1. Introduction
Spinal metastases are a frequent diagnosis in about 15% of patients treated for an oncologic disease, and most likely this incidence is systematically underestimated [
1,
2,
3]. Typically, spinal metastases progress asymptomatically until the terminal phase. However, 10-20% of those with spinal metastasis experience the destruction of supporting spinal elements and develop symptomatic spinal cord compression [
4]. Thus, symptomatic metastases to the spine, primarily and most often originating from malignancies in the breast, lung, prostate, and kidney, cause significant morbidity and reduced quality of life for those affected.
The spine’s structural complexity, crucial role in weight-bearing, and proximity to neural elements make it a critical site for metastatic infiltration, resulting in vertebral compression fractures, spinal cord compression, and neurological deficits. Amid the therapeutic options available, decompressive surgery combined with posterior stabilization emerges as an important intervention for managing the intricate interplay of mechanical instability and neural compromise that is inherent in metastatic spine disease. Thus, surgical treatment is a valid option to ensure an improved quality of life in cases where mechanical instability or spinal cord compression is imminent or has already occurred [
5,
6]. In most cases where surgery is performed, reducing axial pain leads to an enhanced quality of life [
7]. However, surgical treatment is only part of an interdisciplinary treatment including (neo-)adjuvant radiation as well as systemic therapy. In cases of spinal metastases without impending mechanical instability or spinal cord compression, solitary radiation therapy may be a sufficient treatment as well [
8]. However, tumor entity and the general health status of the patient are further important factors to consider when deciding on the best treatment [
9]. Noteworthy, it is apparent that surgery cannot stand alone as a solution for metastatic spine disease when the demand for surgical techniques to improve biomechanical stability and neurologic function is considered. Although interdisciplinary treatment regimens may improve quality of life, there are significant complications including the development of novel neurological deficits. This retrospective study of a large single-center cohort aimed to determine the risk factors for occurrence of novel neurological deficits and local tumor recurrence.
2. Materials and Methods
All consecutive patients who underwent surgery due to spinal metastases at our interdisciplinary spine center between January 2012 and March 2022 were assessed for inclusion into this study. The relevant data were collected from the center’s electronic database, which included medical records and radiologic images. Approval for the study was granted by the local ethics committee (approval code: 20-1643).
The following patient-related parameters were recorded: Age, gender, primary tumor histology, location within the spine and medical comorbidities such as diabetes mellitus, coronary heart disease, history of smoking and chronic obstructive pulmonary disease (COPD), a history of deep vein thrombosis, obesity (defined as a body mass index > 30), and osteoporosis. Multiple myeloma and lymphoma were summarized as hematopoietic cancers. Spinal instability was assessed using the Spinal Instability Neoplastic Score (SINS) and classified into a stable (SINS 0 to 6), intermediate (SINS 7 to 12) and unstable (SINS 13 to 18) groups accordingly [
10].
Surgical, techniques such as decompression, hemi-laminectomy, laminectomy as well as corpectomy were assessed. In cases of revision-surgery due to local recurrence and novel neurologic deficits “debulking-surgery” was registered as well. Additional dorsal instrumentation, kyphoplasty or radiofrequency ablation were registered, too.
The time period between initial surgery and start of radiation therapy at the surgically treated area was monitored, as well as perioperative complications such as wound healing disorders, wound infections, material dislocation, implant failure, epidural hematoma, and so on. Furthermore, the study assessed the Frankel grade (A = complete impairment; B = incomplete, sensory but no motor function below neurological level; C = incomplete, motor function preserved but majority of key muscles muscle grade < 3; D = incomplete, motor function preserved and majority of key muscles muscle grade > 3; E = normal), as well as the appearance of spinal cord syndrome, both before and immediately after surgery and during follow-up (more than 10 days after surgery) [
11]. Finally, the degree of spinal cord compression before surgery and during follow-up was assessed by MRI using the Epidural Spinal Cord Compression (ESCC) scale and divided into a low (ESCC 0 to 1) and a high (ESCC 2 to 3) cord compression group [
12].
All scores were collected independently by at least two investigators. In cases of initial non-agreement, consensus was reached through case-based discussions.
The main outcome measures for this study were occurrence of novel neurologic deficits or symptoms (motoric, sensory or vegetative dysfunction as well as pain) during the course of follow-up as well as local recurrence detected by MRI or CT imaging in symptomatic patients. Hence, subgroup analysis was performed for all patients with spinal cord/cauda syndrome (SCS) during follow-up and those without (NSCS).
2.1. Statistical Analysis
Clinical characteristics were analyzed using descriptive statistics. Categorical variables were compared by a Chi-Square and a Fisher’s Exact test, when appropriate. Continuous variables were tested for normal distribution using the Kolmogorov-Smirnov test and for homoscedasticity using the White test. Data are reported as mean ± standard deviation or with confidence intervals (CI). Group means from normally distributed data were compared using a two-sided unpaired Student’s t-test while a Mann–Whitney U test was used in the case of non-normal or heteroscedastic distribution of data. All calculations were performed using SPSS software (Version 27, IBM SPSS Statistics for Windows, Armonk, NY, USA). A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Demographics
In total, there were 810 patients that were assessed for final analysis. Of those, 64 had to be excluded due to incomplete records. Follow-up of all remaining 746 patients was 296.8 d (95% CI (263.5, 330.1)). The most common secondary diagnoses were COPD (23.4%) type II diabetes (19.6%), and atherosclerosis (15.8%). Overall survival one year after surgery was 62.2%.
3.2. Spinal scores
Initial ESCC was grade I (a-c) in 28.9% of cases. 36.1 % of cases were grade II and 34.9% were grade III. There was no significant difference in distribution between patients that did not develop spinal cord syndrome (NSCS) during follow-up and those that did (SCS; p = 0.29;
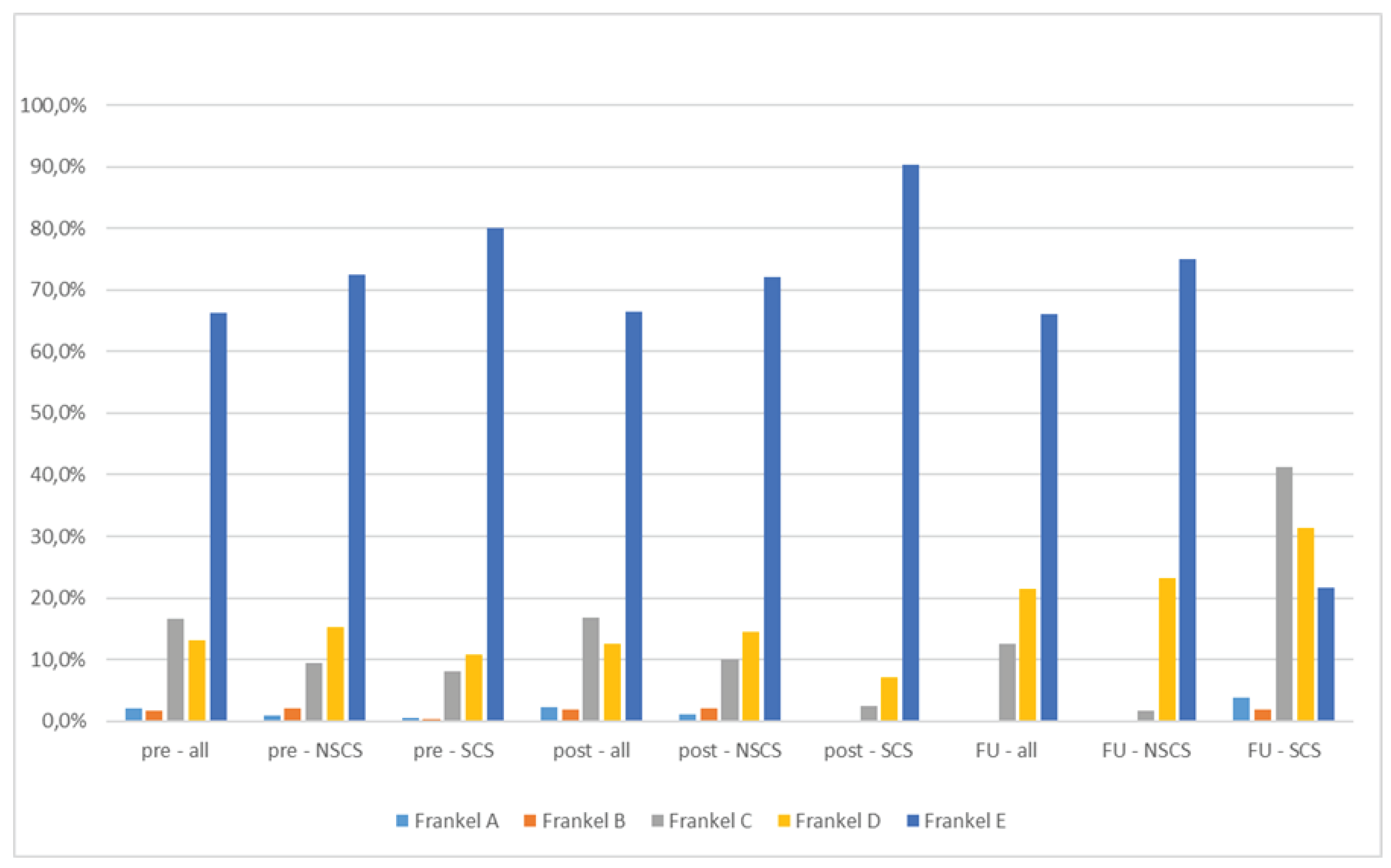
Table 1). Preoperative SINS score was intermediate on average (10.6 ± 4.9). SINS score was not significantly different between the NSCS and the SCS subgroups (10.5 ± 3.1 respective 10.0 ± 2.8; p = 0.55). Frankel grades are depicted in
Figure 1.
3.3. Treatment regimes
In this cohort 53.4% of patients received adjuvant systemic therapy (chemotherapy: 42.1%, targeted therapy: 11.3%). 12.1% of patient received preoperative embolization of the affected vertebral body. There was no significant difference between the NSCS and the SCS subgroups (p = 0.73).
3.4. Complications
In total, 188 patients (25.2%) of patients required revision or secondary surgery, and there was no significant difference between the NSCS and the SCS subgroups (p = 0.67). 42.5% of these cases were stabilized ventrally through piecemeal vertebrectomy and implantation of a vertebral body implant via secondary surgery. 84.3% of revision surgeries were performed on the same vertebral segment. Deep wound infections (6.0%), implant loosening or breakage (8.9%), epidural hematoma (5.2%), dura injury (2.5%), as well as surgical intervention for superficial wound infections (epi-fascial; 14.3%) were further reasons for revision surgery, with no significant differences between both subgroups (p = 0.31).
Other perioperative complications were deep vein thrombosis (2.0%), pneumonia (3.1%), cardiac events (1.1%) and central nervous system events (0.7%).
4. Discussion
Improving the quality of life for patients with metastatic spinal disease is the major goal of surgical treatment [
13]. Most often quality of life is enhanced through pain relief, controlling metastatic disease at the treated site, improving neurologic deficits, maintaining or improving functional status, and minimizing further mechanical instability. Surgically this is achieved by removal of the metastatic deposit, prevention or correction of deformity with stabilization and/or decompressive neurolysis. The latter may be essential for the recovery of an impaired neurological status if a spinal cord syndrome has already occurred.
Patients undergoing this kind of treatment are highly vulnerable and at high risk of a plethora of medical problems. In fact, complication rates are reported to be as high as 47% [
14,
15,
16,
17,
18]. Therefore, decision making on which patients to operate on is essential to ensure patients benefit from surgery [
19]. In all cases, the aims of the surgical intervention need to be considered and the invasiveness of surgery needs to be weighed against the patient’s physiological condition and prognosis.
In addition to immediate and perioperative complications, patients may develop a local recurrence that may become apparent with novel neurological compromise [
20]. To the best of our knowledge this is the largest study to investigate risk factors for the development of novel neurologic comprise after surgical treatment of metastatic spine disease. In this retrospective study 746 cases treated for metastatic spine disease over the course of more than 10 years were reviewed for development of a spinal cord syndrome without temporal correlation to previous surgical measures. 56 (7.5%) cases developed a spinal cord syndrome during follow-up and allowed thus for a comparison with 690 (92.5%) cases that did not experience spinal cord syndrome during follow-up. Both subgroups were comparable in terms of demographic characteristics, and there were no significant differences in comorbidities, surgical techniques, initial KPS, ESCC, or SINS.
In this cohort, no significant difference in complication rates was observed between both subgroups. The complication rates were comparable to those previously reported in the literature [
17,
21].
The main endpoint of this study was development of neurologic comprise due to local recurrence. As parameter the Frankel score was used, revealing a significant difference (p = 0.02) between both subgroups with a deterioration in the SCS subgroup during follow-up, which was not the case immediately before respective after surgery (Fig 1 and
Table 2). Deteriorations in Frankel grade within the SCS subgroup were due to local recurrence -objectified by novel CT or MRI scans. The main risk factor identified for development of a symptomatic local recurrence was delayed radiation therapy. In fact, time to radiation was significantly prolonged in the SCS group (p < 0.001) and it should be discussed whether this timeframe should not exceed five to six weeks. As complications and revision surgery rates were not significantly higher in the SCS group, prolonged time to radiation can be contributed to decentral patient management (e.g. patients that were treated surgically at our center and planned to receive radiation therapy externally) and missing awareness of health care providers to schedule early radiation therapy. It has to be stated that adjuvant radiation does not necessarily excludes the risk of local recurrence but rates are lower than reported in this cohort [
22]. These results once again emphasize the importance of a scheduled interdisciplinary treatment for achieving favorable midterm outcomes.
To our knowledge, no randomized controlled trials exist for this patient cohort to determine the optimal time for adjuvant radiation therapy. Possibly, neoadjuvant radiation therapy may not be inferior, but data is still scarce [
23].
Besides the inherent limitations of a retrospective study, our results are from a single center. Nevertheless, the overall number of cases included is high.
5. Conclusions
Surgical treatment of spinal metastatic disease provides significant benefits to patients’ quality of life but radiation therapy must be scheduled within a few weeks in order to reduce the risk of tumor-induced neurological comprise.
Author Contributions
Conceptualization, S.G.W., P.K.; methodology, P.K., M.L., J.R.; investigation, N.v.S., S.O., M.L. (Moritz Lenschow), M.L. (Maximilian Lenz), M.W., S.L.; formal analysis, S.G.W. and S.O.; writing—original draft preparation P.K, S.G.W.; writing—review and editing, N.v.S., V.N., J.R., S. T., M.W., P.E., P.K., S.G.W.; visualization, S.G.W.; supervision, P.E., S.G.W. All authors have read and agreed to the published version of the manuscript.
Funding
No funding was received for the conduction of this study.
Institutional Review Board Statement
The study was approved by the Ethics Committee of the Medical Faculty of the University of Cologne (approval code: 20-1643; date of approval: 29 December 2020) and conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Patient consent was waived due to the retrospective nature of this study.
Data Availability Statement
The datasets generated and/or analyzed in this study are available upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ortiz Gómez JA The incidence of vertebral body metastases. Int Orthop 1995, 19, 309–311. [CrossRef]
- Walter SG, Gaisendrees C, Kernich N, et al Epidemiology of Surgically Treated Spinal Tumors: A Multicenter Surveillance Study of 9686 Patients from the German Spine Registry (DWG Register). Z Orthop Unfall. 2023. [CrossRef]
- Van den Brande R, MJ Cornips E, Peeters M, et al Epidemiology of spinal metastases, metastatic epidural spinal cord compression and pathologic vertebral compression fractures in patients with solid tumors: A systematic review. J bone Oncol 2022, 35. [CrossRef]
- Kakutani K, Kanda Y, Yurube T, et al The Identification of Risk Factors for Symptomatic Spinal Metastasis Onset: A Prospective Cohort Study of 128 Asymptomatic Spinal Metastasis Patients. Cancers (Basel) 2023, 15, 1251. [CrossRef]
- Dea N, Versteeg AL, Sahgal A, et al Metastatic Spine Disease: Should Patients With Short Life Expectancy Be Denied Surgical Care? An International Retrospective Cohort Study. Neurosurgery 2020, 87, 303. [CrossRef] [PubMed]
- Wai EK, Finkelstein JA, Tangente RP, et al Quality of life in surgical treatment of metastatic spine disease. Spine (Phila Pa 1976) 2003, 28, 508–512. [CrossRef] [PubMed]
- Quan GMY, Vital JM, Aurouer N, et al Surgery improves pain, function and quality of life in patients with spinal metastases: a prospective study on 118 patients. Eur Spine J 2011, 20, 1970. [CrossRef]
- Rades D, Küchler J, Graumüller L, et al Radiotherapy with or without Decompressive Surgery for Metastatic Spinal Cord Compression: A Retrospective Matched-Pair Study Including Data from Prospectively Evaluated Patients. Cancers (Basel) 2022, 14. [CrossRef]
- Laufer I, Rubin DG, Lis E, et al The NOMS Framework: Approach to the Treatment of Spinal Metastatic Tumors. Oncologist 2013, 18, 744. [CrossRef]
- Fisher CG, Dipaola CP, Ryken TC, et al A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine (Phila Pa 1976) 2010, 35. [CrossRef]
- Frankel HL, Hancock DO, Hyslop G, et al The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Spinal Cord 1969, 73, 179–192. [CrossRef]
- Bilsky MH, Laufer I, Fourney DR, et al Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine 2010, 13, 324–328. [CrossRef]
- Paulino Pereira NR, Groot OQ, Verlaan JJ, et al Quality of Life Changes After Surgery for Metastatic Spinal Disease: A Systematic Review and Meta-analysis. Clin spine Surg 2022, 35, 38–48. [CrossRef]
- Luksanapruksa P, Buchowski JM, Zebala LP, et al Perioperative Complications of Spinal Metastases Surgery. Clin spine Surg 2017, 30, 4–13. [CrossRef]
- Paulino Pereira NR, Ogink PT, Groot OQ, et al Complications and reoperations after surgery for 647 patients with spine metastatic disease. Spine J 2019, 19, 144–156. [CrossRef]
- Sundaresan N, Rothman A, Manhart K, Kelliher K Surgery for solitary metastases of the spine: rationale and results of treatment. Spine (Phila Pa 1976) 2002, 27, 1802–1806. [CrossRef]
- Igoumenou VG, Mavrogenis AF, Angelini A, et al Complications of spine surgery for metastasis. Eur J Orthop Surg Traumatol 2019, 301, 37–56. [CrossRef]
- Walter SG, Lenz M, Gaisendrees C, et al Complications associated to wound drainages in tumor spine surgery: a multicenter surveillance study from the German Spine Registry (DWG-Register). Sci Rep 2022, 12, 19983. [CrossRef]
- Curtin M, Piggott RP, Murphy EP, et al Spinal Metastatic Disease: A Review of the Role of the Multidisciplinary Team. Orthop Surg 2017, 9, 145. [CrossRef]
- Kotecha R, Dea N, Detsky JS, Sahgal A Management of recurrent or progressive spinal metastases: reirradiation techniques and surgical principles. Neuro-Oncology Pract 2020, 7, i45. [CrossRef]
- Wise JJ, Fischgrund JS, Herkowitz HN, et al Complication, survival rates, and risk factors of surgery for metastatic disease of the spine. Spine (Phila Pa 1976) 1999, 24, 1943–1951. [CrossRef] [PubMed]
- Bishop AJ, Tao R, Rebueno NC, et al Outcomes for Spine Stereotactic Body Radiation Therapy and an Analysis of Predictors of Local Recurrence. Int J Radiat Oncol 2015, 92, 1016–1026. [CrossRef] [PubMed]
- Hsu F-M, Xiao F, Lin P-C, Chen Y-H Neoadjuvant Stereotactic Body Radiation Therapy for Spine Metastases Medical Images View project Neurocognitive Outcome of Conformal WBRT w/wo Hippocampal Avoidance for Brain Metastases View project Neoadjuvant Stereotactic Body Radiation Therapy for Spine Metastases. Artic J Spine Neurosurg 2018, 7. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).