1. Introduction
Excessive body iron stores are a risk factor for insulin resistance and diabetes, and adipose tissue is among the tissues exhibiting the most significant effects of iron (2). Effects of iron include regulation of leptin and adiponectin, two adipocytokines with key role in the regulation of metabolism (2). High adipocyte iron causes insulin resistance in mice (3) while low adipocyte iron protects from high-fat-diet-induced metabolic disorders (4). We recently reported in an African-American cohort phenotyped for insulin sensitivity, body mass index, and gene expression profiling in muscle and adipose tissue (5), adipocyte expression of 30 transcripts involved in iron regulation were correlated with insulin resistance, with expression of the iron carrier protein transferrin (TF) being most negatively associated with insulin resistance (1). These observations were replicated in two independent European ancestry adipose data sets. The strongest cis-regulatory variant for TF transcript expression (rs6785596; p=7.84×10-18) was identified in adipose tissue but not muscle or liver, and the variants significantly affected the normal positive relationship of serum ferritin to insulin resistance.
We were able to demonstrate in a cultured adipocyte model that decreased intracellular iron resulting from decreased TF expression led to impaired insulin responsiveness and caused differential expression of 465 genes, including genes involved in metabolism, insulin action, and obesity (1). While suggestive, these studies did not definitively prove in an intact organism that altering TF expression only in adipocytes was sufficient to affect glucose homeostasis and insulin resistance. We therefore have created a mouse model of adipocyte-specific decreased transferrin expression and show that its phenotype mirrors that of the human cohorts, consistent with the hypothesis that iron trafficking in adipose tissue is sufficient to affect insulin sensitivity.
2. Results
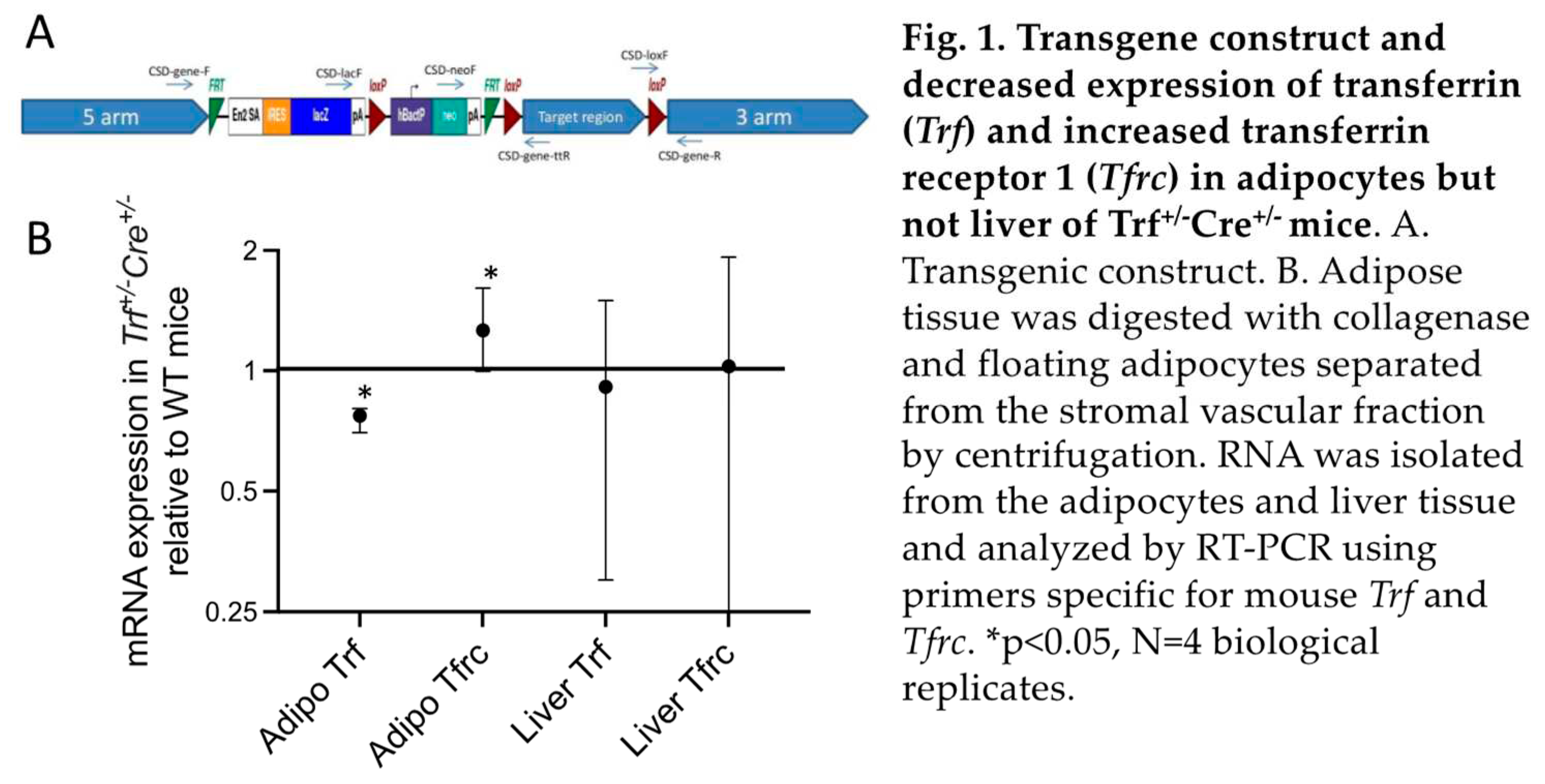
We performed targeted deletion of the transferrin (
Trf) gene in adipocytes of mice by inserting loxP sites in the 5th exon of
Trf (Figure 1A) and crossing mice with those expressing
cre recombinase in adipose tissue under control of the adiponectin promoter. Interestingly, we were unable to obtain mice homozygous for the deletion in over 100 offspring, so only heterozygotes were studied. These
Trf+/-Cre+/- mice exhibited decreased
Trf expression compared to wild type mice (WT,
Trf+/+Cre-/-) in adipocytes (Figure 1B). We also measured mRNA expression of the Transferrin Receptor 1 gene (
Tfrc), whose levels are controlled by iron through iron regulatory proteins (6). Expression of
Tfrc was increased in
Trf+/-Cre+/- adipocytes, indicating decreased intracellular iron and demonstrating that adipocyte TF is involved in regulating adipose tissue iron levels. Neither
Trf nor
Tfrc mRNA levels were altered in liver, the main source of serum transferrin (Figure 1B). Consistent with this result, serum transferrin did not differ between WT and
Trf+/-Cre+/- mice (WT: 38.3±3.5 ng/mL, N=23;
Trf+/-Cre+/-: 38.6±2.3, N=53; p=0.95).
We next examined glucose tolerance. Area under the glucose curve (AUCg) after intraperitoneal glucose administration was increased in the
Trf+/-Cre+/- mice on a normal chow diet, and the mice were significantly heavier (Figure 2A, 2B, p=0.02 for both). Fasting glucose trended higher but was not significant f- (p=0.08, not shown). Homeostasis Model of Insulin Resistance (HOMA-IR) values were significantly higher in the
Trf+/-Cre+/- mice (Figure 2C, p<0.05).
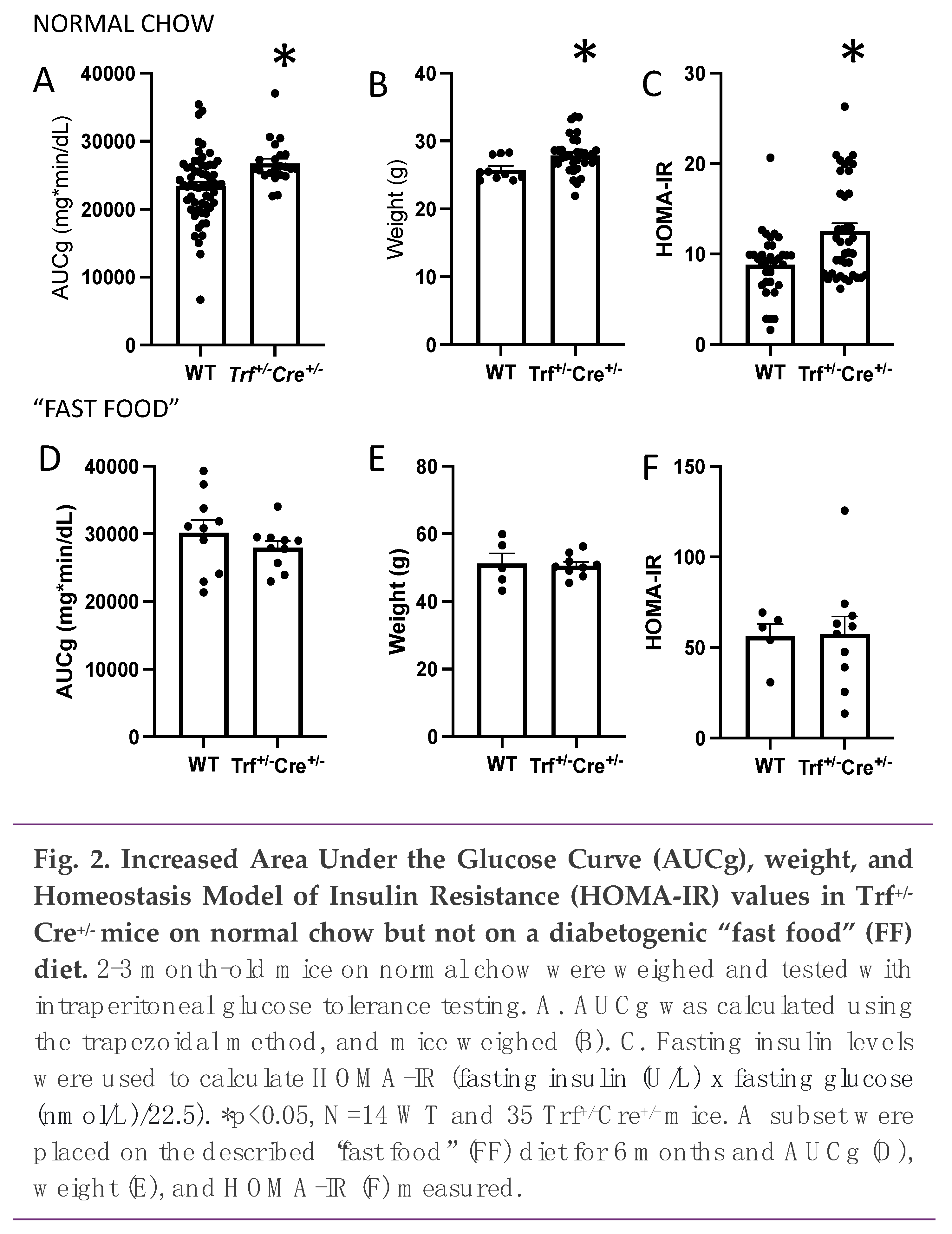
A subset of the WT (N=5) and Trf+/-Cre+/- mice (N=9) were then put on a “Fast Food” (FF) diet. After 6 months on the diabetogenic diet, both groups had higher AUCg, weight, and HOMA-IR compared to the mice on normal chow (Figure 2D-F, p<0.001 by ANOVA). Interestingly, the significant effects of Trf gene deletion on AUCg, weight, and HOMA-IR were erased when mice were switched to a FF diet, resulting in no observed difference between Trf+/-Cre+/- and WT mice.
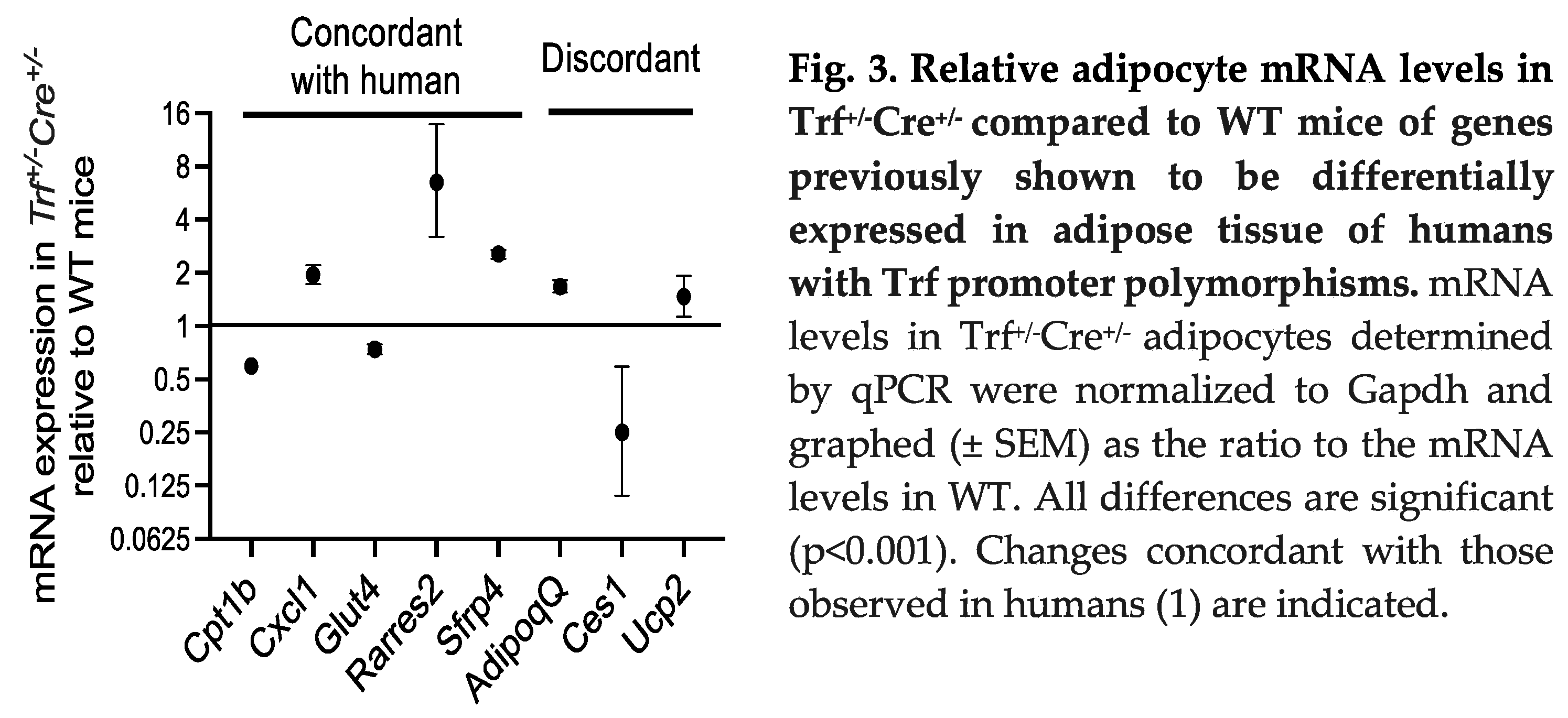
Humans with the
TF gene polymorphisms that decrease TF expression in adipocytes had several associated changes in the expression of a set of genes involved in metabolism, metabolic regulation and insulin action (1). We therefore tested if these were recapitulated in the
Trf+/-Cre+/- mice (Figure 3). Five of the eight genes were regulated concordantly in the humans with decreased transferrin and the
Trf+/-Cre+/- mice. Upregulated genes were
Cxcl1 (CXC motif chemokine ligand 1, which is also upregulated in diabetes (7)),
Sfrp4 (secreted frizzled related protein 4, also upregulated in diabetes and prediabetes (8)), and
Rarres2 (retinoic acid receptor responder 2, also known as chemerin, that is expressed in stressed adipocytes and associated with insulin resistance (9)). Downregulated genes in both mice and humans were
Cpt1B (carnitine palmitoyltransferase 1B, involved in beta oxidation of fatty acids) and
Glut4 (glucose transporter 4, also downregulated in adipocytes in insulin resistance (10). Discordantly regulated were
Adipoq (adiponectin) and
Ucp2 (uncoupling protein 2), both upregulated in the
Trf+/-Cre+/- mice though downregulated in the humans with insulin resistance and/or obesity we studied.
Ces1 (carboxylesterase 1, an enzyme that hydrolyzes triglycerides and also lowers glucose levels and improves insulin sensitivity (11)) was downregulated in the
Trf+/-Cre+/- mice and upregulated in the humans.
3. Discussion
In summary, analysis of mice with decreased expression of the iron carrier protein TF restricted to adipocytes share the increased weight and insulin resistance observed in humans with the genetic polymorphism associated with adipocyte transferrin expression (5). Thus, a decrease in adipocyte TF is sufficient to affect insulin resistance, weight, and glucose homeostasis. Our findings therefore represent one of the relatively few cases in which a biologic mechanism has been determined for the effect of a human genetic polymorphism on diabetes-related quantitative metabolic traits.
Adipose tissue plays a crucial role in the pathogenesis of T2DM (12, 13). There are multiple pathways through which this is hypothesized to occur. For example, the role of the adipocyte as an endocrine organ with major effects on whole body metabolism is illustrated by its production of key hormones such as leptin (14) and adiponectin (15), whose roles in metabolic regulation have been recently reviewed (16, 17). The fact that high iron is an important negative regulator of both leptin (18) and adiponectin (3) is therefore consistent with a role for iron in integrating the macro- and micronutrient status of the organism with overall metabolic regulation. The physiological relevance of this regulation has been demonstrated by, for example, leptin-dependent changes in feeding behavior (18) and adiponectin-dependent increases in serum triglycerides (19) in mice exposed to higher yet nonpathologic levels of iron. To achieve this function, several genes involved in iron metabolism that are otherwise restricted in their expression including ferroportin, hemojuvelin, transferrin, the HFE human hemochromatosis protein, and hepcidin are expressed in adipose tissue (3, 20-22).
Perhaps even more impressive is the effect of low iron to protect from diabetes. This direct and causal antidiabetic effect of low adipocyte iron, as was suggested by higher adiponectin levels in low-hepcidin states such as hemochromatosis, has also been demonstrated in animal models. Scherer’s group has shown that overexpression of mitoNEET, an iron-sulfur cluster binding protein in the outer mitochondrial membrane, lowers intracellular iron and mitochondrial iron transport (23). This results in increased adiponectin, decreased rates of fatty acid oxidation, massive obesity, yet preserved insulin sensitivity. At least some of this salutary phenotype is directly linked to adiponectin insofar as it is ameliorated in adiponectin knockout mice, and is consistent with the phenotype of mice overexpressing adiponectin (24). This “healthy obesity” phenotype was validated with transcriptional profiling that revealed a better vascularized, anti-inflammatory and less fibrotic environment in the fat with higher mitoNEET expression (25). These results also parallel the observation that low serum ferritin in humans is a predictor of the lack of markers of metabolic syndrome (hypertension, dyslipidemia, and diabetes) despite extreme obesity (BMI>40 kg/m2) (3).
The precise molecular mechanism by which adipocyte TF confers the insulin resistance phenotype in our model, however, remains uncertain. TF is the principal carrier of iron to most tissues. The fact that serum TF, largely derived from liver, and hepatic expression of TF were unaffected by the polymorphisms in humans (1) suggests that adipocyte TF does not play a role in whole-body iron regulation, but rather may be playing a paracrine role in adipose tissue and mediating local iron trafficking. Consistent with this hypothesis, work from Dr. Hasty’s laboratory has demonstrated delivery of iron from macrophages to adipocytes within adipose tissue upon high-fat feeding, a process that is also associated with macrophage polarization (26). Other investigators have shown expression of the transferrin receptor in adipocytes is required for normal differentiation and thermogenesis (27). The results shed light on the fact that the adipocyte expresses (3) and highly regulates (1) several proteins involved in iron regulation (the iron export channel ferroportin, transferrin, hepcidin), and additional proteins that are otherwise restricted in expression to the tissues that determine iron homeostasis (2). The results also further support the hypothesis that the adipocyte is an iron-sensing cell that uses not only macronutrient status but also the levels of the micronutrient iron, which is essential for many metabolic processes, to regulate metabolism in other tissues through the secretion of hormones and adipokines.
A curious aspect of the relation of the human polymorphisms regulating TF expression was that although they were strongly associated with insulin resistance, one of the principal risk factors for type 2 diabetes (T2D), those same polymorphisms have not emerged in large genome-wide association studies as risk alleles for T2D itself. The response of the Trf+/-Cre+/- mice to a high fat diet reveal that they are less affected by the diet than the WT in terms of changes in glucose tolerance. Our previous work on the polymorphism in humans also demonstrated a dependence of the insulin sensitivity phenotype on tissue iron stores, suggesting a highly complex interplay among diet, adipocyte iron, total body iron stores and diabetes-related phenotypes.
The majority of genes previously shown to be regulated by TF polymorphisms in humans showed concordant regulation in the Trf+/-Cre+/- mice. In our model, we observed both upregulation of Tfrc and Cxcl1 (cytokine upregulated in diabetes and Alzheimer’s disease (7, 28)). TFRC-mediated iron accumulation has previously been shown to require a CXLC1/CXCR2 signaling cascade in neural circuits for pain (29). Primarily established as an immune regulator, our mouse (and human) results suggest that Cxcl1 is also a crucial part of the transcriptional signature for iron regulation. Similar relationships have been made linking iron metabolism/regulation to Sfrp4 and Rarres2 (30, 31), whose upregulation in our mouse model was also concordant to human data.
Aberrations in Glut4 protein translocation as well as Glut4 transcription in both muscle and adipose tissue can disrupt total-body glucose homeostasis (32). Additionally, adipose-specific reduction of Glut4 mRNA expression has been shown to be sufficient in causing whole-body glucose intolerance and insulin resistance (33). Thus, the observed glucose tolerance and insulin sensitivity abnormalities in the Trf+/-Cre+/- mice on normal chow can, in part, be explained mechanistically by the downregulation of Glut4. Observation of Glut4 downregulation only in Trf+/-Cre+/- suggests significant, yet less explored, interplay between iron regulation and glucose homeostasis. High iron (700 mg/kg) diet supplementation has been shown to reduce Glut4 mRNA expression in mouse adipose (but not muscle) tissue (34). The ability of both iron-underloading and iron-overloading to have an effect on a key player of glucose tolerance insulin sensitivity (Glut4) highlights the importance of iron regulation and understanding the optimal range of iron accumulation.
Although concordance of genes between our human and mouse studies was observed, this was not true for all of the genes examined. The discordantly regulated proteins, however, share regulation also by obesity and/or diet, which may explain the different results observed in humans with the TF polymorphisms: Unlike the Trf+/-Cre+/- mice that showed only modestly increased weight on normal chow compared to WT mice, humans with decreased TF expression also exhibited significant increases in BMI, possibly diet- and/or age-related. Adipoq, for example, is upregulated in low iron situations (3), which is consistent with the diminished intracellular adipocyte iron revealed by upregulation of the transferrin receptor (Tfrc) in the mice (Figure 1). Adiponectin is also downregulated in obesity, however, perhaps accounting for an overriding downregulation in the humans with the TF polymorphisms. The other discordant mRNAs (Ucp2 and Ces1) are similarly regulated by nutrient intake, obesity, and diabetes. In general, however, the pattern of regulation of this set of genes in the Trf+/-Cre+/- mice, where diabetes and obesity have not supervened to counter iron-related regulation, is consistent with the low iron status and a shift away from oxidative metabolism.
4. Materials and Methods
Animals: Animal studies and procedures were approved by the Wake Forest Institutional Animal Care and Use Committee.Mice expressing cre recombinase in adipocytes under control of the adiponectin promoter (B6;FVB-Tg(adipoq-cre)iEvdr/J) were purchased from Jackson Labs (Bar Harbor, ME). Mice were housed in an individual ventilated caging (IVC) system with bed-o’cob bedding and paper nesting material to offer enrichment. Weanling mice were fed a normal laboratory diet for 2 months followed by normal chow or a “fast-food” (FF) diet (35) containing 35mg/kg and 2g/Kg Fe (Harlan Teklad TD.140526 and TD.140527). FF diets contained 49.2% carbohydrate, 21.2% fat, and 17.3% protein by weight. While on the FF diet, fructose (23.1 g/L) and glucose (18.1 g/L) were added to the drinking water. After 8 weeks on FF diet, body weight was taken weekly. Food and water were also monitored weekly. Fasting glucose was determined every four weeks for 6-8 months
Generation of the transgenic mice was established with the modified endogenous transferrin (Trf) locus embryonic stem cell [Trftm1a(KOMP)Wtsi ] (MGI:98821) purchased from Mutant Mouse Resource & Research Centers (MMRC) University of California Davis (CA, USA), with flox sites flanking exon 5 of the mouse transferrin gene (Trf). Mouse generation was carried out through Health Service Center (HSC Core) Transgenic gene Targeting Mouse facility, University of Utah (UT, USA). Resulting Trf chimera were genotype for transferrin (Trfm1a) insert using the gene and cassette specific primers (Figure 1A). The Tfr mice were initially bred with Flp mice (B6.Cg-Tg(ACTFLPe)9205Dym/J Stock #005703, Jackson lab. Bar Harbor, ME, USA) to make a conditional knockout allele by removing the trapping cassette while keeping the critical exon “floxed” (Trf Post-Flp). These mice were backcrossed to wildtype B6N to reestablish the original genetic background. Conditional removal of Trf exon was accomplished by breeding with adipocyte cre recombinase expressing mice (B6;FVB-Tg(adipoq-cre)iEvdr/J Stock#028020 Jackson lab. Bar Harbor, ME, USA). Attempts to generate a homozygous colony by crossing heterozygotes failed, after having no homozygotes in >100 pups from eight breeding pairs. All studies were performed in male mice.
Isolation of primary adipocytes. Epididymal and retroperitoneal fat pads were isolated and incubated in HBSS with 1% BSA and 20mg collagenase type I for 1 hour at 37˚C with rotation. Adipocytes were then filtered through 100-uM nylon mesh, washed using HBSS with 1% BSA, and centrifuged at 500g. The adipocyte layer was then transferred to a clean tube, washed, and pelleted.
Glucose tolerance test. Mice were fasted for 6-8 hours. Tail vein blood was collected at t=0 for baseline glucose using Contour Next EZ glucometer (Contour Next, Ascensia Diabetes Care US Inc. NJ, USA. Mice were then injected with 1g/kg glucose intraperitoneally and glucose readings were taken at t=15,30,60, and 120min. Area under the glucose curve (AUCg) was calculated by the trapezoid method. Tail vein blood (100 μl) was collected for each mouse prior to glucose injection for blood analyses.
Blood analyses. Mice were fasted for 6-8 hours, and tail vein blood was collected. Plasma insulin and transferrin levels were measured using commercially available kits (CrystalChem, #90800 and #80661, Elk Grove Village, IL, USA).
Homeostatic Model Assessment for Insulin Resistance (HOMA-IR). Insulin resistance was assessed using the following formula: fasting insulin (microU/L) x fasting glucose (nmol/L)/22.5. Glucose and insulin values were derived from the oral glucose tolerance test.
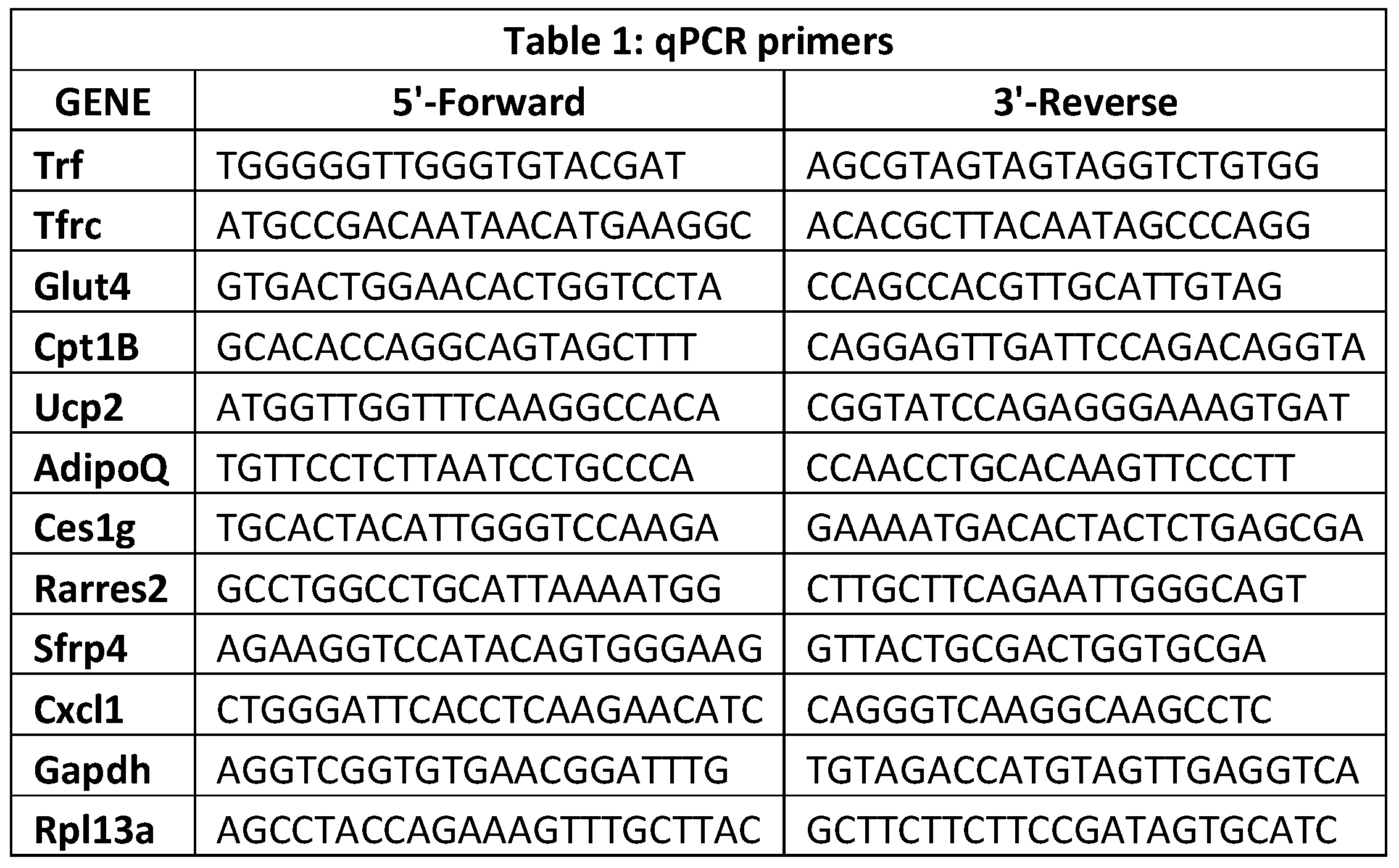
Quantification of transcripts. Quantitative RT-PCR was carried out as previously described (36). Briefly, RNA was extracted from the isolated primary adipocytes using TRizol (Invitrogen) and purified using RNeasy column (QIAgen). RNA quality assessment was performed by subjecting RNA sample aliquots to agarose gel electrophoresis and analyzing using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). RNA purity (A
260/280) and concentration were determined using the Nanodrop 2000 (Thermo Fisher Scientific, Wilmington, DE, USA). RNA samples were synthesized into cDNA using VILO superscript master mix (Invitrogen). Real-time PCR was performed with the 7500 Fast PCR system (Applied Biosystems) using the primers indicated (Table 1). Levels of mRNA for each gene were normalized to the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (Gapdh). The geNorm software algorithms were used to confirm stability of chosen housekeeping gene for mouse tissue samples. The qPCR experiments were conducted under minimum information for publication of quantitative real-time PCR experiments (MIQE) guidelines.
Data and Resource Sharing. Datasets for the current study are available from the corresponding author upon reasonable request.
Statistical Procedures. Results are presented as the mean ± SEM. The significance of variability between groups was determined by 2-way ANOVA with a Tukey post-hoc analysis. Data were analyzed with GraphPad Prism software (v.6).
Author Contributions
All authors contributed to the study conception and design, data collection, and analysis. The manuscript was drafted by Donald A. McClain and commented on and approved by all authors.
Funding
This work was supported by the US Department of Veterans Affairs (5 I01 BX 001140, DAM)
Institutional Review Board Statement
Animal studies and procedures were approved by the Wake Forest Institutional Animal Care and Use Committee.
Informed Consent Statement
Not Applicable.
Data Availability Statement
None.
Acknowledgments
This work was supported by the US Department of Veterans Affairs (5 I01 BX 001140, DAM)
Conflict of interest
The authors declare that they have no conflict of interest.
References
- McClain, D.A.; Sharma, N.K.; Jain, S.; Harrison, A.; Salaye, L.N.; et al. Adipose Tissue Transferrin and Insulin Resistance. J Clin Endocrinol Metab 2018.
- Harrison, A.V.; Lorenzo, F.R.; McClain, D.A. Iron and the Pathophysiology of Diabetes. Annu. Rev. Physiol. 2022, 85, 339–362. [Google Scholar] [CrossRef]
- Gabrielsen, J.S.; Gao, Y.; Simcox, J.A.; Huang, J.; Thorup, D.; Jones, D.; Cooksey, R.C.; Gabrielsen, D.; Adams, T.D.; Hunt, S.C.; et al. Adipocyte iron regulates adiponectin and insulin sensitivity. J. Clin. Investig. 2012, 122, 3529–3540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Funcke, J.-B.; Zi, Z.; Zhao, S.; Straub, L.G.; Zhu, Y.; Zhu, Q.; Crewe, C.; An, Y.A.; Chen, S.; et al. Adipocyte iron levels impinge on a fat-gut crosstalk to regulate intestinal lipid absorption and mediate protection from obesity. Cell Metab. 2021, 33, 1624–1639. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.K.; Sajuthi, S.P.; Chou, J.W.; Calles-Escandon, J.; Demons, J.; Rogers, S.; Ma, L.; Palmer, N.D.; McWilliams, D.R.; Beal, J.; et al. Tissue-Specific and Genetic Regulation of Insulin Sensitivity-Associated Transcripts in African Americans. J. Clin. Endocrinol. Metab. 2016, 101, 1455–1468. [Google Scholar] [CrossRef]
- Wallander, M.L.; Leibold, E.A.; Eisenstein, R.S. Molecular control of vertebrate iron homeostasis by iron regulatory proteins. Biochim. et Biophys. Acta (BBA) - Mol. Cell Res. 2006, 1763, 668–689. [Google Scholar] [CrossRef]
- Nunemaker, C.S.; Chung, H.G.; Verrilli, G.M.; Corbin, K.L.; Upadhye, A.; Sharma, P.R. Increased serum CXCL1 and CXCL5 are linked to obesity, hyperglycemia, and impaired islet function. J. Endocrinol. 2014, 222, 267–276. [Google Scholar] [CrossRef]
- Brix, J.M.; Krzizek, E.C.; Hoebaus, C.; Ludvik, B.; Schernthaner, G.H. Secreted Frizzled-Related Protein 4 (SFRP4) is Elevated in Patients with Diabetes Mellitus. Horm. Metab. Res. 2016, 48, 345–348. [Google Scholar] [CrossRef]
- Helfer, G.; Wu, Q.-F. Chemerin: a multifaceted adipokine involved in metabolic disorders. J. Endocrinol. 2018, 238, R79–R94. [Google Scholar] [CrossRef]
- Kahn, B.B. Lilly lecture 1995. Glucose transport: pivotal step in insulin action. Diabetes 1996, 45, 1644–1654. [Google Scholar]
- Xu, J.; Yin, L.; Xu, Y.; Li, Y.; Zalzala, M.; Cheng, G.; Zhang, Y. Hepatic Carboxylesterase 1 Is Induced by Glucose and Regulates Postprandial Glucose Levels. PLOS ONE 2014, 9, e109663. [Google Scholar] [CrossRef]
- Lee, Y.S.; Olefsky, J. Chronic tissue inflammation and metabolic disease. J. Bone Jt. Surg. 2021, 35, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Scherer, P.E. The many secret lives of adipocytes: implications for diabetes. Diabetologia 2019, 62, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.; Liang, P.; Spiegelman, B.M. AdipoQ Is a Novel Adipose-specific Gene Dysregulated in Obesity. J. Biol. Chem. 1996, 271, 10697–10703. [Google Scholar] [CrossRef] [PubMed]
- Münzberg, H.; Singh, P.; Heymsfield, S.B.; Yu, S.; Morrison, C.D. Recent advances in understanding the role of leptin in energy homeostasis. F1000Research 2020, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Straub, L.G.; Scherer, P.E. Metabolic Messengers: adiponectin. Nat. Metab. 2019, 1, 334–339. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Z.; Gabrielsen, J.S.; Simcox, J.A.; Lee, S.-H.; Jones, D.; Cooksey, B.; Stoddard, G.; Cefalu, W.T.; McClain, D.A. Adipocyte iron regulates leptin and food intake. J. Clin. Investig. 2015, 125, 3681–3691. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, D.; Zhang, H.; Zhang, Y.; Wang, J.; Qi, R.; Yang, J.; Shen, H.; Xu, Y.; Li, M. Rapid responses of adipocytes to iron overload increase serum TG level by decreasing adiponectin. J. Cell. Physiol. 2021, 236, 7544–7553. [Google Scholar] [CrossRef]
- Coimbra, S.; Catarino, C.; Santos-Silva, A. The role of adipocytes in the modulation of iron metabolism in obesity. Obes. Rev. 2013, 14, 771–779. [Google Scholar] [CrossRef]
- Gotardo, M.F.; Dos Santos, A.N.; Miyashiro, R.A.; Gambero, S.; Rocha, T.; Ribeiro, M.L.; Gambero, A. Mice That Are Fed a High-Fat Diet Display Increased Hepcidin Expression in Adipose Tissue. J. Nutr. Sci. Vitaminol. 2013, 59, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Sharma, N.K. Expression quantitative trait analyses to identify causal genetic variants for type 2 diabetes susceptibility. World J. Diabetes 2014, 5, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Kusminski, C.M.; Holland, W.L.; Sun, K.; Park, J.; Spurgin, S.B.; et al. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat Med 2012, 18, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; van de Wall, E.; Laplante, M.; Azzara, A.; Trujillo, M.E.; Hofmann, S.M.; Schraw, T.; Durand, J.L.; Li, H.; Li, G.; et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Investig. 2007, 117, 2621–2637. [Google Scholar] [CrossRef]
- Kusminski, C.M.; Park, J.; Scherer, P.E. MitoNEET-mediated effects on browning of white adipose tissue. Nat Commun 2014, 5, 3962. [Google Scholar] [CrossRef]
- Ameka, M.K.; Hasty, A.H. Fat and Iron Don't Mix. Immunometabolism 2020, 2. [Google Scholar] [CrossRef]
- Li, J.; Pan, X.; Pan, G.; Song, Z.; He, Y.; et al. Transferrin Receptor 1 Regulates Thermogenic Capacity and Cell Fate in Brown/Beige Adipocytes. Adv Sci (Weinh) 2020, 7, 1903366. [Google Scholar] [CrossRef]
- Guedes, J.R.; Lao, T.; Cardoso, A.L.; El Khoury, J. Roles of Microglial and Monocyte Chemokines and Their Receptors in Regulating Alzheimer’s Disease-Associated Amyloid-beta and Tau Pathologies. Front Neurol 2018, 9, 549. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, P.; Liu, C.; Chen, W.; Wang, P.; Jiang, L. Hydrogen-Rich Saline Attenuates Chronic Allodynia after Bone Fractures via Reducing Spinal CXCL1/CXCR2-Mediated Iron Accumulation in Mice. Brain Sci 2022, 12. [Google Scholar] [CrossRef]
- Massaiu, I.; Campodonico, J.; Mapelli, M.; Salvioni, E.; Valerio, V.; Moschetta, D.; Myasoedova, V.A.; Cappellini, M.D.; Pompilio, G.; Poggio, P.; et al. Dysregulation of Iron Metabolism-Linked Genes at Myocardial Tissue and Cell Levels in Dilated Cardiomyopathy. Int. J. Mol. Sci. 2023, 24, 2887. [Google Scholar] [CrossRef]
- Suzuki, T.; Komatsu, T.; Shibata, H.; Tanioka, A.; Vargas, D.; Kawabata-Iwakawa, R.; Miura, F.; Masuda, S.; Hayashi, M.; Tanimura-Inagaki, K.; et al. Crucial role of iron in epigenetic rewriting during adipocyte differentiation mediated by JMJD1A and TET2 activity. Nucleic Acids Res. 2023, 51, 6120–6142. [Google Scholar] [CrossRef] [PubMed]
- van Gerwen, J.; Shun-Shion, A.S.; Fazakerley, D.J. Insulin signalling and GLUT4 trafficking in insulin resistance. Biochem. Soc. Trans. 2023, 51, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.D.; Peroni, O.D.; Kim, J.K.; Kim, Y.-B.; Boss, O.; Hadro, E.; Minnemann, T.; Shulman, G.I.; Kahn, B.B. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 2001, 409, 729–733. [Google Scholar] [CrossRef] [PubMed]
- He MJ, J.; Liu, S.; Cheng, H. Effect of iron supplementation on glucose transporter 4 expression in adipose tissue and skeletal muscle of pregnant rats. Open Journal of Obstetrics and Gynecology 2013, 3, 500–507. [Google Scholar]
- Charlton, M.; Krishnan, A.; Viker, K.; Sanderson, S.; Cazanave, S.; McConico, A.; Masuoko, H.; Gores, G. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G825–G834. [Google Scholar] [CrossRef]
- Huang, J.; Gabrielsen, J.S.; Cooksey, R.C.; Luo, B.; Boros, L.G.; Jones, D.L.; Jouihan, H.A.; Soesanto, Y.; Knecht, L.; Hazel, M.W.; et al. Increased Glucose Disposal and AMP-dependent Kinase Signaling in a Mouse Model of Hemochromatosis. J. Biol. Chem. 2007, 282, 37501–37507. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).








