Submitted:
31 August 2023
Posted:
04 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Mouse colony
2.2. GSH evaluation
2.3. LOOH measurement
2.4. RNA extraction and cDNA preparation
2.5. Real time PCR
2.6. Western blot analysis
2.7. Histopathological analysis
2.8. Statistics
3. Results
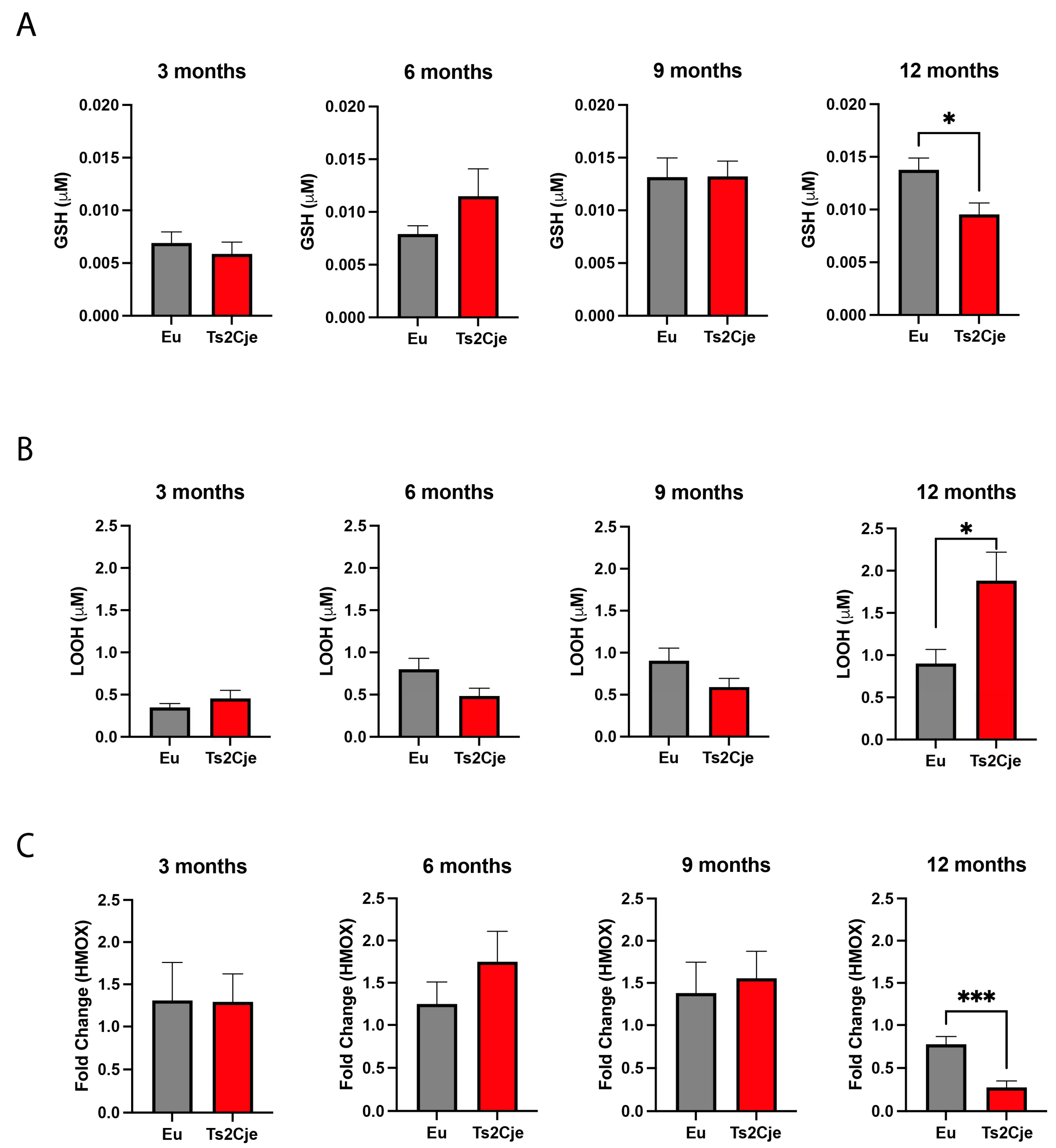
3.1. DS liver display an enhanced exposure to oxidative stress
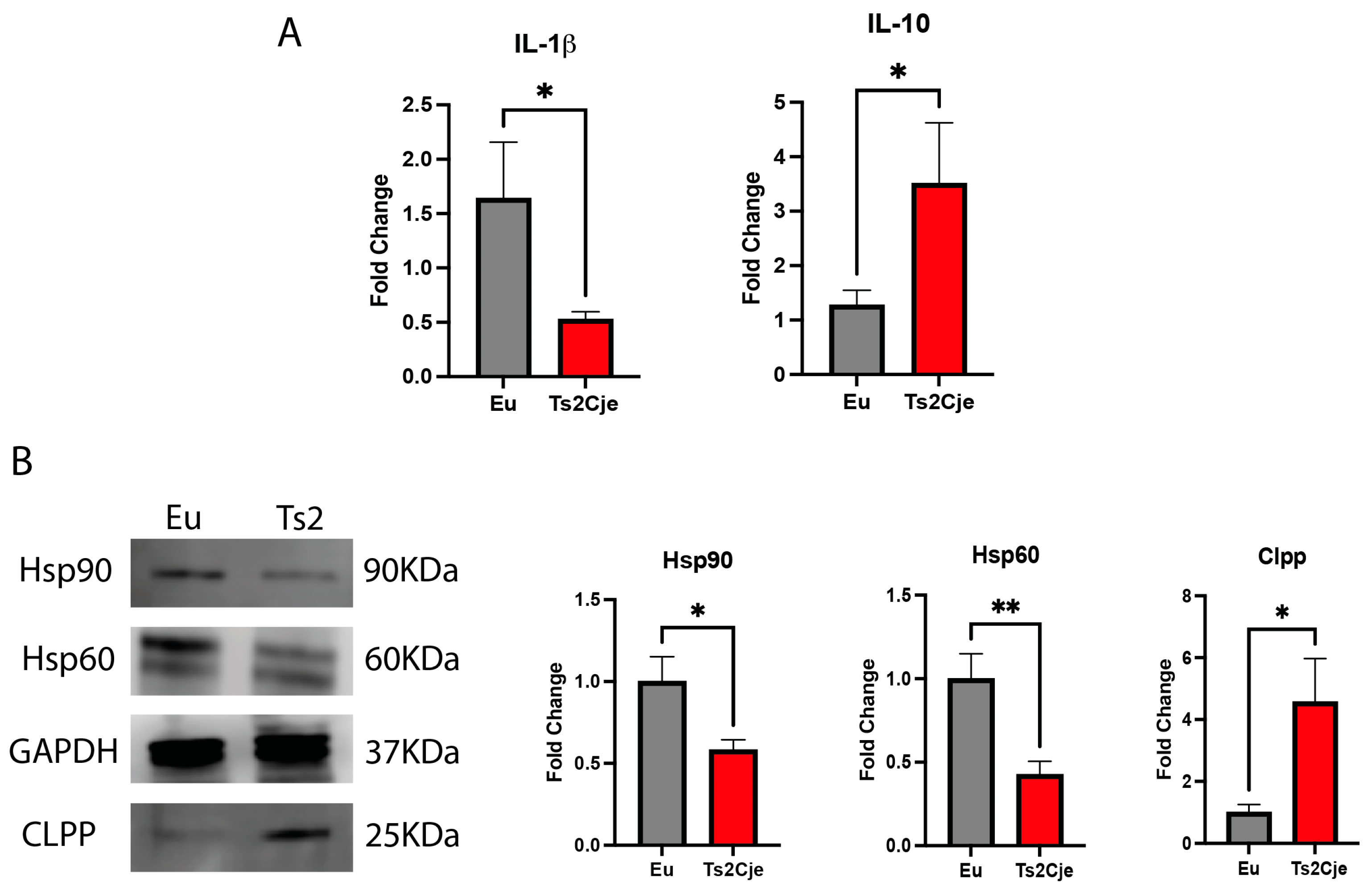
3.2. DS mice livers show an increase of inflammatory markers
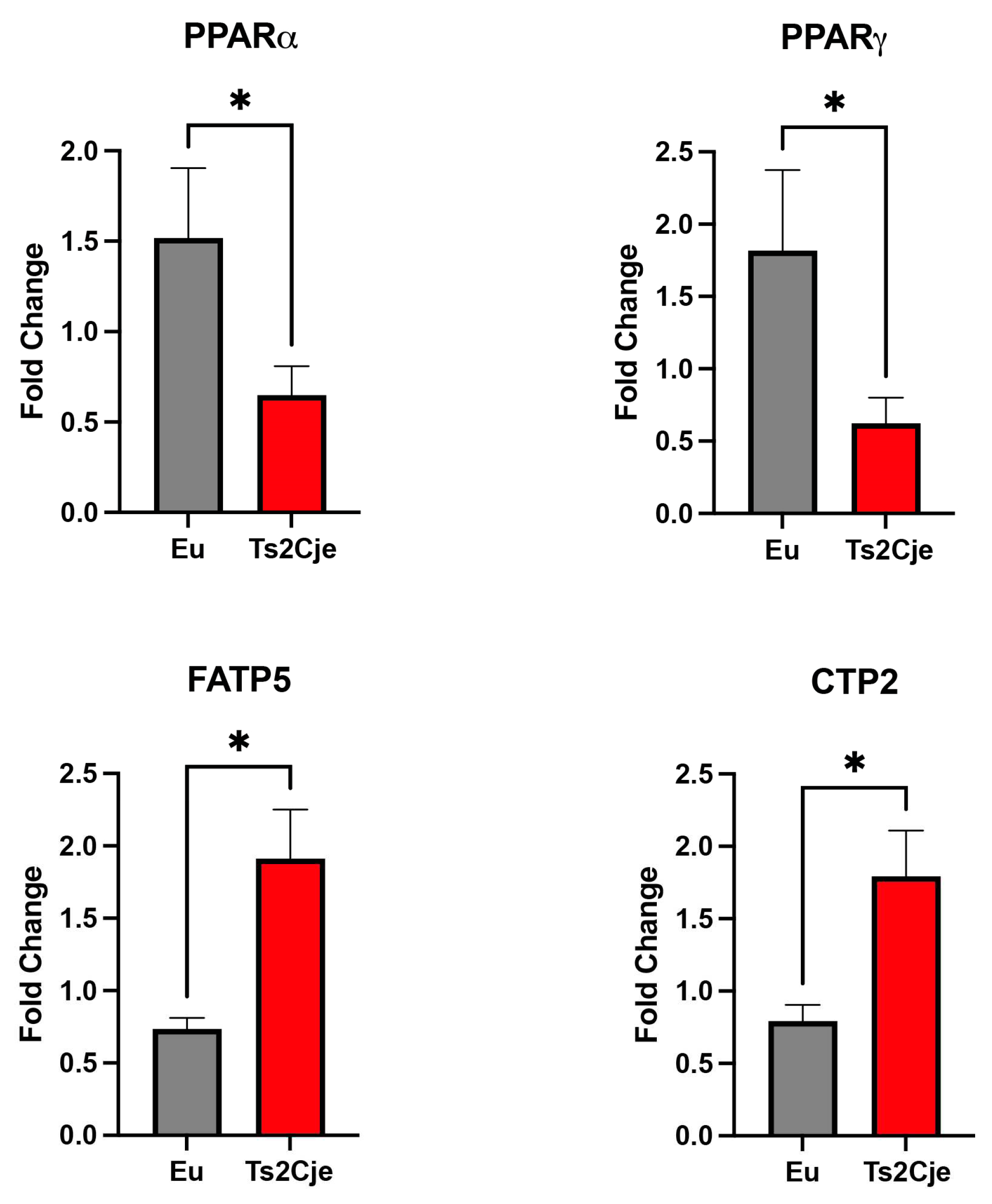
3.3. DS liver increase lipid metabolism
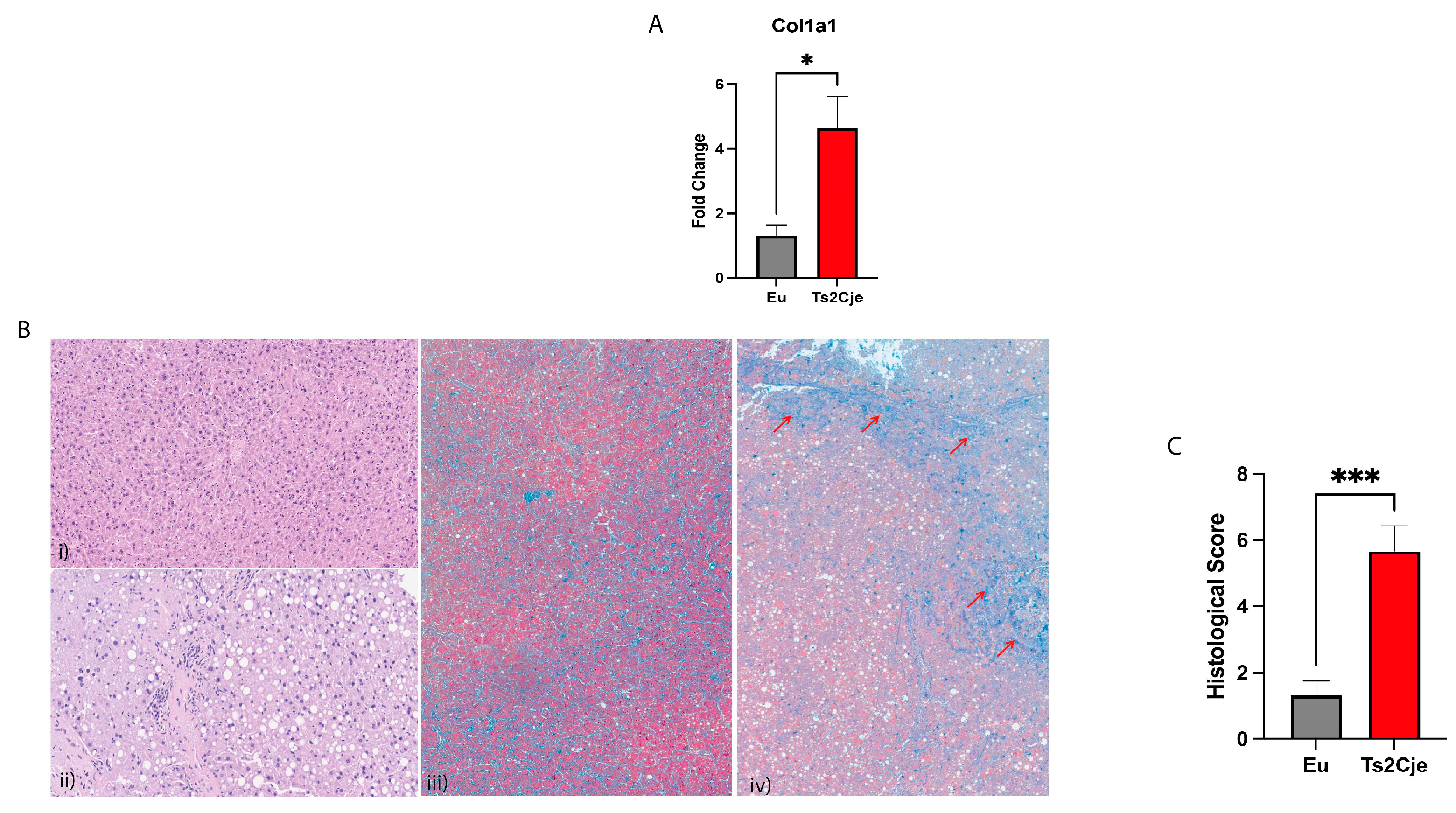
3.4. Ds livers display an increased fibrosis
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Antonarakis, S.E.; Skotko, B.G.; Rafii, M.S.; Strydom, A.; Pape, S.E.; Bianchi, D.W.; Sherman, S.L.; Reeves, R.H. Down syndrome. Nat Rev Dis Primers 2020, 6, 9. [Google Scholar] [CrossRef]
- Campos, C.; Casado, A. Oxidative stress, thyroid dysfunction & Down syndrome. Indian J Med Res 2015, 142, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Perluigi, M.; Butterfield, D.A. Oxidative Stress and Down Syndrome: A Route toward Alzheimer-Like Dementia. Curr Gerontol Geriatr Res 2012, 2012, 724904. [Google Scholar] [CrossRef]
- Giallongo, S.; Rehakova, D.; Raffaele, M.; Lo Re, O.; Koutna, I.; Vinciguerra, M. Redox and Epigenetics in Human Pluripotent Stem Cells Differentiation. Antioxid Redox Signal 2021, 34, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Perluigi, M.; Tramutola, A.; Pagnotta, S.; Barone, E.; Butterfield, D.A. The BACH1/Nrf2 Axis in Brain in Down Syndrome and Transition to Alzheimer Disease-Like Neuropathology and Dementia. Antioxidants (Basel) 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Glasson, E.J.; Sullivan, S.G.; Hussain, R.; Petterson, B.A.; Montgomery, P.D.; Bittles, A.H. The changing survival profile of people with Down’s syndrome: implications for genetic counselling. Clin Genet 2002, 62, 390–393. [Google Scholar] [CrossRef]
- Giallongo, S.; Longhitano, L.; Denaro, S.; D’Aprile, S.; Torrisi, F.; La Spina, E.; Giallongo, C.; Mannino, G.; Lo Furno, D.; Zappala, A.; et al. The Role of Epigenetics in Neuroinflammatory-Driven Diseases. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, K.; Constantine, A.; Clift, P.; Condliffe, R.; Moledina, S.; Jansen, K.; Inuzuka, R.; Veldtman, G.R.; Cua, C.L.; Tay, E.L.W.; et al. Cardiovascular Complications of Down Syndrome: Scoping Review and Expert Consensus. Circulation 2023, 147, 425–441. [Google Scholar] [CrossRef]
- Rabin, K.R.; Whitlock, J.A. Malignancy in children with trisomy 21. Oncologist 2009, 14, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Kieran, M.W.; Vekemans, M.; Robb, L.J.; Sinsky, A.; Outerbridge, E.W.; Der Kaloustian, V.M. Portohepatic shunt in a Down syndrome patient with an interchange trisomy 47,XY,-2,+der(2),+der(21)t(2;21)(p13;q22.1)mat. Am J Med Genet 1992, 44, 288–292. [Google Scholar] [CrossRef]
- Burdall, O.C.; Grammatikopoulos, T.; Sellars, M.; Hadzic, N.; Davenport, M. Congenital Vascular Malformations of the Liver: An Association With Trisomy 21. J Pediatr Gastroenterol Nutr 2016, 63, e141–e146. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.P.; Kaye, G.; Clarke, A.C. Autoimmune hepatobiliary disease in trisomy 21. J Clin Gastroenterol 2000, 30, 330–332. [Google Scholar] [CrossRef]
- Dierssen, M.; Fructuoso, M.; Martinez de Lagran, M.; Perluigi, M.; Barone, E. Down Syndrome Is a Metabolic Disease: Altered Insulin Signaling Mediates Peripheral and Brain Dysfunctions. Front Neurosci 2020, 14, 670. [Google Scholar] [CrossRef] [PubMed]
- Valentini, D.; Alisi, A.; di Camillo, C.; Sartorelli, M.R.; Crudele, A.; Bartuli, A.; Nobili, V.; Villani, A. Nonalcoholic Fatty Liver Disease in Italian Children with Down Syndrome: Prevalence and Correlation with Obesity-Related Features. J Pediatr 2017, 189, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Valentini, D.; Mosca, A.; Di Camillo, C.; Crudele, A.; Sartorelli, M.R.; Scoppola, V.; Tarani, L.; Villani, A.; Raponi, M.; Novelli, A.; et al. PNPLA3 gene polymorphism is associated with liver steatosis in children with Down syndrome. Nutr Metab Cardiovasc Dis 2020, 30, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Crudele, A.; Dato, S.; Re, O.L.; Maugeri, A.; Sanna, P.; Giallongo, S.; Oben, J.; Panera, N.; De Rango, F.; Mosca, A.; et al. Pediatric Non-Alcoholic Fatty Liver Disease Is Affected by Genetic Variants Involved in Lifespan/Healthspan. J Pediatr Gastroenterol Nutr 2021, 73, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, C.; Zhan, Y.T. Nonalcoholic Fatty Liver Disease Cirrhosis: A Review of Its Epidemiology, Risk Factors, Clinical Presentation, Diagnosis, Management, and Prognosis. Can J Gastroenterol Hepatol 2018, 2018, 2784537. [Google Scholar] [CrossRef] [PubMed]
- Reinholdt, L.G.; Ding, Y.; Gilbert, G.J.; Czechanski, A.; Solzak, J.P.; Roper, R.J.; Johnson, M.T.; Donahue, L.R.; Lutz, C.; Davisson, M.T. Molecular characterization of the translocation breakpoints in the Down syndrome mouse model Ts65Dn. Mamm Genome 2011, 22, 685–691. [Google Scholar] [CrossRef]
- Tramutola, A.; Lanzillotta, S.; Aceto, G.; Pagnotta, S.; Ruffolo, G.; Cifelli, P.; Marini, F.; Ripoli, C.; Palma, E.; Grassi, C.; et al. Intranasal Administration of KYCCSRK Peptide Rescues Brain Insulin Signaling Activation and Reduces Alzheimer’s Disease-like Neuropathology in a Mouse Model for Down Syndrome. Antioxidants (Basel) 2023, 12. [Google Scholar] [CrossRef]
- Nibali, L.; Rizzo, M.; Li Volti, G.; D’Aiuto, F.; Giglio, R.V.; Barbagallo, I.; Pelekos, G.; Donos, N. Lipid subclasses profiles and oxidative stress in aggressive periodontitis before and after treatment. J Periodontal Res 2015, 50, 890–896. [Google Scholar] [CrossRef]
- Longhitano, L.; Giallongo, S.; Orlando, L.; Broggi, G.; Longo, A.; Russo, A.; Caltabiano, R.; Giallongo, C.; Barbagallo, I.; Di Rosa, M.; et al. Lactate Rewrites the Metabolic Reprogramming of Uveal Melanoma Cells and Induces Quiescence Phenotype. Int J Mol Sci 2022, 24. [Google Scholar] [CrossRef] [PubMed]
- Longhitano, L.; Broggi, G.; Giallongo, S.; Failla, M.; Puzzo, L.; Avitabile, T.; Tibullo, D.; Distefano, A.; Pittala, V.; Reibaldi, M.; et al. Heme Oxygenase-1 Overexpression Promotes Uveal Melanoma Progression and Is Associated with Poor Clinical Outcomes. Antioxidants (Basel) 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Cambria, D.; Longhitano, L.; La Spina, E.; Giallongo, S.; Orlando, L.; Giuffrida, R.; Tibullo, D.; Fontana, P.; Barbagallo, I.; Nicoletti, V.G.; et al. IGFBP-6 Alters Mesenchymal Stromal Cell Phenotype Driving Dasatinib Resistance in Chronic Myeloid Leukemia. Life (Basel) 2023, 13. [Google Scholar] [CrossRef]
- Broggi, G.; Lo Giudice, A.; Di Mauro, M.; Asmundo, M.G.; Pricoco, E.; Piombino, E.; Caltabiano, R.; Morgia, G.; Russo, G.I. SRSF-1 and microvessel density immunohistochemical analysis by semi-automated tissue microarray in prostate cancer patients with diabetes (DIAMOND study). Prostate 2021, 81, 882–892. [Google Scholar] [CrossRef]
- Mangano, K.; Cavalli, E.; Mammana, S.; Basile, M.S.; Caltabiano, R.; Pesce, A.; Puleo, S.; Atanasov, A.G.; Magro, G.; Nicoletti, F.; et al. Involvement of the Nrf2/HO-1/CO axis and therapeutic intervention with the CO-releasing molecule CORM-A1, in a murine model of autoimmune hepatitis. J Cell Physiol 2018, 233, 4156–4165. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front Physiol 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Moseley, P.L. Heat shock proteins and the inflammatory response. Ann N Y Acad Sci 1998, 856, 206–213. [Google Scholar] [CrossRef]
- Missiroli, S.; Genovese, I.; Perrone, M.; Vezzani, B.; Vitto, V.A.M.; Giorgi, C. The Role of Mitochondria in Inflammation: From Cancer to Neurodegenerative Disorders. J Clin Med 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.E.; Hwang, Y.; Lee, S.J.; Jung, H.; Shin, T.H.; Son, Y.; Park, S.; Han, S.J.; Kim, H.J.; Lee, K.W.; et al. Mitochondrial protease ClpP supplementation ameliorates diet-induced NASH in mice. J Hepatol 2022, 77, 735–747. [Google Scholar] [CrossRef]
- Pei, K.; Gui, T.; Kan, D.; Feng, H.; Jin, Y.; Yang, Y.; Zhang, Q.; Du, Z.; Gai, Z.; Wu, J.; et al. An Overview of Lipid Metabolism and Nonalcoholic Fatty Liver Disease. Biomed Res Int 2020, 2020, 4020249. [Google Scholar] [CrossRef]
- Pagnotta, S.; Tramutola, A.; Barone, E.; Di Domenico, F.; Pittala, V.; Salerno, L.; Folgiero, V.; Caforio, M.; Locatelli, F.; Petrini, S.; et al. CAPE and its synthetic derivative VP961 restore BACH1/NRF2 axis in Down Syndrome. Free Radic Biol Med 2022, 183, 1–13. [Google Scholar] [CrossRef]
- Zuliani, I.; Lanzillotta, C.; Tramutola, A.; Francioso, A.; Pagnotta, S.; Barone, E.; Perluigi, M.; Di Domenico, F. The Dysregulation of OGT/OGA Cycle Mediates Tau and APP Neuropathology in Down Syndrome. Neurotherapeutics 2021, 18, 340–363. [Google Scholar] [CrossRef] [PubMed]
- Lanzillotta, C.; Zuliani, I.; Tramutola, A.; Barone, E.; Blarzino, C.; Folgiero, V.; Caforio, M.; Valentini, D.; Villani, A.; Locatelli, F.; et al. Chronic PERK induction promotes Alzheimer-like neuropathology in Down syndrome: Insights for therapeutic intervention. Prog Neurobiol 2021, 196, 101892. [Google Scholar] [CrossRef] [PubMed]
- Averill-Bates, D.A. The antioxidant glutathione. Vitam Horm 2023, 121, 109–141. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, F.; Pupo, G.; Mancuso, C.; Barone, E.; Paolini, F.; Arena, A.; Blarzino, C.; Schmitt, F.A.; Head, E.; Butterfield, D.A.; et al. Bach1 overexpression in Down syndrome correlates with the alteration of the HO-1/BVR-a system: insights for transition to Alzheimer’s disease. J Alzheimers Dis 2015, 44, 1107–1120. [Google Scholar] [CrossRef]
- Barbagallo, I.; Marrazzo, G.; Frigiola, A.; Zappala, A.; Li Volti, G. Role of carbon monoxide in vascular diseases. Curr Pharm Biotechnol 2012, 13, 787–796. [Google Scholar] [CrossRef]
- Barbagallo, I.; Galvano, F.; Frigiola, A.; Cappello, F.; Riccioni, G.; Murabito, P.; D’Orazio, N.; Torella, M.; Gazzolo, D.; Li Volti, G. Potential therapeutic effects of natural heme oxygenase-1 inducers in cardiovascular diseases. Antioxid Redox Signal 2013, 18, 507–521. [Google Scholar] [CrossRef]
- Barbagallo, I.; Nicolosi, A.; Calabrese, G.; David, S.; Cimino, S.; Madonia, M.; Cappello, F. The role of the heme oxygenase system in the metabolic syndrome. Curr Pharm Des 2014, 20, 4970–4974. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, J.; Li, D.; Li, P.; Zhou, X.; Li, Y.; He, Z.; Qin, L.; Liang, L.; Luo, X. Interleukin-10 inhibits interleukin-1beta production and inflammasome activation of microglia in epileptic seizures. J Neuroinflammation 2019, 16, 66. [Google Scholar] [CrossRef] [PubMed]
- Broers, C.J.; Gemke, R.J.; Morre, S.A.; Weijerman, M.E.; van Furth, A.M. Increased production of interleukin-10 in children with Down syndrome upon ex vivo stimulation with Streptococcus pneumoniae. Pediatr Res 2014, 75, 109–113. [Google Scholar] [CrossRef]
- Yoo, B.C.; Vlkolinsky, R.; Engidawork, E.; Cairns, N.; Fountoulakis, M.; Lubec, G. Differential expression of molecular chaperones in brain of patients with Down syndrome. Electrophoresis 2001, 22, 1233–1241. [Google Scholar] [CrossRef]
- Ambade, A.; Catalano, D.; Lim, A.; Kopoyan, A.; Shaffer, S.A.; Mandrekar, P. Inhibition of heat shock protein 90 alleviates steatosis and macrophage activation in murine alcoholic liver injury. J Hepatol 2014, 61, 903–911. [Google Scholar] [CrossRef]
- Bozner, P.; Wilson, G.L.; Druzhyna, N.M.; Bryant-Thomas, T.K.; LeDoux, S.P.; Wilson, G.L.; Pappolla, M.A. Deficiency of chaperonin 60 in Down’s syndrome. J Alzheimers Dis 2002, 4, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Horwich, A.L.; Hartl, F.U. Prevention of protein denaturation under heat stress by the chaperonin Hsp60. Science 1992, 258, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Dabravolski, S.A.; Bezsonov, E.E.; Orekhov, A.N. The role of mitochondria dysfunction and hepatic senescence in NAFLD development and progression. Biomed Pharmacother 2021, 142, 112041. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, S.; Pharaoh, G.; Ranjit, R.; Murphy, A.; Matsuzaki, S.; Nair, B.C.; Forbes, B.; Gispert, S.; Auburger, G.; Humphries, K.M.; et al. Loss of mitochondrial protease ClpP protects mice from diet-induced obesity and insulin resistance. EMBO Rep 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Gervois, P.; Torra, I.P.; Fruchart, J.C.; Staels, B. Regulation of lipid and lipoprotein metabolism by PPAR activators. Clin Chem Lab Med 2000, 38, 3–11. [Google Scholar] [CrossRef]
- Wang, M.; Yan, Y.; Zhang, Z.; Yao, X.; Duan, X.; Jiang, Z.; An, J.; Zheng, P.; Han, Y.; Wu, H.; et al. Programmed PPAR-alpha downregulation induces inflammaging by suppressing fatty acid catabolism in monocytes. iScience 2021, 24, 102766. [Google Scholar] [CrossRef] [PubMed]
- Leonardini, A.; Laviola, L.; Perrini, S.; Natalicchio, A.; Giorgino, F. Cross-Talk between PPARgamma and Insulin Signaling and Modulation of Insulin Sensitivity. PPAR Res 2009, 2009, 818945. [Google Scholar] [CrossRef]
- Doege, H.; Baillie, R.A.; Ortegon, A.M.; Tsang, B.; Wu, Q.; Punreddy, S.; Hirsch, D.; Watson, N.; Gimeno, R.E.; Stahl, A. Targeted deletion of FATP5 reveals multiple functions in liver metabolism: alterations in hepatic lipid homeostasis. Gastroenterology 2006, 130, 1245–1258. [Google Scholar] [CrossRef] [PubMed]
- Enooku, K.; Tsutsumi, T.; Kondo, M.; Fujiwara, N.; Sasako, T.; Shibahara, J.; Kado, A.; Okushin, K.; Fujinaga, H.; Nakagomi, R.; et al. Hepatic FATP5 expression is associated with histological progression and loss of hepatic fat in NAFLD patients. J Gastroenterol 2020, 55, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Parimbelli, M.; Pezzotti, E.; Negro, M.; Calanni, L.; Allemano, S.; Bernardi, M.; Berardinelli, A.; D’Antona, G. Nutrition and Exercise in a Case of Carnitine Palmitoyl-Transferase II Deficiency. Front Physiol 2021, 12, 637406. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Sotomatsu, M.; Ohki, K.; Arai, K.; Maruyama, K.; Kobayashi, T.; Nishi, A.; Sameshima, K.; Takagi, T.; Hayashi, Y. Liver disease is frequently observed in Down syndrome patients with transient abnormal myelopoiesis. Int J Hematol 2014, 99, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Ruchelli, E.D.; Uri, A.; Dimmick, J.E.; Bove, K.E.; Huff, D.S.; Duncan, L.M.; Jennings, J.B.; Witzleben, C.L. Severe perinatal liver disease and Down syndrome: an apparent relationship. Hum Pathol 1991, 22, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- De Matteo, A.; Vajro, P. Down Syndrome and Pediatric Nonalcoholic Fatty Liver Disease: A Causal or Casual Relationship? J Pediatr 2017, 189, 11–13. [Google Scholar] [CrossRef] [PubMed]
| Gene | Forward 5′ --> 3′ | Reverse 5′ --> 3′ | Accession Number |
|---|---|---|---|
| Hmox1 | TGACACCTGAGGTCAAGCAC | CAGCTCCTCAAACAGCTCAATG | NM_010442.2 |
| Il1β | TGCCACCTTTTGACAGTGATG | CGTCACACACCAGCAGGTTA | NM_008361.4 |
| Il10 | GTAGAAGTGATGCCCCAGGC | GACACCTTGGTCTTGGAGCTTATT | NM_010548.2 |
| Ppar𝛂 | TGCCTTCCCTGTGAACTGAC | CACAGAGCGCTAAGCTGTGA | NM_001113418.1 |
| Ppar𝛄 | GGTCAGTCATGGAACAGCCA | TTCTGGGAGAGGTCTGCAC | NM_001411509.1 |
| Fatp5 | TGTAACGTCCCTGAGCAACC | TAAGCCCACATTGCCCTCTG | NM_009512.2 |
| Cpt2 | GAATGACAGCCAGTTCAGGAAG | GCATGCAGCTCCTTCCCAAT | NM_009949.2 |
| Col1a1 | CCCTGGTCCCTCTGGAAATG | GGACCTTTGCCCCCTTCTTT | NM_007742.4 |
| Gapdh | AACCCTTAAGAGGGATGCTGC | TCTACGGGACGAGGAAACAC | NM_001289726.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





