Submitted:
30 August 2023
Posted:
31 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
- Using the OptQuest optimizer, included in AnyLogic software, to validate a stochastic ABM model for the spread of COVID-19 and use it for short- and long-term forecasting
- Determination of age groups and public places of locations most susceptible to the spread of infection in a large settlement of Kazakhstan
- Assess how the preventive measures taken by the regulator affect material and human resources compared to six hypothetical scenarios: no intervention, school clause, mask veering, vaccination, combined measures.
2. Materials and Methods
2.1. Study Area
2.2. ABM Model Implementation
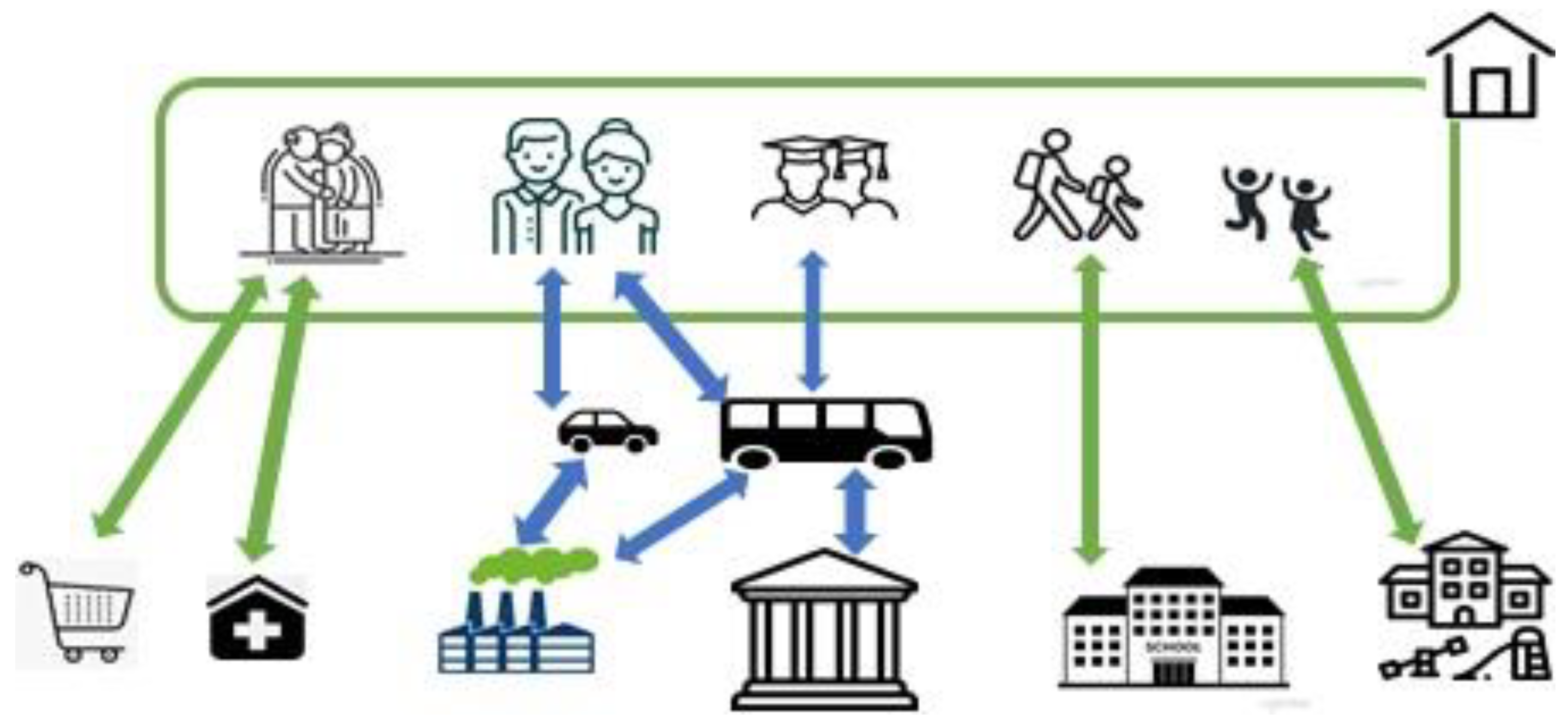
2.2.1. Social Network
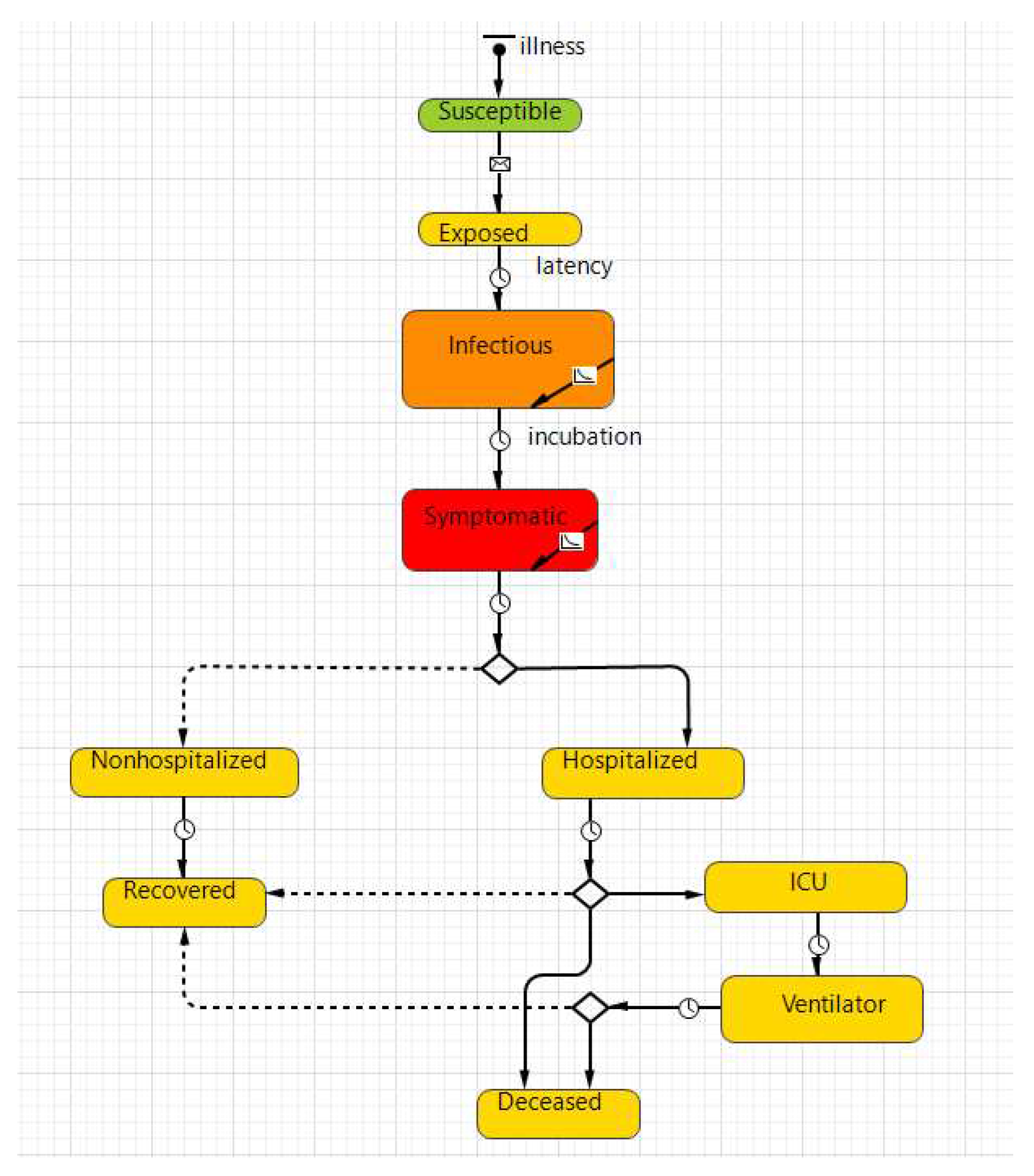
2.2.2. Disease Transmission
2.2.3. Model Parameters
2.3. Data Collection
2.4. Scenario Setting
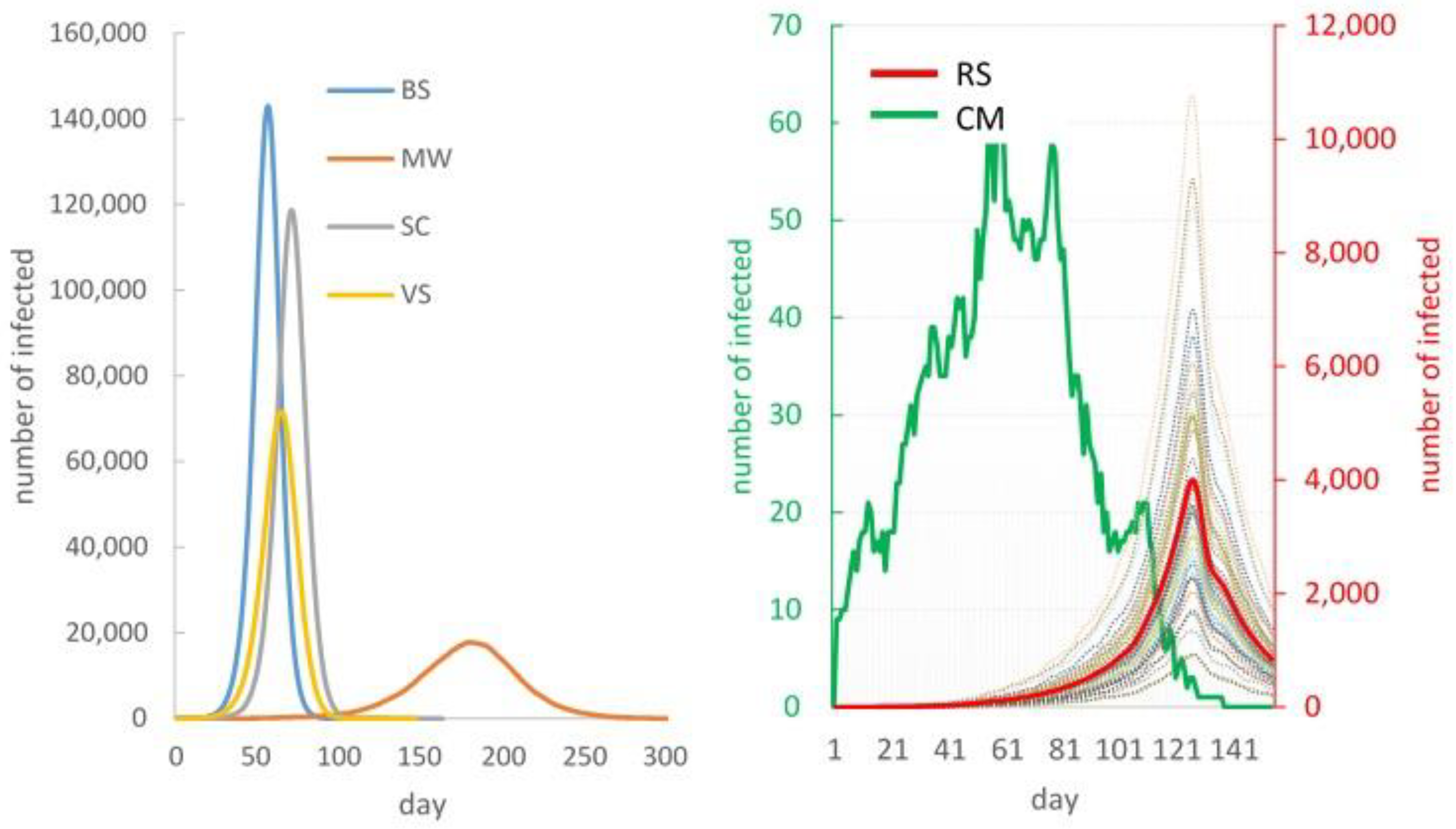
Base Scenario (BS) – No Intervention
Scenario 2 – School Closure (SC).
Scenario 3 – Mask Wearing (MW)
Scenario 4 – Vaccination (VS).
Scenario 5 – Combined Measures (CM)
Scenario 6 – Real Situation Simulation (RS)
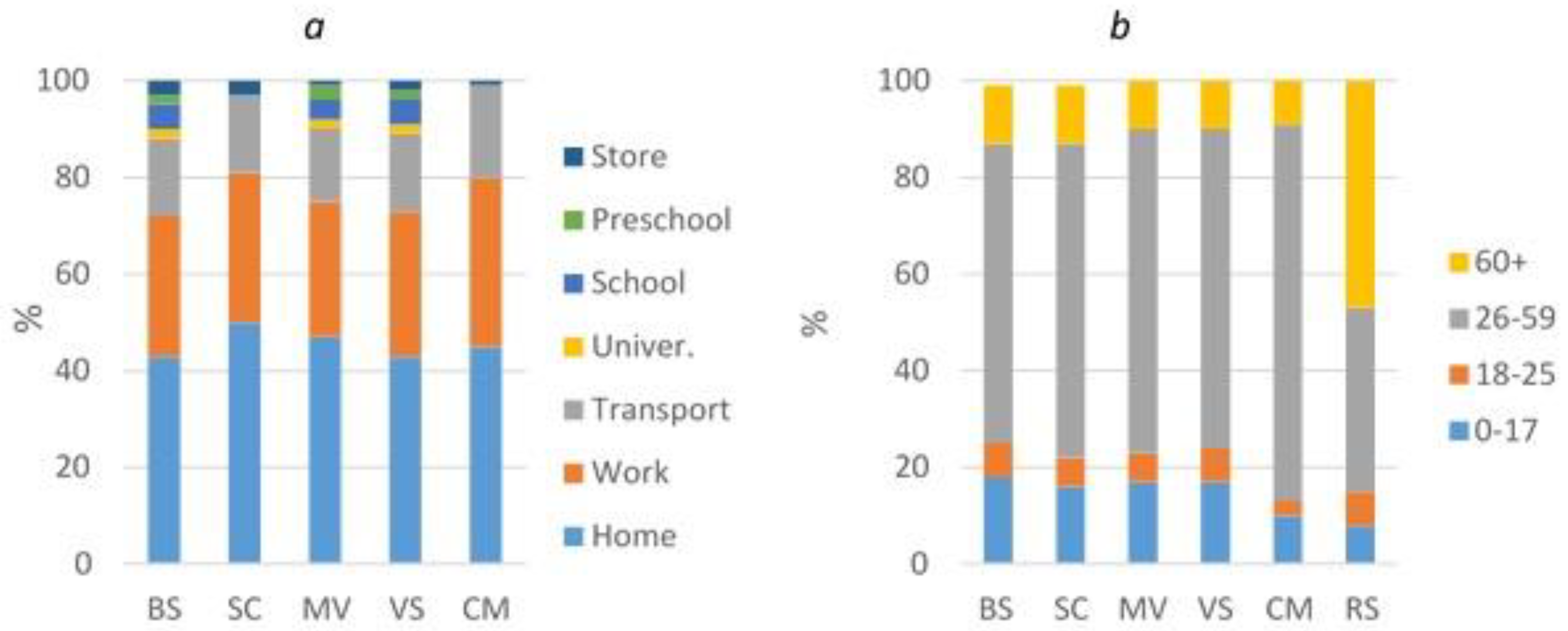
- the number of infected people at home, in educational institutions, at work, in transport, in stores
- the number of infected people among different age groups of the population
- number of doctors, auxiliary medical and technical personnel needed
- number of beds required for hospitalized persons
- the cost of devices, medicines, chemicals and other materials
3. Results
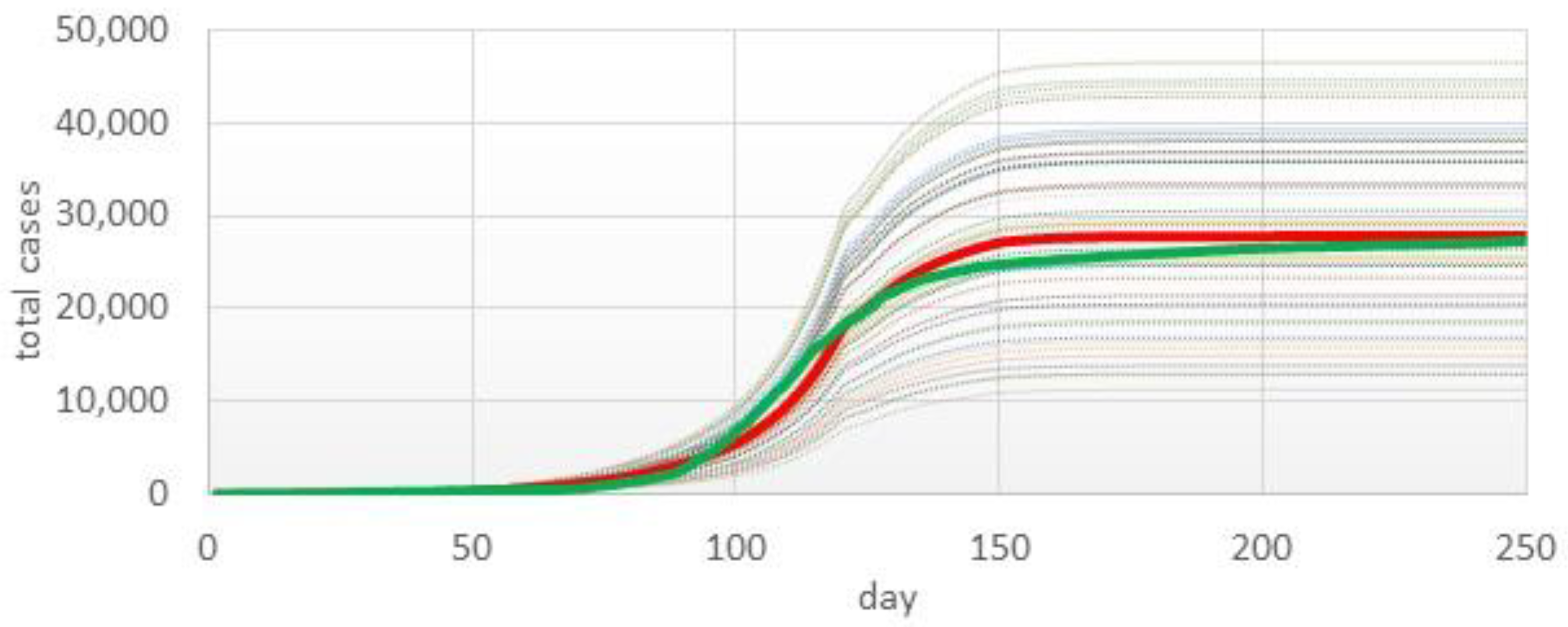
3.1. Model Validation
3.2. Prediction Simulation
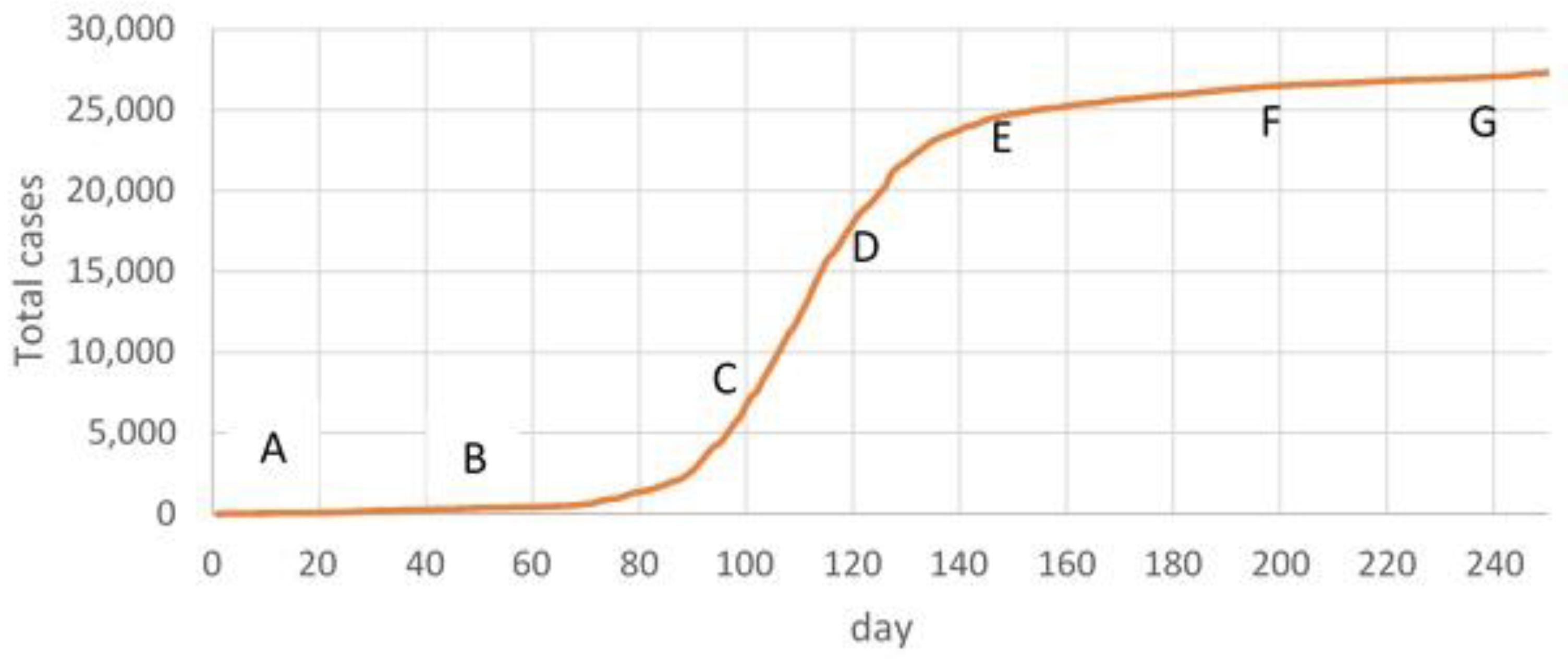
3.2.1. Forecasting Based on Time Frame AB
3.2.2. Forecasting Based on Time Frame AC
3.3. Epidemiological Impact of COVID-19
3.4. Economic Impact of COVID-19
3.4.1. Healthcare Human Resource (Medical Practitioners, including Physicians, Nursing Professionals, and Paramedical Practitioners)
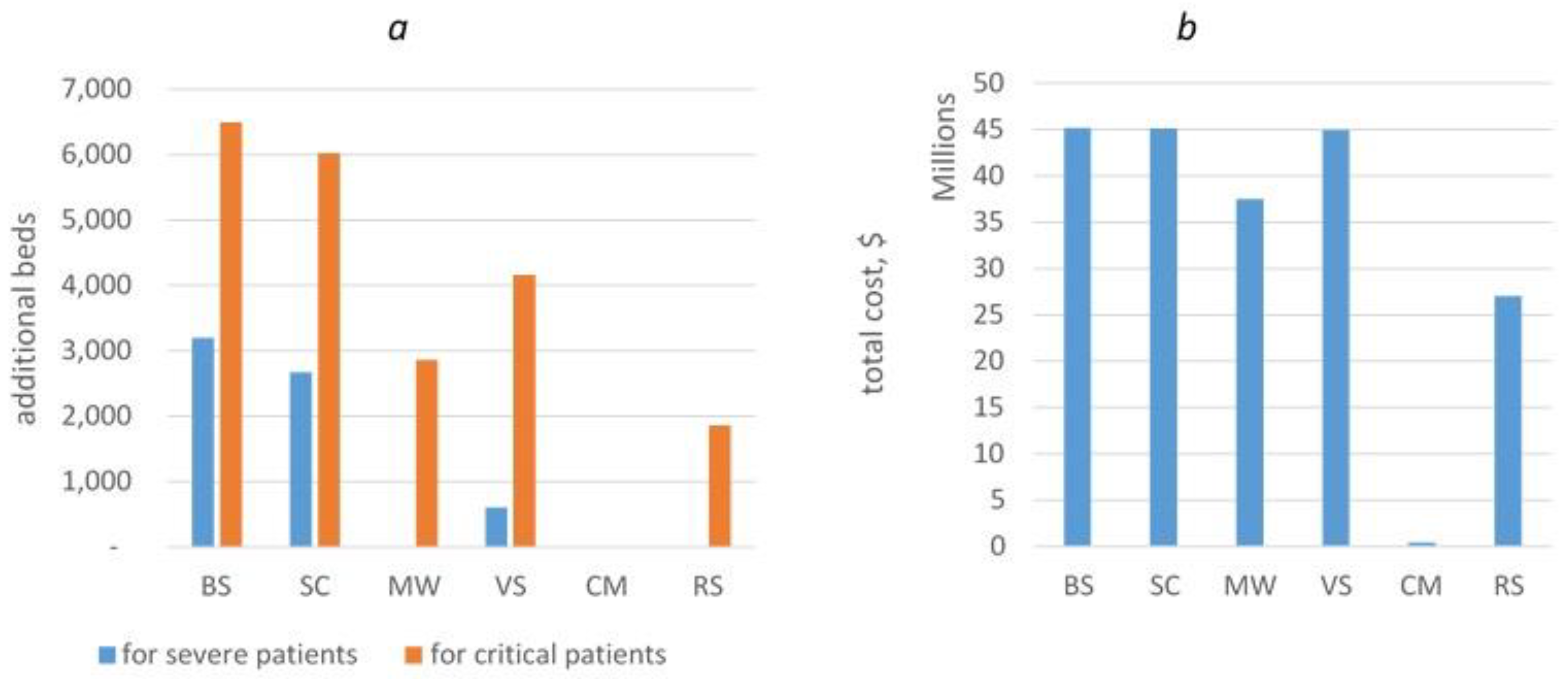
3.4.2. Hospital Beds
3.4.3. Equipment, Pharmaceuticals, Consumables, and Accessories
4. Discussion
4.1. The Findings and Their Implications
4.2. Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Stefano B, Fuks H, Lawniczak AT. Object-Oriented Implementation of CA-LGCA Modelling Applied to the Spread of Epidemics. 2000 Canadian Conference on Electrical and Computer Engineering, IEEE 2000; 1:26-31. [CrossRef]
- Sirakoulis GC, Karafyllidis I, Thanailakis A. A cellular automaton model for the effects of population movement and vaccination on epidemic propagation. Ecological Modelling 2000; 133:209-233. [CrossRef]
- Zhen J, Quan-Xing L. A cellular automata model of epidemics of a heterogeneous susceptibility. Chinese Physics 2006; 15:1248-1256. [CrossRef]
- Macal CM, North MJ. Tutorial on agent-based modelling and simulation. Journal of Simulation. 2010;4(3):151-162. [CrossRef]
- Bonabeau, E. Agent-based modeling: Methods and techniques for simulating human systems. Proc Natl Acad Sci USA. 2002;99(suppl_3):7280-7287. [CrossRef]
- Beutels P, Shkedy Z, Aerts M, Damme PV. Social mixing patterns for transmission models of close contact infections: exploring self-evaluation and diary-based data collection through a web-based interface. Epidemiology & Infection. 2006;134(6):1158-1166. [CrossRef]
- Halloran ME, Longini IM, Nizam A, Yang Y. Containing bioterrorist smallpox. Science. 2002;298(5597):1428-1432. [CrossRef]
- Cooley P, Lee BY, Brown S, et al. Protecting health care workers: a pandemic simulation based on Allegheny County. Influenza and Other Respiratory Viruses. 2010;4(2):61-72. [CrossRef]
- Enanoria WTA, Liu F, Zipprich J, et al. The effect of contact investigations and public health interventions in the control and prevention of measles transmission: a simulation study. PLOS ONE. 2016;11(12):e0167160. [CrossRef]
- Ferguson NM, Cummings DAT, Cauchemez S, et al. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature. 2005;437(7056):209-214. [CrossRef]
- Epstein JM, Pankajakshan R, Hammond RA. Combining computational fluid dynamics and agent-based modeling: a new approach to evacuation planning. PLOS ONE. 2011;6(5):e20139. [CrossRef]
- Braun B, Taraktaş B, Beckage B, Molofsky J. Phase transitions and control measures for network epidemics caused by infections with presymptomatic, asymptomatic,and symptomatic stages. Published online July 9, 2020. Accessed August 3, 2023. http://arxiv.org/abs/2005.09751.
- Kai D, Goldstein GP, Morgunov A, Nangalia V, Rotkirch A. Universal masking is urgent in the COVID-19 pandemic: seir and agent based models, empirical validation, policy recommendations. Published online April 22, 2020. Accessed August 3, 2023. http://arxiv.org/abs/2004.13553.
- Gopalan A, Tyagi H. How reliable are test numbers for revealing the COVID-19 ground truth and applying interventions? Published online April 24, 2020. Accessed August 3, 2023. http://arxiv.org/abs/2004.12782.
- D’Orazio M, Bernardini G, Quagliarini E. How to restart? An agent-based simulation model towards the definition of strategies for COVID-19 “second phase” in public buildings. Build Simul. 2021;14(6):1795-1809. [CrossRef]
- Hoertel N, Blachier M, Blanco C, et al. Facing the COVID-19 Epidemic in NYC: A Stochastic Agent-Based Model of Various Intervention Strategies. Public and Global Health; 2020. [CrossRef]
- Chen J, Vullikanti A, Santos J, et al. Epidemiological and economic impact of COVID-19 in the US. Sci Rep. 2021;11(1):20451. Published 2021 Oct 14. [CrossRef]
- Inoue, H. , Todo Y.. The propagation of the economic impact through supply chains: the case of a mega-city lockdown against the spread of COVID-19. Available at SSRN 35648982020.
- Dignum, F. , Dignum V., Davidsson P., Ghorbani A., van der Hurk M., Jensen M., et al. Analyzing the combined health, social and economic impacts of the coronavirus pandemic using agent-based social simulation. arXiv:2004.12809 2020.
- Silva PCL, Batista PVC, Lima HS, Alves MA, Guimarães FG, Silva RCP. COVID-ABS: An agent-based model of COVID-19 epidemic to simulate health and economic effects of social distancing interventions. Chaos Solitons Fractals. 2020;139:110088. [CrossRef]
- Zhang T, Wang Q, Leng Z, et al. A Scenario-Based Evaluation of COVID-19-Related Essential Clinical Resource Demands in China. Engineering (Beijing). 2021;7(7):948-957. [CrossRef]
- Moynihan R, Sanders S, Michaleff ZA, et al. Impact of COVID-19 pandemic on utilisation of healthcare services: a systematic review. BMJ Open 2021;11:e045343. [CrossRef]
- Pandey KR, Subedee A, Khanal B, Koirala B. COVID-19 control strategies and intervention effects in resource limited settings: A modeling study. Gerberry D, ed. PLoS ONE. 2021;16(6):e0252570. [CrossRef]
- Gnanvi JE, Salako KV, Kotanmi GB, Glèlè Kakaï R. On the reliability of predictions on COVID-19 dynamics: A systematic and critical review of modelling techniques. Infectious Disease Modelling. 2021;6:258-272. [CrossRef]
- Heath B, Hill R., Ciarallo F. (). A survey of agent-based modeling practices (January 1998 to July 2008). Journal of Artificial Societies and Social Simulation. 2009;12(4), 9.
- Lorig F, Johansson E, Davidsson P. Agent-based social simulation of the COVID-19 pandemic: a systematic review. JASSS. 2021;24(3):5. [CrossRef]
- Luke DA, Stamatakis KA. Systems science methods in public health: dynamics, networks, and agents. Annu Rev Public Health. 2012;33(1):357-376. [CrossRef]
- Berkman LF, Glass T, Brissette I, Seeman TE. From social integration to health: Durkheim in the new millennium. Social Science & Medicine. 2000;51(6):843-857. [CrossRef]
- Macintyre S, Ellaway A, Cummins S. Place effects on health: how can we conceptualise, operationalise and measure them? Social Science & Medicine. 2002;55(1):125-139. [CrossRef]
- Rutter H, Savona N, Glonti K, et al. The need for a complex systems model of evidence for public health. The Lancet. 2017;390(10112):2602-2604. [CrossRef]
- Reveil M, Chen YH. Predicting and preventing COVID-19 outbreaks in indoor environments: an agent-based modeling study. Sci Rep. 2022;12(1):16076. [CrossRef]
- Cramer EY, Ray EL, Lopez VK et al. Evaluation of individual and ensemble probabilistic forecasts of COVID-19 mortality in the united states. Proceedings of the National Academy of Sciences 2022;119. [CrossRef]
- Pei S, Kandula S, Shaman J. Differential effects of intervention timing on COVID-19 spread in the United States. Sci Adv. 2020;6(49):eabd6370. [CrossRef]
- Gostic KM, McGough L, Baskerville EB, et al. Practical considerations for measuring the effective reproductive number, Rt. Pitzer VE, ed. PLoS Comput Biol. 2020;16(12):e1008409. [CrossRef]
- Hasan A, Putri ERM, Susanto H, Nuraini N. Data-driven modeling and forecasting of COVID-19 outbreak for public policy making. ISA Transactions. 2022;124:135-143. [CrossRef]
- Zhang Z, Karniadakis G. Numerical Methods for Stochastic Partial Differential Equations with White Noise; Springer: Cham, Switzerland, 2017; 394 p. [Google Scholar]
- Bernal F, Acebrón JA. A comparison of higher-order weak numerical schemes for stopped stochastic differential equations. Communications in Computational Physics. 2016;20(3):703-732. [CrossRef]
- Biagini F, Øksendal B, Sulem A, Wallner N. An introduction to white–noise theory and Malliavin calculus for fractional Brownian motion. Proceedings of the Royal Society of London. Series A: Mathematical, Physical and Engineering Sciences. 2004; Jan 8;460(2041):347-72.
- Burdzy, K. Brownian motion. In: Burdzy K, ed. Brownian Motion and Its Applications to Mathematical Analysis: École d’Été de Probabilités de Saint-Flour XLIII – 2013. Lecture Notes in Mathematics. Springer International Publishing; 2014:1-10. [CrossRef]
- Hussain S, Madi EN, Khan H, et al. Investigation of the stochastic modeling of COVID-19 with environmental noise from the analytical and numerical point of view. Mathematics. 2021;9(23):3122. [CrossRef]
- Niño-Torres D, Ríos-Gutiérrez A, Arunachalam V, Ohajunwa C, Seshaiyer P. Stochastic modeling, analysis, and simulation of the COVID-19 pandemic with explicit behavioral changes in Bogotá: A case study. Infectious Disease Modelling. 2022;7(1):199-211. [CrossRef]
- Tesfaye AW, Satana TS. Stochastic model of the transmission dynamics of COVID-19 pandemic. Advances in Difference Equations. 2021;2021(1):457. [CrossRef]
- Balsa C, Lopes I, Guarda T, Rufino J. Computational simulation of the COVID-19 epidemic with the SEIR stochastic model. Comput Math Organ Theory. Published online March 30, 2021. [CrossRef]
- Zhang Z, Zeb A, Hussain S, Alzahrani E. Dynamics of COVID-19 mathematical model with stochastic perturbation. Adv Differ Equ. 2020;2020(1):451. [CrossRef]
- Alenezi MN, Al-Anzi FS, Alabdulrazzaq H, Alhusaini A, Al-Anzi AF. A study on the efficiency of the estimation models of COVID-19. Results in Physics. 2021;26:104370. [CrossRef]
- Hansen CH, Michlmayr D, Gubbels SM, Mølbak K, Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. The Lancet. 2021;397(10280):1204-1212. [CrossRef]
- Keeling, Matt J. and Rohani, Pejman, Modeling Infectious Diseases in Humans and Animals, Princeton University Press, 2008, 366 p.
- Ebrahimi A H, Alesheikh A A, Hooshangi N. Spatio-temporal agent based simulation of COVID-19 disease and investigating the effect of vaccination (case study: Urmia). JGST 2023; 12 (2) :206-221.
- Zhang L, Zhu J, Wang X, Yang J, Liu XF, Xu XK. Characterizing COVID-19 transmission: incubation period, reproduction rate, and multiple-generation spreading. Front Phys. 2021;8:589963. [CrossRef]
- Litvinova M, Liu QH, Kulikov ES, Ajelli M. Reactive school closure weakens the network of social interactions and reduces the spread of influenza. Proc Natl Acad Sci USA. 2019;116(27):13174-13181. [CrossRef]
- Guo X, Gupta A, Sampat A, Zhai C. A stochastic contact network model for assessing outbreak risk of COVID-19 in workplaces. Mukherjee A, ed. PLoS ONE. 2022;17(1):e0262316. [CrossRef]
- He S, Tang SY, Rong L. A discrete stochastic model of the COVID-19 outbreak: Forecast and control. Math Biosci Eng. 2020;17(4):2792-2804. [CrossRef]
- Klepac P, Kucharski AJ, Conlan AJ, et al. Contacts in context: large-scale setting-specific social mixing matrices from the BBC Pandemic project. Published online March 5, 2020. [CrossRef]
- Gallo LG, Oliveira AF de M, Abrahão AA, et al. Ten epidemiological parameters of COVID-19: use of rapid literature review to inform predictive models during the pandemic. Frontiers in Public Health. 2020;8. Accessed August 3, 2023. https://www.frontiersin.org/articles/10.3389/fpubh.2020.598547.
- McAloon C, Collins Á, Hunt K, et al. Incubation period of COVID-19: a rapid systematic review and meta-analysis of observational research. BMJ Open. 2020;10(8):e039652. [CrossRef]
- Cheng C, Zhang D, Dang D, et al. The incubation period of COVID-19: a global meta-analysis of 53 studies and a Chinese observation study of 11 545 patients. Infectious Diseases of Poverty. 2021;10(1):119. [CrossRef]
- Wu Y, Kang L, Guo Z, Liu J, Liu M, Liang W. Incubation period of COVID-19 caused by unique sars-cov-2 strains: a systematic review and meta-analysis. JAMA Network Open. 2022;5(8):e2228008. [CrossRef]
- Lewis, C.D. Industrial and Business Forecasting Methods: A Practical Guide to Exponential Smoothing and Curve Fitting; Butterworth-Heinemann: Oxford, UK, 1982.
- Diekmann O, Heesterbeek JAP, Metz JAJ. On the definition and the computation of the basic reproduction ratio R 0 in models for infectious diseases in heterogeneous populations. J Math Biol. 1990;28(4). [CrossRef]
- Lipsitch M, Cohen T, Cooper B, et al. Transmission dynamics and control of severe acute respiratory syndrome. Science. 2003;300(5627):1966-1970. [CrossRef]
- Semenova Y, Pivina L, Khismetova Z, et al. Anticipating the need for healthcare resources following the escalation of the COVID-19 outbreak in the republic of kazakhstan. J Prev Med Public Health. 2020;53(6):387-396. [CrossRef]
- Tian L, Li X, Qi F, et al. Calibrated intervention and containment of the COVID-19 pandemic. Published online November 17, 2020. Accessed August 4, 2023. http://arxiv.org/abs/2003.07353.
- Howard J, Huang A, Li Z, et al. An evidence review of face masks against COVID-19. Proc Natl Acad Sci USA. 2021;118(4):e2014564118. [CrossRef]
- https://ourworldindata.org/COVID-vaccinations.
- World Health Organization. COVID-19 essential supplies forecasting tool [cited 2020 Aug 11]. Available from: https://www.who.int/publications/m/item/COVID-19-essential-supplies-fore¬casting-tool.
- Cevik M, Bamford CGG, Ho A. COVID-19 pandemic—a focused review for clinicians. Clinical Microbiology and Infection. 2020;26(7):842-847. [CrossRef]
- Hildenwall H, Luthander J, Rhedin S, et al. Paediatric COVID-19 admissions in a region with open schools during the two first months of the pandemic. Acta Paediatr. 2020;109(10):2152-2154. [CrossRef]
- Hume S, Brown SR, Mahtani KR. School closures during COVID-19: an overview of systematic reviews. BMJ EBM. 2023;28(3):164-174. [CrossRef]
- Hitchings MDT, Ranzani OT, Scaramuzzini Torres MS, et al. Effectiveness of CoronaVac among Healthcare Workers in the Setting of High SARS-CoV-2 Gamma Variant Transmission in Manaus, Brazil: A Test-Negative Case-Control Study. Infectious Diseases (except HIV/AIDS); 2021. [CrossRef]

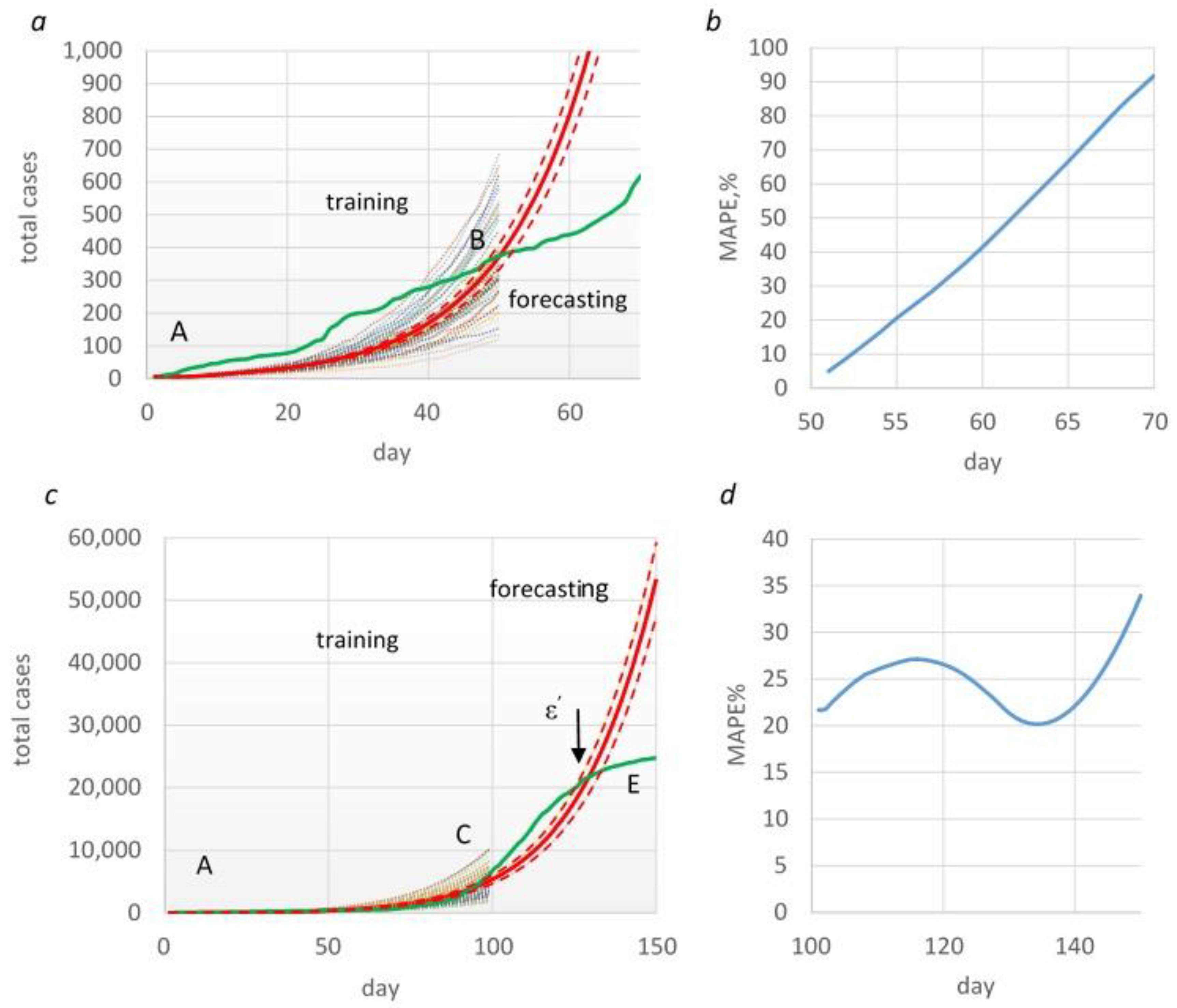
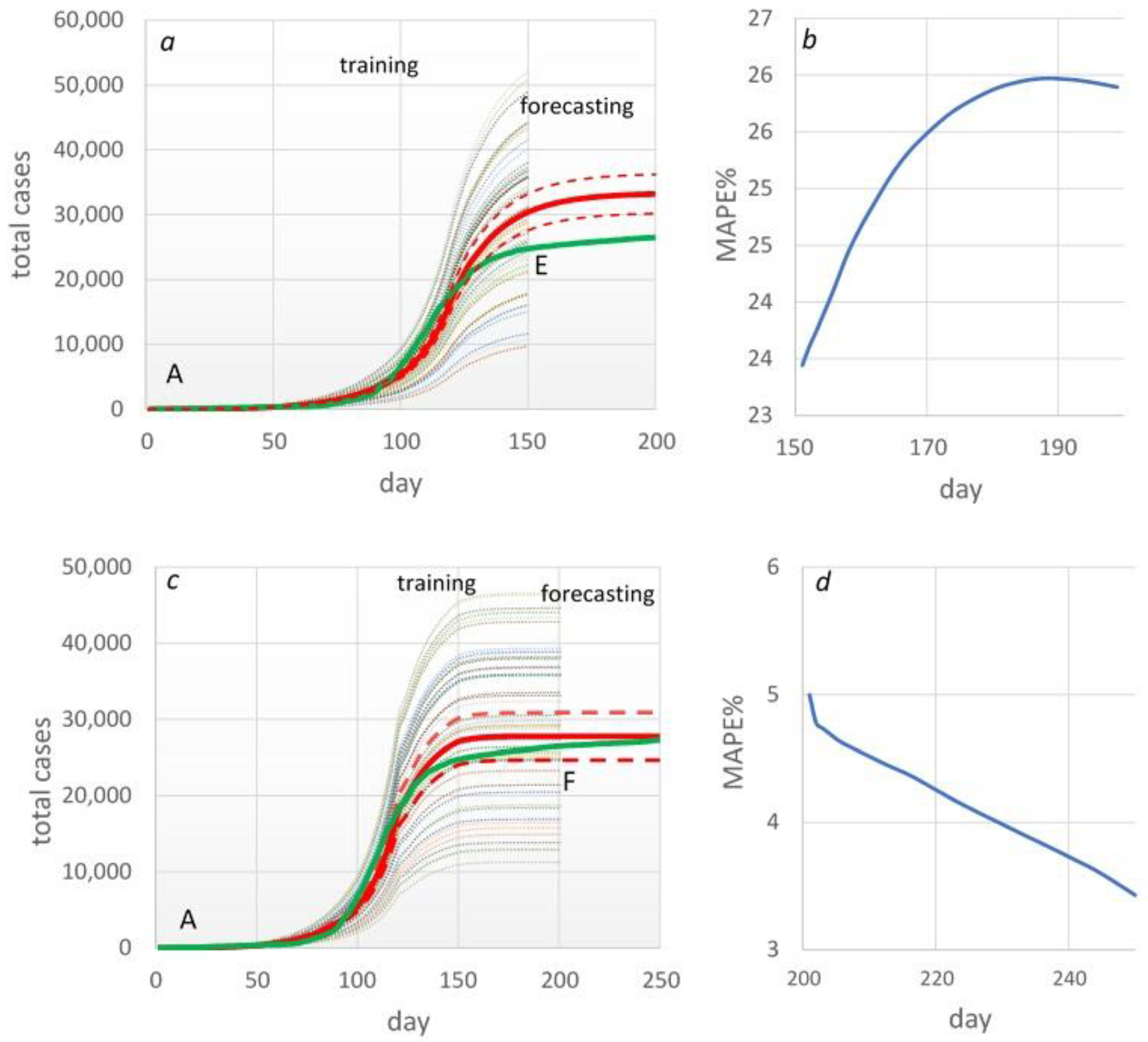
| Table . | p-transmission probability | MAPE% |
|---|---|---|
| 0-50 | 0.09 | 53 |
| 50-100 | 0.069 | 41 |
| 100-120 | 0.084 | 17 |
| 120-150 | 0.024 | 5 |
| 150-200 | 0.011 | 8 |
| Overall | 28 |
| Forecasting period | 5 days | 10 days | 15 days |
|---|---|---|---|
| MAPE% | 20 | 42 | 65.7 |
| Scenario | TotalCases | Outpatient | Hospitalized | ICU | Ventilated |
|---|---|---|---|---|---|
| BS | 471,746 | 349,092 | 122,654 | 7,359 | 1,651 |
| SC | 438,610 | 324,571 | 114,039 | 6,842 | 1,535 |
| MV | 215,715 | 159,629 | 56,086 | 3,365 | 755 |
| VS | 307,381 | 227,462 | 79,919 | 4,795 | 1,076 |
| CM | 604 | 445 | 159 | 10 | 2 |
| RS | 145,044 | 107,757 | 37,287 | 2,294 | 502 |
| BS | SC | MV | VS | CM | RS | ||
|---|---|---|---|---|---|---|---|
| Inpatient | Total number of health care workers | 1,970 | 1,970 | 1,970 | 1,970 | 54 | 1,970 |
| Total number of cleaners | 3,121 | 3,121 | 2,528 | 3,121 | 13 | 1,688 | |
| Total number of ambulance personnel | 4,458 | 4,458 | 3,611 | 4,458 | 18 | 2,412 | |
| Total number of biomedical engineers | 134 | 134 | 108 | 134 | 1 | 72 | |
| Laboratories | Total number of lab staff required | 3 | 3 | 3 | 3 | 3 | 3 |
| Total number of cleaners | 1 | 1 | 1 | 1 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





