Submitted:
30 August 2023
Posted:
31 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
2.1. Materials
2.2. Preparation of magnetic chitosan nanoparticles
2.3. Characterizations of magnetic chitosan nanoparticles
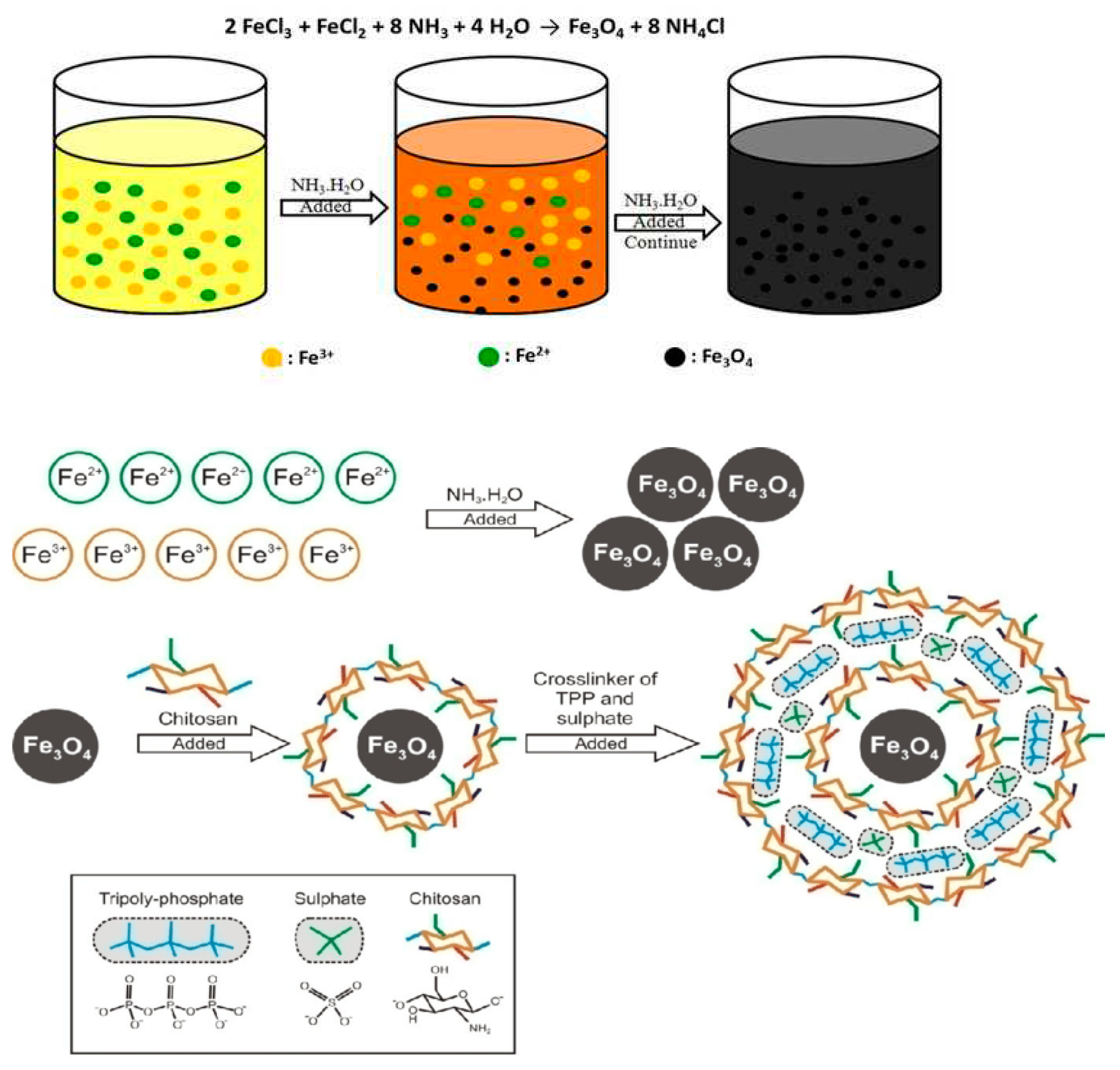
3. Results and discussions
3.1. Nanoparticles with magnetic properties are described
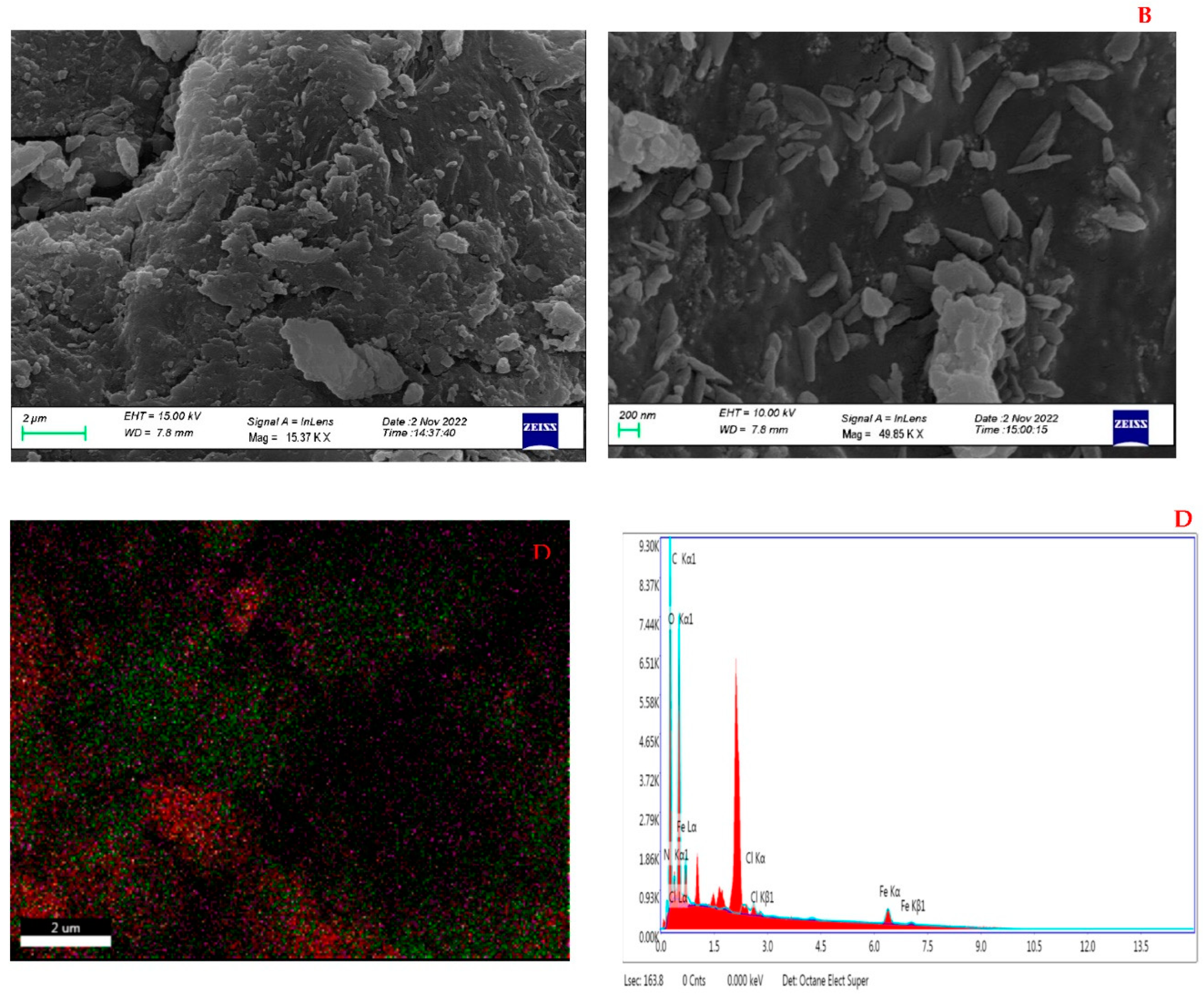
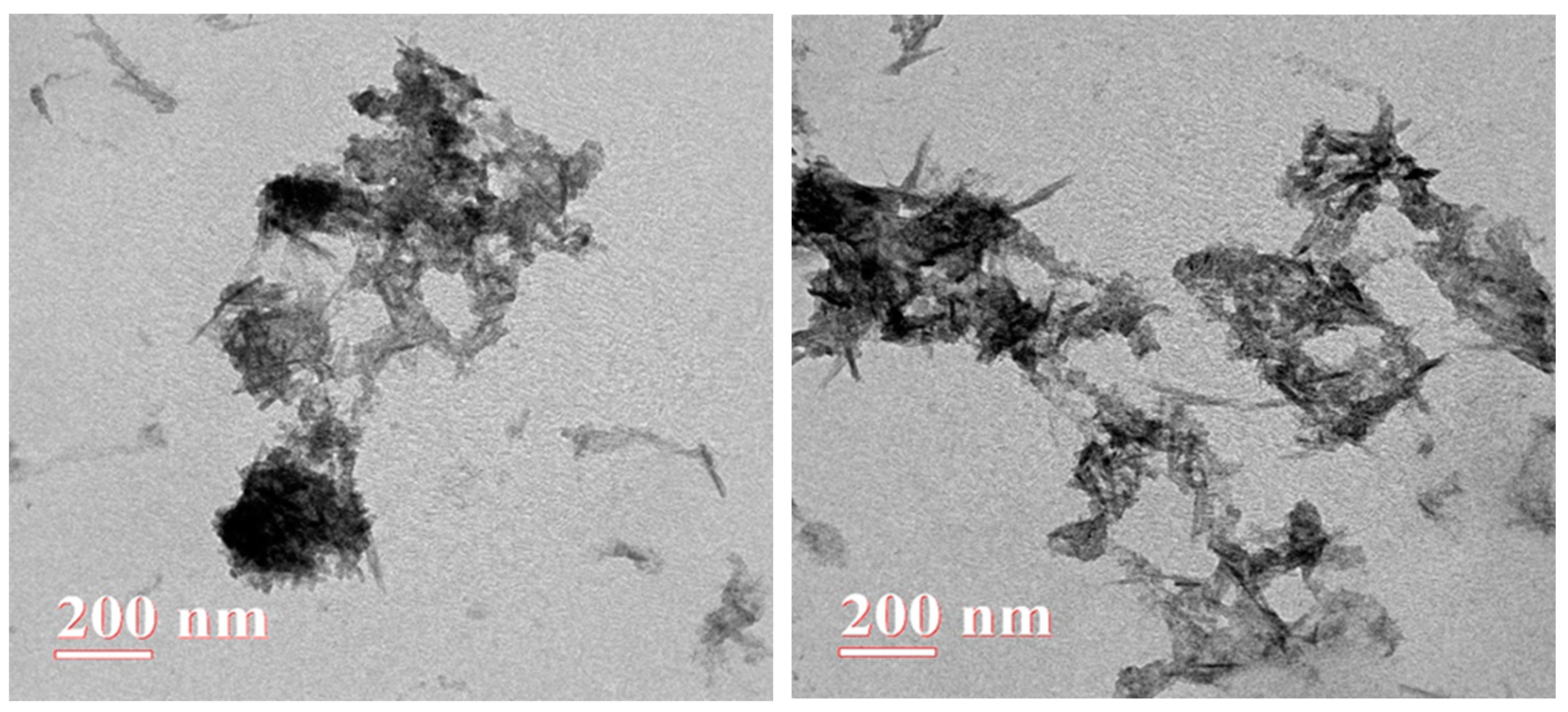
3.1.1. Morphological characterization
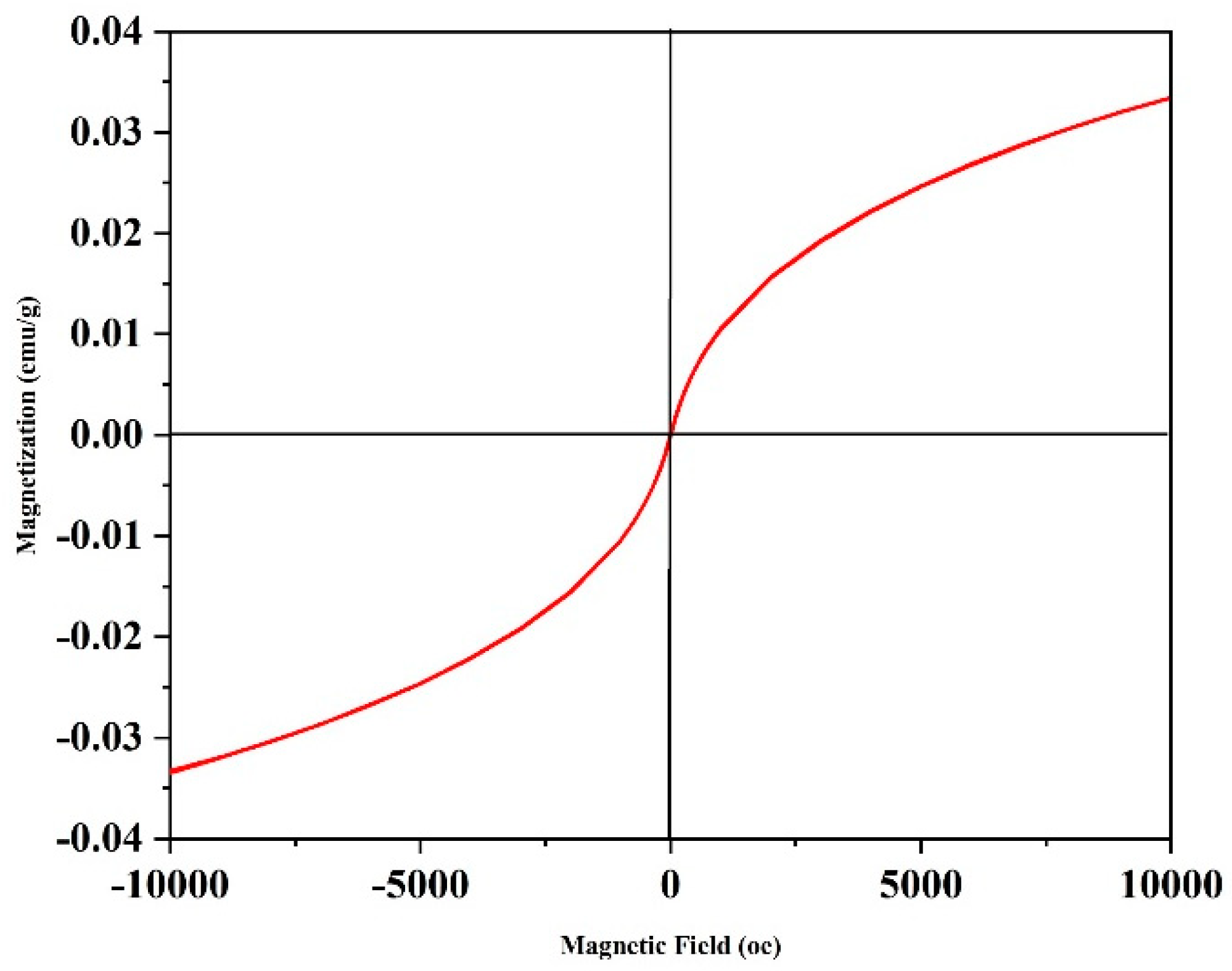
3.1.2. Magnetic properties
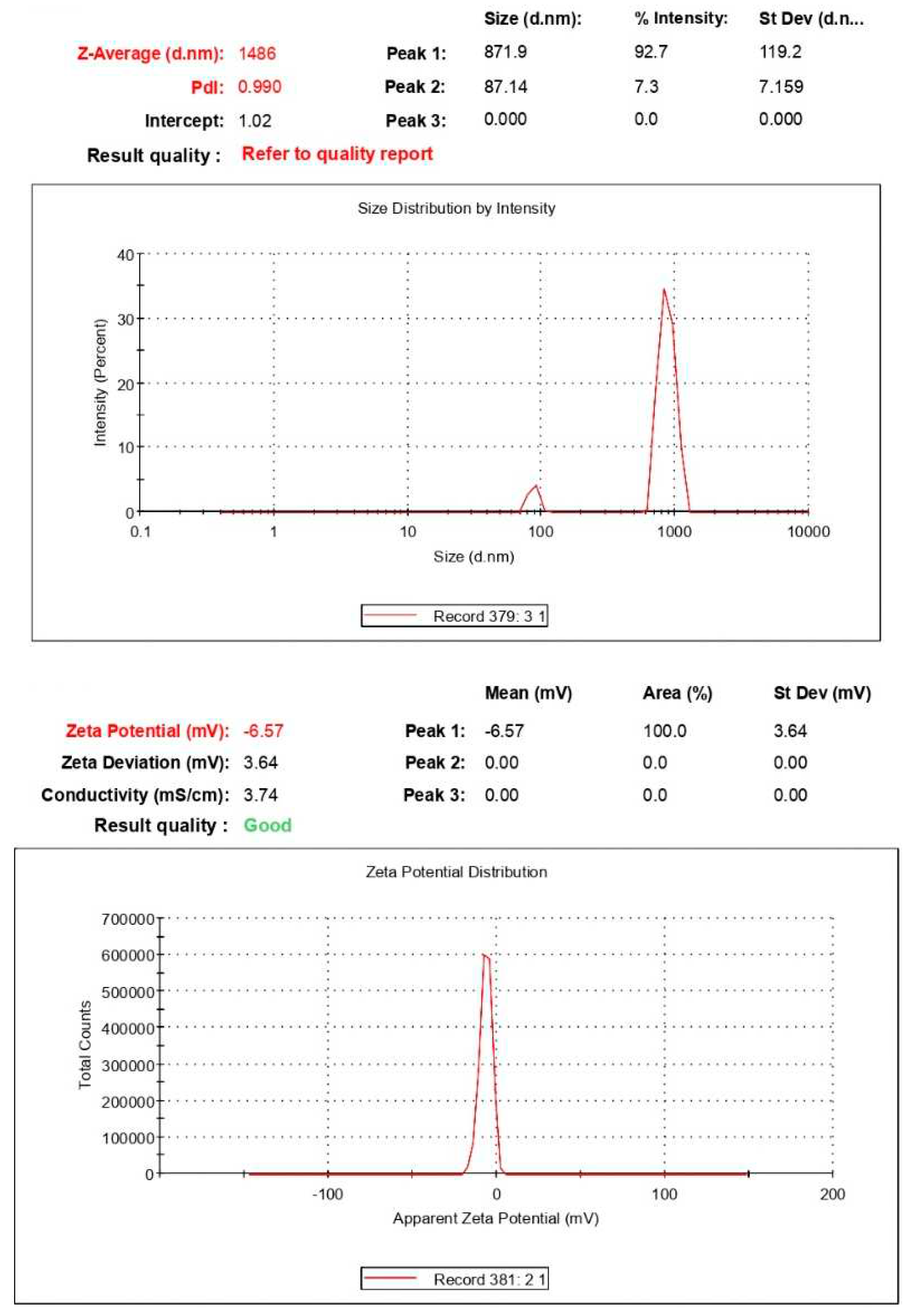
3.1.3. DLS analysis
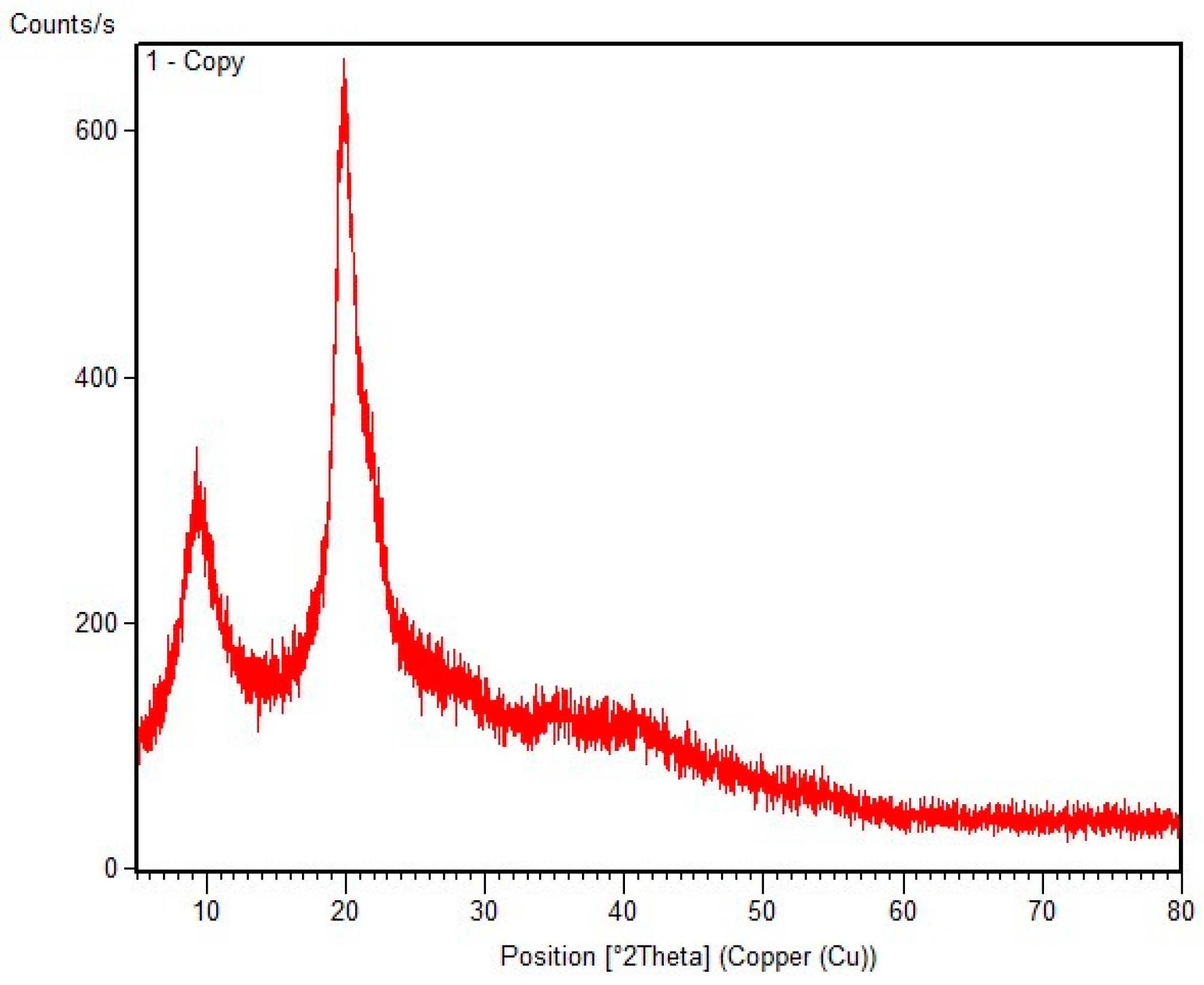
3.1.4. XRD analysis
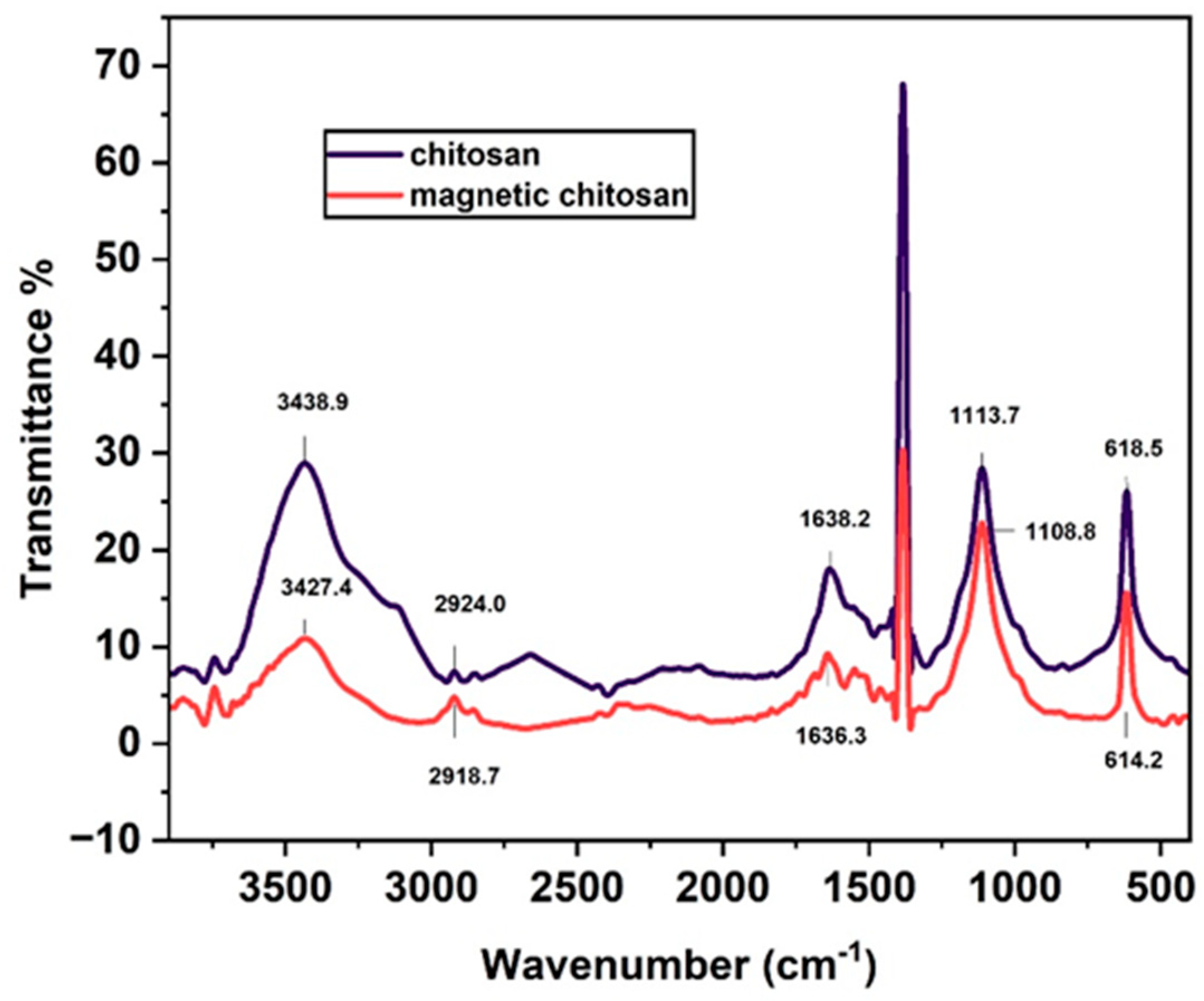
3.1.5. FTIR analysis
3.1.6. Applications of magnetic chitosan nanoparticles
4. Conclusions
References
- Peter, Sherin, Nathalie Lyczko, Deepu Gopakumar, Hanna J. Maria, Ange Nzihou, and Sabu Thomas. Chitin and chitosan based composites for energy and environmental applications: A review. Waste and Biomass Valorization 12, no. 9 (2021): 4777-4804. [CrossRef]
- Joseph, Sherin M., Srinivasan Krishnamoorthy, R. Paranthaman, J. A. Moses, and C. Anandharamakrishnan. A review on source-specific chemistry, functionality, and applications of chitin and chitosan. Carbohydrate Polymer Technologies and Applications 2 (2021): 100036. [CrossRef]
- Wang, Xiangtao, Yifei Guo, Li Yang, Meihua Han, Jing Zhao, and Xiaoliang Cheng. Nanomaterials as sorbents to remove heavy metal ions in wastewater treatment. J. Environ. Anal. Toxicol 2, no. 7 (2012): 154-158. [CrossRef]
- Qasem,Naef AA,Ramy H.Mohammed,and Dahiru U. Lawal. Removal of heavy metal ions from wastewater:A comprehensive and critical review. Npj Clean Water 4,no.1 (2021):1-15. [CrossRef]
- Qin, H., C. M. Wang, Q. Q. Dong, L. Zhang, X. Zhang, Z. Y. Ma, and Q. R. Han. Preparation and characterization of magnetic Fe3O4–chitosan nanoparticles loaded with isoniazid. Journal of Magnetism and Magnetic Materials 381(2015):120-126. [CrossRef]
- Chang, Yang-Chuang, and Dong-Hwang Chen. Adsorption kinetics and thermodynamics of acid dyes on a carboxymethylated chitosan-conjugated magnetic nano-adsorbent. Macromolecular bioscience 5, no. 3 (2005): 254-261. [CrossRef]
- Hosseini, Fatemeh, Somayeh Sadighian, Hassan Hosseini-Monfared, and Niyaz Mohammad Mahmoodi. Dye removal and kinetics of adsorption by magnetic chitosan nanoparticles. Desalination and Water Treatment 57, no. 51 (2016):24378-24386. [CrossRef]
- Carolin, C. Femina, P. Senthil Kumar, A. Saravanan, G. Janet Joshiba, and Mu Naushad. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. Journal of environmental chemical engineering 5, no.3(2017):2782-2799. [CrossRef]
- Prabu, D., P. Senthil Kumar, B. Senthil Rathi, S. Sathish, K. Vijai Anand, J. Aravind Kumar, Osama B. Mohammed, and P. Silambarasan. Feasibility of magnetic nano adsorbent impregnated with activated carbon from animal bone waste: Application for the chromium (VI) removal. Environmental Research 203(2022):111813. [CrossRef]
- Zhang, Lei, Yuexian Zeng, and Zhengjun Cheng. Removal of heavy metal ions using chitosan and modified chitosan: A review. Journal of Molecular Liquids 214 (2016):175-191. [CrossRef]
- Trung, Trang Si, Nguyen Van Tan, Nguyen Van Hoa, Nguyen Cong Minh, Phan Thanh Loc, and Willem F. Stevens. Improved method for production of chitin and chitosan from shrimp shells. Carbohydrate research 489 (2020): 107913. [CrossRef]
- Mahdavinia, Gholam Reza, Moslem Soleymani, Mohammad Sabzi, Hamidreza Azimi, and Ziba Atlasi. Novel magnetic polyvinyl alcohol/laponite RD nanocomposite hydrogels for efficient removal of methylene blue. Journal of environmental chemical engineering 5, no. 3(2017):2617-2630. [CrossRef]
- Sánchez-Aguinagalde, O. , Ainhoa Lejardi, Emilio Meaurio, Rebeca Hernández, Carmen Mijangos, and Jose-Ramon Sarasua. Novel hydrogels of chitosan and poly (Vinyl alcohol) reinforced with inorganic particles of bioactive glass. Polymers 13, no. 5 (2021): 691. [CrossRef]
- Kaloti, Mandeep, and H. B. Bohidar. Kinetics of coacervation transition versus nanoparticle formation in chitosan–sodium tripolyphosphate solutions. Colloids and Surfaces B: Biointerfaces 81, no. 1 (2010): 165-173. [CrossRef]
- Janes, K. A. , and M. J. Alonso. Depolymerized chitosan nanoparticles for protein delivery: preparation and characterization. Journal of applied polymer science 88, no. 12 (2003): 2769-2776. [CrossRef]
- Janes, Kevin A., Marie P. Fresneau, Ana Marazuela, Angels Fabra, and Marı́a José Alonso. Chitosan nanoparticles as delivery systems for doxorubicin. Journal of controlled Release 73, no. 2-3 (2001): 255-267. [CrossRef]
- Yu, Liang, Jie Gong, Changfeng Zeng, and Lixiong Zhang. Synthesis of monodisperse zeolite A/chitosan hybrid microspheres and binderless zeolite A microspheres. Industrial & engineering chemistry research 51,no.5(2012):2299-2308. [CrossRef]
- McGinniss, V. D., F. A. Sliemers, D. K. Landstrom, and S. G. Talbert. Compendium Of information on identification and testing of materials for plastic solar thermal collectors No. DOE/CS/30171-1. Battelle Columbus Labs., OH (USA), 1980. [CrossRef]
- Zhou, Weilie, Robert Apkarian, Zhong Lin Wang, and David Joy. Fundamentals of scanning electron microscopy (SEM). In Scanning microscopy for nanotechnology, pp. 1-40.(2006)Springer,NewYork,NY. [CrossRef]
- Bertrand, Loïc, Marine Cotte, Marco Stampanoni, Mathieu Thoury, Federica Marone, and Sebastian Schöder. Development and trends in synchrotron studies of ancient and historical materials. Physics Reports 519, no. 2 (2012): 51-96. [CrossRef]
- Pine, D. J., D. A. Weitz, J. X. Zhu, and E. Herbolzheimer. Diffusing-wave spectroscopy: dynamic light scattering in the multiple scattering limit. Journal de Physique 51, no. 18 (1990):2101-2127. [CrossRef]
- Clark, Noel A., Joseph H. Lunacek, and George B. Benedek. A study of Brownian motion using light scattering. American Journal of Physics 38, no. 5 (1970): 575-585. [CrossRef]
- Vinod, Sithara, and John Philip. Experimental evidence for the significant role of initial cluster size and liquid confinement on thermo-physical properties of magnetic nanofluids under applied magnetic field. Journal of MolecularLiquids 257(2018):1-11. [CrossRef]
- Pathmamanoharan, Chellappah, and Albert P. Philipse. Preparation and properties of monodisperse magnetic cobalt colloids grafted with polyisobutene. Journal of colloid and interfacescience 205,no.2(1998):340-353. [CrossRef]
- Maji, Ambarish, and Gautam Choubey. Microstructure and mechanical properties of alumina toughened zirconia (ATZ). Materials Today: Proceedings 5, no. 2 (2018): 7457-7465. [CrossRef]
- Khaleda, MZ Farah, Vengadaesvaran Balakrishnan, A. Syafiq, Nasrudin Abd Rahim, and A. K. Pandey. SnO2 and TiO2 coatings toward decreasing the surface temperature of solarcells. (2018):50-5. [CrossRef]
- Kalita, op choudhary pc, pj doley, and a. kalita. 2. uses of transmission electron microscope in microscopy and its advantages and disadvantages by op choudhary, pc kalita, pj doley and a. kalita. Life Sciences Leaflets 85 (2017): 8-to.
- Bogner, Agnes, P-H. Jouneau, Gilbert Thollet, D. Basset, and Catherine Gauthier. A history of scanning electron microscopy developments: Towards wet-STEM imaging. Micron 38,no.4(2007):390-401. [CrossRef]
- Niazi, Asad, Pankaj Poddar, and A. K. Rastogi. A precision, low-cost vibrating sample magnetometer. CURRENT SCIENCE-BANGALORE- 79, no. 1 (2000): 99-109.
- Kirupakar, B. R., B. A. Vishwanath, and M. Padma Sree. Vibrating sample magnetometer and its application in characterisation of magnetic property of the anti cancer drug magnetic microspheres. International Journal of Pharmaceutics and Drug Analysis (2016): 227-233.
- Li, Gui-Yin, Yu-Ren Jiang, Ke-Long Huang, Ping Ding, and Li-Li Yao. Kinetics of adsorption of Saccharomyces cerevisiae mandelated dehydrogenase on magnetic Fe3O4–chitosan nanoparticles. Colloids and Surfaces A: Physicochemical and Engineering Aspects 320, no. 1-3 (2008): 11-18. [CrossRef]
- Brar, Satinder K., and M. Verma. Measurement of nanoparticles by light-scattering techniques. TrAC Trends in AnalyticalChemistry 30,no.1(2011):4-17. [CrossRef]
- Filipe, Vasco, Andrea Hawe, and Wim Jiskoot. Critical evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharmaceutical research 27, no. 5 (2010): 796-810. [CrossRef]
- Caputo, F., J. Clogston, L. Calzolai, M. Rösslein, and A. Prina-Mello. Measuring particle size distribution of nanoparticle enabled medicinal products, the joint view of EUNCL and NCI-NCL. A step by step approach combining orthogonal measurements with increasing complexity. Journal of Controlled Release 299 (2019): 31-43. [CrossRef]
- Mahdavi, Mahnaz, Mansor Bin Ahmad, Md Jelas Haron, Farideh Namvar, Behzad Nadi, Mohamad Zaki Ab Rahman, and Jamileh Amin. Synthesis, surface modification and characterisation of biocompatible magnetic iron oxide nanoparticles for biomedical applications. Molecules 18, no. 7(2013):7533-7548. [CrossRef]
- Ding, Yongling, Shirley Z. Shen, Huadong Sun, Kangning Sun, Futian Liu, Yushi Qi, and Jun Yan. Design and construction of polymerized-chitosan coated Fe3O4 magnetic nanoparticles and its application for hydrophobic drug delivery. Materials Science and Engineering: C 48 (2015): 487-498. [CrossRef]
- Abdollahi, M., S. Zeinali, S. Nasirimoghaddam, and S. Sabbaghi. Effective removal of As (III) from drinking water samples by chitosan-coated magnetic nanoparticles. Desalination and Water Treatment 56, no. 8 (2015):2092-2104. [CrossRef]
- Raeiatbin, P. A. R. I. N. A. Z. , and Y. Sağ Açıkel. Removal of tetracycline by magnetic chitosan nanoparticles from medical wastewaters. Desalin Water Treat 73 (2017): 380-388. [CrossRef]
- Kang, Xin, Yuxiang Gan, Renpeng Chen, and Chao Zhang. Sustainable eco-friendly bricks from slate tailings through geopolymerization: synthesis and characterization analysis. Construction and Building Materials 278 (2021): 122337. [CrossRef]
- Samal, R. K., S. Acharya, M. Mohanty, and M. C. Ray. FTIR spectra and physico-chemical behavior of vinyl ester participated transesterification and curing of jute. Journal of applied polymer science 79, no. 4 (2001): 575-581. https://doi.org/10.1002/1097-4628(20010124)79:4%3C575::AID-APP10%3E3.0.CO;2-U. [CrossRef]
- Wulandari, I. O., D. J. D. H. Santjojo, R. A. Shobirin, and A. Sabarudin. Characteristics and magnetic properties of chitosan-coated Fe3O4 nanoparticles prepared by ex-situ co-precipitation method. Rasayan J. Chem 10 (2017): 1348-1358. [CrossRef]
- Zhang, Meng, Zhi Zhang, Yazhou Peng, Li Feng, Xuhao Li, Chuanliang Zhao, and Khan Sarfaraz. Novel cationic polymer modified magnetic chitosan beads for efficient adsorption of heavy metals and dyes over a wide pH range. International journal of biological macromolecules 156(2020):289-301. [CrossRef]
- Pu, Shengyan, Yaqi Hou, Chun Yan, Hui Ma, Hongyan Huang, Qingqing Shi, Sandip Mandal, Zenghui Diao, and Wei Chu. In situ coprecipitation formed highly water-dispersible magnetic chitosan nanopowder for removal of heavy metals and its adsorption mechanism. ACS Sustainable Chemistry & Engineering 6, no. 12 (2018): 16754-16765. [CrossRef]
- Hazra, S. , and N. N. Ghosh. Preparation of nanoferrites and their applications. Journal of nanoscience and nanotechnology 14, no. 2 (2014): 1983-2000. [CrossRef]
- Shaumbwa, Veino Risto, Dagang Liu, Bright Archer, Jinlei Li, and Fan Su. Preparation and application of magnetic chitosan in environmental remediation and other fields: A review. Journal of Applied Polymer Science 138, no. 42 (2021): 51241. [CrossRef]
- Liu, Changkun, and Renbi Bai. Recent advances in chitosan and its derivatives as adsorbents for removal of pollutants from water and wastewater. Current Opinion in Chemical Engineering 4 (2014): 62-70. [CrossRef]
- Ngah, WS Wan, and MAK Megat Hanafiah. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: a review. Bioresource technology 99,no.10(2008):3935-3948. [CrossRef]
- El-Dib, Fawzia I., Dalia E. Mohamed, Omnia AA El-Shamy, and Marwa R. Mishrif. Study the adsorption properties of magnetite nanoparticles in the presence of different synthesized surfactants for heavy metal ions removal. Egyptian Journal of Petroleum 29, no. 1 (2020): 1-7. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




