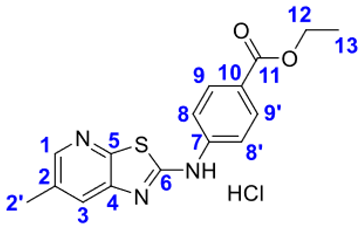
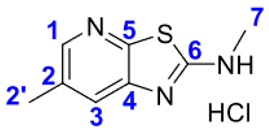
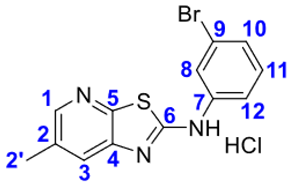
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Campos, J.F.; Scherrmann, M.-C.; Berteina-Raboin, S. Eucalyptol : A new solvent for the synthesis of heterocycles containing oxygen, sulfur and nitrogen. Green Chem. 2019, 21, 1531–1539. [Google Scholar] [CrossRef]
- Campos, J. F.; Berteina-Raboin, S. Eucalyptol, an all-purpose product. Catalysts. 2022, 12, 48. [Google Scholar] [CrossRef]
- Loubidi, M.; Moutardier, A.; Campos, J.F.; Berteina-Raboin, S. Pd-catalyzed Suzuki/Sonogashira cross-coupling reaction and the direct sp3 arylation of 7-chloro-5-methyl-[1,2,4]triazolo[1,5-a]pyrimidine. Tetrahedron Lett. 2018, 59, 1050–1054. [Google Scholar] [CrossRef]
- Campos, J.F.; Berteina-Raboin, S. Eucalyptol as bio-based solvent for Migita–Kosugi–Stille coupling reaction on O,S,N-heterocycles. Catal. Today. 2020, 358, 138–142. [Google Scholar] [CrossRef]
- Campos, J.F.; Berteina-Raboin, S. Eucalyptol as a Bio-Based Solvent for Buchwald-Hartwig Reaction on O,S,N-Heterocycles. Catalysts. 2019, 9, 840. [Google Scholar] [CrossRef]
- Campos, J.F.; Ferreira, V.; Berteina-Raboin, S. Eucalyptol: a bio-based solvent for the synthesis of O,S,N-Heterocycles. Application to Hiyama Coupling, Cyanation, and Multicomponent Reactions. Catalysts. 2021, 11, 222. [Google Scholar] [CrossRef]
- Valente, J.; Zuzarte, M.; Gonçalves, M.J.; Lopes, M.C.; Cavaleiro, C.; Salgueiro, L.; Cruz, M.T. Antifungal, antioxidant and anti-inflammatory activities of Oenanthe crocata L. essential oil. Food Chem. Toxicol. 2013, 62, 349–354. [Google Scholar] [CrossRef]
- Yamasaki, Y.; Kunoh, H.; Yamamoto, H.; Akimitsu, K. Biological roles of monoterpene volatiles derived from rough lemon (Citrus Jambhiri Lush) in citrus defense. J. Gen. Plant. Pathol. 2007, 73, 168–179. [Google Scholar] [CrossRef]
- Asili, J.; Emami, A.; Rahimizadeh, M.; Fazli-Bazzaz, B.S.; Hassanzadeh, M. Chemical and Antimicrobial Studies of Juniperus Sabina L. and Juniperus foetidissima Willd. Essential Oils. J. essent. oil-bear. plants. 2013, 13, 25–36. [Google Scholar] [CrossRef]
- Sacchetti, G.; Maietti, S.; Muzzoli, M.; Scaglianti, M.; Manfredini, S.; Radice, M.; Bruni, R. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food. Chem. 2005, 91, 621–632. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, H.; Liu, H.; Liu, W.; Zhang, R.; Xian, M.; Liu, H. Biosynthesis and Production of Sabinene: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Kader, M.S.; Soliman, G.A.; Alqarni, M.H.; Hamad, M.A.; Foudah, A.I.; Alqasoumi, S.I. Chemical composition and protective effect of Juniperus sabina L. essential oil against CCl4 induced hepatotoxicity. Saudi Pharm. J. 2019, 27, 945–951. [Google Scholar] [CrossRef]
- Verma, Ram S. ; Padalia, Rajendra C.; Chauhan, Amit; Verma, Rajesh K.; Ur Rahman, Laiq; Singh, Anand. "Changes in the Essential Oil Composition of Origanum majoranaL. During Post Harvest Drying". J. Essent. Oil-Bear. Plants. 2016, 19, 1547–1552. [Google Scholar] [CrossRef]
- Dallali, S.; Zouaoui, R.; Dallali, D.; Jdidi, S.; Toumi, L. Determination of some biochemical parameters from leaves of Quercus ilexL.(Fagaceae), collected in Djabel Zagouan (Tunisia). Arab. J. Med. Aromat. Plants. 2021, 7, 1–28. [Google Scholar]
- Jirovetz, L.; Buchbauer, G.; Stoyanova, A.; Metodiev, S. Seasonal Depending Variations of the Composition and Biological Activities of Douglas Fir (Pseudotsuga menziesii) Essential Oils from Bulgaria. Sci. Pharm. 2000, 68, 323–328. [Google Scholar] [CrossRef]
- Snoussi, M.; Noumi, E.; Trabelsi, N.; Flamini, G.; Papetti, A.; De Feo, V. Mentha spicata Essential Oil: Chemical Composition, Antioxidant and Antibacterial Activities against Planktonic and Biofilm Cultures of Vibrio spp. Strains. Molecules. 2015, 20, 14402–14424. [Google Scholar] [CrossRef] [PubMed]
- Fraternale, D.; Flamini, G.; Ricci, D. Essential oil composition and antimicrobial activity of. Angelica archangelica L. (Apiaceae) roots. J. Med. Food. 2014, 17, 1043–1047. [Google Scholar] [CrossRef]
- Haq Raees-ul; Prasad, K. Nutritional and processing aspects of carrot (Daucus carota)- A review. South Asian J. Food Technol. Environ. 2015, 1, 1–14. [Google Scholar] [CrossRef]
- Oliveira, G.L.; Moreira, D.L.; Mendes, A.D.R.; Guimaraes, E.F.; Figueiredo, L.S.; Kaplan, M.A.C.; Martins, E.R. Growth study and essential oil analysis of Piper aduncum from two sites of Cerrado biome of Minas Gerais State, Brazil. Rev. Bras. Farmacogn. 2013, 23, 743–753. [Google Scholar] [CrossRef]
- Omara, T.; Kiprop, A.K.; Kosgei, V.J.; Kagoya, S. Clausena anisata (Willd.) Hook.f. ex Benth. (Rutaceae): ethnomedicinal uses,phytochemistry, pharmacological activities, toxicity, and clinical application. Tradit. Med. Res. 2022, 7, 51–74. [Google Scholar] [CrossRef]
- Renninger, N.S.; Ryder, J.A.; Fisher, K.J. Jet fuel compositions and methods of making and using same. US Patent 794 2940.
- Peralta-Yahya, P.P.; Ouellet, M.; Chan, R.; Mukhopadhyay, A.; Keasling, J.D.; Lee, T.S. Identification and microbial production of a terpene-based advanced biofuel. Nat. Commun. 2011, 2, 483. [Google Scholar] [CrossRef] [PubMed]
- Balaban, A.T. Aromaticity as a Cornerstone of Heterocyclic Chemistry. Chem. Rev. 2004, 104, 2777–2812. [Google Scholar] [CrossRef] [PubMed]
- K. A. Scott and J. T. Njardarson, Analysis of US FDA-Approved Drugs Containing Sulfur Atoms. Top. Curr. Chem. 2018, 376, 5. [Google Scholar] [CrossRef] [PubMed]
- M. T. Chhabria, S. Patel, P. Modi and P. S. Brahmkshatriya, Thiazole: A Review on Chemistry, Synthesis and Therapeutic Importance of its Derivatives. Curr. Top. Med. Chem. 2016, 16, 2841–2862. [CrossRef]
- E. Vitaku, D. T. Smith and J. T. Njardarson, Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [CrossRef]
- Taylor, R.D.; MacCoss, M.; Lawson, A.D.G. Rings in Drugs. J. Med. Chem. 2014, 57, 5845–5859. [Google Scholar] [CrossRef]
- Gibson, S.; McGuire, R.; Rees, D.C. Principal Components Describing Biological Activities and Molecular Diversity of Heterocyclic Aromatic Ring Fragments. J. Med. Chem. 1996, 39, 4065–4072. [Google Scholar] [CrossRef]
- Kalaria, P.N.; Karad, S.C.; Raval, D.K. A Review on Diverse Heterocyclic Compounds as the Privileged Scaffolds in Antimalarial Drug Discovery. Eur. J. Med. Chem. 2018, 5, 917–936. [Google Scholar] [CrossRef]
- Taylor, A.P.; Robinson, R.P.; Fobian, Y.M.; Blakemore, D.C.; Jones, L.H.; Fadeyi, O. Modern Advances in Heterocyclic Chemistry in Drug Discovery. Org. Biomol. Chem. 2016, 14, 6611–6637. [Google Scholar] [CrossRef]
- Cee, V.J.; Frohn, M.; Lanman, B.A.; Golden, J.; Muller, K.; Neira, S.; Pickrell, A.; Arnett, H.; Buys, J.; Gore, A.; Fiorino, M.; Horner, M.; Itano, A.; Lee, M.R.; McElvain, M.; Middleton, S.; Schrag, M.; Rivenzon-Segal, D.; Vargas, H.M.; Xu, H.; Xu, Y.; Zhang, X.; Siu, J.; Wong, M.; Bürli, R.W. Discovery of AMG 369, a Thiazolo[5,4-b]pyridine Agonist of S1P1 and S1P5. ACS Med. Chem. Lett. 2011, 2, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.U.; Palani, A.; Chen, X.; Huang, Y.; Aslanian, R.G.; West, R.E., Jr.; Williams, S.M.; Wu, R.; Hwa, J.; Sondey, C.; Lachowicz, J. Synthesis and Structure–activity Relationships of 2-(1,4′-bipiperidin-1′-yl)thiazolopyridine as H3 Receptor Antagonists. Bioorg. Med. Chem. Lett. 2009, 19, 6176–6180. [Google Scholar] [CrossRef] [PubMed]
- Kale, M.G.; Raichurkar, A.; Hameed, P.S.; Waterson, D.; McKinney, D.; Manjunatha, M.R.; Kranthi, U.; Koushik, K.; Jena, L.K.; Shinde, V.; Rudrapatna, S.; Barde, S.; Humnabadkar, V.; Madhavapeddi, P.; Basavarajappa, H.; Ghosh, A.; Ramya, V.K.; Guptha, S.; Sharma, S.; Vachaspati, P.; Kumar, K.N.; Giridhar, J.; Reddy, J.; Panduga, V.; Ganguly, S.; Ahuja, V.; Gaonkar, S.; Kumar, C.N.; Ogg, D.; Tucker, J.A.; Boriack-Sjodin, P.A.; de Sousa, S.M.; Sambandamurthy, V.K.; Ghorpade, S.R. Thiazolopyridine Ureas as Novel Antitubercular Agents Acting through Inhibition of DNA Gyrase, B. J. Med. Chem. 2013, 56, 8834–8848. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Li, H.; Wang, J.; Mao, S.; Xin, M.; Lu, S.; Mei, Q.; Zhang, S. Synthesis and Anticancer Effects Evaluation of 1-alkyl-3-(6-(2-methoxy-3-sulfonylaminopyridin-5-yl)benzo[d]thiazol-2-yl)urea as Anticancer Agents with Low Toxicity. Bioorg. Med. Chem. 2015, 23, 6477–6485. [Google Scholar] [CrossRef] [PubMed]
- Bebernitz, G.R.; Beaulieu, V.; Dale, B.A.; Deacon, R.; Duttaroy, A.; Gao, J.; Grondine, M.S.; Gupta, R.C.; Kakmak, M.; Kavana, M.; Kirman, L.C.; Liang, J.; Maniara, W.M.; Munshi, S. Nadkarni, S.S.; Schuster, H.F.; Stams, T.; St Denny, I.; Taslimi, P.M.; Vash, B.; Caplan, S.L. Investigation of Functionally Liver Selective Glucokinase Activators for the Treatment of Type 2 Diabetes. J. Med. Chem. 2009, 52, 6142–6152. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, Y.; Zhang, J.; Lin, S.; Zhang, K.; Tian, H.; Dong, Y; Xu, H. Identification of Novel Thiazolo[5,4-b]Pyridine Derivatives as Potent Phosphoinositide 3-Kinase Inhibitors. Molecules. 2020, 25, 4630. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the Phosphoinositide 3-kinase Pathway in Cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef]
- Sahasrabudhe, K.P.; Angels Estiarte, M.; Tan, D.; Zipfel, S.; Cox, M.; O'Mahony, D. J. R.; Edwards, W. T.; Duncton, M. A. J. A single-step preparation of thiazolo[5,4-b]pyridine- and thiazolo[5,4-c]pyridine derivatives from chloronitropyridines and thioamides, or thioureas. J. Het. Chem. 2009, 46, 1125–1131. [Google Scholar] [CrossRef]
- Jemili, R.; Campos, J. F.; Dumuis, N.; Rabat, H.; Semmar, N.; Berteina-Raboin, S. Laser Synthesis: A Solvent-Free Approach for the Preparation of Phenylthiazolo[5,4- b ]Pyridine Derivatives. RSC Adv. 2021, 11, 5003–5007. [Google Scholar] [CrossRef]
- Atland, H.W.; Molander, G.A. A facile synthesis of 2-aminothiazolo[5,4-b] and 2-aminothiazolo[4,5-c]pyridines. J. Heteocyclic Chem. 1977, 14, 129–134. [Google Scholar] [CrossRef]
- Fînaru, A.; Berthault, A.; Besson, T.; Guillaumet, G.; Berteina-Raboin, S. Microwave-Assisted Solid-Phase Synthesis of 5-Carboxamido-N-acetyltryptamine Derivatives. Org. Lett. 2002, 4, 16, 2613–2615. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Xu, X.; Liu, Z.; Sun, L.-P.; You, Q. A general and efficient synthesis of 2-substituted Oxazolopyridines. Synlett 2009, 7, 1172–1174. [Google Scholar] [CrossRef]
| Entry | Solvent | T (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|
| 1 | Acétone | 100 | 4 | 60 |
| 2 | DCM | 100 | 4 | 43 |
| 3 | Toluène | 100 | 4 | 44 |
| 4 | Dioxane | 100 | 4 | 44 |
| 5 | THF | 100 | 4 | 43 |
| 6 | Eucalyptol | 100 | 4 | 59 |
| 7 | Eucalyptol | 100 | 16 | 75 |
| 8 | CPME | 100 | 4 | 63 |
| 9 | CPME | 100 | 16 | 71 |
| 10 | Sabinene | 100 | 4 | 36 |
| 11 | Sabinène | 100 | 16 | 58 |
| 12 | Limonene | 100 | 4 | 65 |
| 13 | Limonene | 100 | 16 | 70 |
| 14 | Citral | 100 | 16 | 21 |
| Entry | Reaction time | Temperature (°C) | Yield (%) | Solvant |
|---|---|---|---|---|
| 1 | 1 h | 160 | 67 | Sabinene |
| 2 | 2 h | 150 | 59 | |
| 3 | 2 h | 130 | 55 | |
| 4 | 4 h | 130 | 62 | |
| 5 | 2 h | 130 | 44 | 75 :25 Sabinene/Ethanol |
| 6 | 2 h | 130 | 64 | 75 :25 Sabinene/ACN |
| Entry | Reaction time | Temperature | Yield |
|---|---|---|---|
| 1 | 30 mn | 150 °C | 40 % |
| 2 | 1 h | 150 °C | 61% |
| 3 | 1 h | 160 °C | 57 % |
| 4 | 2 h | 150 °C | 63 % |
| 6 | 2 h | 110 °C | 37 % |
| 7 | 2 h | 130 °C | 64 % |
| 8 | 1 h | 130°C | 50 % |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





