1. Introduction
Iron is the fourth element in the crust [
1], which is one of the most widely distributed variable valence metal elements on the earth. In the environment, it usually exists in two states: in the oxidized trivalent iron Fe (III), most of them are insoluble in the neutral pH environment and can be reduced, while the reductive iron Fe (II) is usually soluble ion, so it is easier to be used by organisms and can be oxidized [
2]. Microbial mediated dissimilation of iron reduction is the main pathway of Fe (III) oxide reduction in nature [
3]. Most microorganisms from archaea and bacterial domain can metabolize and utilize Fe (III) / Fe (II) redox potential and various electron donors and receptors [
4]. Fe (III) oxides have high element abundance in natural environment and are very sensitive to redox environment [
5].
Fe (III) microbial reduction is of great significance to the treatment of pollutants, and the reduction process not only strongly affects the cycle of C, N, S, P and other elements in the environment, but also plays an important role in the transformation and degradation of organic and inorganic pollution [
6]. Including pollutants released from industry and mining areas [
7,
8]. Iron-reducing bacteria usually live in natural environments such as soil, river sediment, estuary sediments, petroleum layer, groundwater and hot springs [
2]. In many sedimentary environments, the most abundant alternative electron receptor for anaerobic respiration is Fe (III) oxide [
9]. At the same time, there are abundant iron oxides (such as fibrite, goethite, pyrite, hemite, etc.), which is an important iron ore on the earth [
10,
11,
12,
13,
14,
15,
16]. Therefore, the bioreduction of iron widely exists in the underground environment. These forms of iron oxide are basically insoluble, with higher thermodynamic stability and stronger crystal structure, resulting in the decrease of bioavailability and pollutant removal efficiency. However, organic matter with redox activity is common in sediments and aquatic systems [
17], in which quinyl functional groups can act as electron shuttles, forming electronic shuttles between Fe (III) and microorganisms, thus speeding up the electron transfer rate between microorganisms and extracellular electron receptors [
6]. Thus, the bioavailability of Fe (III) can be improved, and the goethite produced in the reaction process can also promote the transformation of iron oxides. This process also affects the migration and transformation of pollutants [
18], It plays an important role in controlling environmental pollution in groundwater and soil.
In this experiment, the effect of electron shuttle AQS and aniline on the biological reduction of Fe (III) was studied. The concentrations of Fe (II), sodium lactate and aniline were dynamically measured during Fe (III) reduction, and the reduced minerals were characterized by X-ray diffraction (XRD), X-ray energy dispersive spectroscopy (XPS) and scanning electron microscopy (SEM).
2. Materials and Methods
2.1. Culture conditions
Shewanella oneidensis MR-1 was purchased from the Marine Microbial Strain Collection and Management Centre of China (MCCC 1A00048), and MR-1 is a parthenogenetic anaerobic bacterium. The MR-1were placed on a sterile operating table before inoculation, and the strains were placed into LB liquid medium (yeast extract 5 g/L, peptone 10 g/L, sodium chloride 10 g/L) [
19] using an inoculation loop for aerobic activation to logarithmic growth phase, poured into 50 mL centrifugal tubes, and centrifuged in a centrifuge for 10 min (8000 r/min). After centrifugation, the supernatant was poured off and the flesh red bacteria were resuspended in sterile water.
2.2. Goethite synthesis and characterization
Prepared according to the method of
Schwertmann and
Cornell [
20], in a polyethylene bottle containing 100 mL of 1M Fe(NO
3)
3 solution, 180 mL of 5M KOH solution was quickly added and stirred, diluted to 2 L with deionised water and then placed in a water bath at 70°C for aging for 60 h. The solid precipitates were collected and washed to neutrality with ultrapure water. Finally, it was dried in an oven at 70°C and ground to less than 200 mesh for spare use.
2.3. Fe (III) reduction experiment design
In order to investigate the effect of AQS on the reduction of Fe(III) by Shewanella oneidensis MR-1, a constant temperature anaerobic incubation was used: sodium lactate was used as the only carbon source, and the concentration of AQS was set to (0.0, 0.1, 0.3, 0.5 mM) while a group without MR-1 was set as the experimental control group. Three sets of parallel experiments were set up for each group, and 2 mL of suspension was taken at fixed intervals to determine the concentrations of Fe(II) and sodium lactate, while the solid mineral acicular ferrite was characterised by SEM, XRD and XPS at the end of the reaction.
To explore the influence of different concentrations of aniline on Fe(III) bioreduction mediated by AQS, an anaerobic incubation at constant temperature was used, and aniline was added to the Fe reduction medium containing 0.5 mM AQS, and the concentrations of aniline were set to be (0.0, 1, 3, and 7 μM); at the same time a group of no-aniline-added was set up as a control group, and the rest of the experimental conditions were consistent. Finally, it was placed in dark light incubation at 30°C in a constant temperature incubator, and three sets of parallel experiments were set up for each group, and 2 mL of suspension was taken at fixed intervals to determine the concentrations of Fe(II), sodium lactate and aniline, while the solid mineral acicular ferrite was characterised by SEM, XRD and XPS at the end of the reaction.
2.4. Chemical analysis
The Fe(II) concentration in the reducing system was determined using the 1,10-phenanthroline spectrophotometric method [
21], where the sample was acidified in 0.5 mM hydrochloric acid for 15 min and then placed in a centrifuge for 10 min to collect the suspension, and the colour was developed by the addition of 0.5% o-phenanthroline chromogenic agent for 15~20 min and then adding acetic acid-ammonium acetate slow flushing solution. Measuring at 510 nm using violet spectrophotometer.
High performance liquid chromatography (Agilent 1260) with C18 column was used to detect the content of sodium lactate in the supernatant during the reduction of Fe(III). After the supernatant sample was taken with a sterile syringe, it was filtered through a 0.22 µM organic filter tip and detected by HPLC with an injection volume of 20 µL. The mobile phase was 95% phosphate (0.1%, pH 3.5) and 5% acetonitrile at a flow rate of 1 mL min-1.
The concentration of aniline was determined by using the method of blowing trap-gas chromatography-mass spectrometry (GC: Atomx, Dentley Tekmar; GC-MS: Thermo Fisher Scientific). The operating conditions were as follows: the purge program was set at a purge gas flow rate of 40 mL/min, a purge time of 11 min, a thermal resolution time of 2 min, a resolution temperature of 190 °C, a baking time of 6 min and a temperature of 200 °C; the temperature of the inlet port of the gas chromatography was 220 °C; the sample was injected by shunt at a flow rate of 30 mL/min, and a shunt ratio of 30:1; the carrier gas was high-purity helium; and the temperature-rising program was set at an initial column temperature of 35 °C, and maintained for 2 hours. The initial column temperature was 35 °C, held for 2 min, then increased to 100°C at 5°C/min, and then increased to 200 °C at 10 °C/min, held for 1 min. The EI ion source was used, and the full scanning range was from 35 to 270; the quantification was done by the combination of external standard and internal standard method.
2.5. Solid Mineral characterization
An X-ray diffractometer (Thermo Fisher Rigaku Ultima IV) was used for the qualitative analysis of the minerals. The experimental samples were centrifuged, dried and then ground into powder by agate mortar and sieved through 200 mesh sieve and then tested on the machine by pressing method. The test conditions were as follows: test voltage 40kV, test current 40mA, Cu Kα ray, test angle range 5°~90°, scanning speed 5°/min, step scanning, step size 0.02°, and the experimental data were analysed in MDI Jade 6.0 software.
In order to determine the changes in the surface iron valence state of iron oxides before and after the experiment, x-ray photoelectron spectroscopy (XPS) (Thermo Fisher Escalab Xi+) was used on the samples.XPS was performed using a monochromatic aluminium card x-ray source. Test conditions: vacuum 3 × 10-7 Pa. The binding energy of the C(1s) energy level at 284.6 eV was taken as the reference binding energy. (b) Surface full-spectrum scanning of the samples was performed to collect high-resolution spectra of Fe2p, which were analysed by subtracting the baseline and performing a least-squares fit using the Gauss-Lorentz equation in Avantage software.
Scanning electron microscopy (SEM) was used to observe the morphology of iron minerals and precipitates after Fe(III) reduction experiments (Oxford Ultim Max65, Thermo Fisher, UK). The reduced minerals were dried by centrifugation and observed.
2.6. Kinetic analyses
The consumption of sodium lactate and aniline in the medium over time was fitted according to a quasi-primary kinetic model.
Where C0 is the initial concentration of aniline or sodium lactate at t=0, Ct(mM) is the concentration of aniline or sodium lactate at t(h), and K is the first-order reaction rate constant mM/h-1 or the first-order reaction rate constant μM/h-1 for aniline.
3. Results
3.1. Characterizations of Goethite
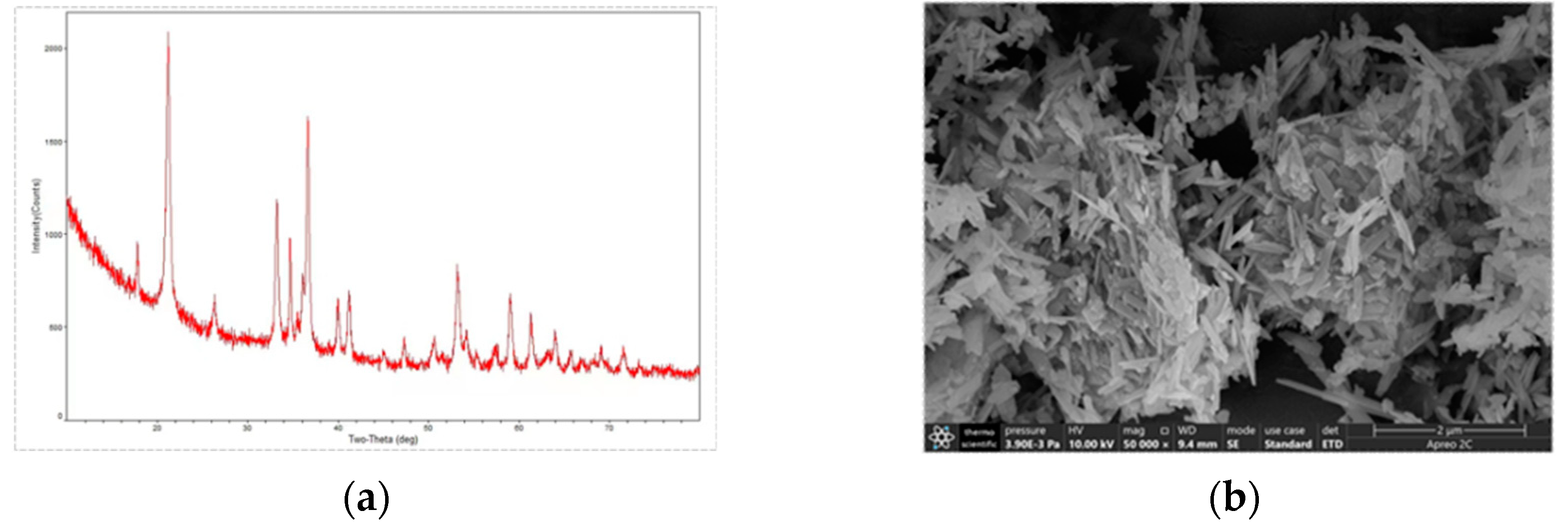
XRD and SEM were used to characterize the synthesized goethite, and the XRD map of the prepared goethite (
Figure 1a) was compared with the standard mineral ( Goethite Standard Card No. 29-0713), and the results of the comparison showed that the synthesized yellow mineral was pure goethite, which did not contain any other crystalline phases; and the morphology of prepared goethite was observed to be long-needle shape under the scanning electron microscope SEM (
Figure 1b).
3.2. Bioreductive transformation of Fe(III)
3.2.1. Effect of AQS on the reduction of Fe(III) by MR-1
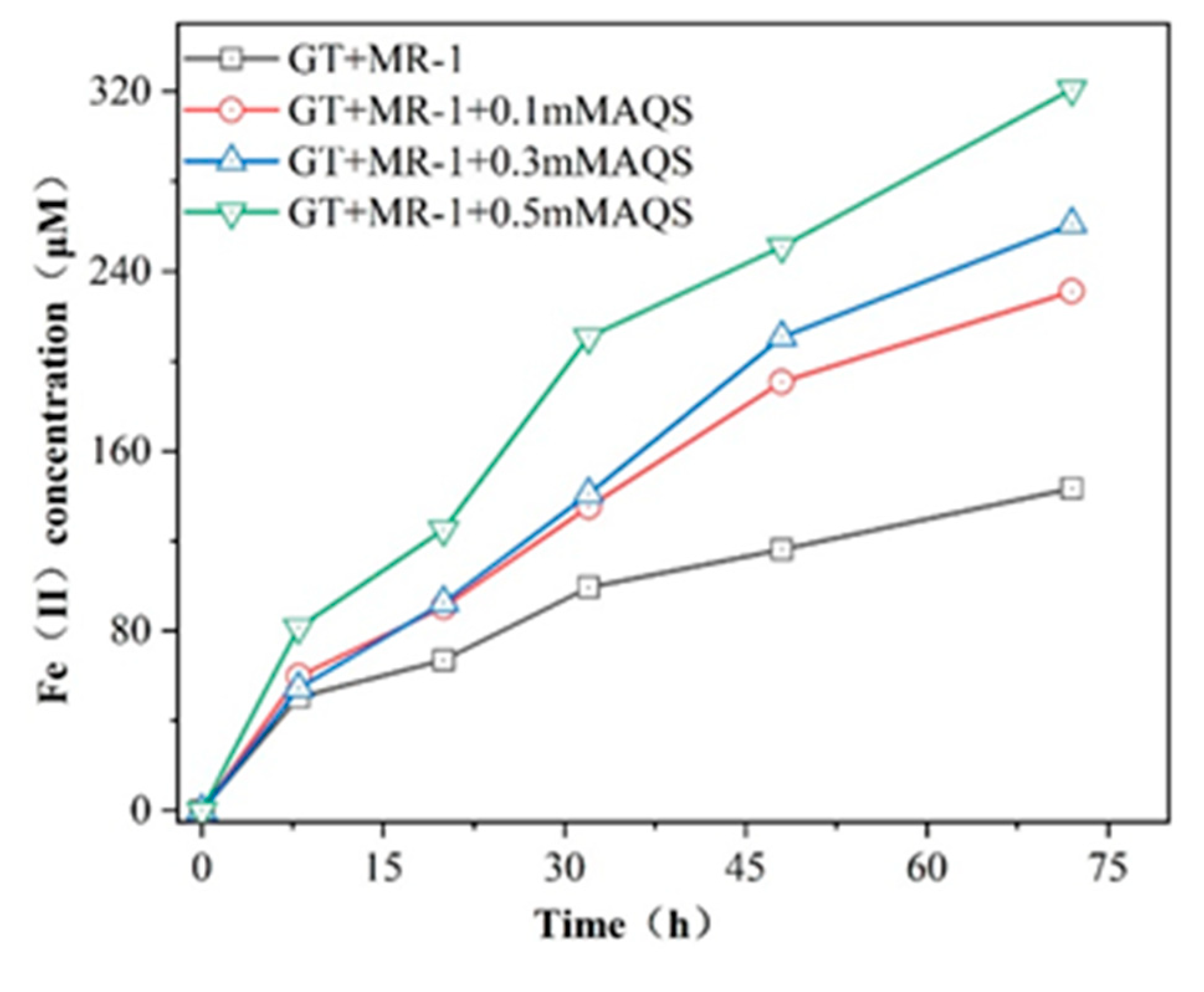
In order to investigate the effect of different concentrations of electron shuttle AQS on the bioreduction of Fe (III), different concentrations of AQS were added to the reduction of goethite by MR-1. The production of Fe (II) during goethite bioreduction mediated by AQS is shown in figure 2. As expected, the electronic shuttle AQS greatly accelerates the reduction process. In the presence of MR-1 alone, the amount of Fe (II) released into the reaction system during goethite reduction was 143.3 μM, while the formation of Fe (II) in the 0.1, 0.3 and 0.5mM AQS reaction group was 1.61, 1.82 and 2.23 times higher than that in the goethite reduction reaction group respectively. This indicates that AQS acts as an electronic shuttle, accelerates the electron transfer between MR-1 and goethite, and leads to the release of a large number of Fe (II) into the reaction system during goethite reduction.
3.2.2. Effect of aniline on AQS-mediated reduction of Fe(III) by MR-1
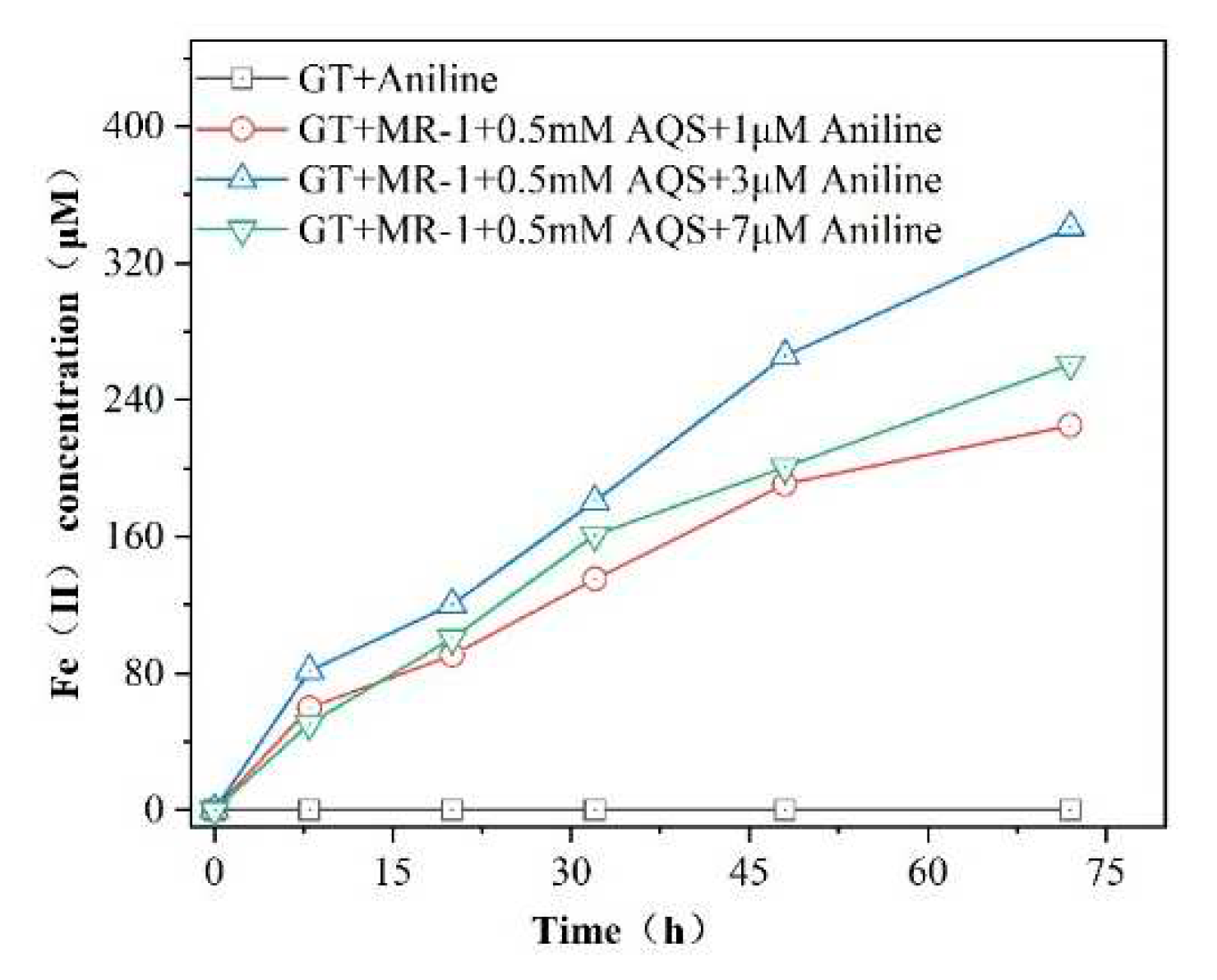
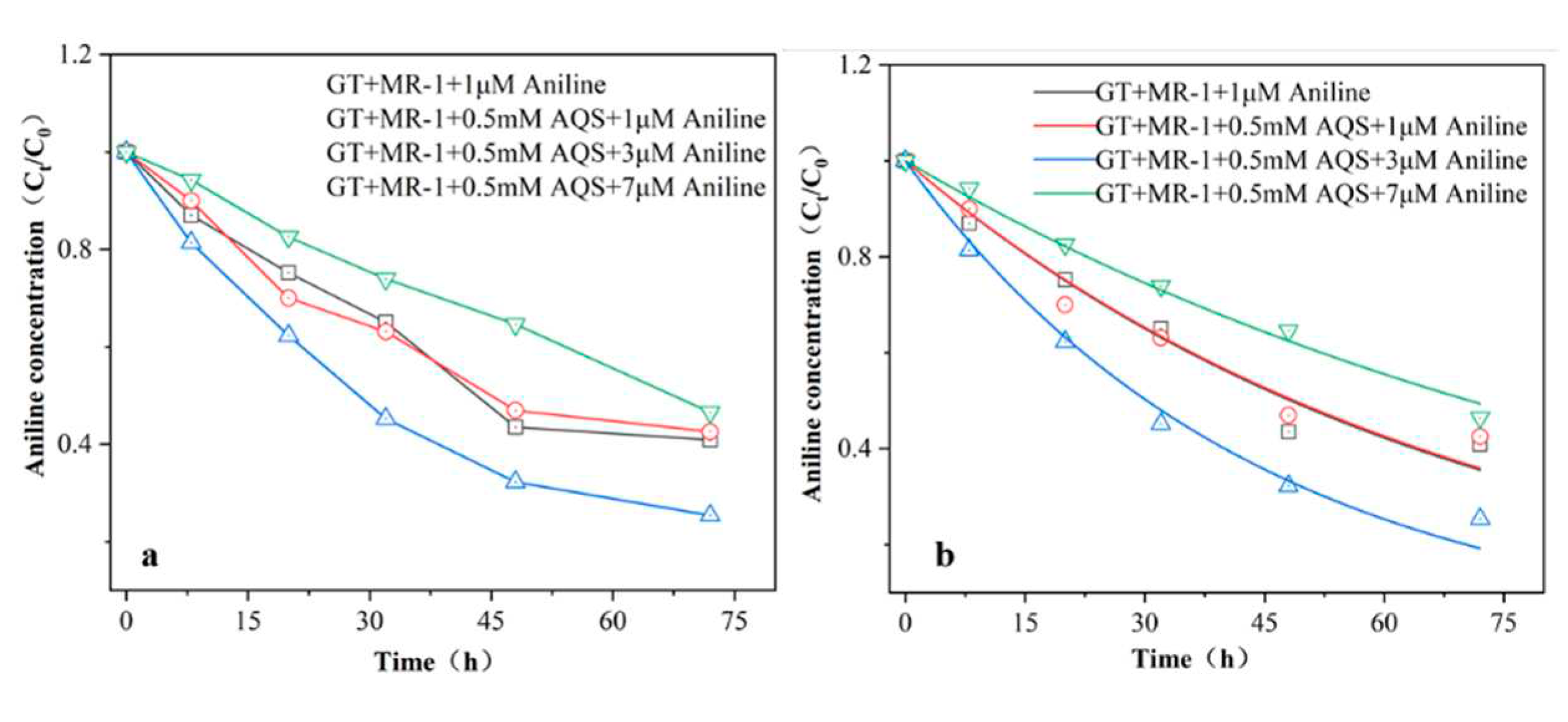
The effect of different concentrations of aniline on the AQS-mediated bioreduction of goethite is shown in
Figure 3. The production of Fe(II) in the reaction system with different concentrations of aniline added increased with time. Among them, the addition of 3μM aniline had the most significant effect on the AQS-mediated promotion of acicular ferrite bioreduction, with Fe(II) production being 1.07 times higher than that of the reaction system containing 0.5 mMAQS .When the concentration of aniline was increased to 7μM, a decreasing trend in Fe(II) production was observed. In the reaction system without the addition of MR-1, no Fe(II) production was detected during the reaction period, indicating that the goethite was not reduced and the pollutant aniline could not directly induce the reduction of Fe(III) oxides, and the addition of aniline was able to promote the reduction of Fe(III), probably due to the interaction with the iron-reducing bacteria.
3.2.3. Consumption and Kinetic Analysis of Sodium Lactate
The bioreduction of Fe (III) oxides is an electron transfer process dominated by microorganisms. The use of sodium lactate as the only source of carbon source and energy in the reduction process is one of the key characteristics of MR-1 metabolism[
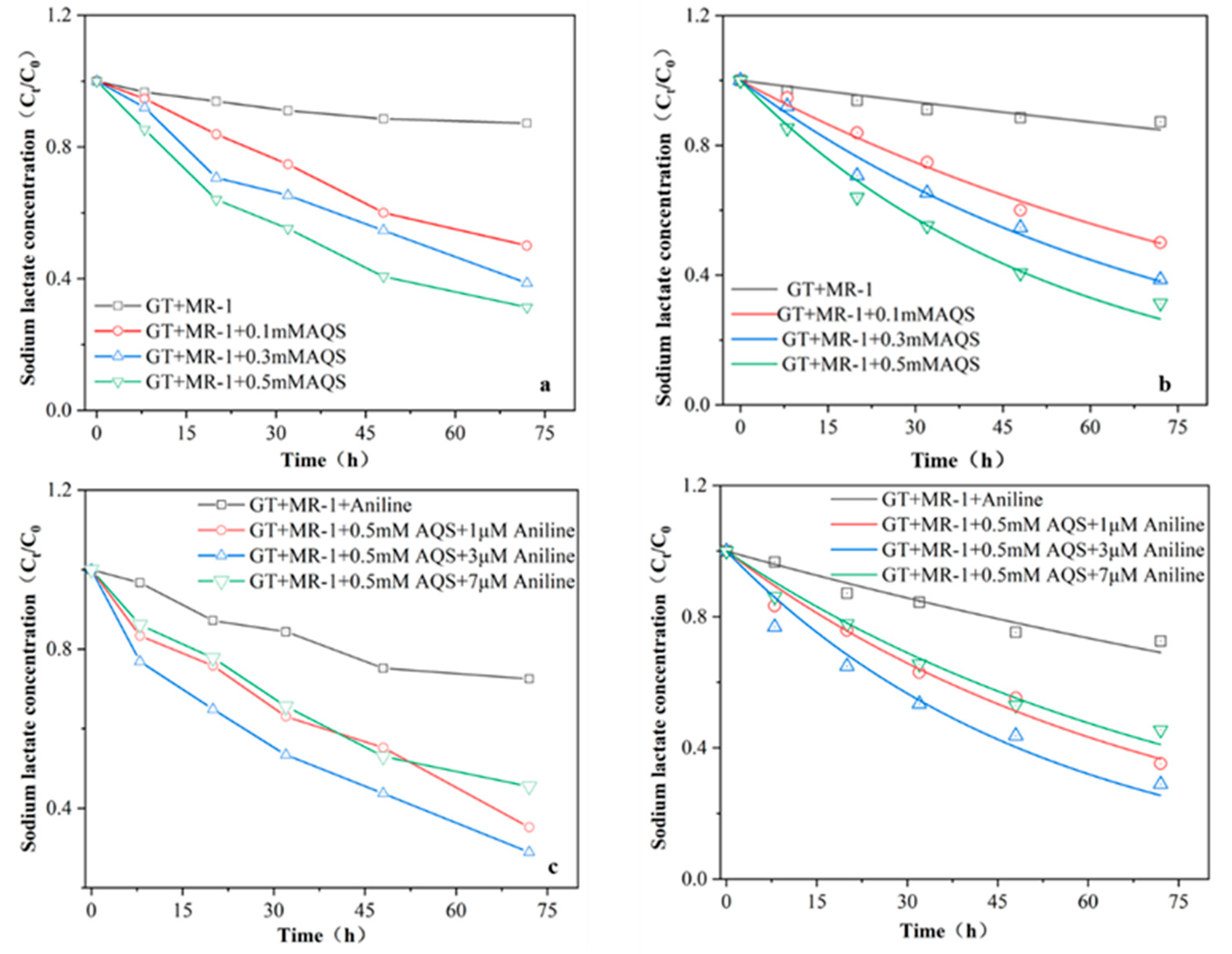
22]. The concentration of sodium lactate in the reaction system was determined to verify whether the electron shuttle AQS and aniline could accelerate the consumption of sodium lactate, the electron donor, and promote the bioreduction of Fe (III). The results showed(
Figure 4) that the consumption of sodium lactate increased with the increase of AQS concentration, and the consumption of sodium lactate in the 0.5mM AQS reaction group within 72 hours was 1.44 times higher than that in the simple microbial reaction group. In the experimental group, the consumption of sodium lactate in the system was the highest with the addition of 3μM. The consumption of sodium lactate was quantitatively analyzed by first-order kinetics. When only microbial MR-1 was used to reduce goethite alone, the first order kinetic constant K of sodium lactate consumption was only 0.0023 ±0.002 M/h-1. When 0.1, 0.3 and 0.5mM AQS were added, the K value increased to 0.0097 ±0.002, 0.013 ±0.001 and 0.018 ±0.002 M/h-1, respectively. These data show that the addition of AQS does stimulate the metabolism of microbial MR-1. The consumption of sodium lactate has been increased so that more electrons can quickly participate in the Fe (III) bioreduction process. When 1,3 μM and 7 μM Aniline was added with 0.5mMAQS, the K values were 0.014 ±7.51 μM /h
-1, 0.019 ±0.001 μM/h
-1 and 0.012 ±6.39 μM/h
-1, respectively. the consumption of sodium lactate in the reaction system supplemented with 3 μM was significantly higher than that in the AQS group. According to previous reports, adding benzene can increase lactic acid consumption, promote microbial metabolism and provide more electrons for Fe (III). After benzene treatment, the permeability of cell membrane is enhanced and contributes to cell metabolism and electron consumption [
23]. Therefore, the addition of Aniline to accelerate the bioreduction of Fe (III) by MR-1 was due to the increase of cell membrane permeability and the consumption of sodium lactate to make more electrons participate in the reaction process.
3.2.4. Degradation and kinetic analysis of aniline
The concentration of aniline varied as shown in
Figure 5a, showing a slow decreasing trend with time. The change in aniline concentration was small at a concentration of 1μM; then it is possible that the concentration of the pollutant was low and the microorganisms were not able to fully utilise it. By fitting the first-order kinetics to the aniline measured in the iron-reduced reaction system (
Figure 5b), the degradation rates of aniline were 0.014±0.001 μM/h
-1, 0.014±0.002 μM/h
-1, 0.023±0.001 μM/h
-1, 0.023±0.001 μM/h
-1, 0.0098±0.001 μM/h
-1 (corresponding to reaction groups GT+1 μM aniline, aniline: 1 μM, 3 μM, 7 μM, respectively), where the addition of 3 μM aniline showed the fastest degradation rate. While 7 μM aniline degradation rate was slower, probably due to the fact that high concentration inhibits microbial reproduction and reduces the bioavailability of aniline to microbial MR-1. However, the reduction was sustained, which also indicated that the iron-reducing bacterium MR-1 had a better tolerance to aniline. It has been shown that the extracellular polymer secreted by microorganisms is a kind of polymer of cellular metabolism, wrapped on the surface of microbial biofilm, which mainly consists of proteins, polysaccharides, humic substances, and DNA [
24], and mainly contains carboxyl, amino, and phosphoryl groups and other reactive groups. Therefore substances with -NH
2, -OH, -COOH functional groups are easily utilised by microorganisms under anaerobic conditions.
3.3. Solid mineral characterisation
3.3.1. Scanning electron microscopy analysis
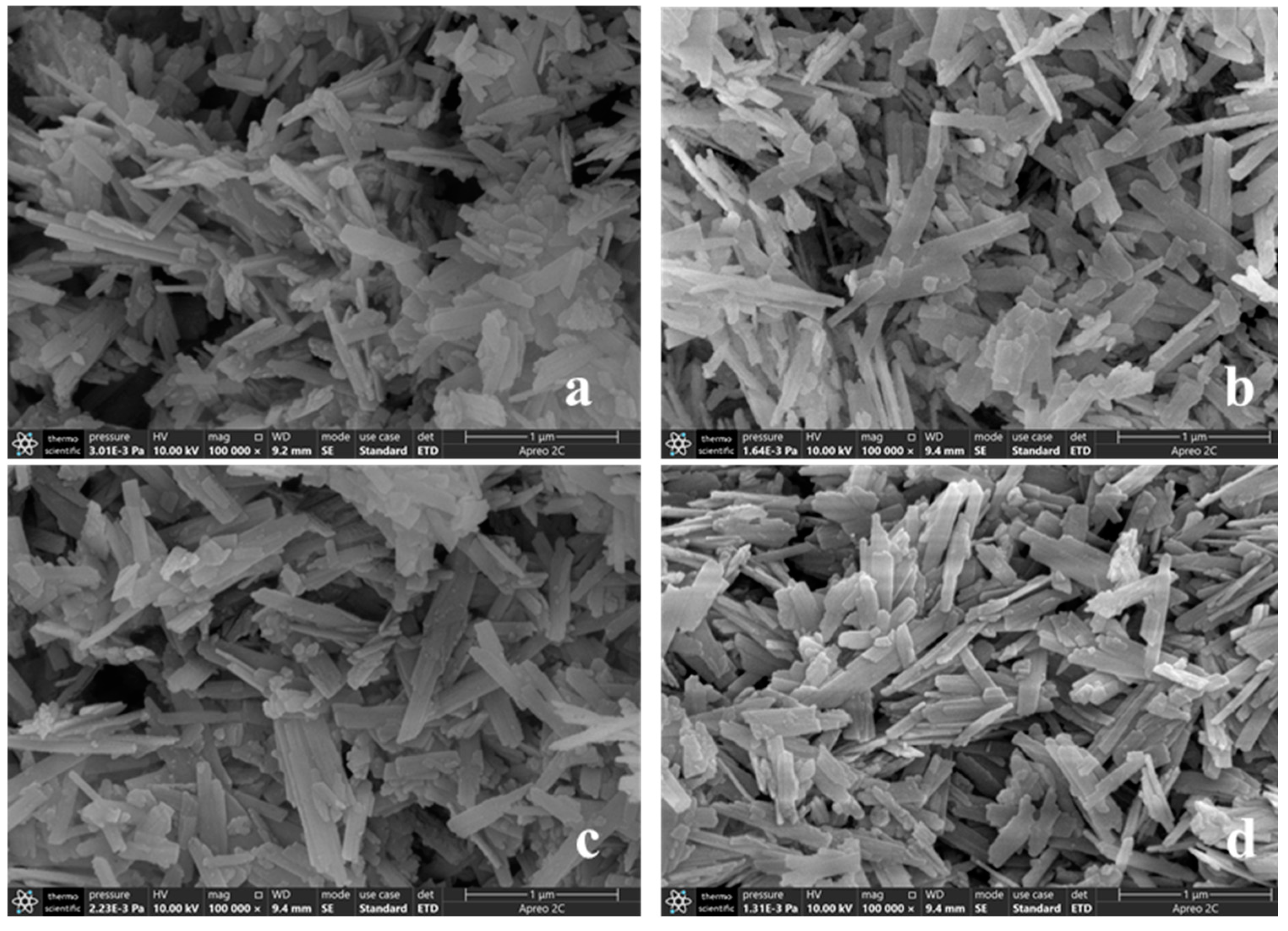
The morphologies of biological reduction of goethite under different conditions were observed by scanning electron microscope (SEM). It can be observed from
Figure 6 that the mineral morphology of the three groups (a), (b) and (c) after the reaction of goethite still presents a typical goethite morphology, The morphology of goethite was not changed as other typical minerals. However, in
Figure 6(d), the goethite was in a "broken" state after bioreduction, and the tail of goethite was uneven and abrasive, which showed that goethite had a certain degree of stability. From the SEM images of (a) and (c), a common phenomenon can be observed: some small solid particles are attached to the surface of goethite after biological reduction; It could be microorganisms attached to the surface of the mineral. This is due to the fact that microorganisms and iron oxides have the opposite surface charge in most environments [
25,
26]. At the same time, the microorganisms attached to goethite surface connect to the surface of Fe (III) oxide through the polysaccharides secreted by themselves to form a dense biofilm, which transmits electrons to Fe (III) through extracellular membrane reducase, thus promoting the reduction of Fe(III) [
27]. Therefore, the reduction of goethite by microorganism is mainly through direct contact. This phenomenon was not observed in (b) and (d), indicating that in the presence of electron shuttles, Fe (III) bioelectron transfer in addition to direct contact, The bioreduction of Fe (III) can also be accelerated through extracellular electron transport and the transfer of electrons from outer membrane C-type cytochrome to insoluble iron minerals [
28,
29].
3.3.2. X-ray photoelectron spectral analysis
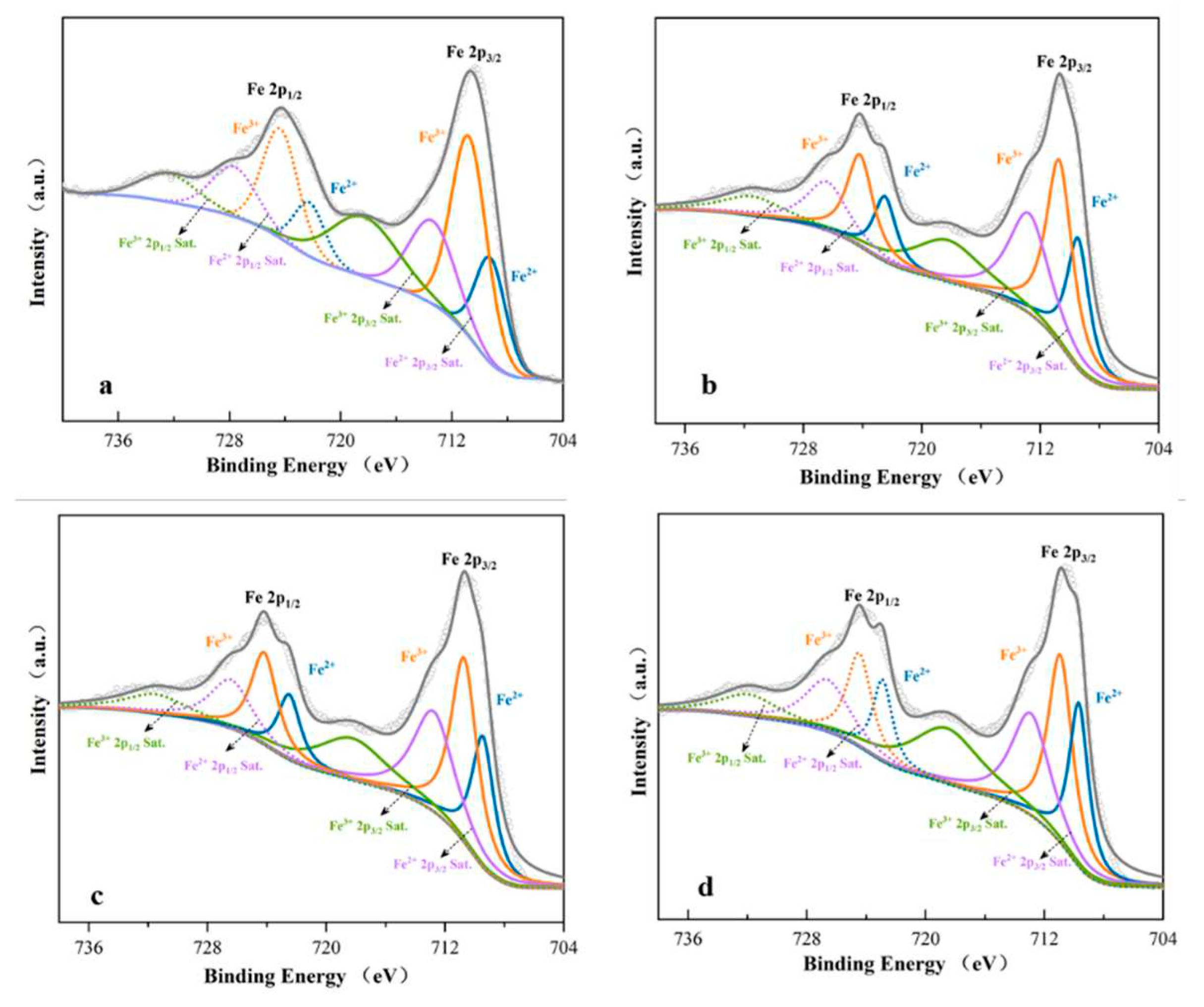
In order to further verify the effect of microbial MR-1 on goethite reduction under different conditions, the chemical state of iron on goethite surface was detected by XPS. It can be seen from
Figure 6 . 709.2 eV and 722.25 eV peaks belonging to Fe (II) oxides were observed from the Fe2p spectra of goethite under the action of single microorganism alone, with the contents of 32.2%. 710.75 eV and 724.33 eV belonging to Fe (III) oxides[
30,
31], with the contents of 67.8%. After the addition of electronic shuttle AQS, the surface Fe (II)-O oxide increased to 40.81%, indicating that the electronic shuttle will reveal the electron transfer from bacteria to goethite, thus reducing Fe (III) to Fe (II). The content of Fe (II) characteristic substance increased to 41.32% after bioreduction of goethite mediated by AQS, which was 1.28 times higher than that of single microorganism, and the specific values were shown in
Table 1. The peak positions of Fe (II)-O and Fe (III)-O have changed, which indicates that the electron density has changed and iron may be involved in the chemical reaction. According to the results of Notini et al. [
32], the survival and reduction rate of goethite with more surface defects is more than twice that of goethite with less defects. According to the above XPS results, the reduction of goethite by MR-1 mediated by electron shuttle increases the defects on the mineral surface. It is beneficial for the electron shuttle to provide more electron aggregation, so that more Fe (II) is released from the reaction system. This step proves that the addition of aniline and the electron shuttle can significantly promote the reduction of Fe (III) by MR-1.
3.3.3. X-ray diffraction analysis
In this experiment, the reduction of Fe (III) oxide by aniline was significantly enhanced by electron shuttle AQS, and the amount of Fe (II) production and surface Fe (II) -O detected in the reaction system were much higher than the reduction of Fe (III) by microorganism alone. In order to observe the mineral phase of the minerals after the biological reduction of goethite, the minerals after the reaction were characterized by XRD (
Figure 8). According to the standard XRD spectra of pure goethite (JCPC29-0713), 21.22 °, 26.32 °, 33.24 °, 34.70 °, 36.65 °, 39.98 °, 41.19 °, 53.24 ° and 59.02 ° diffraction peaks correspond to (110), (120), (130), (021), (111), (121), (140), (221) and (151) planes of goethite, respectively. By contrast, the solid minerals in the system after biological reduction are mainly goethite, and the characteristic peaks of other minerals are not observed. Since the formation of secondary minerals requires sufficient Fe (II) generation, a longer bioreduction cycle is required to produce secondary Fe (II) or Fe (II) / Fe (III) mixed minerals, such as siderite (FeCO
3), kyanite (Fe
3 (PO
4)
2), magnetite and green rust [
33,
34].. In this experiment, aniline promoted the reduction of Fe (III) by MR - 1 through electron shuttle, because the culture period was short. However, the accumulation of Fe (II) in the reaction system is not enough to form new secondary Fe (II) or Fe (II) / Fe (III) mixed minerals, which are still dominated by Fe (III) minerals.
4. Discussion
4.1. AQS enhance the Fe(III) reduction by Shewanella oneidensi MR-1
It was found by this experimental study that the reduction of Fe(III) oxide pinnatite by microorganism MR-1 was mainly through direct contact action. When Fe(III) oxide was used as the sole electron acceptor, the amount of Fe(II) generated in the AQS reaction system with the addition of 0.1, 0.3, and 0.5 mM was significantly more than that of the microbial-only reaction group by 1.61, 1.82, and 2.39 times, respectively. This is mainly due to the fact that quinone groups are the main carriers of electron transfer in the reduction of heterogeneous iron [
35], and thus higher levels are more favourable for the bioreduction of Fe(III).
The addition of the electron shuttle AQS also accelerated the consumption of sodium lactate, which stimulated cell metabolism and provided more electrons to reduce Fe (III). In the study of Zhu Weihuang et al., it was found that after adding AQS to the reaction system, the interaction between microorganisms and iron oxides was easier to proceed. At the same time, it can increase the affinity between the electron donor (sodium lactate) and the electron acceptor Fe (III) oxide[
36]. When the electron shuttle is used as the electron transport carrier, the sodium lactate can be easily utilized by microorganisms and the biological reduction of Fe (III) oxide can be significantly increased.
4.2. Aniline promotes AQS-mediated reduction of Fe(III) oxides by MR-1
Aniline, as a common toxic and harmful substance, may have toxic effects on microorganisms, thereby inhibiting the biological reduction of Fe (III) oxides. According to the results of this experimental study, the addition of aniline significantly promoted the reduction of Fe (III) by MR-1 under AQS mediation, with the addition of 3 μM during the reaction, the amount of Fe (II) generated by M aniline was 2.51 times that of pure microbial action. After the reaction, the solid mineral Fe (II) - O increased to 41.32%, but no other secondary minerals were observed.
Metal-reducing bacteria are the first microorganisms capable of oxidising aromatic compounds using Fe(III) as an electron acceptor. This electron shuttle can catalyse the degradation of various aromatic compounds (e.g., benzene, toluene, phenol, cresol, cresol, p-cresol, phenol, aniline, benzoic acid, p-hydroxybenzoic acid, benzaldehyde, p-hydroxybenzaldehyde, phenylethanol, p-hydroxyphenylethanol, etc.) [
37,
38,
39]. Our experimental results showed that aniline showed different degrees of degradation, possibly due to the fact that aniline may have acted as an electron donor for the metabolism of the iron-reducing bacteria throughout the reaction leading to a gradual decrease in the aniline content.
5. Conclusions
Microbia-mediated Fe (III) reduction plays a key role in the geochemical cycle of iron and is widely used in underground environments. This process not only strongly affects the distribution and speciation of iron[
40,
41] , but also strongly metabolizes iron reducing bacteria that can degrade aromatic carbohydrates during the bioreduction process of Fe (III) oxidation. Including toluene, phenol, methyl phenol, p-methylphenol, Aniline, benzaldehyde and other organic pollutants[
42,
43,
44]. In this study, aniline in the AQS-mediated significantly promoted the increase of MR-1 relative to the pure culture system of "iron reducing bacteria MR-1 + Fe (III) oxide goethite + electron shuttle AQS + pollutant aniline." Because of the complex reaction system in the real environment, it is more practical to apply this study to the geological environment rich in iron minerals, and also provide a working basis for a deeper understanding of the environmental geochemical processes of iron and environmental remediation.
Author Contributions
Conceptualization, M.T. and C.W.; methodology, M.T. and Z.D.; software, M.T.; validation, C.W., Z.D. and Q.C.; formal analysis, M.T.; investigation, M.T., Z.W. and Y.Z.; resources, C.W.; data curation, M.T.; writing—original draft preparation, M.T.; writing—review and editing, M.T.; project administration, C.W.; funding acquisition, C.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (No. 41772129).
Data Availability Statement
The data are available from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- ZHANG Yulong, C.X.W.Y. , Electron shuttle-mediated microbial extracellular electron transfer: Mechanisms and geochemical implications. Ecology and Environmental Sciences, 2021. 30(01): p. 213-222.
- LIU, F.; Chen, L.; ZHENG, S.; ZHANG, H.; Li, Y. The Role of Microorganisms in the Geochemical Iron Cycle. Sci. Sin. Vitae 2016, 46, 1069–1078. [Google Scholar] [CrossRef]
- Yaxian Zhang, Y.W.H.W. , Research advances in the mechanisms of Fe(III) dissimilatory reduction and the reduction products, The Chemical Industry and Engerring Soceiety of China. 2015: BeiJing China. p. 5.
- Schuth, S.; Hurraß, J.; Münker, C.; Mansfeldt, T. Redox-dependent fractionation of iron isotopes in suspensions of a groundwater-influenced soil. Chem. Geol. 2015, 392, 74–86. [Google Scholar] [CrossRef]
- Zhu, W.; Nan, Y.; Huang, T.; Wu, F. The Mechanism, Thermodynamic and Kinetic Characteristics of the Microbial Reduction of Goethite Mediated by Anthraquinone-2-Sulfonate. Geomicrobiol. J. 2013, 30, 928–940. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, L.; Jiang, Y.; Xu, Z.; Gao, S.; Li, M.; Li, R.; Xi, B. Promoting mechanism of electronic shuttle for bioavailability of Fe(III) oxide and its environmental significance. Water Supply 2020, 20, 1157–1166. [Google Scholar] [CrossRef]
- Thomsen, U.; Thamdrup, B.; A Stahl, D.; Canfield, D.E. Pathways of organic carbon oxidation in a deep lacustrine sediment, Lake Michigan. Limnol. Oceanogr. 2004, 49, 2046–2057. [Google Scholar] [CrossRef]
- Straub, K.L., M. Benz and B. Schink, Iron metabolism in anoxic environments at near neutral pH. FEMS microbiology ecology, 2001. 34(3): p. 181-186.
- Lovley, D.R.; Phillips, E.J.P.; Lonergan, D.J. Enzymic versus nonenzymic mechanisms for iron(III) reduction in aquatic sediments. Environ. Sci. Technol. 1991, 25, 1062–1067. [Google Scholar] [CrossRef]
- Usman, M.; Abdelmoula, M.; Faure, P.; Ruby, C.; Hanna, K. Transformation of various kinds of goethite into magnetite: Effect of chemical and surface properties. Geoderma 2013, 197-198, 9–16. [Google Scholar] [CrossRef]
- Till, J.; Guyodo, Y.; Lagroix, F.; Ona-Nguema, G.; Brest, J. Magnetic comparison of abiogenic and biogenic alteration products of lepidocrocite. Earth Planet. Sci. Lett. 2014, 395, 149–158. [Google Scholar] [CrossRef]
- Yang, L.; Steefel, C.I.; Marcus, M.A.; Bargar, J.R. Kinetics of Fe(II)-Catalyzed Transformation of 6-line Ferrihydrite under Anaerobic Flow Conditions. Environ. Sci. Technol. 2010, 44, 5469–5475. [Google Scholar] [CrossRef] [PubMed]
- Roh, Y.; Liu, S.V.; Li, G.; Huang, H.; Phelps, T.J.; Zhou, J. Isolation and Characterization of Metal-Reducing Thermoanaerobacter Strains from Deep Subsurface Environments of the Piceance Basin, Colorado. Appl. Environ. Microbiol. 2002, 68, 6013–6020. [Google Scholar] [CrossRef]
- Orsetti, S.; Laskov, C.; Haderlein, S.B. Electron Transfer between Iron Minerals and Quinones: Estimating the Reduction Potential of the Fe(II)-Goethite Surface from AQDS Speciation. Environ. Sci. Technol. 2013, 47, 14161–14168. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Lee, W. Biotransformation of lepidocrocite in the presence of quinones and flavins. Geochim. et Cosmochim. Acta 2013, 114, 144–155. [Google Scholar] [CrossRef]
- Jiang, Y.; Xi, B.; Li, R.; Li, M.; Xu, Z.; Yang, Y.; Gao, S. Advances in Fe(III) bioreduction and its application prospect for groundwater remediation: A review. Front. Environ. Sci. Eng. 2019, 13, 89. [Google Scholar] [CrossRef]
- Shi, L.; Dong, H.; Reguera, G.; Beyenal, H.; Lu, A.; Liu, J.; Yu, H.-Q.; Fredrickson, J.K. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol. 2016, 14, 651–662. [Google Scholar] [CrossRef]
- Rong Chen, Impact mechanisms of Fe(II) chemical oxidation on Fe(III) bio-reduction. 2018, China University of Geoscience.
- Tor, J.M.; Lovley, D.R. Anaerobic degradation of aromatic compounds coupled to Fe(III) reduction by Ferroglobus placidus. Environ. Microbiol. 2001, 3, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Paterson, E. , Iron oxides in the laboratory. Preparation and characterization. Clay Minerals, 1992. 27(3): p. 393-393.
- Roden, E.E.; Wetzel, R.G. Kinetics of microbial Fe(III) oxide reduction in freshwater wetland sediments. Limnol. Oceanogr. 2002, 47, 198–211. [Google Scholar] [CrossRef]
- Wu, C.Y. , et al., Humic substance-mediated reduction of iron (III) oxides and degradation of 2, 4-D by an alkaliphilic bacterium, C orynebacterium humireducens MFC-5. Microbial Biotechnology, 2013. 6(2): p. 141-149.
- Liu, S.; Liu, H.; Wang, Z.; Cui, Y.; Chen, R.; Peng, Z.; Yuan, S.; Shi, L. Benzene promotes microbial Fe(III) reduction and flavins secretion. Geochim. et Cosmochim. Acta 2019, 264, 92–104. [Google Scholar] [CrossRef]
- Southall, S.C.; Micklethwaite, S.; Wilson, S.A.; Frierdich, A.J. Changes in Crystallinity and Tracer-Isotope Distribution of Goethite during Fe(II)-Accelerated Recrystallization. ACS Earth Space Chem. 2018, 2, 1271–1282. [Google Scholar] [CrossRef]
- Cornell, R.M. and U. Schwertmann, The iron oxides: structure, properties, reactions, occurrences, and uses. Vol. 664. 2003: Wiley-vch Weinheim.
- E Collins, Y.; Stotzky, G. Heavy metals alter the electrokinetic properties of bacteria, yeasts, and clay minerals. Appl. Environ. Microbiol. 1992, 58, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Bonneville, S.; Behrends, T.; Van Cappellen, P.; Hyacinthe, C.; Röling, W.F. Reduction of Fe(III) colloids by Shewanella putrefaciens: A kinetic model. Geochim. et Cosmochim. Acta 2006, 70, 5842–5854. [Google Scholar] [CrossRef]
- Shi, L. , et al., Respiration of metal (hydr) oxides by Shewanella and Geobacter: a key role for multihaem c-type cytochromes. Molecular microbiology, 2007. 65(1): p. 12-20.
- O’Loughlin, E.J. , Effects of electron transfer mediators on the bioreduction of lepidocrocite (γ-FeOOH) by Shewanella putrefaciens CN32. Environmental science & technology, 2008. 42(18): p. 6876-6882.
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Grosvenor, A.P. , et al., Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surface and Interface Analysis: An International Journal devoted to the development and application of techniques for the analysis of surfaces, interfaces and thin films, 2004. 36(12): p. 1564-1574.
- Notini, L.; Byrne, J.M.; Tomaszewski, E.J.; Latta, D.E.; Zhou, Z.; Scherer, M.M.; Kappler, A. Mineral Defects Enhance Bioavailability of Goethite toward Microbial Fe(III) Reduction. Environ. Sci. Technol. 2019, 53, 8883–8891. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, D.; Bu, H.; Deng, L.; Liu, H.; Yuan, P.; Du, P.; Song, H. XRD-based quantitative analysis of clay minerals using reference intensity ratios, mineral intensity factors, Rietveld, and full pattern summation methods: A critical review. Solid Earth Sci. 2018, 3, 16–29. [Google Scholar] [CrossRef]
- Yamamura, S.; Sudo, T.; Watanabe, M.; Tsuboi, S.; Soda, S.; Ike, M.; Amachi, S. Effect of extracellular electron shuttles on arsenic-mobilizing activities in soil microbial communities. J. Hazard. Mater. 2018, 342, 571–578. [Google Scholar] [CrossRef]
- Tinglin Huang, W.Z.T.Y. , The kinetic characteristics of the microbial dissimilatory reduction of lepidocrocite. Acta Scientiae Circumstantiae, 2012. 32(6): p. 1348-1356.
- Zhu, W. , et al., Quinone-mediated microbial goethite reduction and transformation of redox mediator, anthraquinone-2, 6-disulfonate (AQDS). Geomicrobiology Journal, 2017. 34(1): p. 27-36.
- Lovley, D.R.; Baedecker, M.J.; Lonergan, D.J.; Cozzarelli, I.M.; Phillips, E.J.P.; Siegel, D.I. Oxidation of aromatic contaminants coupled to microbial iron reduction. Nature 1989, 339, 297–300. [Google Scholar] [CrossRef]
- Kazumi, J.; Haggblom, M.M.; Young, L.Y. Degradation of Monochlorinated and Nonchlorinated Aromatic Compounds under Iron-Reducing Conditions. Appl. Environ. Microbiol. 1995, 61, 4069–4073. [Google Scholar] [CrossRef]
- Lovley, D.R. and D.J. Lonergan, Anaerobic oxidation of toluene, phenol, and p-cresol by the dissimilatory iron-reducing organism, GS-15. Applied and Environmental Microbiology, 1990. 56(6): p. 1858-1864.
- Bingjie, O.; Xiancai, L.; Huan, L.; Juan, L.; Tingting, Z.; Xiangyu, Z.; Jianjun, L.; Rucheng, W. Reduction of jarosite by Shewanella oneidensis MR-1 and secondary mineralization. Geochim. et Cosmochim. Acta 2014, 124, 54–71. [Google Scholar] [CrossRef]
- Cooper, D.C.; Neal, A.L.; Kukkadapu, R.K.; Brewe, D.; Coby, A.; Picardal, F.W. Effects of sediment iron mineral composition on microbially mediated changes in divalent metal speciation: Importance of ferrihydrite. Geochim. et Cosmochim. Acta 2005, 69, 1739–1754. [Google Scholar] [CrossRef]
- Kenneke, J.F. and E.J. Weber, Reductive dehalogenation of halomethanes in iron-and sulfate-reducing sediments. 1. Reactivity pattern analysis. Environmental science & technology, 2003. 37(4): p. 713-720.
- Rügge, K.; Hofstetter, T.B.; Haderlein, S.B.; Bjerg, P.L.; Knudsen, S.; Zraunig, C.; Mosbæk, H.; Christensen, T.H. Characterization of Predominant Reductants in an Anaerobic Leachate-Contaminated Aquifer by Nitroaromatic Probe Compounds. Environ. Sci. Technol. 1998, 32, 23–31. [Google Scholar] [CrossRef]
- Christensen, T.H.; Kjeldsen, P.; Bjerg, P.L.; Jensen, D.L.; Christensen, J.B.; Baun, A.; Albrechtsen, H.-J.; Heron, G. Biogeochemistry of landfill leachate plumes. Appl. Geochem. 2001, 16, 659–718. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).