1. Introduction
Humeral resurfacing arthroplasties (HRA) have increasingly become a popular alternative to total (TSA) or reverse shoulder arthroplasties (RSA) for the treatment of glenohumeral osteoarthritis (OA), rheumatoid arthritis (RA), and avascular necrosis (AVN) of the humeral head. The main advantage is that HRA permit to restore the articular surface and function without performing neck osteotomy or implanting an intramedullary stem. These pathologies usually affect young patients, and preserving the bone stock is fundamental in the case of further revisions. [
1] In addition, HRA is a less invasive procedure with an almost absent blood loss and shorter recovery times. In the case a revision is needed, the humeral shield can be easily removed and converted into a RSA. Technical difficulties associated with HRA are predominantly a result of incorrectly sizing and orienting the prosthesis, resulting in ‘over-stuffing’ of the joint. In the present paper, we present the long-term results of a retrospective observational study conducted on patients who underwent HRA.
2. Materials and Methods
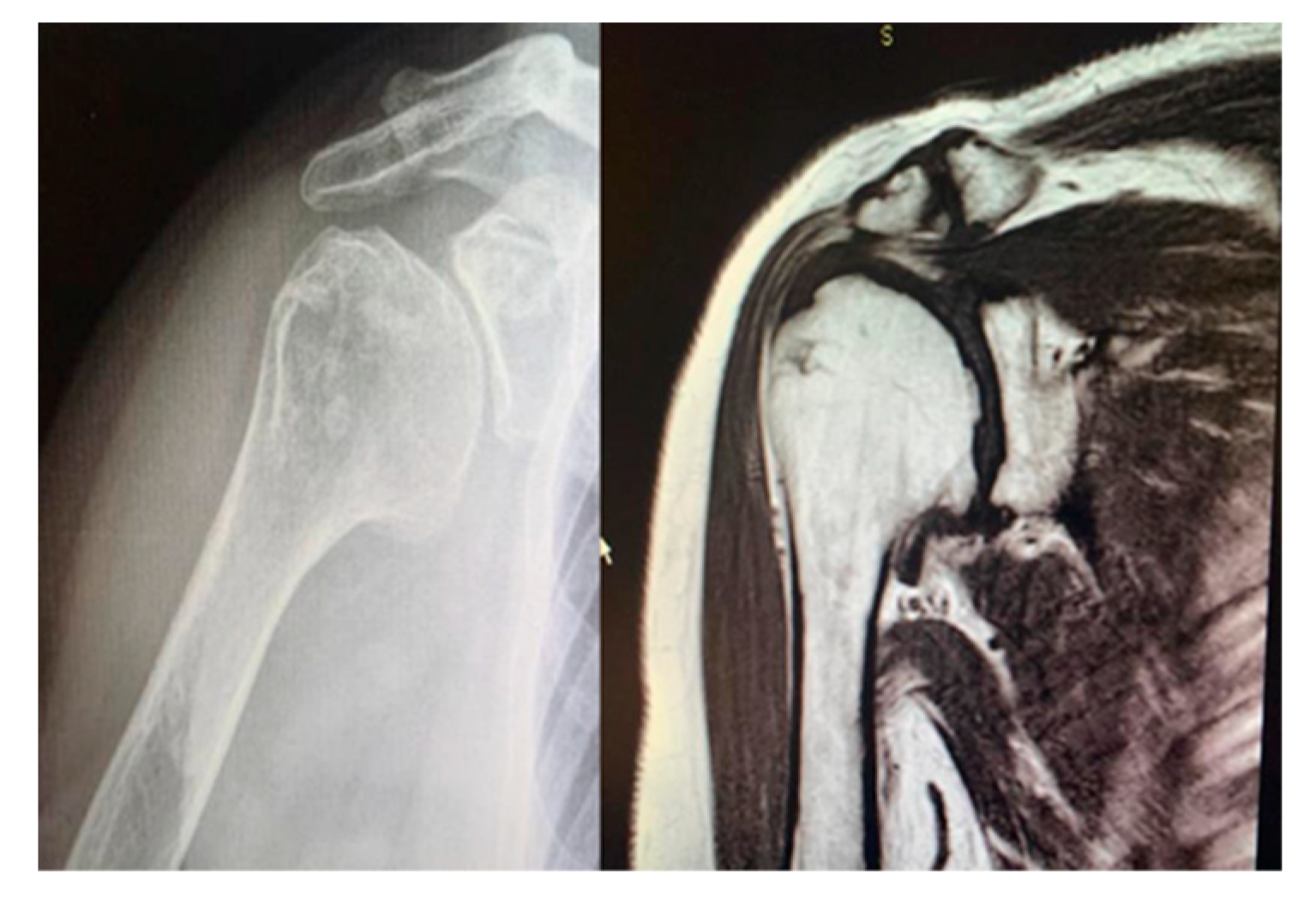
Study design. A retrospective and observational study was performed. Inclusion criteria were as follows: patients with (1) a diagnosis of concentric glenohumeral degeneration (
Figure 1) with rotator cuff integrity following primary OA or RA or humeral head AVN (
Figure 2), (2) glenoid articular surface type A1 or A2, according to Walch’s classification, (3) more than 60% residual humeral head, [2, 3] (4) who underwent HRA.
At our Institution, no Ethical Committee nor Institutional Review Board approval is necessary for retrospective studies, and all patients gave their informed consent to data collection and their anonymous use for scientific and teaching purposes. This study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
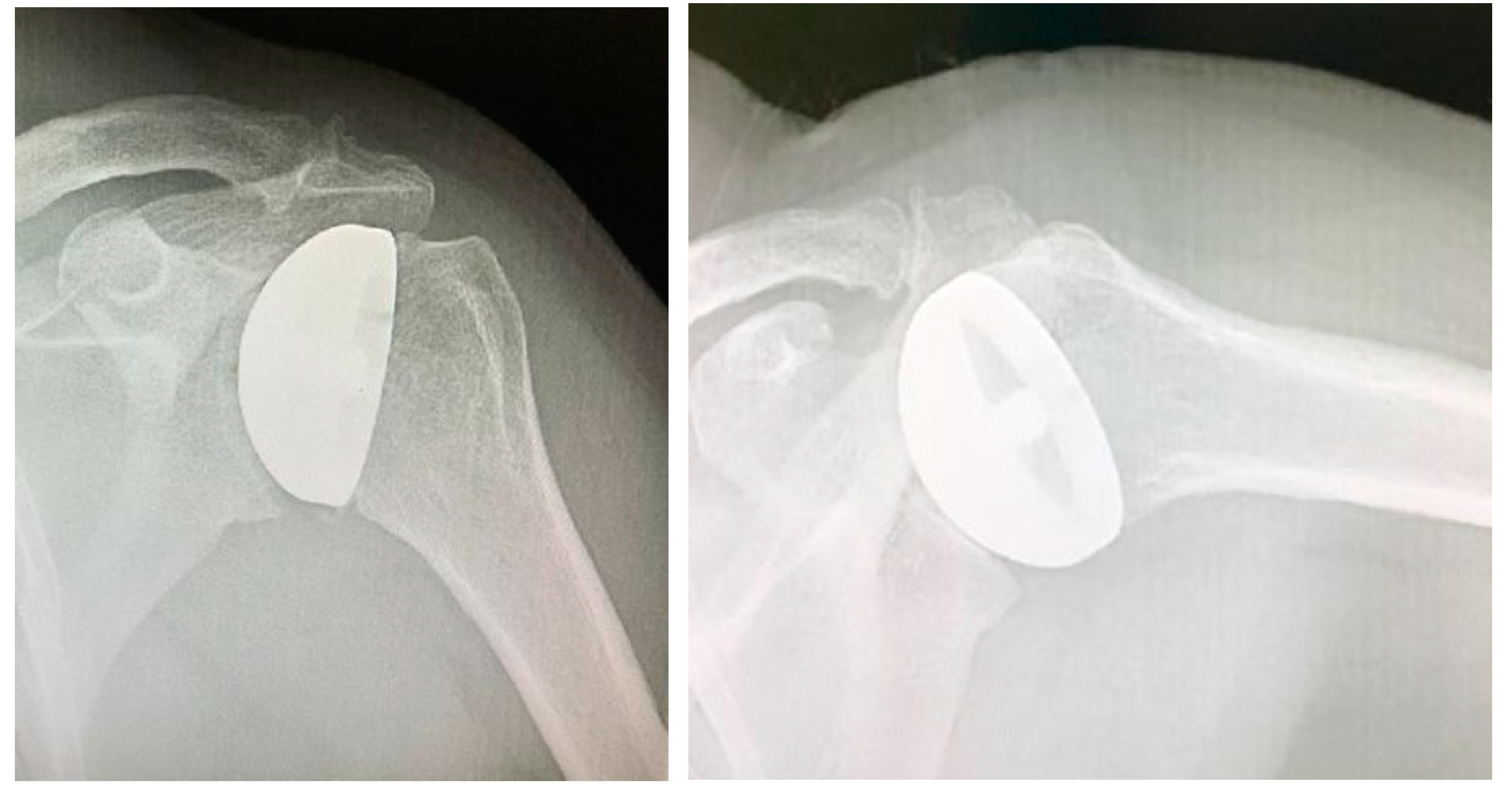
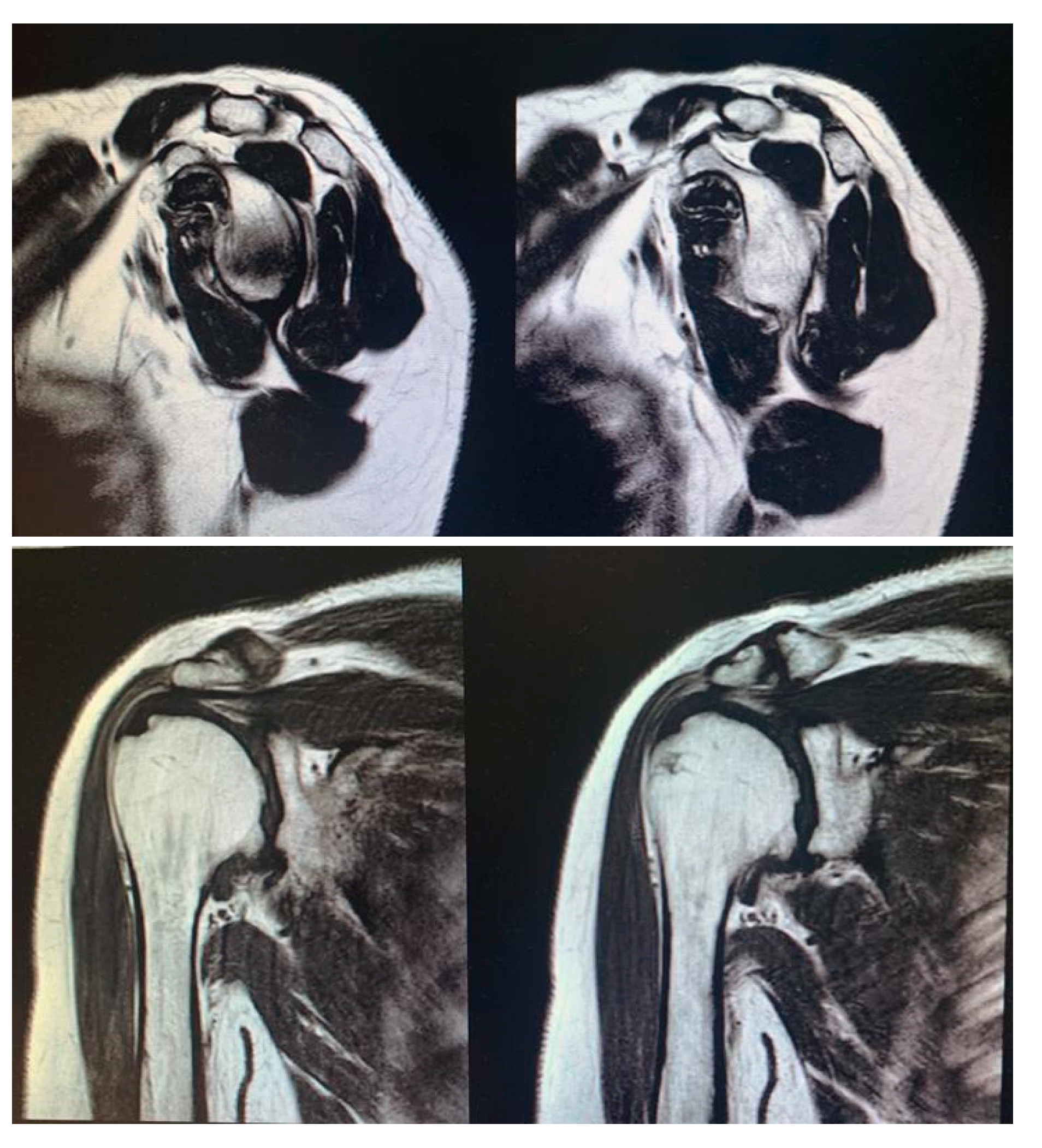
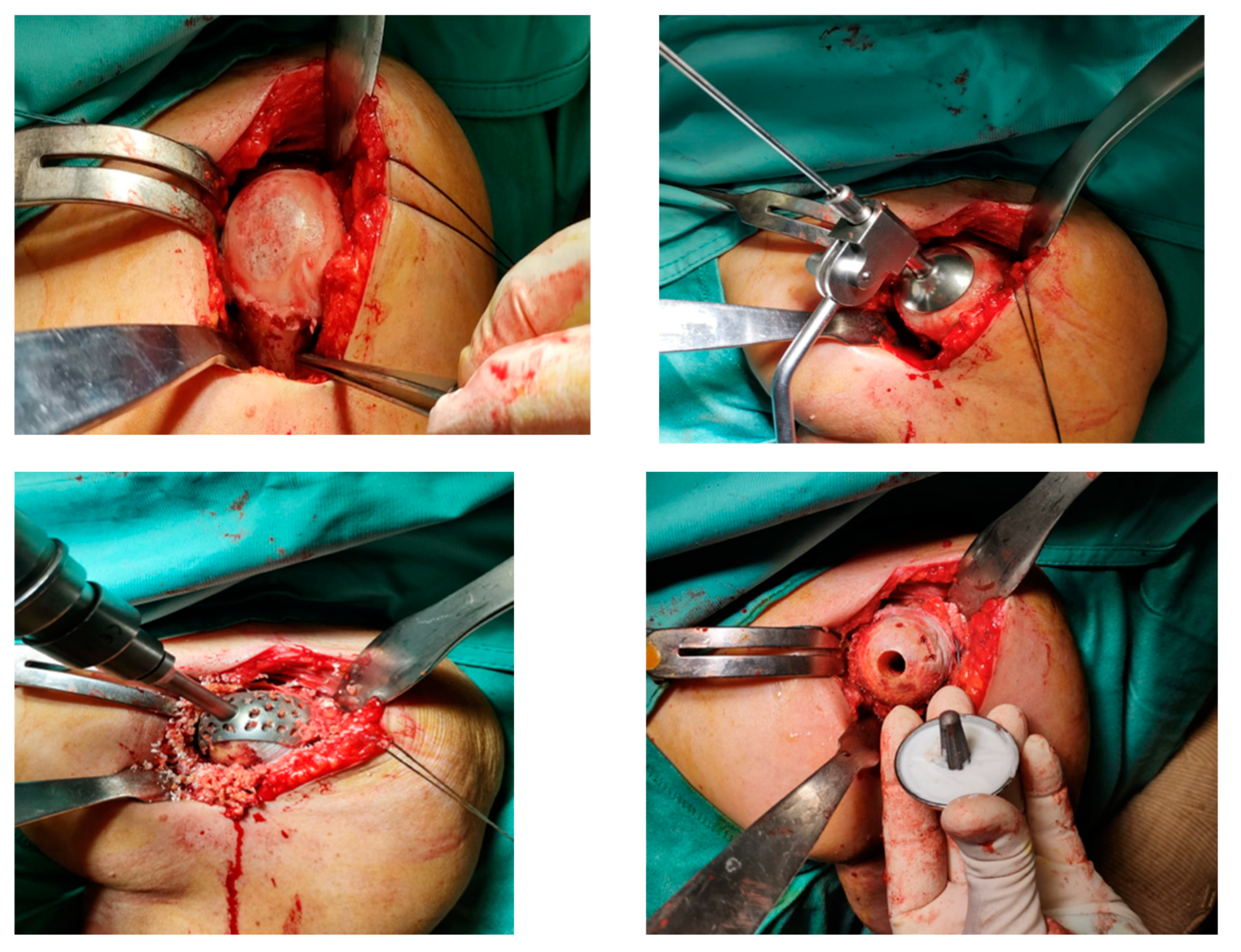
Surgical procedure. All the surgeries were performed by the senior Author (S.G.), implanting a Durom Shoulder Cup (Zimmer Biomet, Warsaw, Indiana, USA), a Copeland Humeral Resurfacing Head Surgery (Zimmer Biomet, Warsaw, Indiana, USA) or a SMR Resurfacing Shoulder Prosthesis System (Lima Corporate, Udine, Italy). All patients were pre-operatively evaluated through a clinical examination, plain radiographs in antero-posterior and axillay view, computed tomography scan and magnetic resonance imaging (
Figure 3) in order to determine the correct indication to HRA.
A deltopectoral approach was used in all cases. [
4] After identification of the subscapularis tendon, its tenotomy was performed at approximately 1 cm from its insertion on the lesser tuberosity. Tenotomy of the long head of the biceps was performed in all the cases. The humeral head was gently dislocated with gradual release of soft tissues, and any osteophyte was removed to precisely identify the humeral neck and the angle between the neck and the diaphysis. Attention was paid to restore the correct retroversion and inclination of the head. Also, the corrected 2 offsets were restored to create the appropriate lever arm for the deltoid and supraspinatus muscles to achieve active mobility of the joint. The 3rd, so-called ‘‘posterior’’, offset was not considered during this procedure, because the implantation of a stemless resurfacing prosthesis is not related to the humeral diaphysis. The surgical instrumentation envisages a standard inclination angle between neck and diaphysis of 135°, which can be modified within a range of 115°–150° depending on the patient’s morphology. Moreover, it allows for 20° to 55° of retroversion, which is decided by the surgeon to suit the axis of the forearm. As the next step, the humeral head is prepared. A pin guide wire is placed at the center of the humeral head parallel to the anatomical neck and passed down through the humeral head to the lateral cortex to provide stability. Using a cannulated humeral surface cutter of the appropriate size over the guide wire, the articular cartilage is completely removed by reaming down to expose the subchondral bone. Afterwards, the central peg hole is executed. Multiple perforations with a drill bit are often performed to improve adhesion of the cement to the bony surface if sclerotic bone is left after the reaming phase. When the humeral surface has been prepared, the trial prosthesis can be positioned on the head and the joint can be reduced to test joint mobility and stability. Last, the trial cup is replaced by the definitive component which is cemented with low-viscosity bone cement. Cement thickness of only 1 mm is required between the bone and the prosthetic component. The glenoid was never resurfaced. Nevertheless, all the osteophytes were removed and, in some cases, microfractures were performed (
Figure 4). The subscapularis was re-attached with multiple non-reabsorbable stitches, and the wound was closed in layers. To control bleeding, in the absence of contraindications, tranexamic acid was administered both intravenously and locally, as previously described.[
5]
Post-operative care. After surgery, the arm was rested in a internal rotation and adduction sling for 3 weeks. Passive range of motion (ROM) exercises from the sling were allowed from the 1st post-operative day. After 3 weeks, active ROM exercises in forward elevation and abduction were allowed. Both passive and active external and internal rotation exercises were not allowed until 3 weeks from surgery. Also, strengthening exercises with light weights was permitted thereafter.
Clinical and radiological assessment. All patients were evaluated clinically and radiographically at 1, 3, 6 and 12 months after surgery, and then annually. Shoulder function was assesses using the Disabilities of the Arm, Shoulder and Hands (DASH) [
6] and Constant scoring system [
7] Plain radiographs in the true antero-posterior and axillary views were obtained at each visit. The distance between the humeral diaphyseal axis and the line through the medial wall of the coracoid process was measured, in order to analyze the morphological changes of the glenoid.
Statistical analysis. The Wilcoxon signed rank test was used to assess statistical differences between the components of the Constant score and DASH score pre-operatively and at last follow-up (FU). The level of significance was set at p < 0.05 and the data were analyzed by use of SPSS software (IBM, Armonk, New York, USA).
3. Results
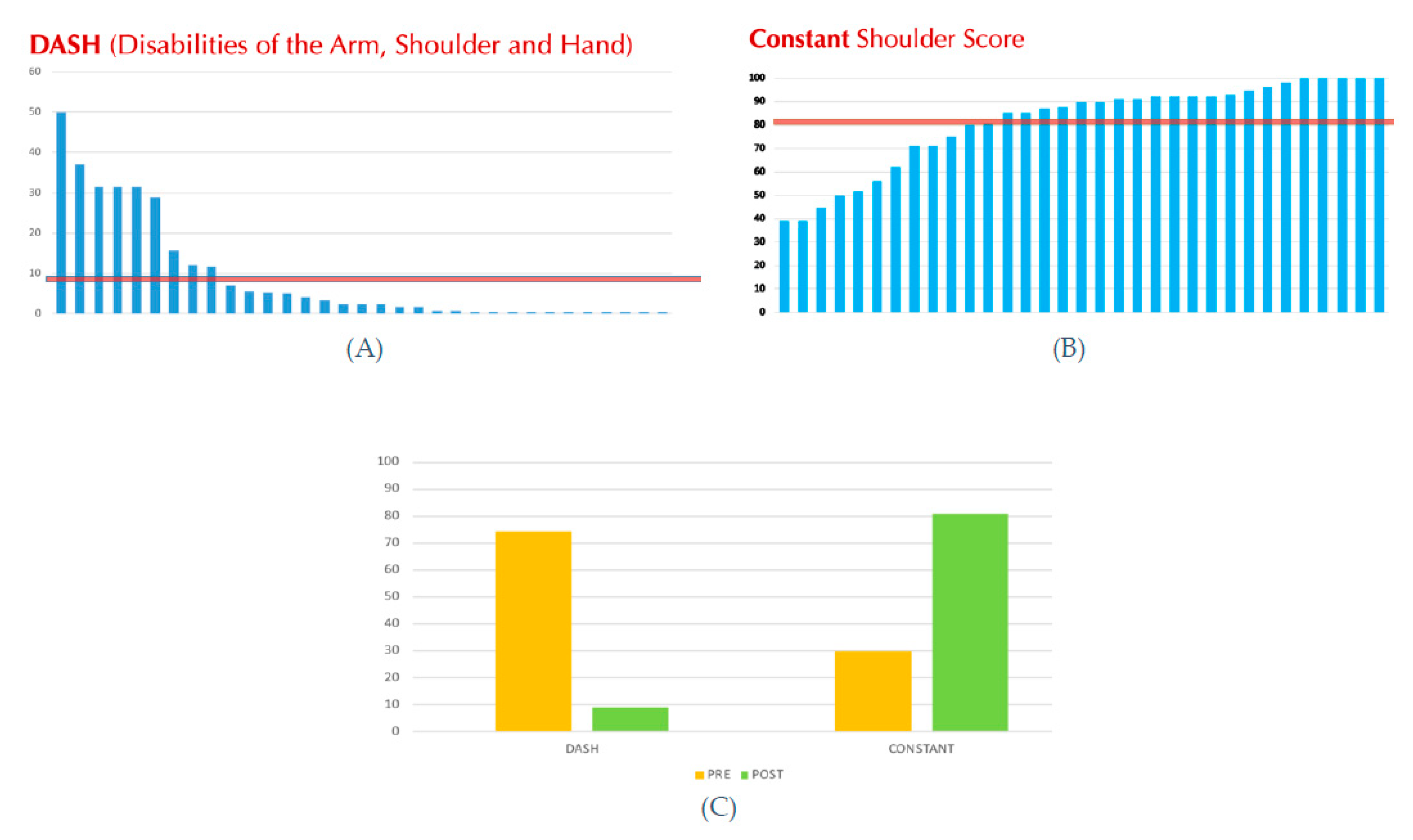
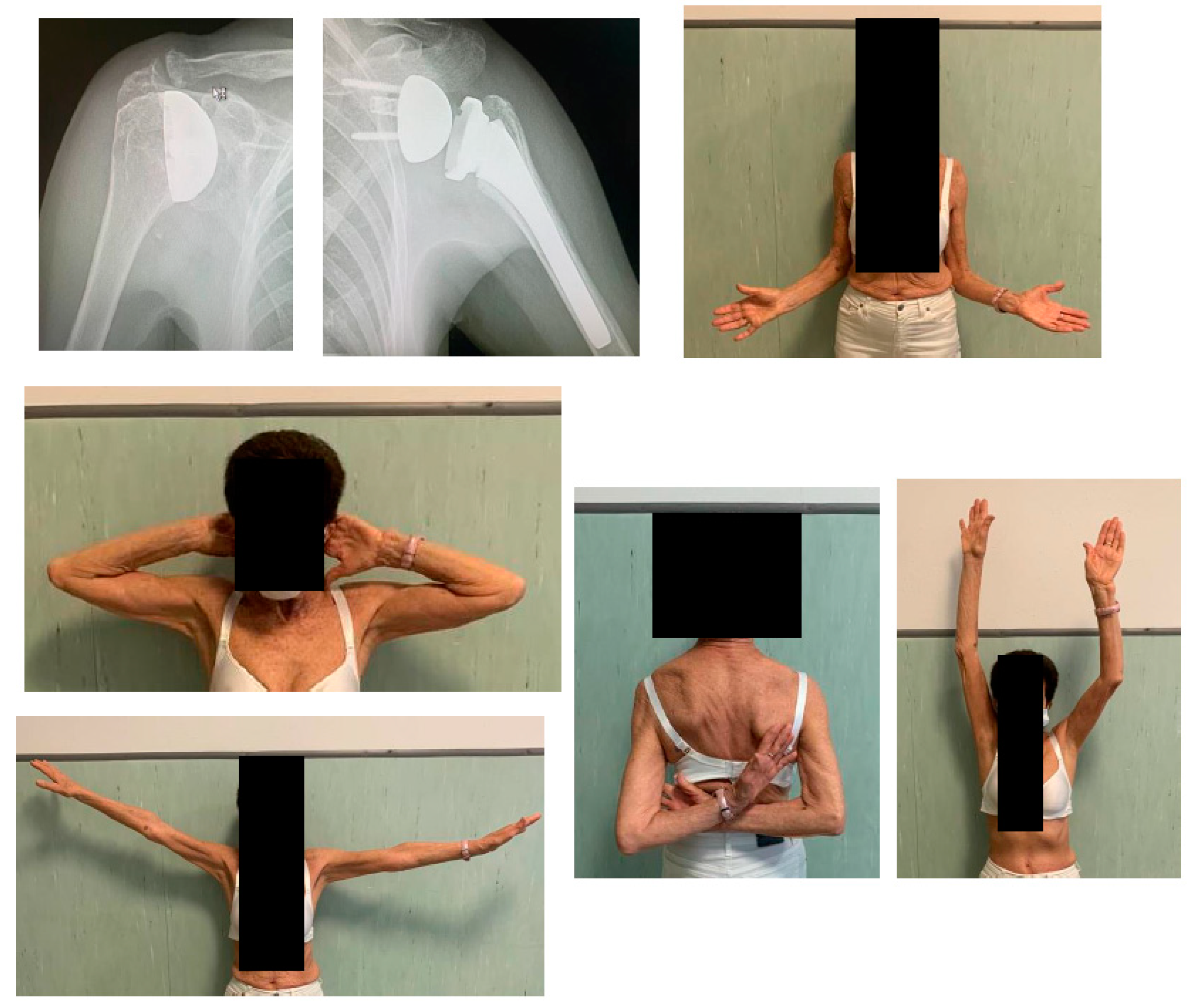
From July 2005 to August 2021 a total of 76 (78 shoulders) patients who underwent HRA were identified, but only 33 (44%) completed the clinical and radiological FU at more than 2 years after surgery, and were included into the study. However, all the 78 patients (or if deceased, their relatives) were contacted by phone to obtain information on their medical history after the prosthetic surgery, namely regarding the need of further surgery. Mean FU ± standard deviation (SD) was 128 ± 61 (range 24 – 216) months. Mean age at surgery was 63.4 ± 12.5 (range 31 – 83) years. There were 17 females and 14 males, two of which had bilateral surgeries (one male and one female). The Constant score significantly improved from a mean pre-operative score of 29.9 ± 12.1 (range 10 – 63) points to a mean post-operative score of 81.2 ± 18.9 (range 39 – 100) points (p < 0.05), and the DASH score also improved significantly from a mean pre-operative score of 46.7 ± 2.9 (range 45 – 50) points to a mean post-operative score of 8.9 ± 13.5 (range 0 – 50) points (p < 0.05). The operative time was less than 1 hour in all surgeries, mean 45 minutes (range 35 – 58) min. As regards perioperative complications, no blood transfusion was required.
Only two out of the 78 cases (3%) had to be subjected to a surgical revision procedure, one as a result of a shoulder instability due to a subscapularis insufficiency observed at 2 years after index suergery, while the other patient developed a rotator cuff arthropathy due to a rotator cuff degenerative tear. In both cases, the surgical revision procedure consisted in RSA.
However, in all 33 implants led to final radiological FU, any radiolucent line was noticed, nor aseptic loosening of the prosthetic cup, nor significant central migration of the humeral head.
Figure 6.
(A) Post-operative DASH score. (B) Post-operative Constant score. The red line is the average of post-operative DASH and Constant values, respectively. (C) Comparison between pre- and post-operative DASH and Constant scores.
Figure 6.
(A) Post-operative DASH score. (B) Post-operative Constant score. The red line is the average of post-operative DASH and Constant values, respectively. (C) Comparison between pre- and post-operative DASH and Constant scores.
4. Discussion
The purpose of this retrospective study was to evaluate and report the results by a subjective, functional, and radiological point of view, obtained with a HRA with a minimum FU of 2 years (mean FU of 11 years) in our consecutive series of patients and the compare our results and revision rate with those present in the literature.
A precise pre-operative planning is essential to evaluate the radius of curvature, the center of rotation (COR) and the angle of inclination of the humeral head to select the optimal prosthetic size. Normal shoulder anatomy can vary considerably among different individuals as well as between the left and right shoulders of the same individual. The normal humeral head is retroverted and is inclined medially relative to the humeral shaft. However, normal humeral head retroversion may range from 0° to 55°, and the inclination may range from 30° to 55°. In addition, the offset of the humerus in relation to the glenoid may vary in three dimensions, and the radius of curvature ranges from 20 to 30 mm. [
8] All of these variations must be considered when an arthroplasty procedure is performed.
Unlike a conventional shoulder joint replacement (being a hemiarthroplasty, a TSA or a RSA), which entails removal of the entire humeral head followed by placement of an intramedullary stem, a resurfacing prosthesis only entails reaming the epiphyseal region of the humeral head and placing a prosthetic cap fixed with or without cement on the residual portion of the humeral head. Hence, it enables reconstruction of the humeral head surface without the need to implant a stem or to perform any osteotomies. This implies that the humeral neck and >50% of the humeral head are retained and the native head-shaft angle remains intact. The resurfacing component may or may not be combined with a glenoid component.[8, 9] Some Authors believe that it is easier to restore the anatomic offset as well as the height of the COR than with a TSA. Thomas et al. [
3] reported that resurfacing of the humerus increased the humeral offset by a mean of 5 mm (from 23 to 28 mm) but there was a mean pre-operative erosion of 6 mm of the lateral offset, so the resurfacing re-established the anatomic offset that had been lost as a result of erosion.
Many Authors have reported that the inclination, version, offset, and head-shaft angles were maintained with resurfacing. [2, 10–12] All those factors are beneficial in order to restore the biomechanics of the shoulder joint as well as patient satisfaction. Changes in the angle or offset of the humerus may result in impingement with the acromion or the glenoid rim, increased tension of the rotator cuff tendons, or a decreased ROM. Alterations of the retroversion or inclination of the humeral head might change the tension and/or the lever arm of the deltoid and rotator cuff muscles, which may lead to a decreased ROM, weaker flexion, or instability of the joint. [
13] An alteration in the radius of curvature of the humeral head by 5 mm may decrease the ROM by 20° to 30°, [14, 15] which may increase the extent of glenohumeral translation during movement.
As regards functional outcomes, good results were observed in the study population at a mean FU of 128 months. The results in terms of DASH and Constant scores are similar or even better than those already present in the Literature.[
16] Lebon et al. [
1] in a study with 41 HRA at a mean FU of 44 months noted a Constant score higher than 75 points in 56% of the patients and a DASH score of 17 ± 15. Levy et al. [
10] in their study analyzed 19 HRA with a mean FU of 6.8 years (5 to 10 years). They found a post-operative Costant score of 73.5 in primary OA and 72.1 in RA. A study conducted from 2005 to 2009 by Mansat et al. [
17] on 64 shoulders at an average FU of 36 months, post-operative Constant score reached 68 points and the post-operative DASH score reached 28 points.
Boileau et al. [
18] showed the great variability of the glenohumeral relationships and dimensions between individuals and underlined the great difficulty in restoring normal anatomy during arthroplasty.
The goal we aimed with HRA was to reproduce the individual anatomy of the humeral head (diameter, radius of curvature, version) and the lateral offset of the proximal humerus by an accurate positioning of the prosthetic humeral head, in order to restore normal glenohumeral kinematics and avoid damage to the rotator cuff and impingement on the glenoid component or the coracoacromial arch, while preserving the bone stock.
Restoring a radius of curvature close to normal is essential because its excessive increase does not change the lateral humeral offset but may induce overstuffing of the joint.[
19] Similarly, the height of the humeral head is of paramount importance, as Harryman et al. [
14] showed: an increase of the humeral height of 5 mm or greater will induce a decrease of 20° to 30°of range of motion.
Also, positioning the resurfacing implant related to the top of the greater tuberosity is fundamental to avoid impingement of the greater tuberosity under the acromion if it is placed too low, or overstuffing the cuff tendons with limited ROM if it is placed too proud.[20, 21]
HRA implants can compensate for the medialization of the humeral head secondary to the wear of the joint surface, with the thickness of the implant, allowing restoration of the lever arm of the rotator cuff tendons.[
17] Thomas et al. [
3] found that the HRA increased humeral offset and the deltoid and cuff lever arm by mean of 22% from the pathologic state. Reconstruction of the lateral humeral offset is important for optimization of the moment arm of the deltoid and rotator cuff and of the normal tension of the soft tissue after prosthetic reconstruction. [22, 23]
Parameters like inclination angle and COR, like also concomitant or previous shoulder pathologies, could also have an impact on revision rate. Alolabi et al. [
24] found in their study that 65.1% of the HRAs demonstrated an inadequate reaming of the humeral head, resulting in overstuffing of the glenohumeral joint.
Geervliet et al. [
25] agreed with Alolabi et al. [
24] that after HRA the normal glenohumeral anatomy, regarding the COR, is not completely reproduced. They found a significant increase in COR in the revision group compared to the non-revision group (8.0 mm versus 4.9 mm, respectively). In other words, the probability of revision will increase significantly with an increased COR. Overstuffing has always been a suspect for failure.
Lebon et al. [
1] in a study with mean FU of 44 months detected a revision rate of 9.8% e they also noted the tendency to varus positioning of the implants and the increase of lateral offset of the humeral head as possible radiologic prognostic factors for future failure.
Delaney et al. [
26] in their study concerning HRA reported patients with no concomitant shoulder pathology showed good clinical outcomes; shoulders in which a concomitant procedure (rotator cuff repair, capsular shift, or biceps tenodesis) was performed still achieved acceptable clinical outcomes; however, shoulders that had undergone prior procedures (for example arthroscopic debridement, Bankart repair, rotator cuff repair, sub- acromial decompression, acromioclavicular joint or distal clavicle resection and biceps tenodesis) had worse clinical outcomes and were at increased risk of failure.
In the present series, revision rate was as 3% (2 out of 78 cases, having being all patients – or their relatives – contacted by telephone, if no clinical and radiological FU was obtained). Both cases were revised to RSA, and in both cases the procedure did not show technical difficulties and it could be performed with bone-stock sparing because of the prosthesis could be easily removed with minimum bone resection.
Rasmussen et al. [
27] analyzed 1210 HRAs between 2006 and 2013 and noticed a revision rate of 8.84%, while the mean time to revision was 27 ± 19 months. Hwang et al. [
28] considered 101 cases between 2007 and 2020 with a median FU of 7 years, and they found a revision rate of 7%. Levy et al. [
10] and in their cohort of 103 HRAs operated from 1990 to 1994, highlighted a revision rate of 8.16% at a mean FU of 6.8 years (5 to 10 years).
Results depend directly on correct indications, which for those of implants are limited and on correct surgical technique with a precise sizing and positioning of the implant. The major difficulty consists in finding adequate landmarks on a pathologic shoulder and in the accurate placement of the guide-pin on which drilling and implant positioning rely. An experienced surgeon, and a complete prosthetic system are key to achieve good results in terms of clinical scores, patient satisfaction and a low revision rate. The humeral component cementation represents a fundamental step during this procedure, as well. In our opinion, cementation prevents the risks of aseptic mobilization and at the same time it does not cause further difficulties during a possible revision surgery. In addition, this surgical procedure is to be considered minimally-invasive for both the saving of the epiphyseal and diaphyseal bone stock and the limited blood loss.
Limitations are present in this study. First of all, it is a retrospective study with a small sample size: it may be difficult to generalize the obtained results. In addition, about half of patients were lost during clinical and radiological FU; this situation can be explained by two factors: the retrospective nature of the study, and the long FU period considered. Surgeries were performed over a 18-years period and many patients became unavailable or died. Nevertheless, the present study presents the longest FU for HRA in Literature.
5. Conclusions
HRA is a feasible solution to improve quality of life of patients with concentric gleno-humeral OA, RA and AVN of the humeral head, that present a morphologically and functionally normal rotator cuff. Notwithstanding the abovementioned limitation, it is possible to affirm that good results at long term FU can only be achieved by an appropriate pre-operative planning, and an accurate surgical technique.
Author Contributions
Conceptualization, N.M and S.G.; data curation, M.S. and N.C.O.; writing—original draft preparation, M.S. and E.T.; writing—review and editing, E.T., G.B.C., N.M.; visualization, G.B.C..; supervision, N.M. and S.G.. All Authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
At our Centre, no Institutional Review Board nor Ethical Committee Approval is necessary for retrospective studies. This study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to data collection and anonymous use of them for scientific and teaching purposes, including to publish this paper.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
Not applicable.
Conflicts of Interest
The Authors declare no conflict of interest.
References
- Lebon J, Delclaux S, Bonnevialle N, et al (2014) Stemmed hemiarthroplasty versus resurfacing in primary shoulder osteoarthritis: a single-center retrospective series of 78 patients. Orthop Traumatol Surg Res 100:S327–S332. [CrossRef]
- Copeland S (2006) The continuing development of shoulder replacement: “reaching the surface.” J Bone Joint Surg Am 88:900–905. [CrossRef]
- Thomas SR, Sforza G, Levy O, Copeland SA (2005) Geometrical analysis of Copeland surface replacement shoulder arthroplasty in relation to normal anatomy. J Shoulder Elb Surg 14:186–192. [CrossRef]
- Steffee A, Moore R (1984) Hemi-resurfacing arthroplasty of the shoulder. Contemp Orthop 9:51–9.
- De Falco L, Troiano E, Cesari M, et al (2021) Intra-operative local plus systemic tranexamic acid significantly decreases post-operative bleeding and the need for allogeneic blood transfusion in total knee arthroplasty. Med Glas (Zenica) 18:1–6. [CrossRef]
- Angst F, Schwyzer HK, Aeschlimann A, et al (2011) Measures of adult shoulder function: Disabilities of the Arm, Shoulder, and Hand Questionnaire (DASH) and Its Short Version (QuickDASH), Shoulder Pain and Disability Index (SPADI), American Shoulder and Elbow Surgeons (ASES) Society Standardized Shoulder. Arthritis Care Res 63:174–188. [CrossRef]
- Constant CR, Gerber C, Emery RJH, et al (2008) A review of the Constant score: Modifications and guidelines for its use. J Shoulder Elb Surg 17:355–361. [CrossRef]
- Pearl ML, Volk AG (1996) Coronal plane geometry of the proximal humerus relevant to prosthetic arthroplasty. J shoulder Elb Surg 5:320–326. [CrossRef]
- Pearl ML (2005) Proximal humeral anatomy in shoulder arthroplasty: Implications for prosthetic design and surgical technique. J Shoulder Elb Surg 14:S99–S104. [CrossRef]
- Levy O, Copeland SA (2001) Cementless surface replacement arthroplasty of the shoulder. 5- to 10-year results with the Copeland mark-2 prosthesis. J Bone Joint Surg Br 83:213–221. [CrossRef]
- Levy O, Funk L, Sforza G, Copeland SA (2004) Copeland surface replacement arthroplasty of the shoulder in rheumatoid arthritis. J Bone Joint Surg Am 86:512–518. [CrossRef]
- Buchner M, Eschbach N, Loew M (2008) Comparison of the short-term functional results after surface replacement and total shoulder arthroplasty for osteoarthritis of the shoulder: a matched-pair analysis. Arch Orthop Trauma Surg 128:347–354. [CrossRef]
- Williams GR, Wong KL, Pepe MD, et al (2001) The effect of articular malposition after total shoulder arthroplasty on glenohumeral translations, range of motion, and subacromial impingement. J shoulder Elb Surg 10:399–409. [CrossRef]
- Harryman DT, Sidles JA, Harris SL, et al (1995) The effect of articular conformity and the size of the humeral head component on laxity and motion after glenohumeral arthroplasty. A study in cadavera. J Bone Joint Surg Am 77:555–563. [CrossRef]
- Jobe CM, Lannotti JP (1995) Limits imposed on glenohumeral motion by joint geometry. J shoulder Elb Surg 4:281–285. [CrossRef]
- Giannotti S, Bottai V, Ghilardi M, et al (2012) Shoulder resurfacing with Durom Cup: clinical and radiological re-assessment. J Orthop Sci 17:545–550. [CrossRef]
- Mansat P, Coutié AS, Bonnevialle N, et al (2013) Resurfacing humeral prosthesis: do we really reconstruct the anatomy? J shoulder Elb Surg 22:612–619. [CrossRef]
- Boileau P, Walch G (1997) The three-dimensional geometry of the proximal humerus. Implications for surgical technique and prosthetic design. J Bone Joint Surg Br 79:857–865. [CrossRef]
- De Leest O, Rozing PM, Rozendaal LA, Van der Helm FCT (1996) Influence of glenohumeral prosthesis geometry and placement on shoulder muscle forces. Clin Orthop Relat Res 330:222–233. [CrossRef]
- Iannotti JP, Spencer EE, Winter U, et al (2005) Prosthetic positioning in total shoulder arthroplasty. J Shoulder Elb Surg 14:S111–S121. [CrossRef]
- Nyffeler RW, Sheikh R, Jacob HAC, Gerber C (2004) Influence of humeral prosthesis height on biomechanics of glenohumeral abduction. An in vitro study. J Bone Joint Surg Am 86:575–580. [CrossRef]
- Rietveld ABM, Daanen HAM, Rozing PM, Obermann WR (1988) The lever arm in glenohumeral abduction after hemiarthroplasty. J Bone Joint Surg Br 70:561–565. [CrossRef]
- Iannotti J, Gabriel J, Schneck S, et al (1992) The normal glenohumeral relationships. An anatomical study of one hundred and forty shoulders. J Bone Jt Surg Am 74:491–500.
- Alolabi B, Youderian AR, Napolitano L, et al (2014) Radiographic assessment of prosthetic humeral head size after anatomic shoulder arthroplasty. J shoulder Elb Surg 23:1740–1746. [CrossRef]
- Geervliet PC, Willems JH, Sierevelt IN, et al (2019) Overstuffing in resurfacing hemiarthroplasty is a potential risk for failure. J Orthop Surg Res 14:. [CrossRef]
- Delaney RA, Freehill MT, Higgins LD, Warner JJP (2014) Durability of partial humeral head resurfacing. J shoulder Elb Surg 23:. [CrossRef]
- Rasmussen J, V. , Olsen BS, Al-Hamdani A, Brorson S (2016) Outcome of Revision Shoulder Arthroplasty After Resurfacing Hemiarthroplasty in Patients with Glenohumeral Osteoarthritis. J Bone Joint Surg Am 98:1631–1637. [CrossRef]
- Hwang N, Modi CS, Drew SJ, Turner SM (2014) Mid-term results of Copeland shoulder cementless surface replacement arthroplasty from an independent centre. Shoulder Elb 6:75–80. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).