1. Introduction
The global pandemic caused by SARS-CoV-2 persists since its recognition in December 2019. Due to continuously emerging immune escape variants, the necessity of booster vaccination of coronavirus disease 2019 (COVID-19) is apparent. Various technologies were used for vaccine delivery such as mRNA, DNA, inactivated, recombinant protein, and adenovirus-based vectors. The BBIBP-CorV (China Sinopharm Bio-Beijing Company) is an inactivated vaccine approved for registration and emergency use. A theoretical advantage of BBIBP-CorV is that unlike other popular vaccines carrying the spike (S) epitopes only, inactivated vaccines retain the integrity of the virus particle envelops, providing immune exposure to a wider range of epitopes. N protein, for example, shows cross-reactivity between coronaviruses and also can induce N-antibody responses in COVID-19 patients [
1,
2].
Neutralizing antibody responses against SARS-CoV-2 correlate with protection efficiency [3-5]. The waning of immunity after vaccination corresponds to the increasing risk of breakthrough infections of SARS-CoV-2. Emerging variants of concern (VOC) have included alpha (B.1.1.7), beta (B.1.351), gamma (P.1), delta (B.1.617 .2) and omicron (B.1.1.529) variants. Omicron has further rapidly evolved many subvariants (BA.1, BA.1.1, BA.2, BA.2.12.1, BA.4, BA.5, BF7, XBB etc.) over time and is the dominant circulating strain globally [
6,
7]. The higher immune escape capacity and greater transmissibility of the Omicron variants has greatly increased the number of breakthrough infections[8-11]. While the booster dose of vaccine can improve immunogenicity of the vaccine series, vaccine effectiveness against Omicron variant remains unclear.
In addition to the humoral immune response, the vaccine-induced cellular immunity is helpful in controlling viral infection. B cells promote the T cell differentiation into T follicular helper (Tfh) cells, improving humoral immune responses [
12,
13]. Specific T cell responses help control SARS-CoV-2 replication, reducing COVID-19 disease severity[
14].
How vaccine protection relates to in vivo antigen specific immunity remains unknown. After inactivated vaccination, the nature and differentiation state of antigen-specific memory and effective T and B cells remain to be elucidated. For example, it is still unclear whether the CD4+ and CD8+Tfh cells can be boosted and whether these cells correlate with memory B cells and neutralizing antibodies. It remains unknown, too, as to how long the subsets of memory cells last and how these cells contribute to long-term immunological memory and protective immunity. In this study, we sought to define the differentiation state of immune cells and address these questions following inactivated vaccine prime and boost in health-care workers who had received inactivated vaccines.
2. Results
2.1. A Longitudinal Cohort of Vaccinees Immunized by BBIBP-CorV
All 205 participating health-care workers had received three doses BBIBP-CorV, with a three weeks interval between the first and second doses vaccination, and an average of 274 days between the second and third vaccine doses. This cohort included 66 men and 139 women; 138 workers were under age 40 years and 67 workers were 40 or more years of age(
Table 1).
2.2. Robust Antibody Responses to SARS-CoV-2 Elicited by Booster Immunization
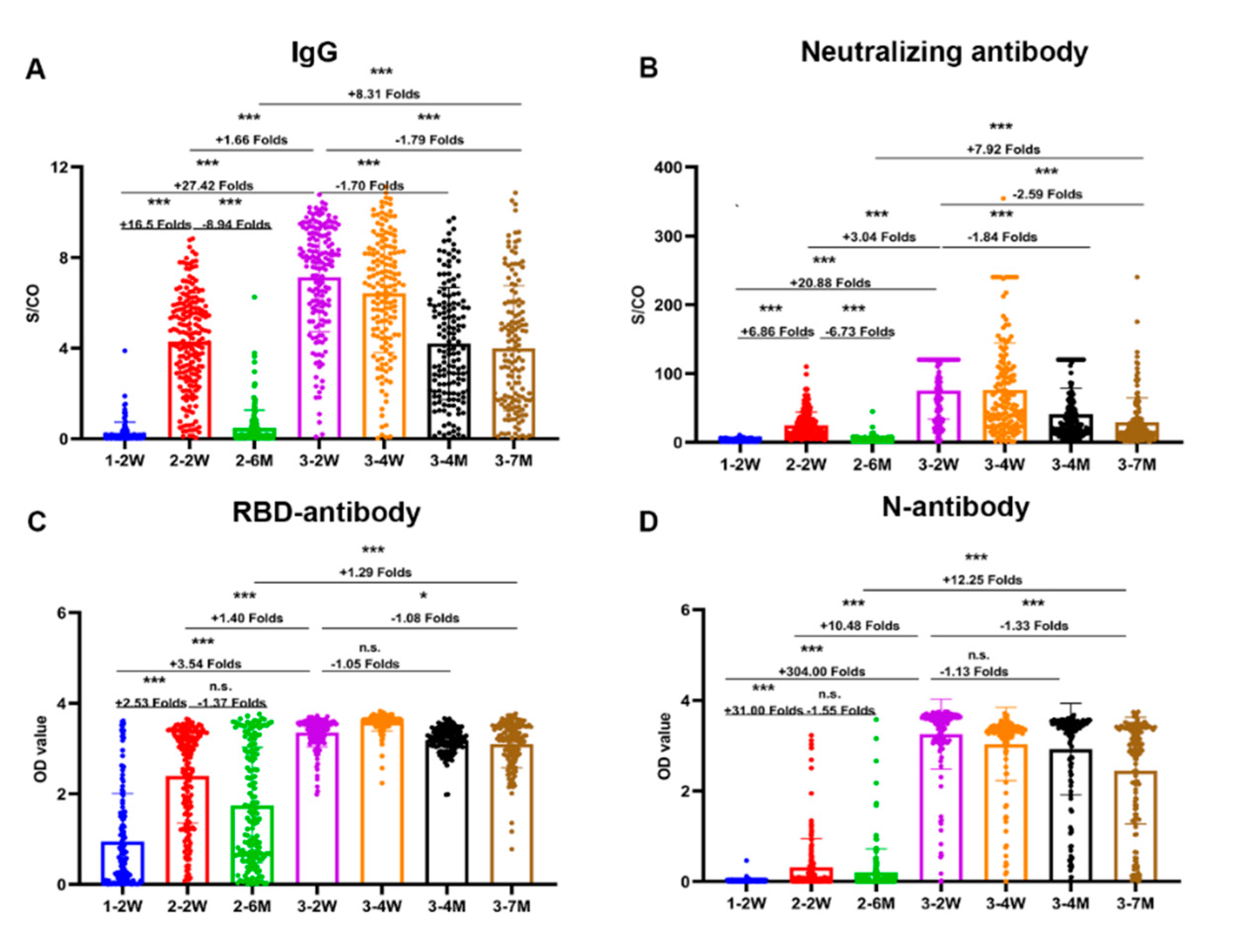
We detected the neutralizing antibody, total antibody, IgG and IgM by chemiluminescence immunoassay assays, nucleoprotein (N) antibody, and receptor-binding domain (RBD) antibody of SARS-CoV-2 by ELISA in plasma samples at all follow-up time points. The antibody magnitude and seropositivity of SARS-CoV-2 after vaccination are shown in
Figure 1 and
Table 2.
Both the peak and durable antibodies response induced by the inactivated vaccine booster dose were significantly stronger than that induced by the second dose. The descending speed of antibodies level after the third dose were also significantly slower than that of the second dose.
Unlike other vaccines, in addition to the potent spike antibody, N antibody response also can be induced by BBIBP-CorV vaccine. We found that the seropositivity of N antibody response was 31.3% after two doses of vaccine. After booster vaccination, the seropositivity of N antibody reached 98.8%, and remained at 92.4% after 7 months.
2.3. Higher, More Durable, and Broadly Neutralizing Antibodies Titers Induced by Booster Immunization
We found neutralizing antibody titers against the wild-type strain and omicron variants of SARS-CoV-2 both by pseudovirus and authentic viral neutralization assays in parallel comparison. The GMT of the neutralization titers to wildtype after third vaccination were about three times than those after second vaccination (
Figure 2). No neutralizing antibodies against omicron variant were induced after only two doses of vaccine. The seropositivity of neutralizing antibodies against omicron was 74%-86% at two weeks after third vaccination and 38%-44% at seven months after third vaccination.
The
Figure 2C shows the strong correlations between neutralization titers by pseudovirus and authentic viral neutralization assays. Consistently, data in
Figure 2D demonstrated positive correlations between neutralization titers against wildtype and omicron.
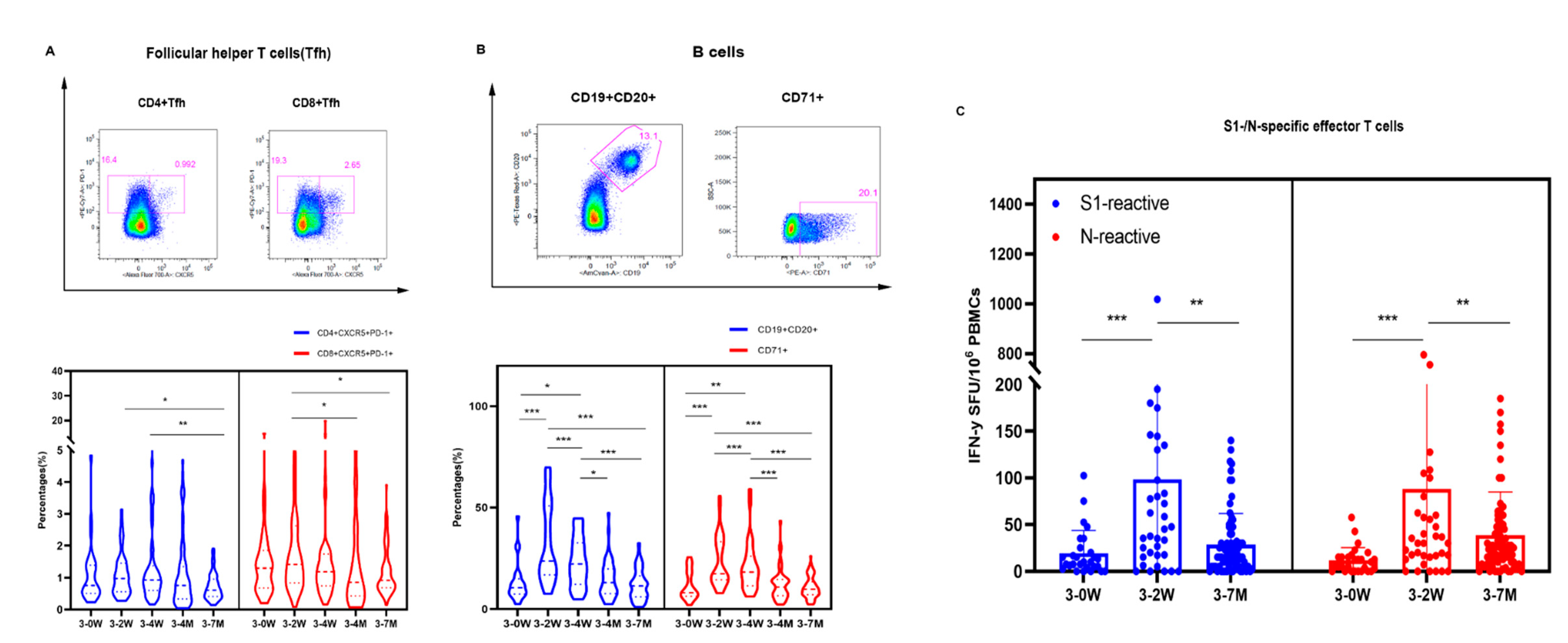
2.4. Specific Effector T Cells, Follicular Helper T Cells, and B Cell Immunity Induced by SARS-CoV-2 Boosters
The follicular helper T cells (Tfh) are involved in the humoral response by triggering the germinal center (GC) B cells to differentiate into antibody-secreting plasma cells and memory B cells. The phenotypic characteristics of Tfh cells were investigated using CXCR5
+PD-1
+ markers in this study. The median frequencies of Tfh cells (0.985% and 1.445% of CD4
+ and CD8
+ Tfh) peaked two weeks after booster vaccination with significant differences seen between groups (
Figure 3A). The frequency of CD19
+CD20
+ and CD71
+ were the highest at 2 weeks after vaccination, then decreased significantly at 4 months and 7 months after vaccination (
Figure 3B).
We performed enzyme-linked immunospot assay (ELISPOT) to detect IFN-γ levels on PBMC cells. The average number of S1-specific IFN-γ effector T cells were 24, 103, and 29 per million PBMCs, and the average number of N-specific IFN-γ effector T cells were 17, 86, and 40 per million PBMCs at the time points before booster vaccination, two weeks, and seven months after booster vaccination, respectively. In addition, both S1 and N-specific effector T cells at 2 weeks vaccination were significantly higher than those before and 7 months after booster immunization (
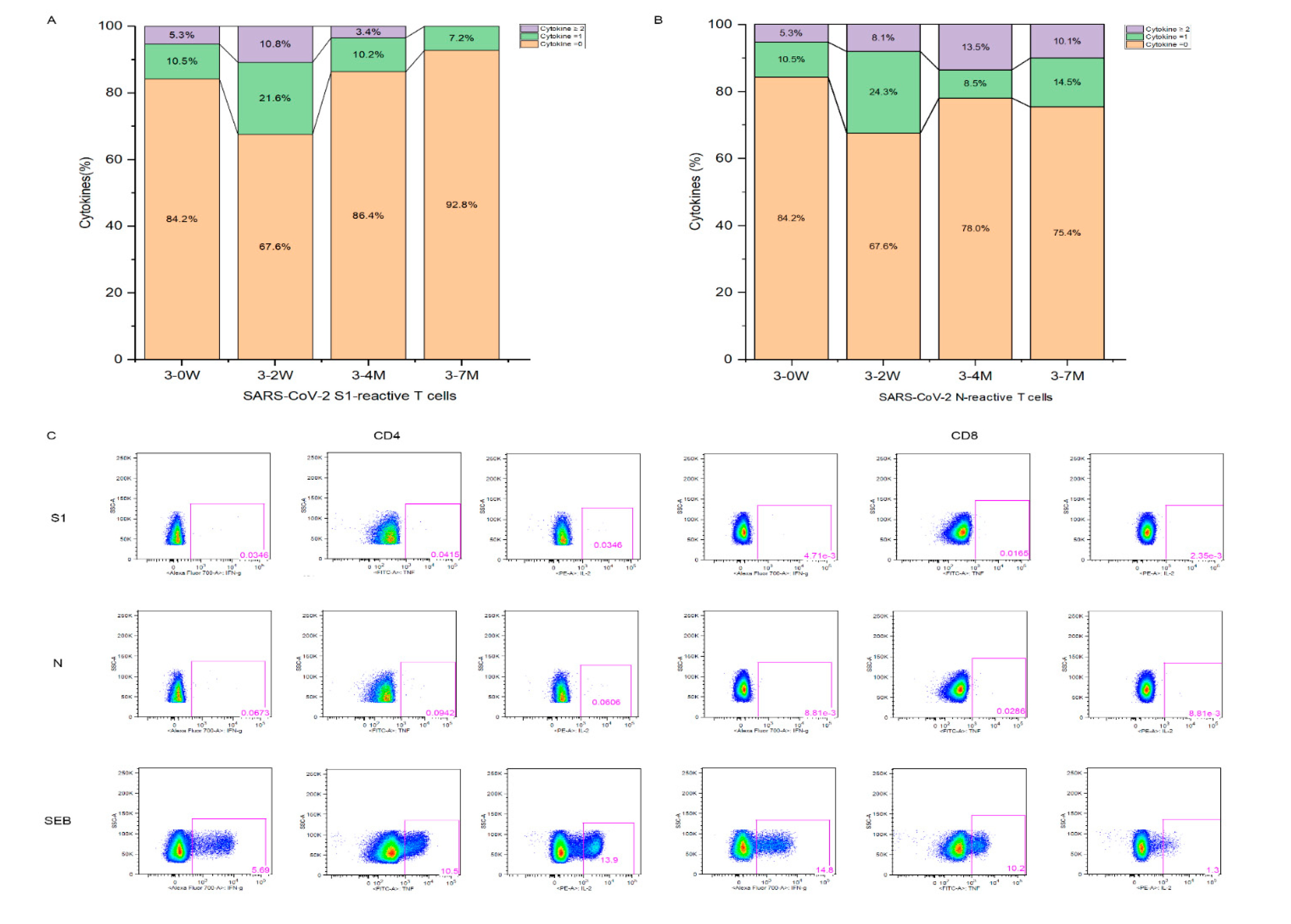
Figure 3C). At 2 weeks after vaccination, about one-third of health-care workers can elicit S1 and N specific effector T cells; only 7.2% and 24.6% population preserved S1 and N specific effector T cells at 7 months after vaccination, respectively (
Figure 4). Thus, Tfh, B cells and specific effector T cells peaked at 2 weeks or 4 weeks, and gradually decreased after booster vaccination.
2.5. Synergistic Effect of Antibody and Cellular Immunity after Vaccination
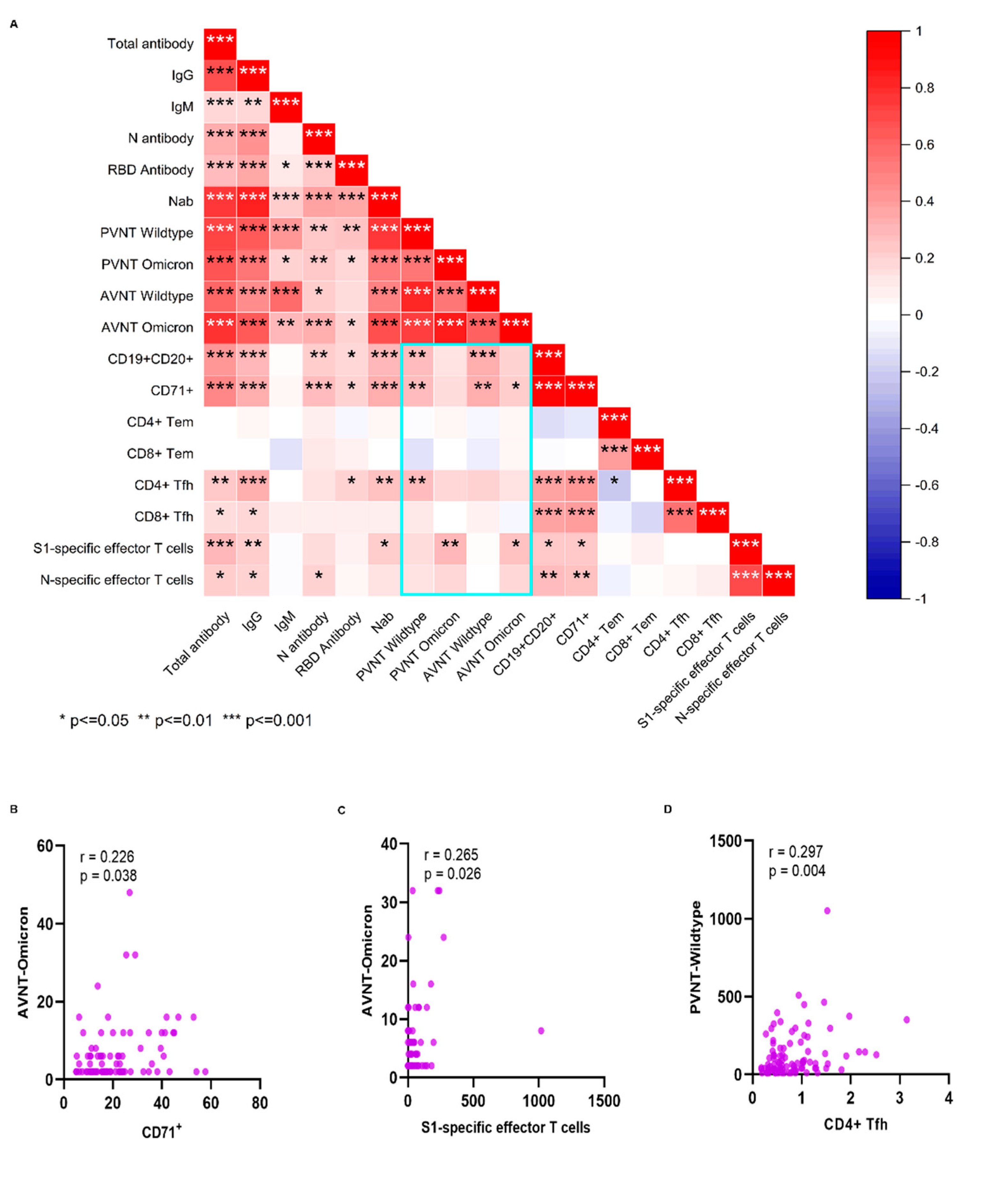
We further analyzed the association between antibodies and cellular response (
Figure 5A). Positive correlations were seen between CD19
+CD20
+ and CD71
+, between CD4
+ Tfh and CD8
+ Tfh, between the N-specific and S1-specific effector T cells, and between B cells and Tfh. CD19
+CD20
+, CD71
+ and CD4
+ Tfh cell subsets showed significant positive correlations with the neutralizing antibody to SARS-CoV-2 wildtype. Also, S1-specific T cells and CD71
+ showed significant positive correlations with the neutralizing antibodies to SARS-CoV-2 Omicron variant (
Figure 5B,C). CD4
+ Tfh cells showed significant positive correlations with the neutralizing antibody to SARS-CoV-2 (
Figure 5D). N antibody levels were also significant associated with neutralizing antibody and N- specific effector T cells.
3. Discussion
SARS-CoV-2 vaccine has significantly reduced the severity and mortality of COVID-19 [
5,
15]. However, due to the fading of the immune protection effect and the emergence of new variants, breakthrough symptomatic infection cases are common. Our longitudinal cohort followed 205 healthcare workers for 16 months, and 7 months after the third vaccine done (booster). Not only humoral antibodies and cellular immunity, also the short-term and durable immunity were examines. About 70% vaccinees retained neutralizing antibody titers against the omicron variant at 2 weeks and 40% at seven months post booster vaccine; no neutralizing antibody capacity against omicron variant detected after only the second dose of vaccine. The booster dose vaccination can induce more potent, durable, and broad antibody responses and neutralization capabilities than those elicited by the second dose of immunization, consistent with prior work [
11,
16].
We discovered N-specific antibody and T cell protective response induced by BBIBP-CorV boosting. The magnitude and seropositivity of N antibody were significant increased after the booster dose vaccine (rarely assessed in prior studies). TH1 or TH2 cell responses cannot be induced by inactivated vaccines in non-human primates and human individuals after only primary vaccination [
17,
18]. In contrast, we found that S1/N-specific effector T cell of SARS-CoV-2 were elicited after the booster vaccination. It is likely that weak specific T cell responses induced by the primary vaccination are enhanced substantially after the booster vaccine dose. Lower S-specific T cell immune responses are noted from inactivated vaccines than from mRNA vaccine. It is possible, however, that the multi-antigen CD4+ T cell response induced by inactivated vaccine may be more protective in improving disease severity [
19].
We found very consistent dynamics and correlations between cellular and humoral immune responses after the booster dose. These results suggest that cellular immunity can provide protective effect indirectly, consistent with previous studies [
20,
21]. The protective T cell response (CD4
+ and CD8
+ T cells producing interferon IFN-γ, commonly referred to as a “type 1” immune-response) against SARS-CoV-2 infection has been noted by others [22-24]. Ineffective IFN-γ, innate immunity has been associated with a failure to control a primary SARS-CoV-2 infection and a high risk of fatal COVID-19 [25-27]. Our findings suggest that antibody and cellular immunity may be demonstrating synergistic effects in the control of SARS-CoV-2 infection. Multi-antigen specific T cell immune responses have also been found in mild and asymptomatic SARS-CoV-2 infected patients, and the rapidly induced antigen-specific response represents the immune protection from structural protein specific T cells [
28,
29]. Compared with S-specific T cells, N-specific T cells induced by inactivated vaccinees are more durable in the face of SARS-CoV-2 newly emerging variants [
19].
The cohort study is a major strength of our study that deployed a wide swath of immunological response indicators. Several limitations are extant. First, while we have analyzed the specific T cells of SARS-CoV-2, specific B cell immunity against the wildtype and variants were not studied. Second, PBMCs were not collected and isolated at the earlier follow-up visits and the cellular immunity was not seen after the first and second doses of vaccine.
The second booster vaccine was implemented rapidly and widely in Israel and was deemed inadequate to prevent symptomatic omicron infection [
30,
31]. Immune responses induced by the secondary booster vaccine were reported to be no higher than those induced by the first booster dose [
32]. Immune attenuation of the booster vaccine was significant slower than that seen in the two-dose vaccine in this study [
32]. The frequency of the second booster vaccine should be based on the substantial and sustained data rather than on short-term immunity. Also, the cross immunity, vaccine supply worldwide should be taken into consideration.
We found that an inactivated vaccine booster dose (third dose) induced substantial potent, durable, and broad immunity, including N-specific immunity. Our data are compatible with a conclusion that humoral and cellular immunity may be synergistic in the control of SARS-CoV-2 infection. To reduce severity of infection, hospitalization, and death from SARS-CoV-2 infection, booster vaccination should be rigorously promoted and implemented.
4. Materials and Methods
4.1. Study Participants
We initiated a 16-month cohort study of health-care workers who had received immunization with BBIBP-CorV vaccine; 205 were enrolled after informed consent was obtained. All participants had been immunized with three doses of vaccines; the interval was 3 weeks between the first and second doses for nearly all participants. The third dose of vaccination was received an average of 274 days (range 146-291 days) after the two-dose vaccination. We drew blood from participants at these times: 2 weeks after first BBIBP-CorV immunization; 2 weeks and 6 months after the second immunization, and; 2 and 4 weeks, and 4 and 7 months after the third immunization (homologous booster). The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Zhejiang Hospital (Reference Number 2021-30K-X1). Written informed consent was obtained from all participants.
4.2. Enzyme-Linked Immunosorbent Assay (ELISA)
We described methodological details previously [
33]. We measured anti-RBD and anti-N antibodies of SARS-CoV-2 using SARS-CoV-2 RBD/N ELISA kits in accordance with manufacturers’ instructions (Wantai Biological Pharmacy, China).
4.3. Chemiluminescent Microparticle Immunoassay (CMIA)
The COVID-19 Abs (neutralizing antibodies, total antibody, anti-IgG, anti-RBD and anti-N) detection kits (Maccura Biosystem Co.) were based on chemiluminescent microparticle immunoassay (CMIA). We mixed 10 µL plasma sample, 50 µL magnetic beads, and 50 µL buffer, incubating for 10 min in a reaction cup. We then added 100 µL aridine ester-labelled marker to resuspended magnetic beads that were incubated for 10 min and then washed. We determined the luminescence signal value after adding substrate solution using a matched automatic chemiluminescence immunoassay analyzer. We calculated the concentrations of neutralizing antibodies according to the standard calibration curve; values >6 AU/mL were considered positive. We presented results of total antibody, IgG and IgM as the S/CO (absorbance of sample/cutoff of calibration); S/CO <1 was considered positive and S/CO ≥1 negative.
4.4. Pseudovirus Neutralization Test (PVNT)
We generated the pseudovirus by co-transfection of HEK 293 T cells with pcDNA3.1-S-COVID19 and pNL4-3Luc, which carry the optimized spike (S) gene and a human immunodeficiency virus type 1 (HIV) backbone, respectively. We added 150 µL serial dilutions of human sera (4 serial 3-fold dilutions in Dulbecco's minimum essential medium (DMEM) with an initial dilution 1:20) into 96-well plates. We then added 50 µL pseudoviruses of SARS-CoV-2 with concentration of 1300 TCID50/ml to the plates, incubating them at 37℃ for 1 hour. We added Hu-h7 cells to the plates (1.5×104 cells/100 uL cells per well), incubating them at 37℃ in a humidified atmosphere with 5% CO2. We performed hemiluminescence detection after 48 hours incubation. The Reed-Muench method was used to calculate the virus neutralization titers. The result reported as half maximal inhibitory concentration of PVNT (PVNT50).
4.5. Authentic Viral Neutralization Test (AVNT)
The authentic viral neutralization test of SARS-CoV-2 was performed as detailed previously [
34]. In brief, we measured the neutralizing antibody titers against the wild-type strain and the variants (Beta B.1.1.7, Gamma P.1 and Delta B.1.617) in serum by using a cytopathic effect-based microneutralization assay in Vero cells (National Collection of Authenticated Cell Cultures, National Academy of Science, China). We then mixed serum with the same volume of viral solution to achieve a final concentration of 100 TCID50 per well. The reported titer was the reciprocal of the highest sample dilution that protected at least 50% of cells from cytopathic effects. Serum dilution for the neutralization assay started from 1:4, and seropositivity was defined as titer ≥1:4.
4.6. Flow Cytometry
To measure antigen-specific effector T cells, we performed intracellular staining (ICS) using 15-mers S1 and N peptide pools (overlapping by 11 amino acids) from SARS-CoV-2 strain (Bio-scientific Co., Shanghai, China). We stimulated peripheral blood mononuclear cells (PBMCs) isolated from the subjects with S1 and N peptide pools (2 μg/ml) in the presence of Brefeldin A (Sigma) for 6 h. We used dimethyl sulfoxide (DMSO, Sigma) as a negative control for peptides. As a positive control, cells were stimulated with Staphylococcal enterotoxin B(SEB) from staphy. After stimulation, we washed cells with phosphate-buffered saline (PBS) and stained them with an ultraviolet-excitable, amine-reactive viability dye (LIVE/DEAD, Invitrogen) to exclude dead cells. After 20 minutes of incubation, we further washed the PBMCs with PBS and stained them with anti-CD4-PE-CF594 (RPA-T4), anti-CD8-Pacific Blue (RPA-T8) and anti-CCR5-BB700 (RF8B2), anti-CD56-BV650 (HCD56), anti-CCR7 (G043H7), anti-CD45RA-APC (HI100), anti-CD19-BV510 (SJ25C1), and anti-PD-1-BV605 (EH12.2H7). After an additional 20 minutes of incubation, we again washed the PBMCs with PBS, fixed them with 1*BD FACS lysing (BD) for 10 minutes, and ruptured their cytomembranes with 0.25% saponin. We then stained the PBMCs with anti-CD3-BV570 (UCHT1), anti-CD154-PE-Cy7 (24-31), anti-IFN-γ-A700 (B27), anti-TNF-α-FITC (MAb11), and anti-IL-2-PE (MQ1-17H12) for 30 minutes. The Spike/N-stimulated group subtract negative control data (> 0.05% for CD4+ T cells and CD8+ T cells) were defined as the specific T cells.
To measure the B cells, follicular helper T cells, and effector memory T cells, we stained PBMCs with anti-CD4-BV786 (SK3), anti-CD8-APC (RPA-T8), anti-CD14-BV421 (MφP9), anti-CD20-PE-CF594 (2H7), anti-IgG-BV605 (G18-145), anti-CD95-FITC (DX2), anti-CD71-PE (M-A712), anti-IgM-PercpCy5.5 (G20-127), anti-CD27-APC-Cy7 (M-T271), anti-CXCR5-APC-R700 (RF8B2), anti-CXCR3-PECy5 (1C6), anti-CD45RA-BV650 (HI100), anti-CD19-BV510 (SJ25C1), anti-CD3-BV570 (UCHT1), and anti-PD-1-PECy7 (eBioJ105). We obtained flow cytometry data using a Fortessa LSR flow cytometer (LSRFortessaTM, BD) and performed data analysis using FlowJoTM software (Tree Star).
4.7. ELISPOT Assay
We ran IFN-γ ELISpot assays according to the manufacturer’s instructions (BD, Cat. 349202). We incubated 0.2 million PBMCs per test with SARS-CoV-2 S1 and N peptide pools (2 μg/mL) at a final concentration of 2 ug/mL for 18h. We used 1640 cell medium (containing 10% FBS+1% Penicillin-Streptomycin) as a negative control and used Staphylococcal enterotoxin B(SEB) from staphy as a positive control. To quantify antigen-specific responses, we subtracted mean spots of the DMSO control wells from the peptide-stimulated wells, and expressed the results as spot-forming units (SFU) per 106 PBMCs. We considered results >20 SFU/106 PBMCs following control subtraction as positive.
4.8. Statistical Analysis
Data and statistical analyses were performed in Prism (version 8.0.2), Origin2021b (version 9.8.5) and SPSS software (version 23.0), unless otherwise stated. Two-tailed, nonparametric Mann-Whitney U test and the Kruskal-Wallis test were performed on numerical data. P-values <0.05 were considered as statistically significant.
Author Contributions
Conceptualization, Ying Chen, Caiqin Hu, Dan Li, Hong Wang, Biao Zhu and Yiming Shao; Funding acquisition, Dan Li, Hong Wang, Biao Zhu and Yiming Shao; Methodology, Caiqin Hu, Ying Chen, Zheng Wang, Shuo Wang, Bin Li, Xiang Liu and Zhenzhen Yuan; Resources, Ying Chen, Junwei Su, Hong Wang and Biao Zhu and Yiming Shao; Software, Caiqin Hu, Ying Chen, Zheng Wang and Zhenzhen Yuan; Writing – original draft, Caiqin Hu and Ying Chen; Writing – review & editing, Caiqin Hu, Ying Chen, Zheng Wang, Dan Li, Hong Wang, Biao Zhu and Yiming Shao. I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part. All the authors listed have approved the manuscript that is enclosed. Then, I briefly outlined the significant findings reported and reasons in this manuscript.
Funding
This research was funded by the National Natural Science Foundation of China under Grant 20A20362; and Beijing Municipal Natural Science Foundation (M21015).
Institutional Review Board Statement
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Zhejiang Hospital (Reference Number 2021-30K-X1).
Informed Consent Statement
Written informed consent has been obtained from all patients, their parents, or legal guardians.
Data Availability Statement
The datasets and materials generated during and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Acknowledgments
The authors thank to all study participants.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
References
- Van Elslande, J.; Decru, B.; Jonckheere, S.; Van Wijngaerden, E.; Houben, E.; Vandecandelaere, P.; Indevuyst, C.; Depypere, M.; Desmet, S.; Andre, E.; et al. Antibody response against SARS-CoV-2 spike protein and nucleoprotein evaluated by four automated immunoassays and three ELISAs. Clin Microbiol Infect 2020, 26, 1557.e1–1557.e7. [Google Scholar] [CrossRef] [PubMed]
- Shrock, E.; Fujimura, E.; Kula, T.; Timms, R.T.; Lee, I.H.; Leng, Y.; Robinson, M.L.; Sie, B.M.; Li, M.Z.; Chen, Y.; et al. Viral epitope profiling of COVID-19 patients reveals cross-reactivity and correlates of severity. Science 2020, 370. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.B.; Montefiori, D.C.; McDermott, A.B.; Fong, Y.; Benkeser, D.; Deng, W.; Zhou, H.; Houchens, C.R.; Martins, K.; Jayashankar, L.; et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022, 375, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.S.; Ash, N.; Alroy-Preis, S.; Huppert, A.; Milo, R. Protection and Waning of Natural and Hybrid Immunity to SARS-CoV-2. N Engl J Med 2022, 386, 2201–2212. [Google Scholar] [CrossRef]
- Evans, J.P.; Zeng, C.; Qu, P.; Faraone, J.; Zheng, Y.M.; Carlin, C.; Bednash, J.S.; Zhou, T.; Lozanski, G.; Mallampalli, R.; et al. Neutralization of SARS-CoV-2 Omicron sub-lineages BA.1, BA.1.1, and BA.2. Cell Host Microbe 2022, 30, 1093–1102.e3. [Google Scholar] [CrossRef]
- Hachmann, N.P.; Miller, J.; Collier, A.Y.; Ventura, J.D.; Yu, J.; Rowe, M.; Bondzie, E.A.; Powers, O.; Surve, N.; Hall, K.; et al. Neutralization Escape by SARS-CoV-2 Omicron Subvariants BA.2.12.1, BA.4, and BA.5. N Engl J Med 2022, 387, 86–88. [Google Scholar] [CrossRef]
- Iketani, S.; Liu, L.; Guo, Y.; Liu, L.; Chan, J.F.; Huang, Y.; Wang, M.; Luo, Y.; Yu, J.; Chu, H.; et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature 2022, 604, 553–556. [Google Scholar] [CrossRef]
- Cele, S.; Jackson, L.; Khoury, D.S.; Khan, K.; Moyo-Gwete, T.; Tegally, H.; San, J.E.; Cromer, D.; Scheepers, C.; Amoako, D.G.; et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 2022, 602, 654–656. [Google Scholar] [CrossRef]
- Liu, L.; Iketani, S.; Guo, Y.; Chan, J.F.; Wang, M.; Liu, L.; Luo, Y.; Chu, H.; Huang, Y.; Nair, M.S.; et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature 2022, 602, 676–681. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Yin, S.; Tao, Y.; Zhu, L.; Tong, X.; Mao, M.; Li, M.; Wan, Y.; Ni, J.; et al. The Third dose of CoronVac vaccination induces broad and potent adaptive immune responses that recognize SARS-CoV-2 Delta and Omicron variants. Emerg Microbes Infect 2022, 11, 1524–1536. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Tang, Y.; Jiang, Q.; Jiang, D.; Zhang, Y.; Lv, Y.; Xu, D.; Wu, J.; Xie, J.; Wen, C.; et al. Follicular Helper T Cells in the Immunopathogenesis of SARS-CoV-2 Infection. Front Immunol 2021, 12, 731100. [Google Scholar] [CrossRef] [PubMed]
- Laidlaw, B.J.; Ellebedy, A.H. The germinal centre B cell response to SARS-CoV-2. Nat Rev Immunol 2022, 22, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Lipsitch, M.; Grad, Y.H.; Sette, A.; Crotty, S. Cross-reactive memory T cells and herd immunity to SARS-CoV-2. Nat Rev Immunol 2020, 20, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wang, Z.; Ren, L.; Hao, Y.; Zhu, M.; Jiang, H.; Wang, S.; Li, D.; Shao, Y. Pre-existing anti-HCoV-OC43 immunity influences the durability and cross-reactivity of humoral response to SARS-CoV-2 vaccination. Front Cell Infect Microbiol 2022, 12, 978440. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hou, L.; Guo, X.; Jin, P.; Wu, S.; Zhu, J.; Pan, H.; Wang, X.; Song, Z.; Wan, J.; et al. Heterologous AD5-nCOV plus CoronaVac versus homologous CoronaVac vaccination: a randomized phase 4 trial. Nat Med 2022, 28, 401–409. [Google Scholar] [CrossRef]
- Rearte, A.; Castelli, J.M.; Rearte, R.; Fuentes, N.; Pennini, V.; Pesce, M.; Barbeira, P.B.; Iummato, L.E.; Laurora, M.; Bartolomeu, M.L.; et al. Effectiveness of rAd26-rAd5, ChAdOx1 nCoV-19, and BBIBP-CorV vaccines for risk of infection with SARS-CoV-2 and death due to COVID-19 in people older than 60 years in Argentina: a test-negative, case-control, and retrospective longitudinal study. The Lancet 2022, 399, 1254–1264. [Google Scholar] [CrossRef]
- Wang, K.; Cao, Y.; Zhou, Y.; Wu, J.; Jia, Z.; Hu, Y.; Yisimayi, A.; Fu, W.; Wang, L.; Liu, P.; et al. A third dose of inactivated vaccine augments the potency, breadth, and duration of anamnestic responses against SARS-CoV-2. medRxiv, 2021. [Google Scholar] [CrossRef]
- Gao, Q.; Bao, L.L.; Mao, H.Y.; Wang, L.; Xu, K.W.; Yang, M.N.; Li, Y.J.; Zhu, L.; Wang, N.; Lv, Z.; et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020, 369, 77. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. The Lancet Infectious Diseases 2021, 21, 181–192. [Google Scholar] [CrossRef]
- Lim, J.M.E.; Hang, S.K.; Hariharaputran, S.; Chia, A.; Tan, N.; Lee, E.S.; Chng, E.; Lim, P.L.; Young, B.E.; Lye, D.C.; et al. A comparative characterization of SARS-CoV-2-specific T cells induced by mRNA or inactive virus COVID-19 vaccines. Cell Rep Med 2022, 3, 100793. [Google Scholar] [CrossRef]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Juno, J.A.; Tan, H.X.; Lee, W.S.; Reynaldi, A.; Kelly, H.G.; Wragg, K.; Esterbauer, R.; Kent, H.E.; Batten, C.J.; Mordant, F.L.; et al. Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19. Nat Med 2020, 26, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat Immunol 2022, 23, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef]
- Liu, J.; Yu, J.; McMahan, K.; Jacob-Dolan, C.; He, X.; Giffin, V.; Wu, C.; Sciacca, M.; Powers, O.; Nampanya, F.; et al. CD8 T Cells Contribute to Vaccine Protection Against SARS-CoV-2 in Macaques. Sci Immunol 2022, eabq7647. [Google Scholar] [CrossRef]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med 2020, 26, 1636–1643. [Google Scholar] [CrossRef]
- Williams, E.S.C.P.; Martins, T.B.; Hill, H.R.; Coiras, M.; Shah, K.S.; Planelles, V.; Spivak, A.M. Plasma cytokine levels reveal deficiencies in IL-8 and gamma interferon in Long-COVID. medRxiv, 2022. [Google Scholar] [CrossRef]
- Matchett, W.E.; Joag, V.; Stolley, J.M.; Shepherd, F.K.; Quarnstrom, C.F.; Mickelson, C.K.; Wijeyesinghe, S.; Soerens, A.G.; Becker, S.; Thiede, J.M.; et al. Cutting Edge: Nucleocapsid Vaccine Elicits Spike-Independent SARS-CoV-2 Protective Immunity. J Immunol 2021, 207, 376–379. [Google Scholar] [CrossRef]
- Chen, J.; Deng, Y.; Huang, B.; Han, D.; Wang, W.; Huang, M.; Zhai, C.; Zhao, Z.; Yang, R.; Zhao, Y.; et al. DNA Vaccines Expressing the Envelope and Membrane Proteins Provide Partial Protection Against SARS-CoV-2 in Mice. Front Immunol 2022, 13, 827605. [Google Scholar] [CrossRef]
- Regev-Yochay, G.; Gonen, T.; Gilboa, M.; Mandelboim, M.; Indenbaum, V.; Amit, S.; Meltzer, L.; Asraf, K.; Cohen, C.; Fluss, R.; et al. 4th Dose COVID mRNA Vaccines' Immunogenicity & Efficacy Against Omicron VOC. medRxiv 2022. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Amir, O.; Freedman, L.; Alroy-Preis, S.; Ash, N.; Huppert, A.; Milo, R. Protection by 4th dose of BNT162b2 against Omicron in Israel. medRxiv 2022. [Google Scholar] [CrossRef]
- Wang, J.; Deng, C.; Liu, M.; Liu, Y.; Li, L.; Huang, Z.; Shang, L.; Jiang, J.; Li, Y.; Mo, R.; et al. Four doses of the inactivated SARS-CoV-2 vaccine redistribute humoral immune responses away from the Receptor Binding Domain. medRxiv 2022. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).