Submitted:
30 August 2023
Posted:
31 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
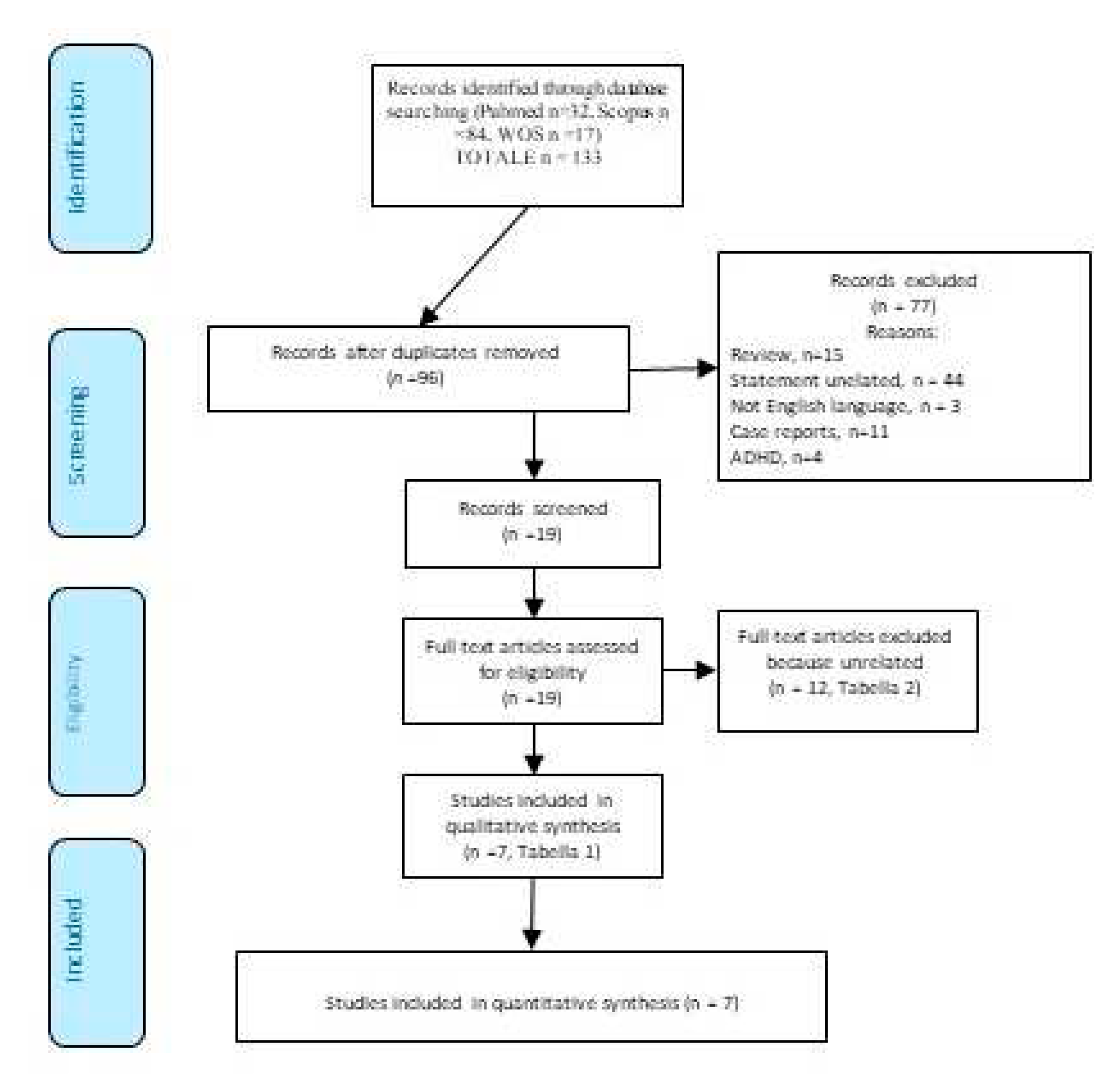
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nosetti, L.; Zaffanello, M.; Katz, E.S.; Vitali, M.; Agosti, M.; Ferrante, G.; Cilluffo, G.; Piacentini, G.; La Grutta, S. Twenty-year follow-up of children with obstructive sleep apnea. J Clin Sleep Med 2022, 18, 1573–1581. [Google Scholar] [CrossRef]
- Nosetti, L.; Zaffanello, M.; De Bernardi, F.; Piacentini, G.; Roberto, G.; Salvatore, S.; Simoncini, D.; Pietrobelli, A.; Agosti, M. Age and Upper Airway Obstruction: A Challenge to the Clinical Approach in Pediatric Patients. Int J Environ Res Public Health 2020, 17. [Google Scholar] [CrossRef]
- Brockmann, P.E.; Gozal, D. Neurocognitive Consequences in Children with Sleep Disordered Breathing: Who Is at Risk? Children (Basel) 2022, 9. [Google Scholar] [CrossRef]
- Zaffanello, M.; Piacentini, G.; La Grutta, S. The cardiovascular risk in paediatrics: the paradigm of the obstructive sleep apnoea syndrome. Blood Transfus 2020, 1–9. [Google Scholar] [CrossRef]
- Nino, G.; Restrepo-Gualteros, S.M.; Gutierrez, M.J. Pediatric sleep apnea and viral respiratory infections: what do clinicians need to know? Expert Rev Respir Med 2022, 16, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Khayat, A.; Bin-Hassan, S.; Al-Saleh, S. Polysomnographic findings in infants with Pierre Robin sequence. Ann Thorac Med 2017, 12, 25–29. [Google Scholar] [CrossRef]
- Schaefer, J.; Davey, M.J.; Nixon, G.M. Sleep-disordered breathing in school-aged children with Prader-Willi syndrome. J Clin Sleep Med 2022, 18, 1055–1061. [Google Scholar] [CrossRef]
- Hill, C.M.; Evans, H.J.; Elphick, H.; Farquhar, M.; Pickering, R.M.; Kingshott, R.; Martin, J.; Reynolds, J.; Joyce, A.; Rush, C.; et al. Prevalence and predictors of obstructive sleep apnoea in young children with Down syndrome. Sleep Med 2016, 27-28, 99–106. [Google Scholar] [CrossRef]
- Hunter, S.J.; Gozal, D.; Smith, D.L.; Philby, M.F.; Kaylegian, J.; Kheirandish-Gozal, L. Effect of Sleep-disordered Breathing Severity on Cognitive Performance Measures in a Large Community Cohort of Young School-aged Children. Am J Respir Crit Care Med 2016, 194, 739–747. [Google Scholar] [CrossRef]
- Menzies, B.; Teng, A.; Burns, M.; Lah, S. Neurocognitive outcomes of children with sleep disordered breathing: A systematic review with meta-analysis. Sleep Med Rev 2022, 63, 101629. [Google Scholar] [CrossRef]
- Zaffanello, M.; Ferrante, G.; Zoccante, L.; Ciceri, M.L.; Nosetti, L.; Tenero, L.; Piazza, M.; Piacentini, G. Predictive Power of Oxygen Desaturation Index (ODI) and Apnea-Hypopnea Index (AHI) in Detecting Long-Term Neurocognitive and Psychosocial Outcomes of Sleep-Disordered Breathing in Children: A Questionnaire-Based Study. J Clin Med 2023, 12. [Google Scholar] [CrossRef]
- Harris, V.C.; Links, A.R.; Kim, J.M.; Walsh, J.; Tunkel, D.E.; Boss, E.F. Follow-up and Time to Treatment in an Urban Cohort of Children with Sleep-Disordered Breathing. Otolaryngol Head Neck Surg 2018, 159, 371–378. [Google Scholar] [CrossRef]
- Zaffanello, M.; Zamboni, G.; Fontana, E.; Zoccante, L.; Tatò, L. A case of partial biotinidase deficiency associated with autism. Child Neuropsychol 2003, 9, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Noto, A.; Fanos, V.; Barberini, L.; Grapov, D.; Fattuoni, C.; Zaffanello, M.; Casanova, A.; Fenu, G.; De Giacomo, A.; De Angelis, M.; et al. The urinary metabolomics profile of an Italian autistic children population and their unaffected siblings. J Matern Fetal Neonatal Med 2014, 27 Suppl 2, 46–52. [Google Scholar] [CrossRef]
- Hodges, H.; Fealko, C.; Soares, N. Autism spectrum disorder: definition, epidemiology, causes, and clinical evaluation. Transl Pediatr 2020, 9, S55–s65. [Google Scholar] [CrossRef]
- Morris-Rosendahl, D.J.; Crocq, M.A. Neurodevelopmental disorders-the history and future of a diagnostic concept. Dialogues Clin Neurosci 2020, 22, 65–72. [Google Scholar] [CrossRef]
- Posar, A.; Visconti, P. Sleep Problems in Children with Autism Spectrum Disorder. Pediatr Ann 2020, 49, e278–e282. [Google Scholar] [CrossRef]
- Schwichtenberg, A.J.; Janis, A.; Lindsay, A.; Desai, H.; Sahu, A.; Kellerman, A.; Chong, P.L.H.; Abel, E.A.; Yatcilla, J.K. Sleep in Children with Autism Spectrum Disorder: A Narrative Review and Systematic Update. Curr Sleep Med Rep 2022, 8, 51–61. [Google Scholar] [CrossRef]
- Youssef, J.; Singh, K.; Huntington, N.; Becker, R.; Kothare, S.V. Relationship of serum ferritin levels to sleep fragmentation and periodic limb movements of sleep on polysomnography in autism spectrum disorders. Pediatr Neurol 2013, 49, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Tudor, M.E.; Walsh, C.E.; Mulder, E.C.; Lerner, M.D. Pain as a predictor of sleep problems in youth with autism spectrum disorders. Autism 2015, 19, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Elrod, M.G.; Nylund, C.M.; Susi, A.L.; Gorman, G.H.; Hisle-Gorman, E.; Rogers, D.J.; Erdie-Lalena, C. Prevalence of Diagnosed Sleep Disorders and Related Diagnostic and Surgical Procedures in Children with Autism Spectrum Disorders. J Dev Behav Pediatr 2016, 37, 377–384. [Google Scholar] [CrossRef]
- Johnson, C.R.; DeMand, A.; Lecavalier, L.; Smith, T.; Aman, M.; Foldes, E.; Scahill, L. Psychometric properties of the children's sleep habits questionnaire in children with autism spectrum disorder. Sleep Med 2016, 20, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Murata, E.; Mohri, I.; Kato-Nishimura, K.; Iimura, J.; Ogawa, M.; Tachibana, M.; Ohno, Y.; Taniike, M. Evaluation of behavioral change after adenotonsillectomy for obstructive sleep apnea in children with autism spectrum disorder. Res Dev Disabil 2017, 65, 127–139. [Google Scholar] [CrossRef]
- Tomkies, A.; Johnson, R.F.; Shah, G.; Caraballo, M.; Evans, P.; Mitchell, R.B. Obstructive Sleep Apnea in Children With Autism. J Clin Sleep Med 2019, 15, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Santapuram, P.; Chen, H.; Weitlauf, A.S.; Ghani, M.O.A.; Whigham, A.S. Investigating differences in symptomatology and age at diagnosis of obstructive sleep apnea in children with and without autism. Int J Pediatr Otorhinolaryngol 2022, 158, 111191. [Google Scholar] [CrossRef]
- Miano, S.; Ferri, R. Epidemiology and management of insomnia in children with autistic spectrum disorders. Paediatr Drugs 2010, 12, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Giannotti, F.; Cortesi, F.; Cerquiglini, A.; Vagnoni, C.; Valente, D. Sleep in children with autism with and without autistic regression. J Sleep Res 2011, 20, 338–347. [Google Scholar] [CrossRef]
- Wright, B.; Sims, D.; Smart, S.; Alwazeer, A.; Alderson-Day, B.; Allgar, V.; Whitton, C.; Tomlinson, H.; Bennett, S.; Jardine, J.; et al. Melatonin versus placebo in children with autism spectrum conditions and severe sleep problems not amenable to behaviour management strategies: a randomised controlled crossover trial. J Autism Dev Disord 2011, 41, 175–184. [Google Scholar] [CrossRef]
- Mazurek, M.O.; Sohl, K. Sleep and Behavioral Problems in Children with Autism Spectrum Disorder. J Autism Dev Disord 2016, 46, 1906–1915. [Google Scholar] [CrossRef]
- Aathira, R.; Gulati, S.; Tripathi, M.; Shukla, G.; Chakrabarty, B.; Sapra, S.; Dang, N.; Gupta, A.; Kabra, M.; Pandey, R.M. Prevalence of Sleep Abnormalities in Indian Children With Autism Spectrum Disorder: A Cross-Sectional Study. Pediatr Neurol 2017, 74, 62–67. [Google Scholar] [CrossRef]
- Mehrazad-Saber, Z.; Kheirouri, S.; Noorazar, S.G. Effects of l-Carnosine Supplementation on Sleep Disorders and Disease Severity in Autistic Children: A Randomized, Controlled Clinical Trial. Basic Clin Pharmacol Toxicol 2018, 123, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Delemere, E.; Dounavi, K. Parent-Implemented Bedtime Fading and Positive Routines for Children with Autism Spectrum Disorders. J Autism Dev Disord 2018, 48, 1002–1019. [Google Scholar] [CrossRef] [PubMed]
- Malhi, P.; Kaur, A.; Singhi, P.; Sankhyan, N. Sleep Dysfunction and Behavioral Daytime Problems in Children with Autism Spectrum Disorders: A Comparative Study. Indian J Pediatr 2019, 86, 12–17. [Google Scholar] [CrossRef]
- da Silveira Cruz-Machado, S.; Guissoni Campos, L.M.; Fadini, C.C.; Anderson, G.; Markus, R.P.; Pinato, L. Disrupted nocturnal melatonin in autism: Association with tumor necrosis factor and sleep disturbances. J Pineal Res 2021, 70, e12715. [Google Scholar] [CrossRef]
- McCrae, C.S.; Chan, W.S.; Curtis, A.F.; Nair, N.; Deroche, C.B.; Munoz, M.; Takamatsu, S.; McLean, D.; Davenport, M.; Muckerman, J.E.; et al. Telehealth cognitive behavioral therapy for insomnia in children with autism spectrum disorder: A pilot examining feasibility, satisfaction, and preliminary findings. Autism 2021, 25, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Estes, A.; Munson, J.; St John, T.; Finlayson, R.; Pandey, J.; Gottlieb, B.; Herrington, J.; Schultz, R.T. Sleep problems in autism: Sex differences in the school-age population. Autism Res 2023, 16, 164–173. [Google Scholar] [CrossRef]
- Distefano, G.; Calderoni, S.; Apicella, F.; Cosenza, A.; Igliozzi, R.; Palermo, G.; Tancredi, R.; Tritto, G.; Craig, F.; Muratori, F.; et al. Impact of sleep disorders on behavioral issues in preschoolers with autism spectrum disorder. Front Psychiatry 2023, 14, 1181466. [Google Scholar] [CrossRef]
- Kamal Nor, N.; Ghozali, A.H.; Ismail, J. Prevalence of Overweight and Obesity Among Children and Adolescents With Autism Spectrum Disorder and Associated Risk Factors. Front Pediatr 2019, 7, 38. [Google Scholar] [CrossRef]
- Veronese, S.; Zoccante, L.; Smania, N.; Sbarbati, A. Stretch marks: a visible expression of connective's involvement in autism spectrum disorders. Front Psychiatry 2023, 14, 1155854. [Google Scholar] [CrossRef]
- Eow, S.Y.; Gan, W.Y.; Lim, P.Y.; Awang, H.; Mohd Shariff, Z. Parental Feeding Practices and Child-Related Factors are Associated with Overweight and Obesity in Children and Adolescents with Autism Spectrum Disorder. J Autism Dev Disord 2022, 52, 3655–3667. [Google Scholar] [CrossRef]
- Zerbo, O.; Leong, A.; Barcellos, L.; Bernal, P.; Fireman, B.; Croen, L.A. Immune mediated conditions in autism spectrum disorders. Brain Behav Immun 2015, 46, 232–236. [Google Scholar] [CrossRef]
- Tonacci, A.; Billeci, L.; Ruta, L.; Tartarisco, G.; Pioggia, G.; Gangemi, S. A systematic review of the association between allergic asthma and autism. Minerva Pediatr 2017, 69, 538–550. [Google Scholar] [CrossRef]
- Zoccante, L.; Ciceri, M.L.; Gozzi, L.A.; Gennaro, G.D.; Zerman, N. The "Connectivome Theory": A New Model to Understand Autism Spectrum Disorders. Front Psychiatry 2021, 12, 794516. [Google Scholar] [CrossRef]
- Georgalas, C. The role of the nose in snoring and obstructive sleep apnoea: an update. Eur Arch Otorhinolaryngol 2011, 268, 1365–1373. [Google Scholar] [CrossRef]
- Ming, X.; Brimacombe, M.; Wagner, G.C. Prevalence of motor impairment in autism spectrum disorders. Brain Dev 2007, 29, 565–570. [Google Scholar] [CrossRef]
- Gabis, L.V.; Shaham, M.; Leon Attia, O.; Shefer, S.; Rosenan, R.; Gabis, T.; Daloya, M. The Weak Link: Hypotonia in Infancy and Autism Early Identification. Front Neurol 2021, 12, 612674. [Google Scholar] [CrossRef] [PubMed]
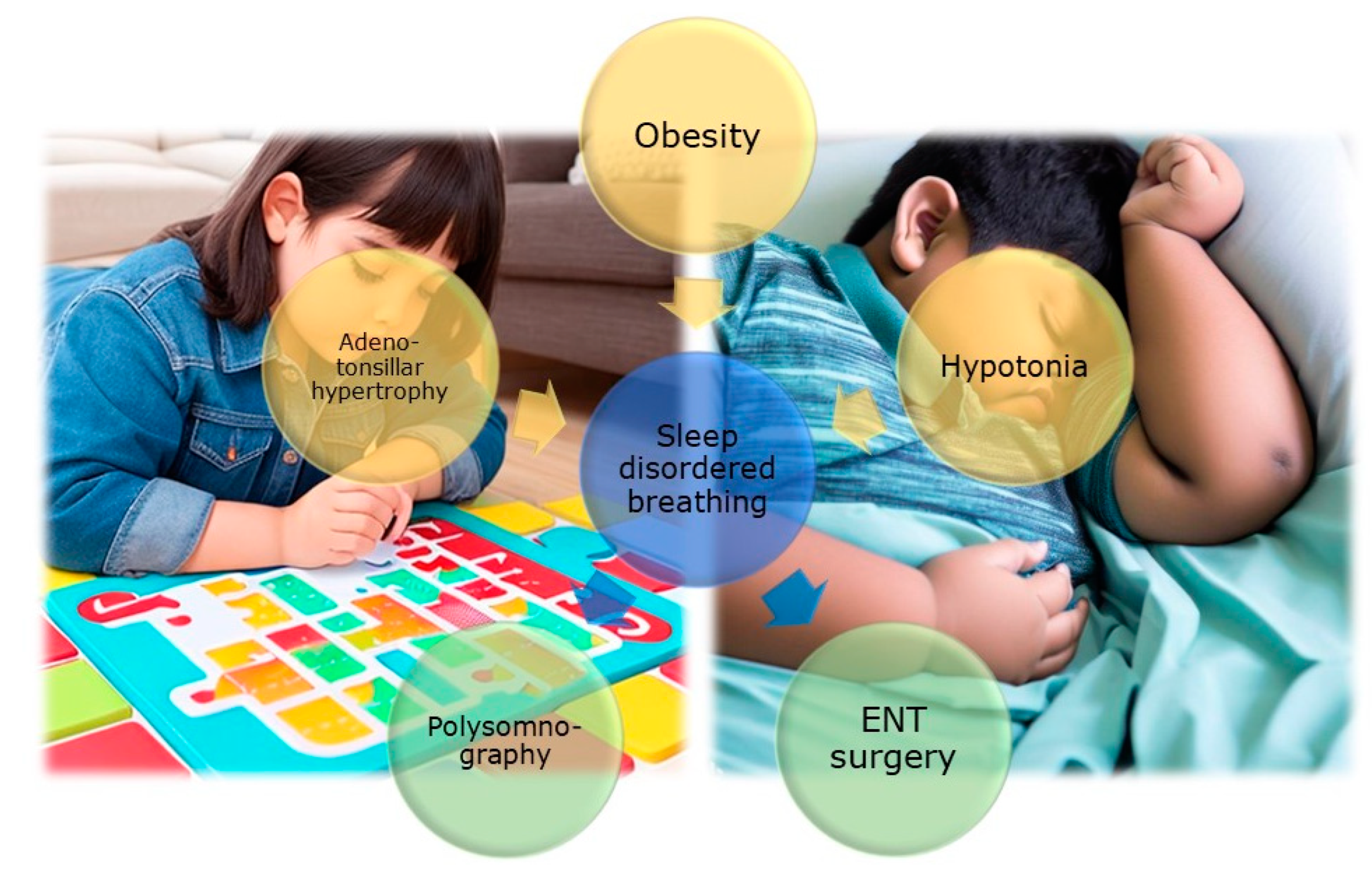
- Nosetti, L.; Zaffanello, M.; De Bernardi di Valserra, F.; Simoncini, D.; Beretta, G.; Guacci, P.; Piacentini, G.; Agosti, M. Exploring the Intricate Links between Adenotonsillar Hypertrophy, Mouth Breathing, and Craniofacial Development in Children with Sleep-Disordered Breathing: Unraveling the Vicious Cycle. Children 2023, 10. [Google Scholar] [CrossRef]
- Kang, K.T.; Chou, C.H.; Weng, W.C.; Lee, P.L.; Hsu, W.C. Associations between adenotonsillar hypertrophy, age, and obesity in children with obstructive sleep apnea. PLoS One 2013, 8, e78666. [Google Scholar] [CrossRef] [PubMed]
- Strollo, P.J., Jr.; Rogers, R.M. Obstructive sleep apnea. N Engl J Med 1996, 334, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Asmika, A.; Oktafiani, L.D.A.; Kusworini, K.; Sujuti, H.; Andarini, S. Autistic Children Are More Responsive to Tactile Sensory Stimulus. Iran J Child Neurol 2018, 12, 37–44. [Google Scholar] [PubMed]
- Moore, M.; Evans, V.; Hanvey, G.; Johnson, C. Assessment of Sleep in Children with Autism Spectrum Disorder. Children (Basel) 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Villa, M.P.; Pietropaoli, N.; Supino, M.C.; Vitelli, O.; Rabasco, J.; Evangelisti, M.; Del Pozzo, M.; Kaditis, A.G. Diagnosis of Pediatric Obstructive Sleep Apnea Syndrome in Settings With Limited Resources. JAMA Otolaryngol Head Neck Surg 2015, 141, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Chervin, R.D.; Weatherly, R.A.; Garetz, S.L.; Ruzicka, D.L.; Giordani, B.J.; Hodges, E.K.; Dillon, J.E.; Guire, K.E. Pediatric sleep questionnaire: prediction of sleep apnea and outcomes. Archives of Otolaryngology--Head & Neck Surgery 2007, 133, 216–222. [Google Scholar]
| Primo autore | Year of publication | Design | Aim | Subjects | Methods | Results | Conclusions |
|---|---|---|---|---|---|---|---|
| Youssef J et al. [19] | 2013 | Retrospective chart review (Massachusetts) | To investigate the relationship between ferritin levels, fragmented sleep disorders, and joint movements in children with ASD. | Out of the 9,791 identified ASD children, 511 had ferritin level data, 377 had PSG data, and 53 had both ferritin and PSG data. | Review of ASD children's records. PSG and ferritin analysis. Assessment of sleep fragmentation, limb movements. Comparison with the control group. |
37% had sleep apnea. There was no significant difference in BMI or ferritin levels between ASD patients with or without OSA (P > 0.1). Ferritin levels did not predict abnormal sleep outcomes (P > 0.1). | No correlation between apnea, ferritin, and BMI. |
| Tudor, M.E, et al. [20] | 2015 | - (USA) | Parental assessment. Correlation between pain and sleep issues. |
Individuals with ASD (N = 62), Child ages ranged from 3 to 18 yrs (9.39±4.19 yrs). | NCCPC-R and CSHQ. Correlations between pain and sleep, including duration, parasomnias, SDB. Impact of pain on sleep issues. | High scores in the SDB subscale were predicted by high scores in the Vocal subscale. SDB: mean subscale 3.99 ± 122; n.(%) scoring > 0.35 (56%). | Sleep behaviours and vocalizations influence duration, parasomnias, and SDB. |
| Elrod MG, et al. [21] | 2016 | Retrospective cohort study. (Bethesda) | Risk assessment between ASD and controls for sleep disorders and diagnostic/surgical procedures. | 48,762 children with ASD and controls (aged 2 to 18 yrs). | ASD (2000-2013). ASD matched 1:5 with controls for age, gender, and enrollment. Analysis of ICD-9-CM sleep disorders. RR and 95% CI were calculated using binary Poisson regression. |
ASD children have a higher risk of sleep disorders, including OSA (RR: 1.97 [95% CI, 1.91-2.02]). Higher risk of PSG (RR: 3.74 [95% CI, 3.56-3.93]) and related surgeries (RR: 1.50 [95% CI, 1.46-1.54]). |
Individuals with ASD have an elevated susceptibility to the emergence of sleep disorders, which includes OSA. They are more likely to have abnormal PSG results and undergo sleep-related surgeries than children without ASD. |
| Johnson CR et al. [22] | 2016 | Multisite RCT (Emory University, Indiana University, Ohio State University, University of Pittsburgh, University of Rochester, and Yale University) | Psychometric properties of the CSHQ in children with ASD. | 310 children with ASD (Age 4.7±1.14 yrs) | The CSHQ (8 subscales): Bedtime Resistance, Sleep Onset Delay, Sleep Duration, Sleep Anxiety, Night Wakings, Parasomnias, SDB, and Daytime Sleepiness. | Loud, persistent snoring (5.1%) and other abnormal breathing behaviours (frequent apnea 0.6%) during sleep are relatively infrequent. | Loud snoring and other abnormal breathing behaviours (apneas) during sleep are rare. |
| Murata E, et al. [23] | 2017 | Short-term retrospective study (Japan) | Behavioural changes after A&T for OSA in children with ASD. | 55 ASD children (30 with OSA). Mean age: 7 yrs and 3 months (SD = 2 years and 5 months, range: 5-14 yrs) in the OSA group, and 7 yrs and 5 months (SD = 2 years and 0 months, range: 5-13 yrs) in the control group. | Children with untreated OSA and ASD control without OSA. OSA diagnosis: PSG, cardiorespiratory monitoring, oximetry. CBCL before and after treatment. | Pre-A&T scores for externalizing (p < 0.01), somatic problems (p < 0.05), anxiety/depression (p < 0.05), social issues (p < 0.01), thought problems (p < 0.01), delinquent behaviour (p < 0.01), and aggressive behaviour (p < 0.05) are significantly higher in the improved group compared to the no-change/deterioration group. Sex, A&T age, obesity indices, and severity of OSA based on AHI/3% and ODI did not differ between the improved group and the no-change/deterioration group. |
OSA in children with ASD should be treated regardless of obesity and age, even in cases of mild OSA, especially when more severe behavioural problems are present. We need to be aware of OSA in children with ASD. |
| Tomkies A, et al. [24] | 2019 | Retrospective study (Texas) | Demographic and clinical characteristics, undergoing PSG, predictors of OSA and severe OSA. | 45 children (age range 2 - 18 yrs, mean age 6.1 yrs). | PSG on children (born between 2009 - Feb. 2015). Excluding severe comorbidities, tonsillectomy, and missing data. Collected age, sex, race, and clinical data. Analysis of OSA predictors. | The mean oAHI in children with OSA was 13.1 ± 18/hr. 58% had OSA (AHI >1). 33% were obese (BMI ≥ 95th percentile). Severe OSA is significantly associated with weight (OR 1.0, 95% CI 1.0-1.1, P = 0.05). The mean AHI is 7.7 /hour. 20% had severe OSA (AHI ≥ 10 /hr). There were no significant predictors for OSA except weight increase for severe OSA. | OSA is quite common in children, with considerable variability in severity. Obesity is associated with greater OSA severity. Weight appears to be a predictive factor for severe OSA. |
| Santapuram P, et al. [25] | 2022 | Retrospective cohort study (USA) | A study comparing symptoms and age of OSA diagnosis. Children with and without ASD. Assessment of symptoms and age of OSA diagnosis. Identification of differences between groups. |
Children with and without ASD. 166 children. The control group comprised 91 patients (54.9% male) with typical development and OSA. Age at OSA diagnosis: ASD 72.8 (45.6) months; Control 73.4 (47.4) months, p = 0.999. | Review of clinical records for OSA (2019-2021). Analysis of diagnosis and treatment. Included children with OSA and A&T. |
Less severity of autism was associated with a later age at OSA diagnosis (p < 0.001). Multivariate regression analysis did not reach statistical significance (p = 0.079). BMI and age at ASD diagnosis were independently associated with age at OSA diagnosis (p = 0.033 and p < 0.001, respectively). | Association between autism severity and age at OSA diagnosis. The association might not be significant when considering other factors simultaneously, such as BMI and age at ASD diagnosis. BMI and age at ASD diagnosis appear to have independent impacts on age at OSA diagnosis. |
| First author (yrs of publication) | Aim | Subjects | Methods | Results | Conclusions |
|---|---|---|---|---|---|
| Miano S, Ferri R. (2010) [26] | Analysis of Insomnia Epidemiology and Management in Children with ASD (Review) | ASD children | Causes of Insomnia in ASD: Neurochemical (Melatonin), Psychiatric (Anxiety), Behavioral (Sleep Habits). ASD-related Insomnia: Common, Difficulty Falling Asleep, Frequent Awakenings, Early Awakenings, Non-Restorative Sleep. Interventions: Behavioral Therapies (Routine, Relaxation, Anxiety), Medications (Melatonin), Multidisciplinary Approaches. | Sleep Issues in Children with ASD. Similar to typical ones, but more prevalent. Common Insomnia: onset, maintenance, restless, resistance, awakenings. PSG: less sleep, altered microstructure. Treatments: Medications, behavioural interventions, promising melatonin. |
Sleep in children with ASD presents issues similar to those in typical children but occurs more frequently. Insomnia, difficulty falling asleep, nighttime awakenings, restlessness, and resistance to sleep are typical. PSG analysis reveals reduced sleep duration and alterations in its structure. Medications, behavioural therapies, and melatonin appear promising for addressing these issues. |
| Giannotti, F., et al. (2011) [27] | Comparison of Sleep in Regressive Autism, Non-Regressive Autism, and Typical Development. NREM Analysis. Understanding Sleep in Autism Compared to Typical Development. |
Subjects with Non-Regressive Autism (22 participants) | 52 children (ages 5-10) - 22 with non-regressive autism, 18 with regressive autism (without comorbidities), 12 typically developing (TD). Instruments: PSG, CSHQ. | Higher CSHQ scores in TD. Regression: less efficient sleep, reduced total sleep time, prolonged REM, increased wakefulness after sleep onset. Lower CAP and A1 index in light sleep in regressive compared to TD and non-regressive. | TD children have higher CSHQ scores than non-regressive. Autism exhibits sleep changes: less sleep, prolonged REM, and wakefulness after sleep onset. These patterns provide insights into insomnia in non-regressives. |
| Wright, B., et al. (2011) [28] | Comparison of Melatonin vs. Placebo in Severe Sleep Disorders in ASD Unresponsive to Behavioral Management | 22 children with ASD, 17 children completed the study | Controlled crossover study: treated with melatonin/placebo for 3 months after behavioural therapies failed, assessment of sleep variables | Improvements with melatonin: Reduced sleep latency (47 minutes) and increased total sleep duration (52 minutes), but no effect on nighttime awakenings. Low and similar side effects between melatonin and placebo. | Melatonin improves sleep: sleep onset -47 min, duration +52 min. Minimal side effects enhance sleep safely and effectively. |
| Mazurek, M.O. & Sohl, K. (2016) [29] | The study examines relationships between sleep disturbances and behavioural issues in children with ASD | 81 children with ASD | Multivariate analysis to correlate sleep disturbances and behavioural problems in children with ASD. Assessment: Utilized Sleep Disturbance Scale and Aberrant Behavior Checklist. |
Sleep disturbances are linked to aggressiveness, attention, and hyperactivity. Analysis: Sleep explains 22-32% of the behavioural variance—nighttime awakenings linked to daytime issues, controlling for age and gender. | Sleep disturbances linked to behaviour: aggressiveness, attention, hyperactivity. Sleep explains 22-32% of behavioural variation—nighttime awakenings tied to daytime issues. Sleep impacts daytime behaviour. |
| Aathira, R. et al. (2017) [30] | Prevalence of Sleep Alterations in Indian Children with ASD | For children with ASD and controls, 109 children with ASD were screened, of which 71 fulfilled the inclusion criteria (age: 3-10 years). | Two-year Study. Sleep evaluated with Children's Sleep Habits Questionnaire. Additional assessments: PSG, Autism Scale, Behavioral Checklist, Developmental Profile 3. | Sleep prevalence: 77.5% in ASD, 29.2% in controls. PSG: Reduced efficiency, less REM, slow waves, desaturation in ASD. Checklist: A high score indicates poor sleepers—no correlation with autism scale or developmental profile 3. | The findings underscore the significance of addressing sleep disorders in ASD through tailored interventions aimed at enhancing overall well-being. |
| Mehrazad-Saber, Z., et al. (2018) [31] | Effects of l-Carnosine Supplementation on SDB and Severity of Core Autism Symptoms in ASD patients | Individuals with ASD (31 males and 12 females), ranging in age from 4 to 16 yrs old. | Double-masked study: carnosine (treatment), placebo (control). 2 months. Sleep assessment and symptoms using Gilliam Autism Rating Scale 2. Effects of carnosine on ASD sleep symptoms. | Carnosine supplement: Reduced sleep duration (p = 0.04). Reduced parasomnias (p = 0.02). Sleep disturbance score -7.59% vs. control (p = 0.006). |
Promising initial results: Carnosine may aid sleep—further research for confirmation, long-term effects, and mechanism of action. |
| Delemere E, Dounavi K (2018) [32] | Studying the effectiveness of stimulus control interventions (bedtime fading and positive routines) on sleep in children with ASD, using multiple designs | 6 children with ASD (2 and 7 yrs) | Two interventions related to stimulus control (bedtime fading and positive routines) were implemented. | Bedtime fading: Increased sleep duration, reduced latency. Positive routines: Mixed effects on sleep latency and duration in some participants. |
Bedtime fading aids sleep in some; positive routines vary—tailoring strategies for personalized adaptation for optimal outcomes. |
| Malhi, P. et al. (2019) [33] | Objective 1: Comparison of ASD sleep vs. controls. Objective 2: Association between sleep and behaviour in children with ASD. | 60 children with ASD (85% males). Mean age: 6.1 yrs (±2.4). Control group: 60 typically developing children, matched for age and socioeconomic status. |
Sleep: Assessed with CSHQ. Behaviour: Assessed with Child Psychopathology Measurement Tool. |
ASD: High prevalence of sleep problems. CSHQ, high resistance and duration. Sleep-related daytime behaviours. CSHQ and wakefulness explain behaviours. | Results: ASD and related sleep disturbances correlated; addressing them improves daytime behaviours and quality of life in ASD. |
| da Silveira Cruz-Machado, S., et al. (2021) [34] | To assess urinary aMT6s and salivary TNF, IL-6 in ASD. Correlation with sleep. |
40 participants: typical group (n = 20; mean age 10.2 yrs) and ASD group (n = 20; mean age 11.0 yrs). | Method: Urinary aMT6s, salivary TNF, IL-6 evaluated—correlation analysis with sleep disturbances. Sleep Disturbance Scale was used to assess sleep—correlation analysis with the same sleep disturbances. | Autism sleep results: 60% ASD: Increased nighttime aMT6s. ASD: Increased nighttime TNF, no change in IL-6. Sleep dysfunction (Scales) correlated with aMT6s. SDB: Decreased aMT6s; Increased TNF. |
Complex interaction between sleep, immunity, and autism, the possible role of melatonin. Further research is needed to understand mechanisms and clinical implications. |
| McCrae, C.S. et al. (2021) [35] | Common insomnia in ASD. CBT improves sleep and autism functioning. Parents benefit: their sleep improves with CBT. | Pilot study: CBT for insomnia via Telehealth. Participants: 17 children (6-12 yrs) with ASD and insomnia + parents. Evaluate the effectiveness of CBT for insomnia, and involve parents in ASD. | Telehealth delivery of eight-session cognitive behavioural treatment for childhood insomnia | High treatment fidelity. Parents satisfied with insomnia CBT. Telehealth: Sleep and functioning improvements. 1 month: Less inappropriate language, stable hyperactivity. | High treatment compliance and effective telehealth. CBT therapy is beneficial for insomnia in autism and improvements in parents and sleep, and some behaviours require further study. |
| Estes, A., et al. (2023) [36] | Evaluate gender differences in sleep problems in school-age autism. | Autistic children (n = 250); typically developing children (n = 114), 6–12 yrs of age | Title: Parent Sleep Problems (CSHQ) - Children 6-12 Years | Females with ASD: resistance, anxiety, drowsiness, reduced sleep. More sleep problems compared to males with ASD and typical children. Males with ASD: anxiety-sleep link. | Gender differences in ASD sleep disturbances: Females - resistance, anxiety, drowsiness; males - anxiety and sleep. Consider managing ASD sleep with gender differences in mind. |
| Distefano G, et al. 2023 [37] | Identifying sleep in preschool ASD, correlations with autism, development, comorbidities. | 163 preschool-age children diagnosed with ASD (43.37±12.56 months) | Applying CSHQ and standard tests to assess children's sleep, intelligence, repetitive behaviours, and psychiatric comorbidities (CBCL 11/2-5 and RBS-R). | Sleep disturbances linked to high scores on CSHQ and CBCL, with connections to anxiety symptoms for repetitive behaviours and CBCL syndromes. | Link between sleep and CSHQ and CBCL scores. Sleep problems are connected to behaviours and symptoms. Sleep treatment can improve well-being in children with behavioural and psychological symptoms. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





