1. Introduction
Humanity's living environment is actually facing the unprecedented environmental challenge. The global crisis like increased warming and desertification together with frequent activities of humans including overexploitation of natural resources have intensified the deterioration of human living environment, which is causing the focus of global attention [
1,
2,
3]. China has actively put forward the implementation of carbon peak and carbon neutral "
Double Carbon Goal" that will adopt more powerful policies and measures, carbon dioxide emissions strive to peak before 2030, and strive to achieve carbon neutrality before 2060 [
4].It is time to discuss the development and application of plant cell biotechnology due to great potential, green and safe.
1.1. The brief history of the development and application of plant cell engineering
Plant cell engineering was born in the 20th century, and it is interesting that it has experienced three stages of exploration, foundation and rapid development with an average gap of almost 30 years. Back to the year of 1902, German plant physiologist Gottlieb Haberlandt carried out the first in vitro cell experiment and published the report of "Plant in vitro cell Culture Experiment" [
5]. Three decades later, two important advances of both medium model and hormone regulation model by three great scientists promoted plant in vitro culture to the stage of foundation. They are White well known as White culture medium, Gautheret revealed the significance of B vitamins and Nobecourt established a continuous growing tissue culture, who are regarded as the founders of plant tissue culture [
5]. It is worth raising that British scientist Frederick Steward successfully obtained whole plants from the callus cells of carrot root for suspension culture in 1958 [
5], which not only confirmed the theory of cell totipotency for the first time, but also laid the foundation for the technical procedures of tissue culture. Later haploid plants were obtained from the microspore culture of tobacco and carrot[
6], and Cocking et al. treated tobacco cells with purified cellulase and pectinase to obtain protoplasts from which was regenerated plants in the 1960s [
7]. Since then, the research of plant in vitro culture has moved to the stage of large-scale culture and made a major achievements in protoplast culture, microspore culture and micropropagation technology [
8,
9,
10].
With the rapid development of modern plant cell biotechnology in the 21st century, their application has swept rapidly in many fields such as medicine, cosmetics and food industry, besides flower market, and has spawned many well-known companies [
11,
12]. Taking plant breeding as an example, the work of plant breeders has been focused on major staple crop species including wheat, soybean, maize, rice, potato, carrot and sunflower in order to improve the targeted traits in their desired crops [
13,
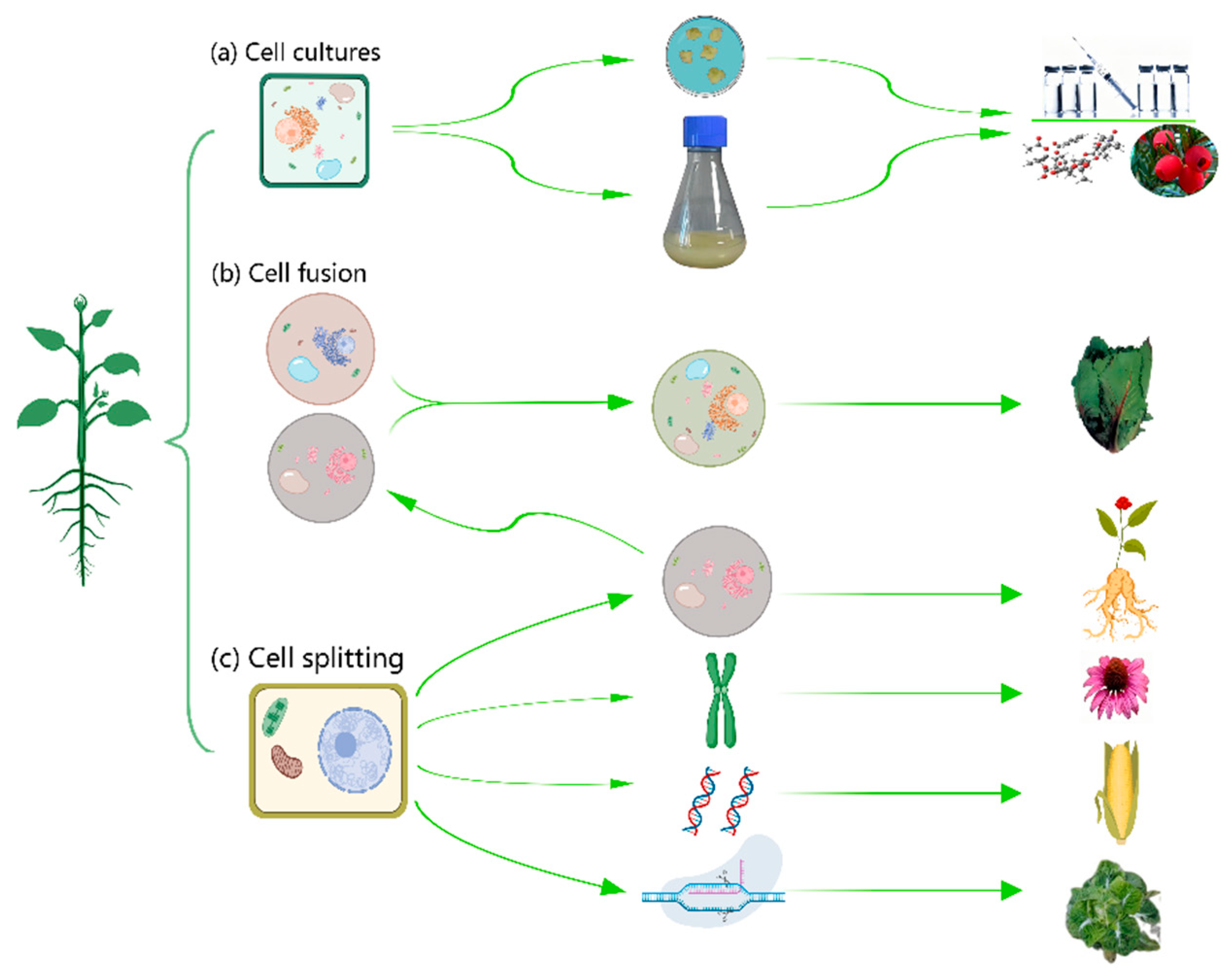
14]. At present, the manipulation technology of plant cells in vitro mainly covers three aspects of cell cultures, cell fusion and cell splitting as shown in
Figure 1, which all have made encouraging progress.
1.2. Medicinal plants is the treasures of nature
There are about 80,000 species plants having medicinal value among the 250,000–500,000 species of higher vascular plants on the planet, and only 0.5% of them have been screened for medicinal properties [
5]. Aiming for survival, reproduction and defense, medical plants synthesize series of specific secondary metabolites estimated to be in excess of 200 000 which are very valuable for human increasingly concerned about health and disease control [
15]. As well known that the novel coronavirus has caused great harm to mankind, and traditional Chinese medicine has played an important role in the battle against the novel coronavirus in the past few years.
As a representative of traditional Chinese medicine, traditional medicine herbs has a long history especially in China for not only maintaining human health but also contributing treatment of lots of diseases including cardiovascular system, cancers, diabetes, etc. According to the performance and function of drugs instead of strengths or weaknesses recorded in
Shennong Materia Medica, Chinese medicinal materials are divided into upper, middle and lower three grades, so called the three-product classification method. One hundred and twenty kinds of medicine belong to top grade for long-term service and nontoxic, such as ginseng, licorice, jujube, etc. One hundred and twenty kinds of toxic or non-toxic medicine herbs are middle grade and used dependent on the condition, like lily and angelica. Another one hundred and twenty-five kinds of medicine are used as an assistant to heal specific sick, but they are too poisonous to be taken for long time, such as rhubarb, aconite, croton and so on [
16,
17,
18].
More than that, medical plants are becoming important group of plant species benefit people for its wide range of activity and high ecological security of production [
14,
19,
20]. In treatment of diabetes, herbal medicines are more effective than synthetic antidiabetic drugs, with insignificant toxicity and no side effects [
19]. The anti-obesogenic drugs of Orlistat and Sibutramine are not popular to the people because of the higher cost and severe side effects, whereas plant-based drugs have been considered as better alternatives due to their lower cost and lower side effects [
20]. Thus, medicinal plants and plant-derived medicines are in the ascendant group for their rich nutritious and produced valuable active components with safety and lesser side effects. We here focus on the development and application of plant cell engineering in medicinal plants.
2. Development and application of cell Cultures in medicinal plants
2.1. Great advantages of Cell Cultures for medicinal plants
For such a natural treasure medicinal plants, that traditional cultivation of seed propagation or cutting seriously restricts herbal medicine production because of high-consuming of time and manpower, at the same time wild biological resources are being depleted due to environmental impact such as warming and desertification [
2].
With the rapid development of modern biotechnology, in vitro cell culture in medicinal plants bring new life in obtaining excellent medicinal plant varieties and thereby the plant-derived medicines. The most important advantage and tempting feature of plant cell cultures of medicinal plants is to obtain man-controlled system by which to avoid environmental stimuli including geographical or seasonal changes, to induce high production of target medicinal ingredients, and to achieve large-scale synthesis of at a lower cost.
In addition, to produce biological medicines using plant cell culture is without those problems of virus, prions or DNA related to mammals during the production process, although few secondary metabolites have been successfully biosynthesized in microbes to date [
21,
22]. Hence, plant cell cultures could not only overcome limitations of traditional cultivation and but also have the advantages of regulatory compliance, safety, carbon reduction and environmental protection [
23,
24]. These advantages have prompted the rapid development of in vitro cell culture technology especially in medical plants.
2.2. Current Application of Cell Cultures in Medicinal Plants
Plant in vitro cell culture is a technology in which living cells from explants including roots, stems, leaves and so on are placed in media under artificially created sterile conditions, and are continuously cultivated in a controlled environment to obtain steady vigorous callus or suspension cells for further producers of valuable secondary synthesis substances. There are hundreds of medicinal plants has made progress in vitro cell culture and providing kinds of secondary metabolites [
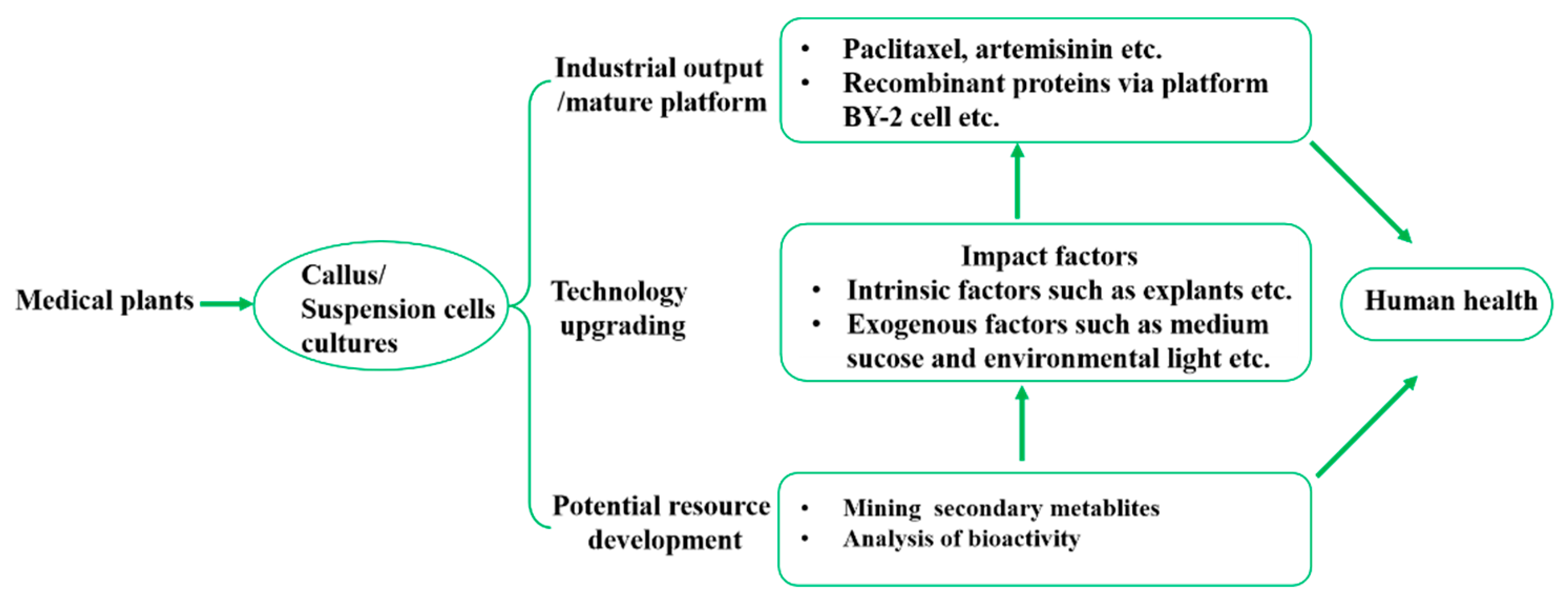
15]. According to the content of the research and the stage of development, current cell cultures of medical plants could be roughly divided into three main application trends of industrial output, technology upgrading and potential resource development as shown in
Figure 2.
2.2.1. Application Trends of Industrial Output in Plants Cell Cultures
Plants cell cultures technology of the application trends of industrial output means in a relatively leading development stage and that has established a mature cell cultures platform such as tobacco BY-2 cell, Arabidopsis cell and Nicotiana tabacum cell, and even stride forward industrial level like production of paclitaxel, artemisinin, ginsenosides, etc. [
23,
24,
25]. As model plants, in vitro culture of both tobacco BY-2 cell and Arabidopsis cell have demonstrated that they are promising bioproduction platform for therapeutic proteins due to many advantages of easily handle, fast growing, mature engineering methods and so on [
23,
26]. Hence, Plant-based pharmaceutical production has gained more attentions because of great advantages that plant platforms such as BY-2 cell have more substantial technical benefits over mammalian cell-based system including high-throughput screening and development of target recombinant protein, and low-cost process [
23,
27,
28,
29].
Speaking of the representatives of industrial production, paclitaxel, artemisinin, ginsenosides have their own hotspots. Artemisinin, originally used for its antimalarial activity, has received much attention since Tu Youyou was awarded the Nobel Prize in 2015 for her enormous contribution to human, and more studies about artemisinin are wildly reported concerning different directions such as cancer therapy related biomaterials, chemical synthesis and biosynthetic gene expression in Artemisia annua [
30,
31,
32].
Panax ginseng C.A.M, a genus of ginseng plant in Acanthaceae,its dried roots are commonly used for being listed as top quality medical herbs in
Shennong Materia Medica [
16,
17,
18]. Although there were reports of ginseng callus culture in the 1960s, in vitro culture of ginseng cell are relatively delayed due to the slow growth and low content of target active ingredients in ginseng callus [
33,
34]. While Research on hairy root of ginseng has made great progress like regulating endogenous methyl jasmonate biosynthesis [
35,
36].
As for another representative worth mentioning is paclitaxel, a potent antitumor alkaloid, is widely used for the treatment of several cancer types including ovarian, breast, esophageal, lung and pancreatic cancer. And such valuable secondary metabolite naturally exists in the inner bark of several Taxus species at quite low levels which leads to that unsustainable extraction. Although there has been some success in its synthesis using hybrid approaches, the production of paclitaxel remains expensive [
37]. Hence, eco-friendly biotechnological platforms based on Taxus sp. cell suspension cultures are developed and several elicitors on yields improvement are investigated. Paclitaxel biosynthesis was obviously increased due to the effect of coronatine and methyl-β-cyclodextrin elicitation, leading to the production level of 5.62 times higher than that of the untreated cultures. Four key genes of DBAT, BAPT, DBTNBT and PAM that encode rate-limiting enzymes in paclitaxel biosynthesis pathway were identified among 13 related genes in cell suspension cultures [
38].To improved biotechnological production of paclitaxel in Taxus media cell cultures, the effect of the elicitor coronatine and the macrocyclic nanoparticles calix[
8]arenes were studied. These nanoparticles can facilitate their excretion to the medium by forming complexes with paclitaxel, and the nanoparticles also could protect cells from their toxicity. The highest taxane production was 15-fold greater than that in control after coronatine and calix[
8]arenes treatment [
39]. Moreover, a promising alternative to paclitaxel production was put forward. In Corylus avellana cell suspension culture, C. palmarum CW was reported as an efficient elicitor that promoting paclitaxel biosynthesis [
40].
2.2.2. Application Trends of Medicinal Plants Cell Cultures Technology Upgrading
Kinds of effect factors on callus, suspension cells cultures of medical plant are wildly reported for technology upgrading especially for those medicinal plants with clearly effective ingredients to enhance the yield. In the process of medicinal plant cell culture, intrinsic factors such as explants and exogenous factors are crucial for the accumulation of target secondary metabolites [
41,
42,
43,
44]. Here are some recent reports. The callus of Curcuma longa was induced by leaf sheath, leaf base, top bud and lateral bud respectively. The results showed that the contents of curcumin and total phenol in the callus with lateral bud as explants were significantly higher than those of others [
45]. The callus of Solanum nigrum were cultured with different concentrations of sucrose. It was found that the content of solanine in callus increased significantly with the increase of sucrose content in the medium [
46]. The content and distribution of secondary metabolite in medicinal plants are also closely related to environmental factors, especially light [
47]. The effects of different spectral light on the biosynthesis of phenylpropanoid metabolites in the callus of
Ocimum basilicum were studied, and results showed that total content of phenol and flavonoid under blue light treatment were the highest compared with the control group [
48].
2.2.3. Application and Development of Potential Medicinal Plants Resource
Mining valuable secondary metabolites of medical plant and analyzing their bioactivities are another application trends and are core content of secondary metabolites engineering. There are large majority medical plants with unsure/unknown bioactive constituents, which are natural gifts and responsible for therapeutic properties due to valuable secondary metabolites [
5,
15,
16,
17,
18]. That further exploration of the potential effective ingredients from medicine plants resources and investigating their anti-inflammation, antibacterial, anti-tumor activities are meaningful for whole human health and hence one of the current research hotspots [
49,
50,
51]. Taking human immunodeficiency virus (HIV) as an example, HIV-positive people are also susceptible to candida albicans. Extracts of Luffa cylindrical were found not only containing alkaloids, saponins but also showing antimicrobial activity against Candida albicans [
52]. In addition, infection with HIV is the most common cause of mycobacterium tuberculosis, which can accelerate the risk of latent tuberculosis reactivation by 20-fold. Recently, Habibi Peyman et.al. highlighted the potential of plant system as a promising approach for the production of relevant pharmaceuticals for HIV and tuberculosis treatment [
53]. More examples, Kowalczyk firstly reported the protective antioxidant and anti-inflammatory properties of
Menyanthes trifoliata L., Menyanthaceae, which has been used as a folk medicine for various ailments [
54]. and the extracts from Epilobium sp. were shown to possess antimicrobial anti-proliferative anti-inflammatory analgesic and antioxidative activities [
55]. Given that, phytomedicine holds great promise for the treatment of many disease including AIDS cross disease in the near future [
53,
56].
The above three research trends are relative, and they are always not clearly separated. The research overlap is often carried out between technology upgrading and potential resource development. Take a typical example,
Moringa oleifera Lam. family Moringaceae is a rich nutritious plant and is known as the "miracle tree" for its various edible and medicinal uses [
57,
58]. Kinds of analysis of extraction and identification and function in moringa oleifera have spread out, which indicate that such treasure plants could reduce blood lipids, improve intestinal microflora of dairy cows, improve immune tolerance etc. [
58,
59,
60]. More than that,
Moringa oleifera extracts also have anti-inflammatory, antiviral and antibacterial bioactivity [
61,
62,
63]. It is emerging as health plants in common and even designated as the most important food substitute [
64]. Given that wide range of biological activities of, suspension culture protocols for calli induced from seeds, leaf and roots was explored and further evaluation reveal that superior biological activities were identified for the produced calli compared to plant. These reports provide a feasible suspension cell culture system of
Moringa oleifera utilization [
65,
66]. Thus the next step is getting ready for industrial production tendency after further improvement of the system. Above all, plant cell culture is attracted great concern as a promising platform especially with the increased international demands of health, investment of countries and fast development of various technological innovation [
67,
68,
69].
3. Cell Fusion
3.1. Advantages of Somatic Hybridization
The principle of somatic hybridization is cell fusion, which is the technique by which two protoplasts deprived from somatic cells from different sources are artificially combined to form a single cell from which later differentiate and regenerate into new plant variety. The successful application of somatic cell hybridization is of great significance due to the major advantages as follows: [
70,
71,
72]
- ✧
Overcome the barrier of species and genus sexual hybridization incompatibility.
- ✧
Provide novel approaches to strengthen the gene pool unavailable naturally in the environment by introducing genes from wild species. Produce fertile somatic hybrids with target traits of wild plant species.
- ✧
Result in recombination of nuclear and cytoplasmic genomes.
- ✧
Avoid biosafety regulatory issues associated with transgenics.
Although somatic hybridization research program was questioned failed due to the promises instead fulfilled by advances in recombinant DNA technology, the technique should be considered a dormant biotechnology beyond genetic modification and therefore noteworthy of the application progress achieved and the prospect worth discussing [
73].
3.2. Major development stage of somatic hybridization
Formulation of hypothesis. After Rudolf Ludwig Karl Virchow perfecting the cell theory by stating that cells can only originate from cells in 1858, people tend to explore not only inner structure but also culture and manipulation of cells in vitro. Based on the core idea of cell theory that a cell is a relatively independent unit, scientists attempted to perform in vitro fusion of two cultured somatic cells, which was successfully achieved first in animals and later in plants after removing cell walls and was known as somatic hybridization.
Protoplast isolation and culture are premise condition. The term of protoplast representing the specific entire plant cell stripped of their cell wall was first coined by Hanstein in 1880. We could conceptually know that there are two important features of protoplasts in which are easier entry of foreign genes into naked plant cells and are still entire totipotent cell. That tomato protoplast achieved by enzymatic methods was firstly reported by Edward Cocking [
7]. Thereafter, significance of protoplasts has been fully realized when new plants successfully regenerated from tobacco protoplasts was obtained by Takebe ten years later [
74].
Fusion methods promotes the development of somatic hybridization. Systematically, there are three ways to induce cell fusion in animals. They are biological methods such as inactivated Sendai virus induced cell fusion, chemical methods like PEG induced fusion and commonly used physical methods of electric fusion. As for plants, the fusion methods of protoplasts have undergone a transformation from NaNO3 fusion, PEG fusion, PEG-high Ca2+-high pH combination fusion, and electrofusion [
75,
76,
77,
78,
79]. As a typical representative of chemical method, compound Polyethylene glycol (PEG) induced protoplast fusion is widely used because of user-friendly and high induction frequency. With the development of electrofusion method, reports on somatic hybrid regenerants were more prevalent [
76,
77] due to little damage to protoplasts, strong repeatability and easy control of parameters [
78,
79].
The main technique process of somatic hybridization is shown in
Figure 3. In general, the basic technical process mainly includes protoplast technology, somatic hybrid cell technology and new plants regeneration from fused somatic hybrids. It is crucial parts of successful somatic hybridization that obtaining vigorous target protoplast after the serial operation of isolation, purification, culture and identification. Both PEG and electrofusion methods are commonly used to induce target protoplasts fusion. Then specific approaches including drug resistance screening, nutritional complementation screening and fluorescent labeling could be adopted for screening and identification of desired hybrid cells among homokaryons, heterokaryons and non-fused protoplasts of parents. Finally, candidate somatic hybrid plants regenerated from the hybrid cell are obtained after identification.
3.3. Current Application of Somatic Hybridization in Medicinal and Edible Plants
The most important feature of somatic hybridization is that it can successfully cross the incompatible parents in sexual hybridization to broaden the base of accessible germplasm and to offer additional opportunities for introgression of desirable traits from wild resource plants into cultivars. Given that, somatic cell hybridization has been widely studied in various types of plants and has made great progress. "
Medicine is not as good as food" this sentence is widely spread because that there are another old saying "
medicine and food homology" in traditional Chinese medicine [
16,
17].In modern society, enhancing both yield and quality of medicinal and edible plants deserves much attentions because of the rapidly growing population and healthy life concept of people.
3.3.1. Somatic Hybridization in Department of Cereal Crops of Gramineae.
Gramineous crops are of great economic value because they provide human rations and maintain stability in global food production as cereal crops mainly including rice wheat, maize, barley, sorghum and so on. Rice is one of the important nutritionally cereal crops and provides food of flat nature with the role of supplementing the body in traditional Chinese medicine [
16,
17] for half of the world's population. However, the yield of rice crop is generally decreased by the salinity stress due to aquatic environment. The somatic hybridization was reported useful in the development of salt tolerant rice varieties because the hybrid calli from
Oryza sativa and
Myriostachya wightiana showed better tolerance to salt stress [
80] , although distant crosses between gramineous plants is usually difficult to succeed because of the small affinity between the parents.
Another noteworthy example in Gramineae crop is wheat due to that not only one of the most popular food providing calories of carbohydrate to decrease starvation in the world but also typical model of genomic asymmetry in allopolyploid plants. It is well known that the genome of wheat is complex and even more complicated in the classification of wheat relatives. Cultivated wheat are derived from an intricate history of three genomes both diploid and polyploid species. Further results showed that more than half of the species descend from an ancient hybridization event but with a more complex scenario involving a different parent than previously thought that
Aegilops mutica are polyploids (especially allopolyploids) [
81]. It was reported that symmetric somatic hybridization induced a whole-genome scale shift of synonymous codon usage bias (SCUB) via whole genomic shock rather than local chromosomal shock. And there is a complicated association between genetic and epigenetic variation like DNA methylation [
82].The process of distant somatic hybridization was recently reported to alter key regulating genes and altered the structure and function of the root bacterial community in somatic hybrid wheat introgression lines SR3 and SR4 [
83]. Hence, asymmetric somatic hybridization could be an efficient strategy for wheat breeding.
3.3.2. Somatic Hybridization in Department of Vegetables Crops
Vegetables are not only an important component of the agriculture production systems and but also an important part of a healthy diet. In this part, we focus on the application of somatic hybridization in vegetables of Solanaceae, Brassicaceae, Apiaceae and so on.
Somatic hybridization in Solanaceae, such as potato, sweet potato, potato, eggplant,has a long history since electrofusion was first reported in potato between Solanum tuberosum and semi-cultivated [
79,
84,
85]. As the fourth most cultivated crop after wheat, rice and corn, potato is not only a staple food in many west countries but also a common delicious vegetable on Chinese tables [
85]. Moreover, potato was also selected as a candidate plant species for space bioregeneration life support system because of high nutritional value, small space occupancy and easy propagation. Wild Solanum species have been used in potato breeding for various available desirable traits [
86]. Development of somatic hybrids could effectively overcome the reproductive isolation caused by ploidy difference between cultivated major diploid and wild potato species almost tetraploid and widen the narrow genetic base of the cultivated Solanum plants to further raise yield and quality [
87]. Researchers have made great efforts on potato somatic hybridization such as screening of donor materials, methods of culture, fusion and selection, development of new germplasm resistant to fungi like late blight and so on [
88,
89,
90,
91,
92,
93,
94]. For example, selection of somatic hybrids (
S. tuberosum +
S. chacoense) based on callus growth tagged with green fluorescent protein has been observed [
92]. Progress in somatic hybridization research and their application in potato improvement were discussed and summarized in several excellent reviews [
95,
96,
97]. Meanwhile, new breeding technologies like CRISPR/Cas9 editing in potato trait improvement and potential application is rising [
84,
85].
Brassica plants in the Brassicaceae Family are not only a rich reservoir of genes that are valuable for the improvement of cultivated species but also potential medicinal resources as well as beloved table vegetables for containing lots of nutrients such as carotene, dietary fiber and various trace elements [
98,
99]. Somatic hybridization via protoplast culture of many Brassica plants such as Chinese cabbage, cabbage, cabbage rape, cauliflower and turnip has been reported hitherto concerning germplasm resource, and resistant improvement and multi-parent fusion so on [
100,
101,
102,
103]. Broad phenotypic variations were obtained from the asymmetric somatic hybridization of cauliflower “Korso” (
Brassica oleracea var. botrytis) and black mustard “G1/1” (
Brassica nigra). The underlying mechanisms of these variations were investigated and further revealed that asymmetric somatic hybridization induced genetic and epigenetic alterations and produced a new germplasm resource for cauliflower improvement [
101]. Two symmetric hybrids one asymmetric hybrids between
Brassica juncea and
Sinapis alba were generated through protoplast fusion, and further studies revealed the hybrids are highly resistant to
Alternaria brassicae and heat tolerance [
102]. In addition, somatic hybrids produced from three-parent protoplasts fusion showed high genetic and phenotypic variation of brassica were [
103].
The availability of high-quality male sterile lines is of paramount importance and remains currently a bottleneck for hybrid breeding due to flower emasculation in such open pollinated cultivar. [
70]. Take Apiaceae vegetables as an example, for their rich nutrition, nice color and special aroma, species of the family Apiaceae are common table side vegetables such as carrot (
Daucus carota), celery and celeriac (
Apium graveolens var. dulce and var.
rapaceum, respectively) [
18]. Compared to sexual reproduction, somatic hybridization has many advantages to transfer or generate the cytoplasmic male sterility condition de novo, in particular because it avoids unwanted traits coming from the simultaneous transmission of genes other than those responsible for cytoplasmic male sterility [
70,
104]. In order to solve the difficulties of lack uniformity, yield regularity and development of novel plants with unique characteristics, the technology of somatic hybridization are developed in Apiaceae vegetables like carrot improvement [
70,
71]. Real-time detection of somatic hybrid cells during electrofusion of carrot protoplasts with stably labelled mitochondria was reported. Differential fluorescence staining of fused protoplast as routinely used can be replaced by new tagging approaches using non-toxic proteins by this approach [
105]. Recently, somatic hybridization in the family Apiaceae is comprehensively reviewed concerning cytoplasmic male sterility and molecular mechanisms. They focused on protoplast technology of donor materials sources, enzyme mixtures for digestion, and cell wall re-generation in detail. They also emphasized the significance of somatic hybridization in Apiaceae plants to human diet and are looking forward to the future of somatic hybridization associated with robotic platforms, CRISPR, artificial intelligence technology for trait identification and selection [
71,
104,
105,
106,
107].
3.3.3. Somatic Hybridization in Other Medicinal and Edible Plants
Medicinal and edible homologous plants include but are not limited to grains and vegetables, as well as fruits and herbs. Take citrus fruits as an example, they have attracted researchers' interest because of not only delicious and nutritious but also the extensive health-promoting effects of the peel of citrus, known as citri reticulatae pericarpium (CRP). CRP is the dry and mature peel of
citrus reticulata blanco and its cultivated varieties, which can regulate “Qi” and strengthen spleen, dry dampness and eliminate phlegm and belong to top quality medical herbs [
16,
17,
18]. Moreover, recent research has shown that flavonoids found in CRP have the potential to normalize blood glucose, cholesterol levels, and liver lipid levels. And these flavonoids exhibit anti-inflammatory properties, prevent oxidation and prevent liver injury [
108]. The difference in environment, varieties and origin of cultivated varieties thereby CRP are closely related to the content of bioactive compounds mostly secondary metabolites [
109,
110]. Studies on bioactive effect of flavonoids and polysaccharide and environmental influence of CRP are wildly reported [
108,
109,
110,
111,
112,
113]. The recent advances on health benefits, microbial transformations, and authenticity identification of CRP bioactive compounds was systematically summarized [
114].
Somatic hybridization has a significant advantage in citrus improvement because citrus plants could regenerate into whole plants [
115]. Researchers were initially focused on produce male sterile and seedless citrus cybrids then tried more explorations of somatic hybridization covering improvement of techniques, citrus species, rootstocks resistance and so on [
116,
117,
118,
119]. Hybrid of somatic embryos between
citrus and
murraya puniculata were obtained by electrofusion, and further study revealed that percentage of multinucleated protoplast increasing linearly with increase the alternating voltage [
117]. Aiming to develop new tetraploid rootstocks harboring virtues of both parents, new intergeneric somatic hybrids were obtained by electrofusion between protoplasts of citrus, which adapt to abiotic stresses, and P. trifoliata hybrids, which exhibit resistances or tolerances to major diseases. Diploid cybrids and nuclear somatic hybrids were found, while symmetrical addition of the nuclear genomes of both parents was predominant at the whole genome level [
118]. Asymmetrical somatic hybridization between two complementary diploid parents are thought be an efficient way to develop new tetraploid rootstocks for citrus stress adaptation [
117,
118,
119].
So somatic hybridization provides excellent opportunities for plant improvement by attempting to transfer the available genetic information from one species to another via protoplasts fusion and have great progress in many medical and edible homologous crops, vegetables and fruits, etc. With the rapid development of modern technology such as CRISPR/Cas9 editing, artificial intelligence and functional genomics, somatic hybridization may well break the temporarily dormant period and makes new contributions for the future health care nutrition and medicine and food homologous plant improvement [
73,
120].
4. Cell Splitting
Besides techniques such as cell culture and cell fusion, cell manipulation moves towards fine operations at the subcellular and molecular level, which leads to cell separation biotechnology like protoplast isolation, chromosome manipulation, genetically modification and CRISPR editing. Among the above separation technology, chromosome engineering, is a technology that systematically subtracts, adds and replaces homologous or heterologous chromosomes according to a proposed design, so as to achieve directional changes in genetic traits and breed new varieties. Application of chromosome manipulation have great significance in variety and quality improvement of medicinal and edible plants.
4.1. Artificial Polyploidy Induction is an Important Part of Chromosome Engineering
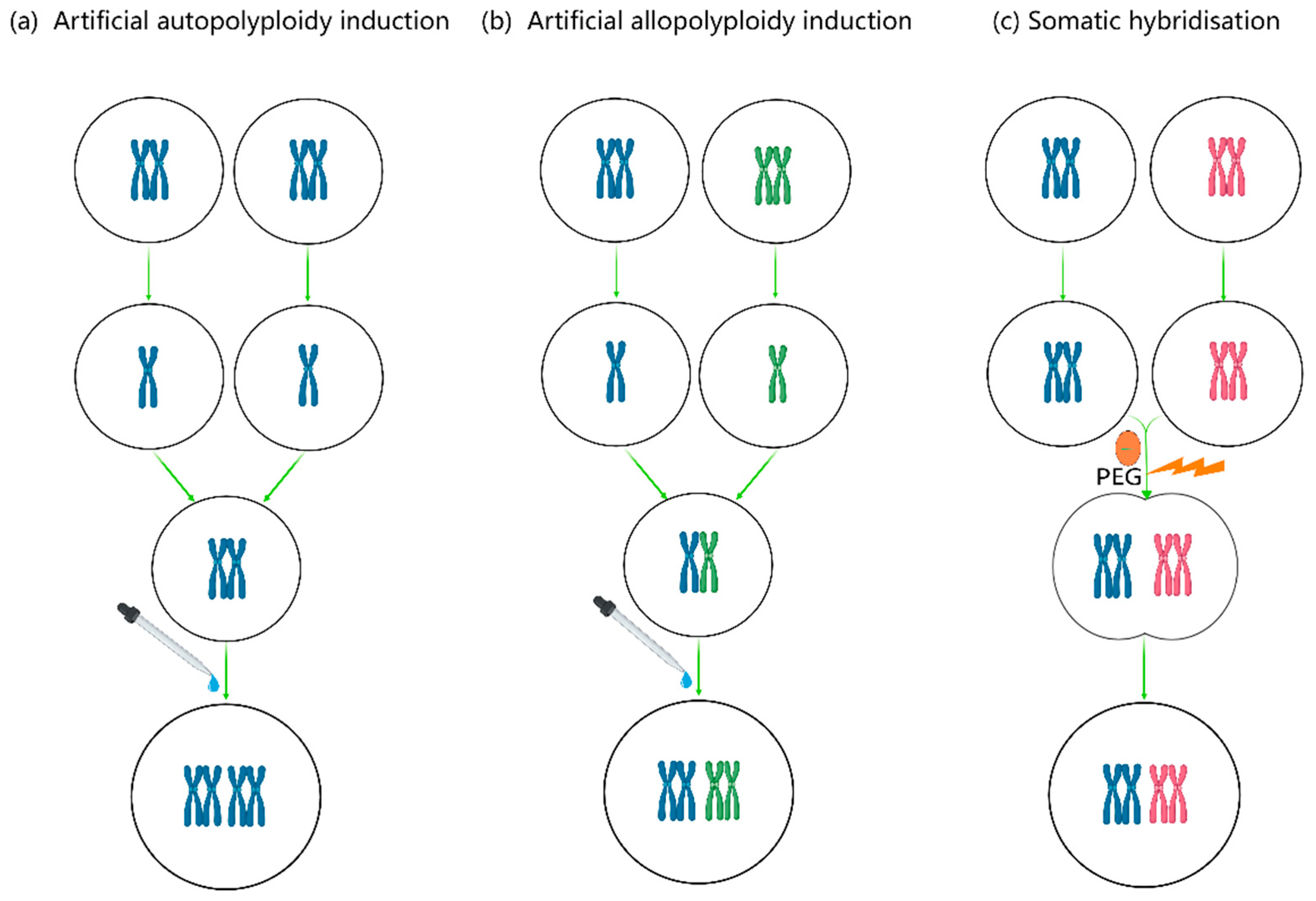
Genetically, chromosome manipulation may result in chromosome changes of both structure and numbers variation. Chromosomal structural variation can occur in callus cells, regenerated plants and the regenerated offspring via somatic hybridization mentioned before. As a major force in the evolution of both wild and cultivated plants, only polyploidy resulted from numbers variation of chromosome are concerned here. To be specific, there are types of euploidy change which is distinguished as polyploid and haploid or autopolyploid and allopolyploid, and aneuploid change which contains chromosome reduction like monosomic and chromosome additions like trisomy. Allopolyploids have more than two chromosome sets derived from different plant species and are generated by hybrid polyploidization or somatic hybridization, while autopolyploid plants have duplicated chromosome sets derived from same plant species [
121] (
Figure 4). Of these, autopolyploid of medicinal and edible plants deserve much attention, because that autopolyploid with duplicated whole sets of chromosomes have more distinctive characteristics of desired traits such as huge flower or root size [
122,
123],enhanced resistance to stresses and higher content of secondary metabolite like desired fragrance [
14,
124,
125,
126,
127,
128]. Moreover, the genome doubling in a newly formed sterile hybrid allows the restoration of its fertility. Therefore, artificial polyploidy induction is one of the remarkable breeding strategies to improve the valuable properties of medicinal and edible plants.
4.2. Application of Artificial Polyploidy Induction in Medicinal and Edible Plants
4.2.1. Artificial Polyploidy Induction in Medicinal Herbs of Compositae
As the largest Family of the subclass Daisy in Dicotyledonous plant, there are almost three hundred health medical herbs in the family Compositae. Some ingredients in Compositae like sesquiterpene lactones have broad biological functions of cardiotonic, anticancer, repellent and analgesic [
16,
17,
18]. To name only a few, Ginger tuber can be processed into soy sauce to be accessary food, and chrysanthemum is popular table vegetable in China. In addition, many herbs of Compositae becomes healthy drink tea or herbal cuisine such as
Cichorium intybus L.,
Artemisia annua L.,
Chamomilla recutita L. and that polyploidy in all the three health medical herbs were reported concerning morphological, physiological, cytological and phytochemical studies [
129,
130,
131,
132,
133]. For instance, level of the total phenolic and chlorogenic acid content in the leaves of tetraploid
Cichorium intybus was reported significantly enhanced [
129]. Autotetraploid chicory plants had more phenolic compounds and produced 10-fold more chlorogenic acid than the controls [
132]. And the content of cichoric acid and chlorogenic acid increased 71% and 45% respectively in the leaves of tetraploid
Echinacea purpurea L [
131], which were also reported for artemisinin [
133].
4.2.2. Artificial Polyploidy Induction in Medicinal Herbs of Other Family
Scutellaria baicalensis is a perennial herb of the family Labiatae, which benefits for treatment of many kinds diseases including heat, yellow pox, edema, and so on according to "
Compendium of Materia Medica" [
16,
17,
18]. And it was used in the prevention and treatment of novel coronavirus pneumonia published by local authorities. In vitro production and identification of autotetraploid of
Scutellaria baicalensis were reported [
134]. Induced polyploidization and morphological traits of medical herbs of other family have also been reported in
Lobularia maritime Cruciferae and
Plantago psyllium of Plantaginaceae. In addition, types and concentration of anti-mitotic agents and plant receptor such as seeds, apical growing points of seedlings in
Lobularia maritima and terminal buds in
Plantago psyllium have also been discussed [
135,
136,
137].
4.2.3. Artificial Polyploidy Induction in Department of Edible Vegetables and Fruits
There are lots of valuable medical and edible herbs recorded in
Shen Nong Materia Medical, which is tempting to increase biomass of medical and edible herbs through artificial polyploidy induction. For example, the root of Chinese sage (
Salvia miltiorrhiza Bunge) was regarded as top-grade Chinese medicine two thousand years ago [
16,
17,
18]. Aiming to develop an easy and reliable means for obtaining tetraploid
Salvia miltiorrhiza, both colchicine and thidiazuron were together used for induction of tetraploids with doubling of chromosomes. And the produced 4x plants showed significantly increased the biomass of leaf, pollen, roots, and the yield of dihydrotanshinone I was also increased. Moreover, a simple and efficient system for inducing and subculture of tetraploid which have stable ploidy level, enhanced growth characteristics as well as the content of dihydrotanshinone I in the root of
S. miltiorrhiza [
122]. A new synthetic B. napus was produced through interspecific hybridization between cultivar
B. rapa and
B. oleracea. The hybrids were then treated with colchinine for artificial polyploidy induction. Results showed that somatic cells of generated synthetic plants contained 38 chromosomes which were the sum of the somatic chromosomes of both
B. rapa and
B. oleracea, and which were also the double times of the untreated hybrids containing 19 somatic chromosomes. It is a successful artificial polyploidy induction which lead to similar results that both production of allopolyploid but different technology of somatic hybridization [
138]. As medical and edible herbs, Perilla is a young allotetraploid Lamiaceae species widely used in East Asia [
139,
140]. Genomes comparative analyses of the tetraploid (
Perilla frutescens) and the wild diploid progenitor (
Perilla citriodora) suggested that a section of Myb113 transcription factor on chromosome 8 was related to anthocyanin synthesis and accumulation, and several sudden changes affected the expression and function of Myb113 gene thereby affecting the synthesis and accumulation of α-linolenic acid during seeds developmental. It has become a key breeding factor for new varieties of oil Perilla [
124,
141]. Therefore, creation allooctoploid Perilla with gigantic biomass via artificial chromosomes doubling on the background of allotetraploid Perilla could be a viable strategy.
Artificial polyploidy induction has been used to increase plant secondary metabolite production or to improve the metabolite profile for their duplicated genes [
140,
141,
142]. By contrast with increased morphological biomass, there also evidences supporting inconsistency traits change appeared in artificial induced polyploidy fruits. For example, broad indexes of morphology, cytology, essential oil composition, gene expression and antioxidant activity of colchicine-induced tetraploid plants of citrus limon were tested. Evidence from all morphological indexes including plant height, number of leaves, root length of tetraploid was increased than that in diploid, while the profile of tetraploid and diploid derived essential oil had a significant difference in their components. The components limonene and lanceol increased drastically while (Z, E)-a-farnesene decreased significantly in tetraploids compare to diploids [
143]. This kind of uneven change with gain or reduction or even loss of components in the essential oil profile have been reported in induced polyploids of ginger bush and ajowan [
144,
145]. Another example used to investigate the effect on secondary metabolite profiles of artificially induced polyploidy is induced tetraploid of
Solanum commersonii, a diploid wild potato species. The tetraploid genotypes (2n=4x=48) of wild S. commersonii (2n=2x=24) were produced by oryzalin treatment. And comparative analysis showed that content changes of secondary metabolite are not consistent in the two species. The tetraploids showed higher content of phenylpropanoid and minor GAs (solanidenediol triose, solanidadienol lycotetraose, and solanidenol lycotetraose) while lower content of major GAs (dehydrodemissine and dehydrocommersonine) than that in diploid
S. commersonii [
146]. All these results supported that artificial induced polyploidy should be tested in terms of desired traits profile.
So far, the research on artificial polyploidy induction has been applied and has made some progress of both medicinal and edible plants to increase the levels of target compounds and improve morphological characteristics. And pathways and the key influence factors of artificial polyploidy induction of medicinal plants are recently discussed in several excellent reviews [
147,
148,
149]. Meanwhile, the research on autopolyploid induction of both medicinal and edible plants is still limited due to lack of the high efficiency of regeneration system of different plants and autopolyploid induction, unmanageable mutation rates and incidence of chimeras and different expression patterns of desired traits which found in the same duplicated genes in various obtained polyploid lines [
143,
150,
151,
152,
153].
5. Conclusions
In brief, facing the unprecedented environmental challenge and growing populations, application and development of modern plant cell biotechnology concerning cell cultures in vitro, protoplast fusion based somatic hybridization and artificial induced chromosome polyploidy are summarized in typical medical and edible plants. Like any other science, plant cell biotechnology began as a purely academic study to answer certain theoretical questions about plant cell growth and development regulation. While, the result of its rapid development has shown unexpected surprises via directly or indirectly producing significant benefits in many fields. In a dialectically view, although there are many problem such as exploration of suitable cell culture reactors, establishment of high efficient model system wildly used for protoplast fusion and hybrids regeneration, more accurate controlled monitor and analysis tools, development of the natural gifted medicinal plants in an ecological green scientific way will benefits whole human society especially with rapid development of CRISPR/Cas9 editing, artificial intelligence, functional genomics and more interdisciplinary technologies.
Author Contributions
F.M. Shi and C. Wang contributed equally to this work. F.M. Shi designed and wrote the manuscript, and C. Wang reviewed and edited the final manuscript. Both authors agreed to the published manuscript.
Funding
We are grateful to the National Nature Science Foundation of China (31240035) and Key Research Project of Shandong Province Graduate Education and Teaching Reform (SDYJG21047).
Data Availability Statement
The data that supports the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
References
- Ramamoorthy, P.; Bheemanahalli, R.; Meyers, S.L.; Shankle, M.W.; Reddy, K.R. Drought, low nitrogen stress, and ultraviolet-b radiation effects on growth, development, and physiology of sweetpotato cultivars during early season. Genes. 2022, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Chupakhin, E.; Babich, O.; Prosekov, A.; Asyakina, L.; Gureev, M.; Krasavin, M. Plants of the russian federation pharmacopeia: an unexhausted natural products research opportunity? Nat Prod Res. 2021, 35, 3525–3527. [Google Scholar] [CrossRef] [PubMed]
- Guiné, R.P.F.; Florença, S.G.; Barroca, M.J.; Anjos, O. The link between the consumer and the innovations in food product development. Foods. 2020, 9, 1317. [Google Scholar] [CrossRef]
- Administration, N.E. National energy administration issued the "energy carbon peak carbon neutral standardization promotion action plan". http://www.nea.gov.cn/2022-10/09/c_1310668927.htm (accessed on 0005/11/1).
- Babich, O.; Sukhikh, S.; Pungin, A.; Ivanova, S.; Asyakina, L.; Prosekov, A. Modern trends in the in vitro production and use of callus, suspension cells and root cultures of medicinal plants. Molecules. 2020, 25, 5805. [Google Scholar] [CrossRef] [PubMed]
- GUHA, S.; MAHESHWARI, S.C. In vitro production of embryos from anthers of datura. Nature. 1964, 204, 497. [Google Scholar] [CrossRef]
- Cocking, E.C. A method for the isolation of plant protoplasts and vacuoles. Nature. 1960, 187, 962–963. [Google Scholar] [CrossRef]
- NAGATA, T.; TAKEBE, I. Cell wall regeneration and cell division in isolated tobacco mesophyll protoplasts. Planta. 1970, 92, 301. [Google Scholar] [CrossRef]
- Ali, J.; Nicolas, K.L.C.; Akther, S.; Torabi, A.; Ebadi, A.A.; Marfori-Nazarea, C.M.; Mahender, A. Improved anther culture media for enhanced callus formation and plant regeneration in rice (oryza sativa l. ). Plants-Basel. 2021, 10, 839. [Google Scholar] [CrossRef]
- Mayo-Mosqueda, A.; Garcia-Hernandez, E.; Noguera-Savelli, E.; Cetzal-Ix, W.; Alatorre-Cobos, F. Advances in breeding, bioprospecting, and in vitro culture of laelia orchid species. Horticulturae. 2022, 8, 103. [Google Scholar] [CrossRef]
- Krasteva, G.; Georgiev, V.; Pavlov, A. Recent applications of plant cell culture technology in cosmetics and foods. Eng Life Sci. 2021, 21, 68–76. [Google Scholar] [CrossRef]
- Eibl, R.; Meier, P.; Stutz, I.; Schildberger, D.; Hühn, T.; Eibl, D. Plant cell culture technology in the cosmetics and food industries: current state and future trends. Appl Microbiol Biot. 2018, 102, 8661–8675. [Google Scholar] [CrossRef]
- Muyonga, J.; Nansereko, S.; Ilona Steenkamp, M.M.; Okoth, J. Linking agricultural universities with civil society, the private sector, governments and other stakeholders in support of agricultural development in africa. In potential role of traditional african foods in food security, nutrition and health, proceedings of the fifth african higher education week and ruforum biennial conference 2016; Cape Town, South Africa, 2016/10/17, 2016; p 311-318.
- Niazian, M. Application of genetics and biotechnology for improving medicinal plants. Planta. 2019, 249, 953–973. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G.; Berni, R.; Armando Munoz-Sanchez, J.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J. , et al. Production of plant secondary metabolites: examples, tips and suggestions for biotechnologists. Genes. 2018, 9, 309. [Google Scholar] [CrossRef]
- Shizhen, L. Compendium of materia medica (bencao gangmu). People's Medical Publishing House: Beijing, 1979; p.
- Emperor, Y. Shennong's herbal classic. China Medical Science and Technology Press: Beijing, 2018; p.
- China, E.C.O.F.; Sciences, C.A.O. Flora of china. Science Press: Beijing, 2004; p.
- Kooti, W.; Farokhipour, M.; Asadzadeh, Z.; Ashtary-Larky, D.; Asadi-Samani, M. The role of medicinal plants in the treatment of diabetes: a systematic review. Electronic Physician. 2016, 8, 1832–1842. [Google Scholar] [CrossRef] [PubMed]
- Mopuri, R.; Islam, M.S. Medicinal plants and phytochemicals with anti-obesogenic potentials: a review. Biomed Pharmacother. 2017, 89, 1442–1452. [Google Scholar] [CrossRef]
- Arturo Zavala-Ortiz, D.; Ebel, B.; Guedon, E.; Marc, A.; Maria Barradas-Dermitz, D.; Margaret Hayward-Jones, P.; Guadalupe Aguilar-Uscanga, M. In situ cell differentiation monitoring of catharanthus roseus suspension culture processes by nir spectroscopy. Bioproc Biosyst Eng. 2020, 43, 747–752. [Google Scholar] [CrossRef]
- Pyne, M.E.; Narcross, L.; Martin, V.J.J. Engineering plant secondary metabolism in microbial systems. Plant Physiol. 2019, 179, 844–861. [Google Scholar] [CrossRef]
- Wu, T.; Kerbler, S.M.; Fernie, A.R.; Zhang, Y. Plant cell cultures as heterologous bio-factories for secondary metabolite production. Plant Commun. 2021, 2, 100235. [Google Scholar] [CrossRef]
- Efferth, T. Biotechnology applications of plant callus cultures. Engineering. 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Li, Y.C.; Jiang, Z.G. Cell suspension culture of medicinal plants and its application. Chemical Industry Press: Beijing, 2015; p.
- Menges, M.; Murray, J. Cryopreservation of transformed and wild-type arabidopsis and tobacco cell suspension cultures. Plant J. 2004, 37, 635–644. [Google Scholar] [CrossRef]
- Holtz, B.R.; Berquist, B.R.; Bennett, L.D.; Kommineni, V.J.M.; Munigunti, R.K.; White, E.L.; Wilkerson, D.C.; Wong, K.I.; Ly, L.H.; Marcel, S. Commercial-scale biotherapeutics manufacturing facility for plant-made pharmaceuticals. Plant Biotechnol J. 2015, 13, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Daniell, H.; Rai, V.; Xiao, Y. Cold chain and virus-free oral polio booster vaccine made in lettuce chloroplasts confers protection against all three poliovirus serotypes. Plant Biotechnol J. 2019, 17, 1357–1368. [Google Scholar] [CrossRef] [PubMed]
- Habibi, P.; Daniell, H.; Soccol, C.R.; Grossi-de-Sa, M.F. The potential of plant systems to break the hiv-tb link. Plant Biotechnol J. 2019, 17, 1868–1891. [Google Scholar] [CrossRef]
- Yue, L.; Pan, Y.; Wang, J.; Yue, L.; Luo, Y.; Lv, F.; Lv, J.; Chen, J.; Zhao, Q.; Lin, H. Design, synthesis, and antitumor activities of isomers of artemisinin dimer derivatives. Chem Biodivers. 2023, 20, e202300615. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, J.; Deng, K.; Zhou, W.; Li, K.; Wang, C.; Wang, Q.; Wu, M.; Huang, S. Gpx4 inhibition synergistically boosts mitochondria targeting nanoartemisinin-induced apoptosis/ferroptosis combination cancer therapy. Biomater Sci-Uk. 2023. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, Q.; Zhang, Y.; Wu, C.; Zheng, Y.; Tong, F.; Zhang, L.; Lu, R.; Pan, X.; Tan, H. , et al. Effects of exogenous indole-3-acetic acid on the density of trichomes, expression of artemisinin biosynthetic genes, and artemisinin biosynthesis in artemisia annua. Biotechnol Appl Bioc. 2023. [Google Scholar] [CrossRef]
- Paek, K.Y.; Murthy, H.N.; Zhong, J.J. Production of biomass and bioactive compounds using bioreactor technology. Springer: Netherlands, 2014; p.
- Wang, J.; Li, J.X.; Li, J.L.; Gao, W.Y. Application of plant tissue culture in field of chinese medicine resources. China J Chin Mater Med. 2017, 42, 2236–2246. [Google Scholar] [CrossRef]
- Zhang, R.; Tan, S.; Zhang, B.; Hu, P.; Li, L. Cerium-promoted ginsenosides accumulation by regulating endogenous methyl jasmonate biosynthesis in hairy roots of panax ginseng. Molecules. 2021, 26, 5623. [Google Scholar] [CrossRef]
- Jin, S.; Bang, S.; Ahn, M. ; LEEKYUBIN; Kyunghwan, K. ; Kyung, H.T. Overproduction of anthocyanin in ginseng hairy roots enhances their antioxidant, antimicrobial, and anti-elastase activities. Journal of Plant Biotechnology. 2021, 48, 100–105. [Google Scholar] [CrossRef]
- Schwab, W.; Lange, B.M.; Wüst, M. Commercial-scale tissue culture for the production of plant natural products: successes, failures and outlook. Springer: Germany, 2018; p.
- Kashani, K.; Javaran, M.J.; Sabet, M.S.; Moieni, A. Identification of rate-limiting enzymes involved in paclitaxel biosynthesis pathway affected by coronatine and methyl-beta-cyclodextrin in taxus baccata l. Cell suspension cultures. Daru. 2018, 26, 129–142. [Google Scholar] [CrossRef]
- Farhadi, S.; Moieni, A.; Safaie, N.; Sabet, M.S.; Salehi, M. Fungal cell wall and methyl-beta-cyclodextrin synergistically enhance paclitaxel biosynthesis and secretion in corylus avellana cell suspension culture. Sci Rep-Uk. 2020, 10, 5427. [Google Scholar] [CrossRef] [PubMed]
- Escrich, A.; Almagro, L.; Moyano, E.; Cusido, R.M.; Bonfill, M.; Hosseini, B.; Palazon, J. Improved biotechnological production of paclitaxel in taxus media cell cultures by the combined action of coronatine and calix[8]arenes. Plant Physiol Bioch. 2021, 163, 68–75. [Google Scholar] [CrossRef]
- Khare, S.; Singh, N.B.; Singh, A.; Hussain, I.; Niharika, K.; Yadav, V.; Bano, C.; Yadav, R.K.; Amist, N. Plant secondary metabolites synthesis and their regulations under biotic and abiotic constraints. J Plant Biol. 2020, 63, 203–216. [Google Scholar] [CrossRef]
- Fan, Z.T.; Wei, F.; Qiao, Z.; Liang, Y.; Xie, T.G.; Liu, J.H.; Wei, K.H. Research progress in the production of secondary metabolites by tissue culture of medicinal plants. Biotic Resources. 2022, 44, 130–140. [Google Scholar] [CrossRef]
- Kulus, D.; Tymoszuk, A. Induction of callogenesis, organogenesis, and embryogenesis in non-meristematic explants of bleeding heart and evaluation of chemical diversity of key metabolites from callus. Int J Mol Sci. 2020, 21, 5826. [Google Scholar] [CrossRef]
- Irshad, M.; Debnath, B.; Mitra, S.; Arafat, Y.; Li, M.; Sun, Y.; Qiu, D. Accumulation of anthocyanin in callus cultures of red-pod okra [abelmoschus esculentus (l. ) Hongjiao] in response to light and nitrogen levels. Plant Cell Tiss Org. 2018, 134, 29–39. [Google Scholar] [CrossRef]
- Gurav, S.S.; Gurav, N.S.; Patil, A.T.; Duragkar, N.J. Effect of explant source, culture media, and growth regulators on callogenesis and expression of secondary metabolites of curcuma longa. Journal of Herbs, Spices & Medicinal Plants. 2020, 26, 172–190. [Google Scholar] [CrossRef]
- Mu, A.A.A.K.; Shibli, R.A.; Tahtamouni, R.W.; Alqudah, T.S.; Abu-Iramaileh, B.B. Improving solanine production in in vitro cultures of solanum nigrum l. Using different chemical and physical factors. Sultan Qaboos University. 2020, 24, 51–62. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Zou, H.; Qiu, L.; Zheng, Y.; Yang, D.; Wang, Y. Effects of light on secondary metabolite biosynthesis in medicinal plants. Front Plant Sci. 2021, 12. [Google Scholar] [CrossRef]
- Nazir, M.; Ullah, M.A.; Younas, M.; Siddiquah, A.; Shah, M.; Giglioli-Guivarc'H, N.; Hano, C.; Abbasi, B.H. Light-mediated biosynthesis of phenylpropanoid metabolites and antioxidant potential in callus cultures of purple basil (ocimum basilicum l. Var purpurascens). Plant Cell Tiss Org. 2020, 142, 107–120. [Google Scholar] [CrossRef]
- Lei, X.; Yu-shi, Z.; Wei-dong, L. Current situation and prospect of development and utilization of non-medicinal parts of medicinal plants. Journal of Chinese Medicinal Materials. 2019, 42, 470–473. [Google Scholar] [CrossRef]
- D'Amelia, V.; Docimo, T.; Crocoll, C.; Rigano, M.M. Specialized metabolites and valuable molecules in crop and medicinal plants: the evolution of their use and strategies for their production. Genes-Basel. 2021, 12, 936. [Google Scholar] [CrossRef]
- Mundo, J.; Villedahernández, J.; Herreraruiz, M. Neuro pharmacological and neuroprotective activities of some metabolites produced by cell suspension culture of waltheria americana linn. Biomed Pharmacother. 2017, 94, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Aboh, M.I.; Okhale, S.E.; Ibrahim, K. Preliminary studies on luffa cylindrica: comparative phytochemical and antimicrobial screening of the fresh and dried aerial parts. African Journal of Microbiology Research. 2012, 6, 3088–3091. [Google Scholar] [CrossRef]
- Habibi, P.; Daniell, H.; Soccol, C.R.; Grossi-de-Sa, M.F. The potential of plant systems to break the hiv-tb link. Plant Biotechnol J. 2019, 17, 1868–1891. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Sitarek, P.; Skala, E.; Rijo, P.; Andrade, J.M.; Synowiec, E.; Szemraj, J.; Krajewska, U.; Sliwinski, T. An evaluation of the dna-protective effects of extracts from menyanthes trifoliata l. Plants derived from in vitro culture associated with redox balance and other biological activities. Oxid Med Cell Longev. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Granica, S.; Piwowarski, J.P.; Czerwińska, M.E.; Kiss, A.K. Phytochemistry, pharmacology and traditional uses of different epilobium species (onagraceae): a review. J Ethnopharmacol. 2014, 156, 316–346. [Google Scholar] [CrossRef]
- Mohaddab, M.; El Goumi, Y.; Gallo, M.; Montesano, D.; Zengin, G.; Bouyahya, A.; Fakiri, M. Biotechnology and in vitro culture as an alternative system for secondary metabolite production. Molecules. 2022, 27, 8093. [Google Scholar] [CrossRef]
- Olson, M.E.; Sankaran, R.P.; Fahey, J.W.; Grusak, M.A.; Odee, D.; Nouman, W. Leaf protein and mineral concentrations across the "miracle tree" genus moringa. Plos One. 2016, 11, e159782. [Google Scholar] [CrossRef]
- Barbagallo, I.; Vanella, L.; Distefano, A.; Nicolosi, D.; Maravigna, A.; Lazzarino, G.; Di Rosa, M.; Tibullo, D.; Acquaviva, R.; Li Volti, G. Moringa oleifera lam. Improves lipid metabolism during adipogenic differentiation of human stem cells. Eur Rev Med Pharmaco. 2016, 20, 5223–5232. [Google Scholar]
- Sun, J.; Zeng, B.; Chen, Z.; Yan, S.; Huang, W.; Sun, B.; He, Q.; Chen, X.; Chen, T.; Jiang, Q. , et al. Characterization of faecal microbial communities of dairy cows fed diets containing ensiled moringa oleifera fodder. Sci Rep-Uk. 2017, 7, 41403. [Google Scholar] [CrossRef]
- Tuorkey, M.J. Effects of moringa oleifera aqueous leaf extract in alloxan induced diabetic mice. Interventional Medicine & Applied Science. 2016, 8, 109–117. [Google Scholar] [CrossRef]
- Liao, P.M.K.Y. Identification of beta-sitosterol as in vitro anti-inflammatory constituent in moringa oleifera. J Agr Food Chem. 2018, 66, 10748–10759. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, G.; Martorell, M.; Ramirez-Alarcon, K.; Salehi, B.; Sharifi-Rad, J. Phytochemical screening of moringa oleifera leaf extracts and their antimicrobial activities. Cell Mol Biol. 2020, 66, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Nwonuma, C.O.; Adelani-Akande, T.A.; Osemwegie, O.O.; Olaniran, A.F.; Adeyemo, T.A. Preliminary in vitro antimicrobial potential and phytochemicals study of some medical plants. F1000 Research. 2020, 8, 81. [Google Scholar] [CrossRef]
- Tian, Y.; Zeng, Y.; Zhang, J.; Yang, C.; Yan, L.; Wang, X.; Shi, C.; Xie, J.; Dai, T.; Peng, L. , et al. High quality reference genome of drumstick tree (moringa oleifera lam.), A potential perennial crop. Sci China Life Sci. 2015, 58, 627–638. [Google Scholar] [CrossRef]
- Yue, H.; QIin, R.; Gong, H.Y.; Song, X.Y.; Xiong, H.R.; Liu, H.; Yu, G.H. Suspension cell culture of moringa oleifera resource utilization. Biotic Resource. 2018, 40, 31–35. [Google Scholar] [CrossRef]
- Mustafa, R.; El-Naggar, E.M.B.; Svajdlenka, E.; Omran, G.; ELFiky, F.; El-Hawiet, A. Enhancement of phenolic content, antioxidant and cytotoxic activities of moringa oleifera leaf and seed by suspension culture. Nat Prod Res. 2021, 35, 5233–5237. [Google Scholar] [CrossRef]
- Jha, S.G.; Borowsky, A.T.; Cole, B.J.; Fahlgren, N.; Farmer, A.; Huang, S.; Karia, P.; Libault, M.; Provart, N.J.; Rice, S.L. , et al. Science forum: vision, challenges and opportunities for a plant cell atlas. Elife. 2021, 10, e66877. [Google Scholar] [CrossRef]
- Karki, U.; Fang, H.; Guo, W.; Unnold-Cofre, C.; Xu, J. Cellular engineering of plant cells for improved therapeutic protein production. Plant Cell Rep. 2021, 40, 1087–1099. [Google Scholar] [CrossRef]
- Bajwa, M.N.; Khanum, M.; Zaman, G.; Ullah, M.A.; Farooq, U.; Waqas, M.; Ahmad, N.; Hano, C.; Abbasi, B.H. Effect of wide-spectrum monochromatic lights on growth, phytochemistry, nutraceuticals, and antioxidant potential of in vitro callus cultures of moringa oleifera. Molecules. 2023, 28. [Google Scholar] [CrossRef] [PubMed]
- Suenaga; Leiko. Basic studies on transfer of cytoplasmic male sterility by means of cytoplasmic hybridization in carrot : (daucus carota l. ). J. Fac. Agric. Hokkaido Univ. 1991, 65, 62–118.
- Chadipiralla, K.; Gayathri, P.; Rajani, V.; Reddy, P.V.B. Plant tissue culture and crop improvement. Sustainable agriculture in the era of climate change; Roychowdhury, R., Choudhury, S., Hasanuzzaman, M., Srivastava, S., Eds.; Springer: Cham: Switzerland, 2020; pp. 391–412. [Google Scholar]
- Helgeson, H.C.; Knox, A.M.; Owens, C.E.; Shock, E.L. Petroleum, oil field waters, and authigenic mineral assemblages are they in metastable equilibrium in hydrocarbon reservoirs. Geochim Cosmochim Ac. 1993, 57, 3295–3339. [Google Scholar] [CrossRef]
- Holmes, M. Somatic hybridization: the rise and fall of a mid-twentieth-century biotechnology. Hist Stud Nat Sci. 2018, 48, 1–23. [Google Scholar] [CrossRef]
- Takebe, I.; Labib, G.; Melchers, G. Regeneration of whole plants from isolated mesophyll protoplasts of tobacco. Sci Nat-Heidelberg. 1971, 58, 318–320. [Google Scholar] [CrossRef]
- Carlson, P.S.; Smith, H.H.; Dearing, R.D. Parasexual interspecific plant hybridization. P Natl Acad Sci Usa. 1972, 69, 2292–2294. [Google Scholar] [CrossRef]
- Fish, N.; Karp, A.; Jones, M. Production of somatic hybrids by electrofusion in solanum. Theor Appl Genet. 1988, 76, 260–266. [Google Scholar] [CrossRef]
- Mattheij, W.M.; Puite, K.J. Tetraploid potato hybrids through protoplast fusions and analysis of their performance in the field. Theor Appl Genet. 1992, 83, 807–812. [Google Scholar] [CrossRef]
- Wallin, A.; Glimelius, K.; Eriksson, T. The induction of aggregation and fusion of dancus carota protoplasts by polyethylene glycol. Zeitschrift Fü Pflanzenphysiologie. 1974, 74, 64–80. [Google Scholar] [CrossRef]
- Puite, K.J.; Roest, S.; Punacker, L.P. Somatic hybrid potato plants after electrofusion of diploid solanum-tuberosum and solanum-phureja. Plant Cell Rep. 1986, 5, 262–265. [Google Scholar] [CrossRef]
- Kiran, K.M.; Sandeep, B.V.; Sudhakar, R.P. Development of salt tolerant callus cultures by somatic hybridization between oryza sativa and mangrove grass myriostachya wightiana. Annals of Agrarian Ence. 2018, 16, 396–404. [Google Scholar] [CrossRef]
- Glemin, S.; Scornavacca, C.; Dainat, J.; Burgarella, C.; Viader, V.; Ardisson, M.; Sarah, G.; Santoni, S.; David, J.; Ranwez, V. Pervasive hybridizations in the history of wheat relatives. Sci Adv. 2019, 5. [Google Scholar] [CrossRef]
- Xu, W.; Li, Y.; Li, Y.; Liu, C.; Wang, Y.; Xia, G.; Wang, M. Asymmetric somatic hybridization affects synonymous codon usage bias in wheat. Front Genet. 2021, 12. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, X.; Huang, Y.; Song, J.; Shi, T.; Li, Y.; Tang, X.; Yi, Y.; Li, F. Distant somatic hybridization alters the structure of wheat root bacterial microbiota. Agron J. 2022, 114, 1952–1962. [Google Scholar] [CrossRef]
- Das, D.S.; Barakate, A.; Stephens, J.; Caliskan, M.E.; Bakhsh, A. Genome editing of potato using crispr technologies: current development and future prospective. Plant Cell Tiss Org. 2019, 139, 403–416. [Google Scholar] [CrossRef]
- Hameed, A.; Zaidi, S.S.; Shakir, S.; Mansoor, S. Applications of new breeding technologies for potato improvement. Front Plant Sci. 2018, 9, 925. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, J.E.; Bryan, G.J.; Ramsay, G. Genetic resources (including wild and cultivated solanum species) and progress in their utilisation in potato breeding. Potato Res. 2006, 49, 49–65. [Google Scholar] [CrossRef]
- Spooner, D.M.; Salas, A. Structure, biosystematics, and genetic resources. Handbook of Potato Production Improvement & Postharvest Management. 2006. [CrossRef]
- Jones, H.; Karp, A.; Jones, M.G.K. Isolation, culture, and regeneration of plants from potato protoplasts. Plant Cell Rep. 1989, 8, 307–311. [Google Scholar] [CrossRef]
- Sadia, B. Improved isolation and culture of protoplasts from s. Chacoense and potato: morphological and cytological evaluation of protoplast-derived regenerants of potato cv. Desiree. Pak J Agr Sci. 2015, 52, 51–61. [Google Scholar]
- Dai, C.; Mertz, D.; Lambeth, V. Improved procedures for the isolation and culture of potato protoplasts. Plant Sci. 1987, 50, 79–84. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, L.; Liang, Y.; Lu, X.; Zhang, F. Isolation and culture of pollen tetrad protoplasts from solanum tuberosum. Am J Potato Res. 2017, 417–424. [Google Scholar] [CrossRef]
- Rakosy-Tican, E.; Aurori, A. Green fluorescent protein (gfp) supports the selection based on callus vigorous growth in the somatic hybrids solanum tuberosum l. Plus s. Chacoense bitt. Acta Physiol Plant. 2015, 37, 201. [Google Scholar] [CrossRef]
- Luthra, S.K.; Tiwari, J.K.; Kumar, V.; Lal, M. Evaluation of interspecific somatic hybrids of potato (solanum tuberosum) and wild s. Cardiophyllum for adaptability, tuber dry matter, keeping quality and late blight resistance. Agr Res. 2019, 8, 158–164. [Google Scholar] [CrossRef]
- Smyda-Dajmund, P.; Sliwka, J.; Wasilewicz-Flis, I.; Jakuczun, H.; Zimnoch-Guzowska, E. Bc1 and f-1 progeny from solanum x michoacanum (+) s-tuberosum somatic hybrids, autofused 4x s-michoacanum and cultivated potato. Am J Potato Res. 2017, 94, 323–333. [Google Scholar] [CrossRef]
- Orczyk, W.; Przetakiewicz, J.; Nadolska-Orczyk, A. Somatic hybrids of solanum tuberosum - application to genetics and breeding. Plant Cell Tiss Org. 2003, 74, 1–13. [Google Scholar] [CrossRef]
- Tiwari, J.K.; Devi, S.; Ali, N.; Luthra, S.K.; Kumar, V.; Bhardwaj, V.; Singh, R.K.; Rawat, S.; Chakrabarti, S.K. Progress in somatic hybridization research in potato during the past 40 years. Plant Cell Tiss Org. 2018, 132, 225–238. [Google Scholar] [CrossRef]
- Belete, T. A review on somatic hybridization and its utilization in crop improvement. International Journal of African and Asian Studies. 2018, 43, 24–34. [Google Scholar]
- Wang, Y.; Yang, F.; Liu, T.; Zhao, C.; Gu, F.; Du, H.; Wang, F.; Zheng, J.; Xiao, H. Carotenoid fates in plant foods: chemical changes from farm to table and nutrition. Crit Rev Food Sci. 2022, 11–19. [Google Scholar] [CrossRef]
- Shi, L.C.; Chang, L.; Yu, Y.J.; Zhang, D.S.; Zhao, X.Y.; Wang, W.H.; Li, P.R.; Xin, X.Y.; Zhang, F.L.; Yu, S.C. , et al. Recent advancements and biotechnological implications of carotenoid metabolism of brassica. Plants-Basel 2023, 12. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Germaine, K.; Bourke, P.; Malone, R. Genetic diversity and population structure of brassica oleracea germplasm in ireland using ssr markers. Cr Biol. 2016, 339, 133–140. [Google Scholar] [CrossRef]
- Wang, G.; Lv, J.; Zhang, J.; Han, S.; Zong, M.; Guo, N.; Zeng, X.; Zhang, Y.; Wang, Y.; Liu, F. Genetic and epigenetic alterations of brassica nigra introgression lines from somatic hybridization: a resource for cauliflower improvement. Front Plant Sci. 2016, 7, 1258. [Google Scholar] [CrossRef]
- Kumari, P.; Bisht, D.S.; Bhat, S.R. Stable, fertile somatic hybrids between sinapis alba and brassica juncea show resistance to alternaria brassicae and heat stress. Plant Cell Tiss Org. 2018, 133, 77–86. [Google Scholar] [CrossRef]
- Lian, Y.; Lin, G.; Zheng, Q. Tri-parental protoplast fusion of brassica species to produce somatic hybrids with high genetic and phenotypic variability. Indian J Genet Pl Br. 2015, 75, 497–505. [Google Scholar] [CrossRef]
- Ranaware, A.S.; Kunchge, N.S.; Lele, S.S.; Ochatt, S.J. Protoplast technology and somatic hybridisation in the family apiaceae. Plants-Basel. 2023, 12, 1060. [Google Scholar] [CrossRef] [PubMed]
- Gieniec, M.; Siwek, J.; Oleszkiewicz, T.; Mackowska, K.; Klimek-Chodacka, M.; Grzebelus, E.; Baranski, R. Real-time detection of somatic hybrid cells during electrofusion of carrot protoplasts with stably labelled mitochondria. Sci Rep-Uk. 2020, 10, 18811. [Google Scholar] [CrossRef]
- Klimek-Chodacka, M.; Oleszkiewicz, T.; Lowder, L.G.; Qi, Y.; Baranski, R. Efficient crispr/cas9-based genome editing in carrot cells. Plant Cell Rep. 2018, 37, 575–586. [Google Scholar] [CrossRef]
- Lenaghan, S.C.; Stewart, C.N. An automated protoplast transformation system. Methods in Molecular Biology (Clifton, N.J.). 2019, 1917, 355–363. [Google Scholar] [CrossRef]
- Gao, S.; Chen, X.; Yu, Z.; Du, R.; Chen, B.; Wang, Y.; Cai, X.; Xu, J.; Chen, J.; Duan, H. , et al. Progress of research on the role of active ingredients of citri reticulatae pericarpium in liver injury. Phytomedicine. 2023, 115, 154836. [Google Scholar] [CrossRef]
- Pant, P.; Pandey, S.; Dall'Acqua, S. The influence of environmental conditions on secondary metabolites in medicinal plants: a literature review. Chem Biodivers. 2021, 18, e210035. [Google Scholar] [CrossRef]
- Lv, W.; Lin, T.; Ren, Z.; Jiang, Y.; Zhang, J.; Bi, F.; Gu, L.; Hou, H.; He, J. Rapid discrimination of citrus reticulata 'chachi' by headspace-gas chromatography-ion mobility spectrometry fingerprints combined with principal component analysis. Food Res Int. 2020, 131, 108985. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Chen, B.; Hou, Y.; Wen, Y.; Gan, L.; Jin, J.; Li, C.; Wu, P.; Li, D. , et al. Ultrasonic assisted extraction, characterization and gut microbiota-dependent anti-obesity effect of polysaccharide from pericarpium citri reticulatae ?chachiensis? Ultrason Sonochem. 2023, 95, 106383. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant secondary metabolites as defenses, regulators, and primary metabolites: the blurred functional trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zeng, X.; Peng, W.; Wu, Z.; Su, W. Study on the discrimination between citri reticulatae pericarpium varieties based on hs-spme-gc-ms combined with multivariate statistical analyses. Molecules. 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Qiu, Z.; Chen, Y.; Song, Y.; Zhou, A.; Cao, Y.; Xiao, J.; Xiao, H.; Song, M. Review of recent advances on health benefits, microbial transformations, and authenticity identification of citri reticulatae pericarpium bioactive compounds. Crit Rev Food Sci. 2023, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Xiao, S.; Deng, X. Somatic cybrid production via protoplast fusion for citrus improvement. Sci Hortic-Amsterdam. 2013, 163, 20–26. [Google Scholar] [CrossRef]
- Chetto, O.; Benyahia, H.; Barantin, P.; Ollitrault, P.; Dambier, D. Production and molecular characterization of new citrus hybrids using somatic hybridization coupled with nuclear and chloroplastic microsatellite markers. Acta Horticulturae. 2018, 1230, 15–24. [Google Scholar] [CrossRef]
- Hasan; Basri; Jumin. Somatic hybridization of cells between citrus and murraya paniculata by electro-fusion. Ecology, Environment and Conservation. 2018, 24, 145–152.
- Dambier, D.; Barantin, P.; Boulard, G.; Costantino, G.; Mournet, P.; Perdereau, A.; Morillon, R.; Ollitrault, P. Genomic instability in somatic hybridization between poncirus and citrus species aiming to create new rootstocks. Agriculture-Basel. 2022, 12, 134. [Google Scholar] [CrossRef]
- Khalid, M.F.; Hussain, S.; Anjum, M.A.; Morillon, R.; Ahmad, S.; Ejaz, S.; Hussain, M.; Jaafar, H.Z.E.; Alrashood, S.T.; Ormenisan, A.N. Physiological and biochemical responses of kinnow mandarin grafted on diploid and tetraploid volkamer lemon rootstocks under different water-deficit regimes. Plos One. 2021, 16, e277636. [Google Scholar] [CrossRef]
- Tian, S.; Xing, S.; Xu, Y. Advances in crispr/cas9-mediated genome editing on vegetable crops. In Vitro Cell Dev-Pl. 2021, 57, 672–682. [Google Scholar] [CrossRef]
- Comai, L. The advantages and disadvantages of being polyploid. Nat Rev Genet. 2005, 6, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.G.; Tsai, K.; Chung, H.; Chen, J. Chromosome doubling-enhanced biomass and dihydrotanshinone i production in salvia miltiorrhiza, a traditional chinese medicinal plant. Molecules. 2018, 23, 3106. [Google Scholar] [CrossRef] [PubMed]
- Sattler, M.C.; Carvalho, C.R.; Clarindo, W.R. The polyploidy and its key role in plant breeding. Planta. 2016, 243, 281–296. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Q.; Leng, L.; Zhang, D.; Chen, S.; Shi, Y.; Ning, Z.; Chen, S. Incipient diploidization of the medicinal plant perilla within 10,000 years. Nat Commun. 2021, 12. [Google Scholar] [CrossRef]
- Liu, S.; Chen, S.; Chen, Y.; Guan, Z.; Yin, D.; Chen, F. In vitro induced tetraploid of dendranthema nankingense (nakai) tzvel. Shows an improved level of abiotic stress tolerance. Sci Hortic-Amsterdam. 2011, 127, 411–419. [Google Scholar] [CrossRef]
- Li, M.; Guo, Y.; Liu, S.; Zhao, Y.; Pang, X.; Li, Y. Autotetraploidization in ziziphus jujuba mill. Var. Spinosa enhances salt tolerance conferred by active, diverse stress responses. Environ Exp Bot. 2019, 165, 92–107. [Google Scholar] [CrossRef]
- Parsons, J.L.; Martin, S.L.; James, T.; Golenia, G.; Boudko, E.A.; Hepworth, S.R. Polyploidization for the genetic improvement of cannabis sativa. Front Plant Sci. 2019, 10, 476. [Google Scholar] [CrossRef] [PubMed]
- Jana, E.; Marcela, M.; Pavla, Z.; Eloy, F.C.; Daniel, Z. Identification of phytophthora tolerance in the anemone sylvestris tetraploid. Sci Hortic-Amsterdam. 2019, 256, 108579. [Google Scholar] [CrossRef]
- Ravandi, E.G.; Rezanejad, F.; Zolala, J.; Dehghan, E. The effects of chromosome-doubling on selected morphological and phytochemical characteristics of cichorium intybus l. J Hortic Sci Biotech. 2013, 88, 701–709. [Google Scholar] [CrossRef]
- Svehlikova, V.; Repcak, M. Variation of apigenin quantity in diploid and tetraploid chamomilla recutita (l. ) Rauschert. Plant Biology. 2000, 2, 403–407. [Google Scholar] [CrossRef]
- Abdoli, M.; Moieni, A.; Naghdi Badi, H. Morphological, physiological, cytological and phytochemical studies in diploid and colchicine-induced tetraploid plants of echinaceapurpurea (l. ). Acta Physiol Plant. 2013, 35, 2075–2083. [Google Scholar] [CrossRef]
- Ravandi, E.G.; Rezanejad, F.; Dehghan, E. In vitro regeneration ability of diploid and autotetraploid plants of cichorium intybus l. Cytol Genet+. 2014, 48, 166–170. [Google Scholar] [CrossRef]
- Lin, X.; Zhou, Y.; Zhang, J.; Lu, X.; Zhang, F.; Shen, Q.; Wu, S.; Chen, Y.; Wang, T.; Tang, K. Enhancement of artemisinin content in tetraploid artemisia annua plants by modulating the expression of genes in artemisinin biosynthetic pathway. Biotechnol Appl Bioc. 2011, 58, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.L.; Chen, B.J.; Zhu, D.N. In vitro production and identification of autotetraploids of scutellaria baicalensis. Plant Cell Tiss Org. 2002, 70, 289–293. [Google Scholar] [CrossRef]
- Huang, R.; Liu, D.; Zhao, M.; Li, Z.; Li, M.; Sue, S. Artificially induced polyploidization in lobularia maritima (l. ) Desv. And its effect on morphological traits. Hortscience. 2015, 50, 636–639. [Google Scholar] [CrossRef]
- Sabzehzari, M.; Hoveidamanesh, S.; Modarresi, M.; Mohammadi, V. Morphological, anatomical, physiological, and cytological studies in diploid and tetraploid plants of plantago psyllium. Plant Cell Tiss Org. 2019, 139, 131–137. [Google Scholar] [CrossRef]
- Sabzehzari, M.; Hoveidamanesh, S.; Modarresi, M.; Mohammadi, V. Morphological, anatomical, physiological, and cytological studies in diploid and tetraploid plants of ispaghul (plantago ovata forsk. ). Genet Resour Crop Ev. 2020, 67, 129–137. [Google Scholar] [CrossRef]
- Das, G.G.; Malek, M.A.; Shamsuddin, A.; Sagor, G. Production of synthetic brassica napus through interspecific hybridization between brassica rapa and brassica oleracea and their cross-ability evaluation. Plant Breeding and Biotechnology. 2021, 9, 171–184. [Google Scholar] [CrossRef]
- Chen, J.; Guo, L.; Yang, G.; Yang, A.; Zheng, Y.; Wang, L. Metabolomic profiling of developing perilla leaves reveals the best harvest time. Front Plant Sci. 2022, 13. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, D.; Wang, Q.; Yang, A.; Zheng, Y.; Wang, L. Comprehensive comparison of two color varieties of perillae folium by gc-ms-based metabolomic approach. Molecules. 2022, 27, 6792. [Google Scholar] [CrossRef]
- Wang, Z.; Tu, Z.; Xie, X.; Cui, H.; Kong, K.W.; Zhang, L. Perilla frutescens leaf extract and fractions: polyphenol composition, antioxidant, enzymes (alpha-glucosidase, acetylcholinesterase, and tyrosinase) inhibitory, anticancer, and antidiabetic activities. Foods. 2021, 10. [Google Scholar] [CrossRef]
- Dhawan, O.P.; Lavania, U.C. Enhancing the productivity of secondary metabolites via induced polyploidy: a review. Euphytica. 1996, 87, 81–89. [Google Scholar] [CrossRef]
- Bhuvaneswari, G.; Thirugnanasampandan, R.; Gogulramnath, M. Effect of colchicine induced tetraploidy on morphology, cytology, essential oil composition, gene expression and antioxidant activity of citrus limon (l. ) Osbeck. Physiol Mol Biol Pla. 2020, 26, 271–279. [Google Scholar] [CrossRef]
- Hannweg, K.; Visser, G.; de Jager, K.; Bertling, I. In vitro-induced polyploidy and its effect on horticultural characteristics, essential oil composition and bioactivity of tetradenia riparia. S Afr J Bot. 2016, 106, 186–191. [Google Scholar] [CrossRef]
- Noori, S.A.S.; Norouzi, M.; Karimzadeh, G.; Shirkool, K.; Niazian, M. Effect of colchicine-induced polyploidy on morphological characteristics and essential oil composition of ajowan (trachyspermum ammi l. ). Plant Cell Tiss Org. 2017, 130, 543–551. [Google Scholar] [CrossRef]
- Caruso, I.; Lepore, L.; De Tommasi, N.; Dal Piaz, F.; Frusciante, L.; Aversano, R.; Garramone, R.; Carputo, D. Secondary metabolite profile in induced tetraploids of wild solanum commersonii dun. Chem Biodivers. 2011, 8, 2226–2237. [Google Scholar] [CrossRef] [PubMed]
- Niazian, M.; Shariatpanahi, M.E. In vitro-based doubled haploid production: recent improvements. Euphytica. 2020, 216. [Google Scholar] [CrossRef]
- Niazian, M.; Nalousi, A.M. Artificial polyploidy induction for improvement of ornamental and medicinal plants. Plant Cell Tiss Org. 2020, 142, 447–469. [Google Scholar] [CrossRef]
- Madani, H.; Escrich, A.; Hosseini, B.; Sanchez-Munoz, R.; Khojasteh, A.; Palazon, J. Effect of polyploidy induction on natural metabolite production in medicinal plants. Biomolecules. 2021, 11. [Google Scholar] [CrossRef]
- Fu, L.; Zhu, Y.; Li, M.; Wang, C.; Sun, H. Autopolyploid induction via somatic embryogenesis in lilium distichum nakai and lilium cernuum komar. Plant Cell Tiss Org. 2019, 139, 237–248. [Google Scholar] [CrossRef]
- Adams, K.L.; Wendel, J.F. Novel patterns of gene expression in polyploid plants. Trends Genet. 2005, 21, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Borrill, P.; Harrington, S.A.; Uauy, C. Genome-wide sequence and expression analysis of the nac transcription factor family in polyploid wheat. G3-Genes Genom Genet. 2017, 7, 3019–3029. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ding, B.; Huang, W.; Pan, J.; Ding, Z.; Jiang, F. Induction and characterization of tetraploids from seeds of bletilla striata (thunb.) Reichb.f. Biomed Res Int. 2018, 2018. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).