Submitted:
30 August 2023
Posted:
01 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Test plants
2.2. Test insects
2.3. Oviposition rate
2.4. Settling preference
2.5. Statistical analysis
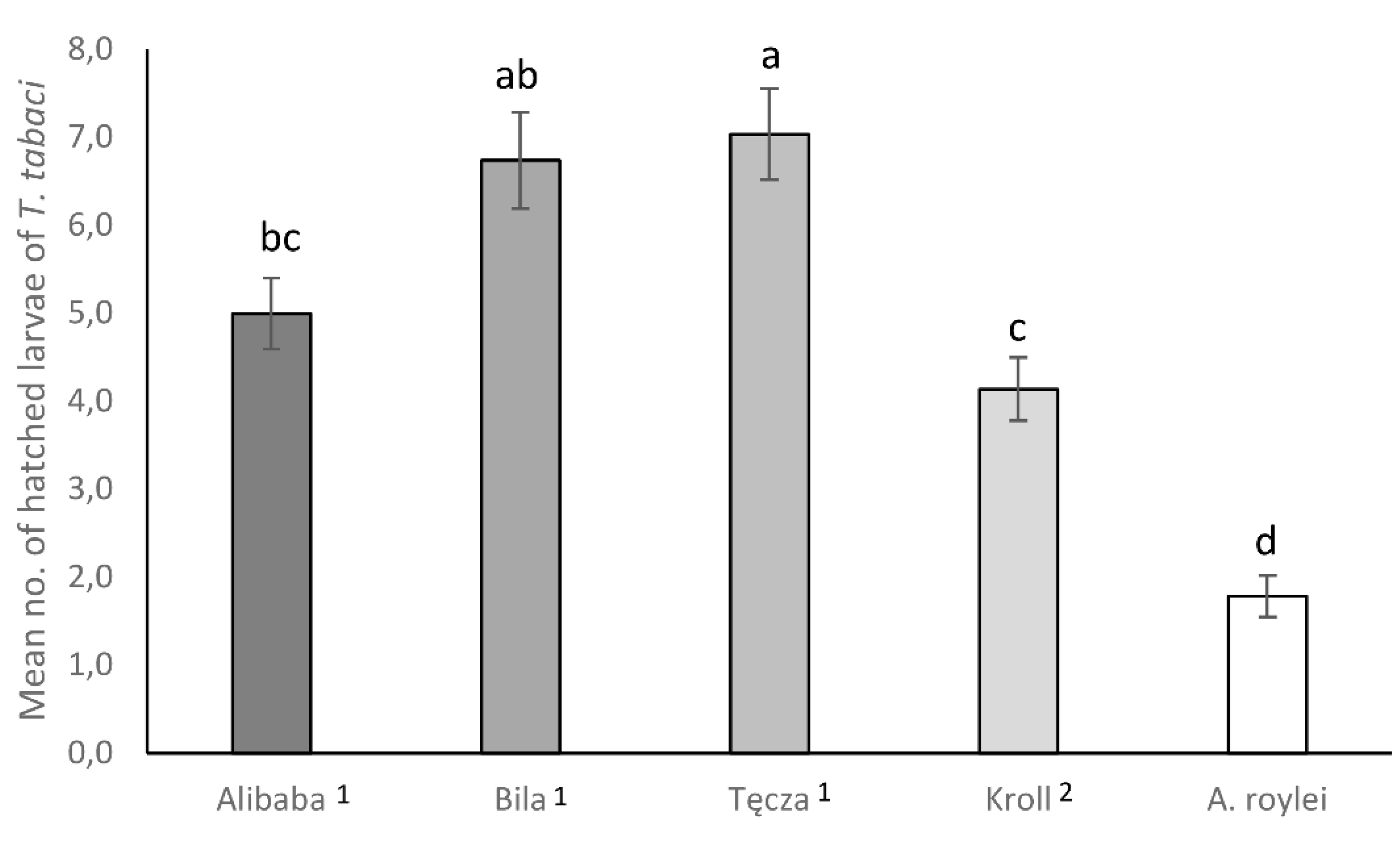
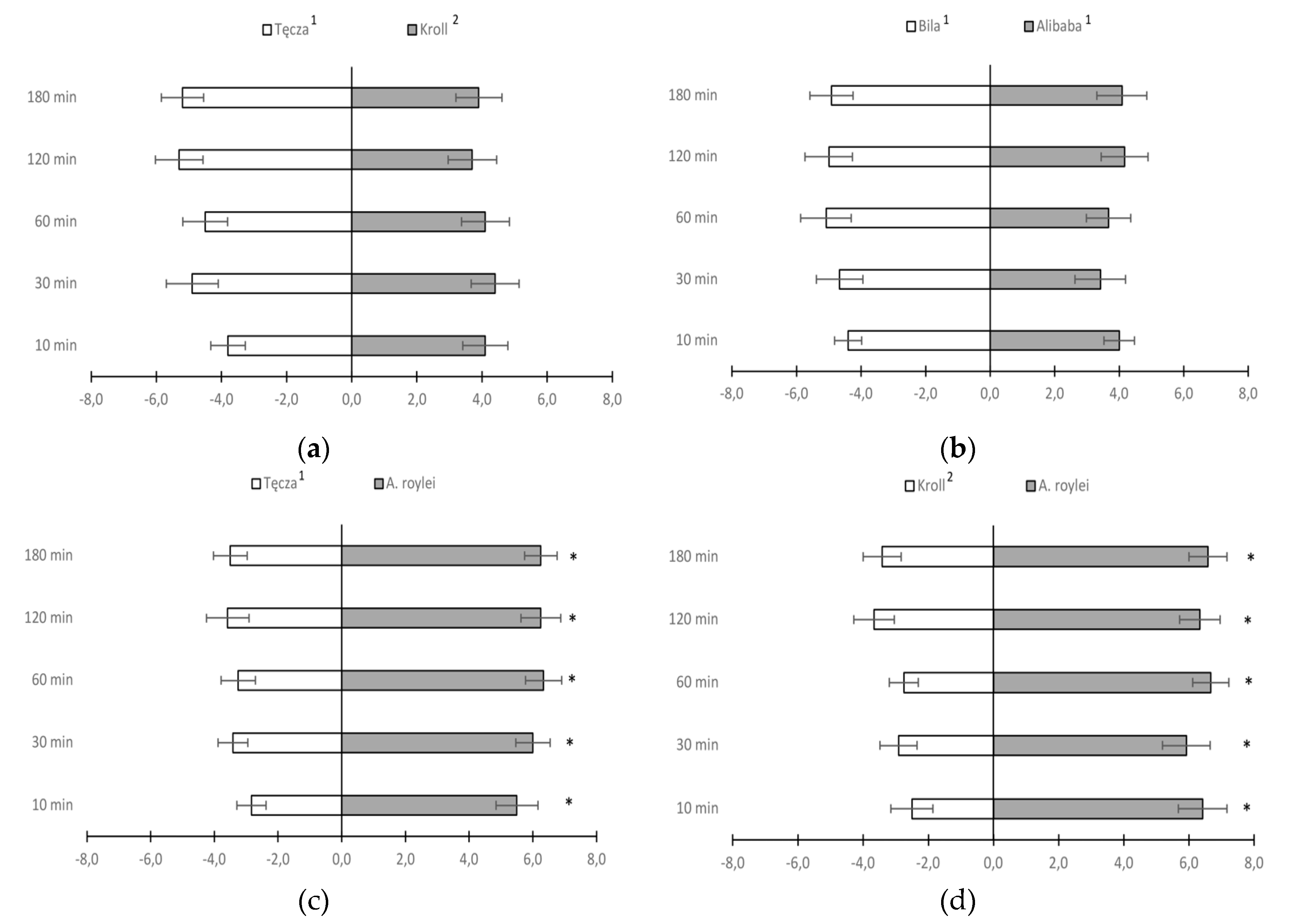
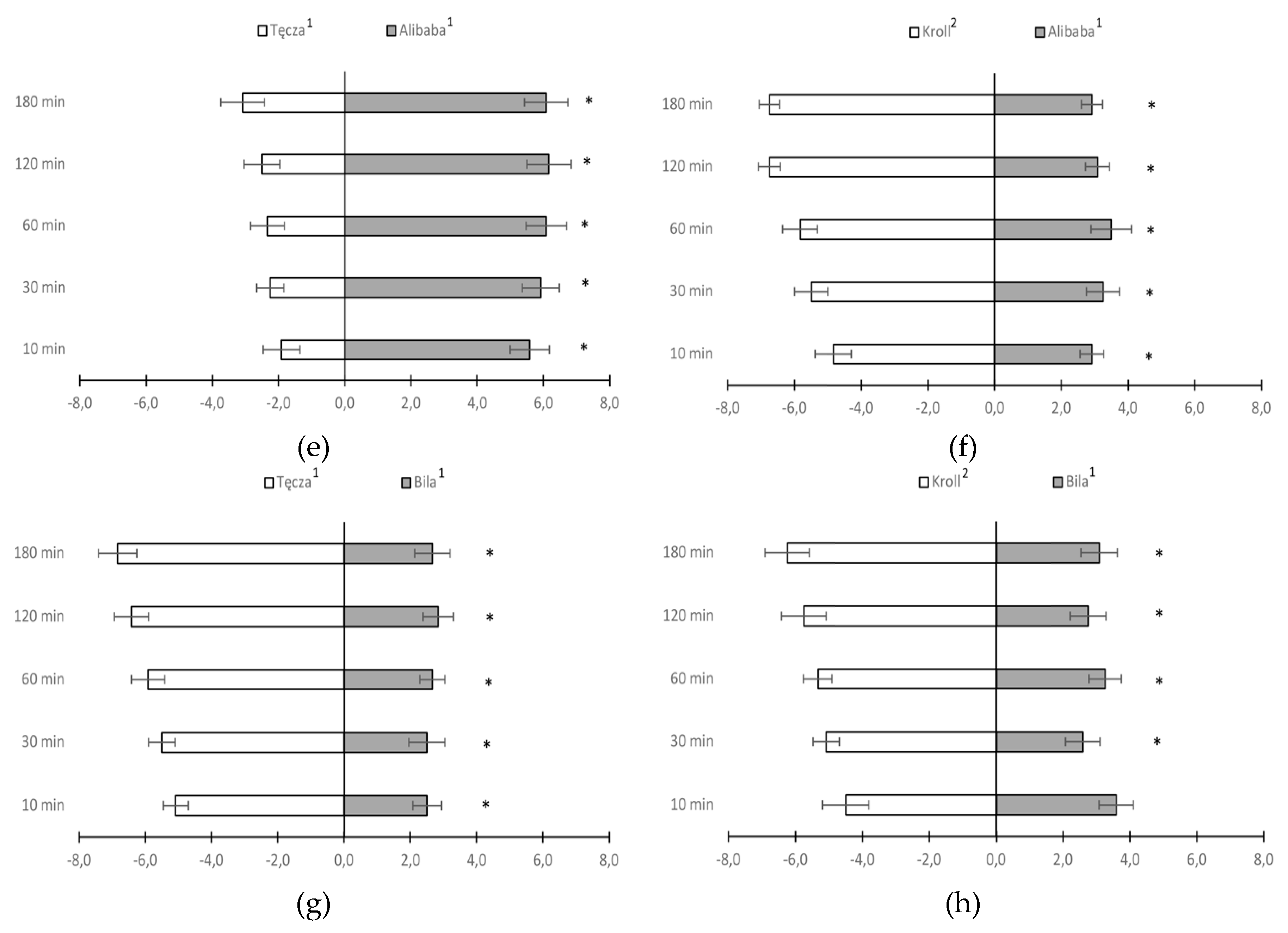
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Commodity Production Data; Food and Agriculture Organization: Rome, Italy, 2021. [Google Scholar]
- Mishra, R.K.; Jaiswal, R.K.; Kumar D.; Saabal P.R; Singh A. Management of major diseases and insect pests of onion and garlic: A comprehensive review. J. Plant Breed. Crop. Sci. 2014, 6, 160–170. [CrossRef]
- Schwartz, H.F; Mohan, S.K. Compendium of Onion and Garlic Diseases; APS Press: St. Paul, MN, USA, 1985.
- Diaz-Montano, J.; Fuchs, M.; Nault, B.A.; Fail, J.; Shelton, A.M. Onion thrips (Thysanoptera: Thripidae): a global pest of increasing concern in onion. J. Econ. Èntomol. 2011, 104, 1–13. [Google Scholar] [CrossRef]
- Gent, D.H.; du Toit, L.J.; Fichtner, S.F.; Mohan, S.K.; Pappu, H.R.; Schwartz, H.F. Iris yellow spot virus: An Emerging Threat to Onion Bulb and Seed Production. Plant Dis. 2006, 90, 1468–1480. [Google Scholar] [CrossRef]
- Grode, A.; Chen, S.; Walker, E.D.; Szendrei, Z. Onion thrips (Thysanoptera: Thripidae) feeding promotes infection by Pantoea ananatis in onion. J. Econ. Entomol. 2017, 110, 2301–2307. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.K.; Garg, H.; Gill, A.K.; Gillett-Kaufman, J.L.; Nault, B.A. Onion Thrips (Thysanoptera: Thripidae) Biology, Ecology, and Management in Onion Production Systems. J. Integr. Pest Manag. 2015, 6, 6–6. [Google Scholar] [CrossRef]
- Lewis, T. Pest thrips in perspective. In Thrips as Crop Pests; Lewis, T., Ed.; CAB International: New York, NY, USA, 1997; pp. 1–13. ISBN 9780851991788. [Google Scholar]
- Martin, N.A.; Workman, P.J. A new bioassay for determining the susceptibility of onion(Allium cepa)bulbs to onion thrips,Thrips tabaci(Thysanoptera: Thripidae). New Zealand J. Crop. Hortic. Sci. 2006, 34, 85–92. [Google Scholar] [CrossRef]
- Adesanya, A.W.; Waters, T.D.; Lavine, M.D.; Walsh, D.B.; Lavine, L.C.; Zhu, F. Multiple insecticide resistance in onion thrips populations from Western USA. Pestic. Biochem. Physiol. 2020, 165, 104553. [Google Scholar] [CrossRef]
- Shelton, A.M.; Zhao, J.-Z.; Nault, B.A.; Plate, J.; Musser, F.R.; Larentzaki, E. Patterns of Insecticide Resistance in Onion Thrips (Thysanoptera: Thripidae) in Onion Fields in New York. J. Econ. Èntomol. 2006, 99, 1798–1804. [Google Scholar] [CrossRef]
- Karar, H.; Abbas, G.; Hameed, A. ; Ahmad. G.; Ali, A. Losses in Onion (Allium cepa) Due to Onion Thrips (Thrips tabaci) (Thysanoptera: Thripidae) and Effect of Weather Factors on Population Dynamics of Thips. World Appl Sci J. 2014, 32 (11), 2250-2258. [Google Scholar] [CrossRef]
- Kendall, D.M.; Capinera, J.L. Susceptibility of Onion Growth Stages to Onion Thrips (Thysanoptera: Thripidae) Damage and Mechanical Defoliation. Environ. Èntomol. 1987, 16, 859–863. [Google Scholar] [CrossRef]
- Diaz-Montano, J.; Fail, J.; Deutschlander, M.; Nault, B.A.; Shelton, A.M. Characterization of Resistance, Evaluation of the Attractiveness of Plant Odors, and Effect of Leaf Color on Different Onion Cultivars to Onion Thrips (Thysanoptera: Thripidae). J. Econ. Èntomol. 2012, 105, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Damon, S.J.; Groves, R.L.; Havey, M.J. Variation for Epicuticular Waxes on Onion Foliage and Impacts on Numbers of Onion Thrips. J. Am. Soc. Hortic. Sci. 2014, 139, 495–501. [Google Scholar] [CrossRef]
- Munaiz, E.D.; Groves, R.L.; Havey, M.J. Amounts and Types of Epicuticular Leaf Waxes among Onion Accessions Selected for Reduced Damage by Onion Thrips. J. Am. Soc. Hortic. Sci. 2020, 145, 30–35. [Google Scholar] [CrossRef]
- Ferreira, G.d.O.; Santos, C.A.F.; Oliveira, V.R.; de Alencar, J.A.; da Silva, D.O.M. Evaluation of onion accessions for resistance to thrips in Brazilian semi-arid regions. J. Hortic. Sci. Biotechnol. 2017, 92, 550–558. [Google Scholar] [CrossRef]
- da Silva, V.C.P.; Bettoni, M.M.; Bona, C.; Foerster, L.A. Morphological and chemical characteristics of onion plants (Allium cepa L.) associated with resistance to onion thrips. Acta Sci. Agron. 2014, 37, 85. [Google Scholar] [CrossRef]
- Njau, G.M.; Nyomora, A.M.S.; Dinssa, F.F.; Chang, J.-C.; Malini, P.; Subramanian, S.; Srinivasan, R. Evaluation of onion (Allium cepa) germplasm entries for resistance to onion thrips, Thrips tabaci (Lindeman) in Tanzania. Int. J. Trop. Insect Sci. 2017, 37, 98–113. [Google Scholar] [CrossRef]
- Pobożniak, M.; Olczyk, M.; Wójtowicz, T.; Kamińska, I.; Hanus-Fajerska, E.; Kostecka-Gugała, A.; Kruczek, M. Anatomical and Biochemical Traits Associated with Field Resistance of Onion Cultivars to Onion Thrips and the Effect of Mechanical Injury on the Level of Biochemical Compounds in Onion Leaves. Agronomy 2022, 12, 147. [Google Scholar] [CrossRef]
- Kamal, N.; Nourbakhsh, S.S.; Cramer, C.S. Reduced Iris Yellow Spot Symptoms through Selection within Onion Breeding Lines. Horticulturae 2021, 7, 12. [Google Scholar] [CrossRef]
- Raut, A.; Pal, S.; Wahengbam, J.; Banu, A.N. Population dynamics of onion thrips (Thrips tabaci lindeman, Thysanoptera; Thripidae) and varietal response of onion cultivars against onion thrips. J. Èntomol. Res. 2020, 44, 547–554. [Google Scholar] [CrossRef]
- Kik, C. Exploitation of wild relatives for the breeding of cultivated Allium species. In Allium Crop Science: Recent Advances; Rabinowitch, H.D., Currah, L., Eds.; CABI Publishing Oxon: Oxford, UK, 2002; pp. 81–100. ISBN 9780851995106. [Google Scholar]
- Chuda, A. Hybridization and Molecular Characterization of F1 Allium Cepa × Allium Roylei Plants. Acta Biol. Cracoviensia Ser. Bot. 2012, 54, 25–31. [Google Scholar] [CrossRef]
- Shigyo, M.; Kik, C. Onion. In Vegetables II: Fabaceae, Liliaceae, Solanaceae, and Umbelliferae; Prohens-Tomás, J., Nuez, F., Handbook of Plant Breeding, Vegetables, Eds.; Springer: New York, NY, USA, 2008; pp. 121–159. ISBN 978-0-387-74108-6. [Google Scholar]
- Kohli, B.; Gohil, R.N. Need to conserve Allium roylei Stearn: a potential gene reservoir. Genet. Resour. Crop. Evol. 2009, 56, 891–893. [Google Scholar] [CrossRef]
- Kofoet, A.; Kik, C.; Wietsma, W.A.; Vries, J.N. Inheritance of Resistance to Downy Mildew (Peronospora destructor [Berk.] Casp.) from Allium roylei Stearn in the Backcross Allium cepa L. x (A. roylei x A. cepa). Plant Breed. 1990, 105, 144–149. [Google Scholar] [CrossRef]
- Scholten, O.E.; van Heusden, A.W.; Khrustaleva, L.I.; Burger-Meijer, K.; Mank, R.A.; Antonise, R.G.C.; Harrewijn, J.L.; Van Haecke, W.; Oost, E.H.; Peters, R.J.; et al. The long and winding road leading to the successful introgression of downy mildew resistance into onion. Euphytica 2007, 156, 345–353. [Google Scholar] [CrossRef]
- de Vries, J.N.; Wietsma, W.A.; de Vries, T. Introgression of leaf blight resistance from Allium roylei Stearn into onion (A. cepa L.). Euphytica 1992, 62, 127–133. [Google Scholar] [CrossRef]
- Rian, V.; Ford-Lloyd, S.; Armstrong, J. 5 - Welsh onion: Allium fistulosum L., In: Genetic Improvement of Vegetable Crops; Kalloo, G., Bergh, B.O., Eds.; Pergamon, 1993, pp. 51-58.
- Peffley, E.B.; Hou, A. Bulb-type onion introgressants posessing Allium fistulosum L. genes recovered from interspecific hybrid backcrosses between A. cepa L. and A. fistulosum L. Theor. Appl. Genet. 2000, 100, 528–534. [Google Scholar] [CrossRef]
- Yamashita, K.-I.; Takatori, Y.; Tashiro, Y. Chromosomal location of a pollen fertility-restoring gene, Rf, for CMS in Japanese bunching onion (Allium fistulosum L.) possessing the cytoplasm of A. galanthum Kar. et Kir. revealed by genomic in situ hybridization. Theor. Appl. Genet. 2005, 111, 15–22. [Google Scholar] [CrossRef]
- Pobożniak, M.; Leśniak, M.; Chuda, A.; Adamus, A. Field assessment of the susceptibility of onion cultivars to thrips attack – preliminary results. Pol. J. Èntomol. 2016, 85, 121–133. [Google Scholar] [CrossRef]
- Loomans, A. J. M.; Murai, T. Culturing thrips and parasitoids. In Thrips as Crop Pests; Lewis, T., Ed.; CAB International: Wallingford, Oxon, UK, 1997; pp. 477–503. [Google Scholar]
- Diaz-Montano, J.; Fuchs, M.; Nault, B.A.; Shelton, A.M. Evaluation of Onion Cultivars for Resistance to Onion Thrips (Thysanoptera: Thripidae) and Iris Yellow Spot Virus. J. Econ. Èntomol. 2010, 103, 925–937. [Google Scholar] [CrossRef]
- Mayhew, P.J. Adaptive Patterns of Host-Plant Selection by Phytophagous Insects. Oikos 1997, 79, 417. [Google Scholar] [CrossRef]
- Thompson, J.N. Evolutionary ecology of the relationship between oviposition preference and performance of offspring in phytophagous insects. Èntomol. Exp. Appl. 1988, 47, 3–14. [Google Scholar] [CrossRef]
- Mc Collum, G.D. Experimental Hybrids between Allium fistulosum and A. roylei. Bot. Gaz. 1982, 143, 238–242. [Google Scholar] [CrossRef]
- Hanafy, A.R.I.; Tahany, R.A.; Nowar, E.E.; Hasan, S.M. Effect of anatomical and phytochemical diversity of two onion cultivars on the infestation with onion thrips (Thysanoptera: Thripidae). Middle East J. Appl. Sci. 2016, 6, 941–948. [Google Scholar]
- da Silva, V.C.P.; Bettoni, M.M.; Bona, C.; Foerster, L.A. Morphological and chemical characteristics of onion plants (Allium cepa L.) associated with resistance to onion thrips. Acta Sci. Agron. 2014, 37, 85. [Google Scholar] [CrossRef]
- Brown, A.S.S.; Simmonds, M.S.J.; Blaney, W.M. Relationship between nutritional composition of plant species and infestation levels of thrips. J. Chem. Ecol. 2002, 28, 2399–2409. [Google Scholar] [CrossRef] [PubMed]
- Steenbergen, M.; Abd-El-Haliem, A.; Bleeker, P.; Dicke, M.; Escobar-Bravo, R.; Cheng, G.; A Haring, M.; Kant, M.R.; Kappers, I.; Klinkhamer, P.G.L.; et al. Thrips advisor: exploiting thrips-induced defences to combat pests on crops. J. Exp. Bot. 2018, 69, 1837–1848. [Google Scholar] [CrossRef]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef]
- Khalid, M.; Rahman, S.U.; Bilal, M.; Huang, D. Role of flavonoids in plant interactions with the environment and against human pathogens — A review. J. Integr. Agric. 2019, 18, 211–230. [Google Scholar] [CrossRef]
- Taylor, A.; Vagany, V.; Barbara, D.J.; Thomas, B.; Pink, D.A.C.; Jones, J.E.; Clarkson, J.P. Identification of differential resistance to sixFusarium oxysporumf. sp. cepaeisolates in commercial onion cultivars through the development of a rapid seedling assay. Plant Pathol. 2012, 62, 103–111. [Google Scholar] [CrossRef]
- Zheng, S. , Henken, B. , Wietsma, W. et al. Development of bio-assays and screening for resistance to beet armyworm (Spodoptera Exigua Hübner) in Allium cepa L. and its wild relatives. Euphytica 2000, 114, 77–85. [Google Scholar] [CrossRef]
- Hudák, K.; Pénzes, B. Factors influencing the population of the onion thrips on onion. Acta Phytopathol. et Èntomol. Hung. 2004, 39, 193–197. [Google Scholar] [CrossRef]
- Ren, X.; Wu, S.; Xing, Z.; Gao, Y.; Cai, W.; Lei, Z. Abundances of thrips on plants in vegetative and flowering stages are related to plant volatiles. J. Appl. Èntomol. 2020, 144, 732–742. [Google Scholar] [CrossRef]
- Jones, H.A.; Bailey, S.F.; Emsweller, S.L. Field Studies of Thrips tabaci Lind. With Especial Reference to Resistance in onions. J. Econ. Èntomol. 1935, 28, 678–680. [Google Scholar] [CrossRef]
- Porter, D.R. , Jones, H., A. Resistance of some cultivated species of Allium to pink root (Phoma terrestrs). Phytopatology 1933, 23(29), 298.
- Jones, H.A.; Mann, L.K. Onions and Their Allies; London Leonard Hill [Books] Limited Interscience Publishers, Inc.: New York, NY, USA, 1963; p. 284. [Google Scholar]
- Cryder, C.M. A study of the associations of heritable traits in progeny from the interspecific backcross (Allium fistulosum x Allium cepa L.) x Allium cepa L. Ph.D. Thesis, New Mexico State University, Las Cruces, USA, 1988. [Google Scholar]
- Sueyoshi, T.; Shimomura, K.; Koga, T.; Yamamura, Y.; Takemoto, H. The varietal difference in resistance to stone leek leaf miner in Welsh onions. Bull. Fukuoka Agric. Res. Cent. 2006, 25, 37–41. [Google Scholar]
- Takeda, M.; Kawai, A.; Mitsunaga, T.; Tsukazaki, H.; Yamashita, K.-I.; Wako, T. A novel method for evaluating the egg killing defenses and varietal resistance of the bunching onion against Liriomyza chinensis (Diptera: Agromyzidae) via the artificial inoculation of eggs. Appl. Èntomol. Zoöl. 2019, 55, 93–103. [Google Scholar] [CrossRef]
- Khandagale, K.; Roylawar, P.; Randive, P.; Karuppaiah, V.; Soumia, P.S.; Shirsat, D.; Gedam, P.; Ade, A.; Gawande, S.; Singh, M. Isolation and Expression Profiling of Insecticidal Lectins from Wild Alliums Against Onion Thrips (Thrips tabaci Lindeman). Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 2020, 92(2), 451–459. [CrossRef]
- Tsukazaki, H.; Honjo, M.; Yamashita, K.-I.; Ohara, T.; Kojima, A.; Ohsawa, R.; Wako, T. Classification and identification of bunching onion (Allium fistulosum) varieties based on SSR markers. Breed. Sci. 2010, 60, 139–152. [Google Scholar] [CrossRef]
- Basri, R.; Ansari, M.S. Analytical study of phenotypic and biochemical attributes of onion cultivars in relation to infestation of onion thrips, Thrips tabaci. J. Asia-Pacific Èntomol. 2021, 24, 529–535. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




